Abstract
This retrospective clinical study determined the association of caries activity and orange/red fluorescence on QLF images of surfaces that progressed to cavitation as determined by clinical visual examination. A random sample of QLF images from 565 children (5-13years) previously enrolled in a longitudinal study was selected. Buccal, lingual and occlusal surfaces’ images obtained after professional brushing at baseline and every 4 months over a 4-year period were analyzed for Red Fluorescence (RF). Surfaces that progressed (N=224) to cavitation according to International Caries Detection and Assessment System (ICDAS 0/1/2/3/4 to 5/6/filling) and surfaces that did not progress (N=486) were included. QA2 image analysis software outputs the percentage increase of the red/green components as ΔR and Area of ΔR (Area△R) at different thresholds. Mixed-model ANOVA was used to compare progressive and non-progressive surfaces to account for correlations of RF (ΔR and AreaΔR) between surfaces within a subject. The first analysis used the first observation for each surface or the first available visit if the surface was unerupted (baseline), while the second analysis used the last observation prior to cavitation for surfaces that progressed and last observation for surfaces that did not progress (final). There was a significant (p<0.05) association between RF and progression to cavitation at thresholds ΔR0, ΔR10, ΔR20, ΔR60, ΔR70, ΔR80, ΔR90 and ΔRMax at baseline and for ΔR0 and ΔR10 at final observation. Quantification of orange/red fluorescence may help to identify lesions that progress to cavitation. Future studies identifying microbiological factors causing orange/red fluorescence and its caries activity are indicated.
Keywords: Biofilm, Caries, Clinical study, Digital image analysis and Red fluorescence
Introduction
The optical phenomenon of tooth autofluorescing on illumination with ultraviolet light was first observed by Hans Stubel [Buchalla, 2005; Stubel, 1911]. This property has been applied in caries detection devices as means of detection of incipient carious lesions [Alfano and Yao, 1981; Benedict, 1928; Bjelkhagen et al., 1982; de Josselin de Jong et al., 1995; Sundstrom et al., 1985; Zandona and Zero, 2006]. The concept held previously was that carious enamel lesions do not fluoresce [Benedict, 1928], and deep dentinal caries appeared dark brown, orange brown, and pinkish orange, when caries was debrided. It was shown later that carious lesions fluoresce in the red region of the spectrum [Buchalla, 2005; Konig et al., 1999; Slimani et al., 2014]. Several studies have demonstrated heterogeneous emission spectral bands in the carious region compared to the sound surface of the tooth [Buchalla, 2005; Buchalla et al., 2004a, b; Zezell et al., 2007]. The long standing question is whether the chromogenic auto fluorescence originates from the tooth structure, from bacteria or do both contribute to this autofluorescence. It has been shown that macromolecular porphyrin might be responsible for the autofluorescence [Buchalla et al., 2008].
Quantitative Light-Induced Fluorescence (QLF-clin, Inspektor Research Systems BV, Amsterdam, Netherlands) employs the optical property of fluorescence for caries detection. Inspektor™ QLF Pro employs an intra-oral camera that illuminates the tooth with light in the violet-blue wavelength (290-450nm) and captures fluorescence above 520 nm by a high pass filter. Under these conditions, the sound areas of the tooth fluoresce green, however an orange to red fluorescence can also be observed on these images. A new device, the QLF-D Biluminator ™ 2, has been introduced using a Single Lens Reflex (SLR) camera with violet LED lights with a peak wavelength of 405±20nm (violet). White LED's are used for standard white-light images, while the violet LED's provide excitation light for fluorescent imaging. The filter in this device allows the images of sound tooth surfaces to have a whitish appearance instead of green, while demineralized areas look darker and bacterially infected areas show a bright red fluorescence. The orange/red fluorescence has been assumed to originate from metabolic byproducts of oral bacteria within the dental biofilm called porphyrins [Konig et al., 1998; van der Veen et al., 2006; Volgenant et al., 2013]. In vitro oral biofilm studies have shown correlation between the red fluorescence captured through QLF-D Biluminator™ with caries risk severity [Kim et al., 2014; Lee et al., 2013]. A proprietary software (Inspektor Pro System) allows analyses of the images reporting three parameters: average loss of fluorescence denoting lesion depth (ΔF [%]); lesion area (A in px2) and lesion volume (ΔQ [%px2]). In the QLF-D Biluminator™ the ratio of red to green fluorescence in percentage (ΔR [%]) and the area of red fluorescence (AΔR) are reported. Red or orange fluorescence (△R) is represented as the percentage ratio increase of the red to green components in comparison to sound surface. RF Area (A△R) in px2 is equal or higher than a specific threshold of △R (Table 1)[Waller E et al., 2012]. Red or orange fluorescence is believed to emanate from the excitation of fluorophores from bacterial byproducts on illumination through blue–violet light [van der Veen MH, 2003]. A preliminary in vitro study [Alammari MR, 2010] showed the relationship between the △R and △F to ICDAS and histology scores, requiring clinical validation.
Table: 1.
Description of QLF-parameter terminologies
| Terminology | Definition | |
|---|---|---|
| 1 | Delta R (ΔR ) | Ratio of red over green in the area of interest compared to a sound area of the tooth. Expressed in percentage (%). ΔR is based on thresholds at ΔR0, ΔR10, ΔR20, ΔR30, ΔR40, ΔR50, ΔR60, ΔR70, ΔR80, ΔR90, ΔR100, ΔR110, ΔR120 and Total. |
| 2 | Area of red fluorescence (AΔR) | Area of ΔR equivalent or higher to a specific threshold at ΔR30, ΔR70, ΔR120 |
| 3 | Plaque wizard patch | A rectangular contour which could be adjusted to any size, and shape. Here it was placed around the lesion area, in the sound area of the tooth. |
| 4 | Delta F(ΔF) | Average loss of green fluorescence in the carious surface compared to the green fluorescence in the sound tooth area. Expressed as percentage (%) and resembling, lesion depth. |
| 5 | Area of lesion (AΔF) | Area of the carious lesion surface expressed as px2 equivalent to a threshold specific at ΔF. |
| 6 | Delta Q(ΔQ ) | Percentage of green fluorescence loss (ΔF) times the area of the lesion (A). It is expressed as %px2 resembling, lesion volume. |
The relationship between the QLF parameters (△F, △A and △Q), the visual appearance of the lesions and its clinical behavior longitudinally has been demonstrated previously by our group [Ferreira Zandona et al., 2010; Ferreira Zandoná A, 2003]. During capture and analyses of these clinical images the red fluorescence observed by others [Bittar et al., 2014; Coulthwaite et al., 2006] was also seen on the images captured in these longitudinal studies. Thus, the objective of this study was to determine if there is an association between the intrinsic orange/red fluorescence seen in QLF images of surfaces that progressed to cavitation over a period of time as compared to surfaces with non-cavitated lesions that did not progress to cavitation as determined by clinical visual examination using the International Caries Detection and Assessment System criteria (ICDAS).
Study Participants and Methods
This is a retrospective study based on the images captured during a longitudinal study previously published [Ferreira Zandona et al., 2013]. Detailed information of participant selection, sample size calculation, examinations conducted and the analyses of fluorescence in QLF images were previously published [Ferreira Zandona et al., 2013; Ferreira Zandona et al., 2010; Ferreira Zandona et al., 2012; Fontana et al., 2011]. In summary, in January 2007 children (N = 565) aged 5 to 13 years from kindergarten to 9th grade enrolled in public schools from the area of Aguas Buenas, Puerto Rico were recruited into the study. Consent and assent if the child was over 7 years old was obtained. The study was approved by the Institutional review Board (IRB) of Indiana University (IU-IRB #0608-15) and the University of Puerto Rico (UPR-IRB#A1340107). The inclusion criteria included having at least one permanent molar with at least one unrestored surface, no medical problems, and have tolerance for the examinations performed.
Comprehensive Oral Examinations
The children underwent examinations at baseline, 8, 12, 18, 24, 28, 32, 36, 40, 44, and 48 months. It is important to note that the exams were conducted after a professional tooth brushing, thus most, if not all biofilm was removed from the surfaces of interest. A visual examination based on ICDAS which ranges from 0 to 6 [Pitts, 2004] was performed at each time point as well as an examination of the teeth with QLF. Fluorescent images of occlusal and buccal surfaces of all permanent molars and lingual surfaces of upper molars were obtained at each exam. At the end of the four-year study fluorescent images of surfaces that progressed to cavitation (ICDAS 0/1/2/3/4 to 5/6 or filling) and a random sample of images of surfaces that did not progress to cavitation were analyzed longitudinally using proprietary software (QLF 2.00g).
Orange/Red Fluorescence Analyses
The same images previously analyzed were analyzed for Orange/Red Fluorescence by RF Analysis (QA2 software, Inspector Research Systems, The Netherlands). Surfaces (N=224) that progressed to cavitation according to ICDAS (ICDAS 0/1/2/3/4 to 5/6 or filling) and surfaces that did not progress (N=486) were included. [Ferreira Zandona et al., 2013]. The Plaque Wizard patch of QA2 of QLF D Biluminator was used to analyze the images obtained from Inspektor ™ QLF Pro. For RF analysis the plaque wizard was applied on the same area of the carious lesion surface which was analyzed previously to monitor the progression of caries. It was applied on the last visit for sites that did not progress, or if there was a filling or a cavitation with ICDAS 5 or 6, it was applied on the visit prior to the filling/cavitation. A similar plaque wizard was applied to all other visits including the first observation, which was at baseline, or the first visit after eruption if the tooth was unerupted at baseline. QA2 outputs the percentage increase of red to green components as △R and Area△R at different thresholds. For intra-examiner reliability 60 surfaces were chosen randomly including surfaces that progressed to cavitation and those that did not progress for repeat analyses by a single, experienced examiner.
Statistical Analyses
Statistical analysis was performed with SAS 9.3 (SAS Institute Inc., Cary, NC). Mixed-model ANOVA was used to compare surfaces that progressed to cavitation and those that did not progress to account for correlations of RF (△R and Area △R) between surfaces within a subject. The ranks of the data were used in the analysis because of non-normal distributions. Two sets of analyses were performed. The first analysis used the first observation for each surface, which was at baseline or the first available visit if the surface was unerupted at baseline. The second analysis used the last observation before progression for surfaces that did progress to cavitation and used the last observation for sites that did not progress. The area under the receiver operating curve (ROC) was calculated to determine the sensitivity and specificity of the threshold for the RF areas. Intraclass Correlation Coefficients (ICCs) were calculated to determine the level of intra-examiner repeatability.
Results
A statistically significant association (p < 0.05) was seen between RF and surfaces that progressed to cavitation. Analysis of the initial or first observation of the tooth surfaces showed that sites that progressed to cavitation had a significantly higher percentage (%) of red fluorescence for specific thresholds of ΔR0, ΔR10, ΔR20, ΔR60, ΔR70, ΔR100, ΔRMax, and Total than sites that did not progress. RF Area (A△R), which is the Area at △70 in px2, correlated with the percentage of RF observed in the first observation (p=0.0191). Simple plaque score (SPS ™) was borderline significant (p=0.0522) at the initial observation (Table 2).
Table: 2.
RF analysis of baseline and final observation of Progressive and Non–Progressive Surfaces
| First or baseline observation | Final observation or last before progression | |||||
|---|---|---|---|---|---|---|
| Progressed to cavitation (N = 224) | Did not progress (N = 486) | Progressed to cavitation (N = 224) | Did not Progress (N = 486) | |||
| RF Parameter | Mean (SE) | Mean (SE) | p-value | Mean (SE) | Mean (SE) | p-value |
| SPS™ | 0.045 (0.021) | 0.019 (0.011) | 0.0522 | 0.067 (0.031) | 0.029 (0.009) | 0.8458 |
| Area ΔR30 | 0.069 (0.035) | 0.039 (0.019) | 0.3948 | 0.142 (0.086) | 0.044 (0.012) | 0.4164 |
| Area ΔR70 | 0.016 (0.010) | 0.009 (0.006) | 0.0191* | 0.022 (0.017) | 0.006 (0.002) | 0.4774 |
| Area ΔR120 | 0.000 (0.000) | 0.000 (0.000) | 0.003 (0.003) | 0.000 (0.000) | 0.1905 | |
| ΔR0 | 1341 (87) | 752 (48) | <.0001* | 1449 (96) | 867 (57) | <.0001* |
| ΔR10 | 43.00 (5.06) | 26.87 (3.14) | 0.0009* | 51.17 (6.27) | 32.00 (3.49) | <.0001* |
| ΔR20 | 5.96 (1.45) | 2.56 (0.48) | 0.0029* | 9.55 (2.70) | 3.72 (0.70) | 0.3863 |
| ΔR30 | 2.37 (0.83) | 0.84 (0.28) | 0.3770 | 4.43 (1.96) | 1.46 (0.44) | 0.3603 |
| ΔR40 | 1.38 (0.58) | 0.49 (0.25) | 0.2837 | 2.76 (1.63) | 1.01 (0.37) | 0.2869 |
| ΔR50 | 0.95 (0.44) | 0.34 (0.20) | 0.0538 | 2.04 (1.35) | 0.62 (0.27) | 0.4398 |
| ΔR60 | 0.64 (0.29) | 0.23 (0.13) | 0.0167* | 1.27 (0.83) | 0.37 (0.18) | 0.2233 |
| ΔR70 | 0.33 (0.16) | 0.12 (0.07) | 0.0191* | 0.71 (0.43) | 0.21 (0.12) | 0.4749 |
| ΔR80 | 0.17 (0.09) | 0.04 (0.03) | 0.0511 | 0.42 (0.25) | 0.13 (0.10) | 0.7095 |
| ΔR90 | 0.07 (0.05) | 0.02 (0.01) | 0.1440 | 0.33 (0.23) | 0.09 (0.08) | 0.3285 |
| ΔR100 | 0.02 (0.01) | 0.00 (0.00) | 0.0373* | 0.28 (0.20) | 0.07 (0.07) | 0.4253 |
| ΔR110 | 0.01 (0.01) | 0.00 (0.00) | 0.1416 | 0.22 (0.17) | 0.06 (0.06) | 0.1905 |
| ΔR120 | 0.00 (0.00) | 0.00 (0.00) | 0.18 (0.15) | 0.04 (0.04) | 0.1905 | |
| ΔRMax | 44.08 (1.43) | 35.85 (0.75) | <.0001* | 47.71 (2.14) | 40.51 (1.05) | 0.0002* |
| Total | 4955 (285) | 3038 (166) | <.0001* | 5113 (287) | 3204 (169) | <.0001* |
Statistically significant at the level of p<0.05; SPS – Simple Plaque Score; RF – Red Fluorescence; SE– Standard Error
At the first observation the mean value (M) and standard error (SE) of RF values were 4955 (285) for surfaces that progressed to cavitation while the mean value and standard error was 3038 (166) for surfaces that did not progress. As shown on Table 2 the sites that progressed to cavitation had significantly higher ΔR0, ΔR10, △R20, △R60, △R70, △R100, Δ RMax, and Total at the first observation than sites that did not progress. Even at the thresholds where there were no significant differences between the sites, there was a tendency for the sites that did not progress to have lower values than those that did progress to cavitation. The area at threshold △R70 (Area △R70) was significant for lesions that progressed to cavitation compared to area at △R30 and area at △R120. Analysis of the final observation showed that sites that progressed to cavitation had significantly higher ΔR0, ΔR10, ΔRMax, and Total than sites that did not progress (p<0.0001). SPS™ scores were not significant at the last visit. The mean RF values at various thresholds during the final observation are included in Table 2. Area of thresholds at △R30, △R70 and △R120 were not significantly different.
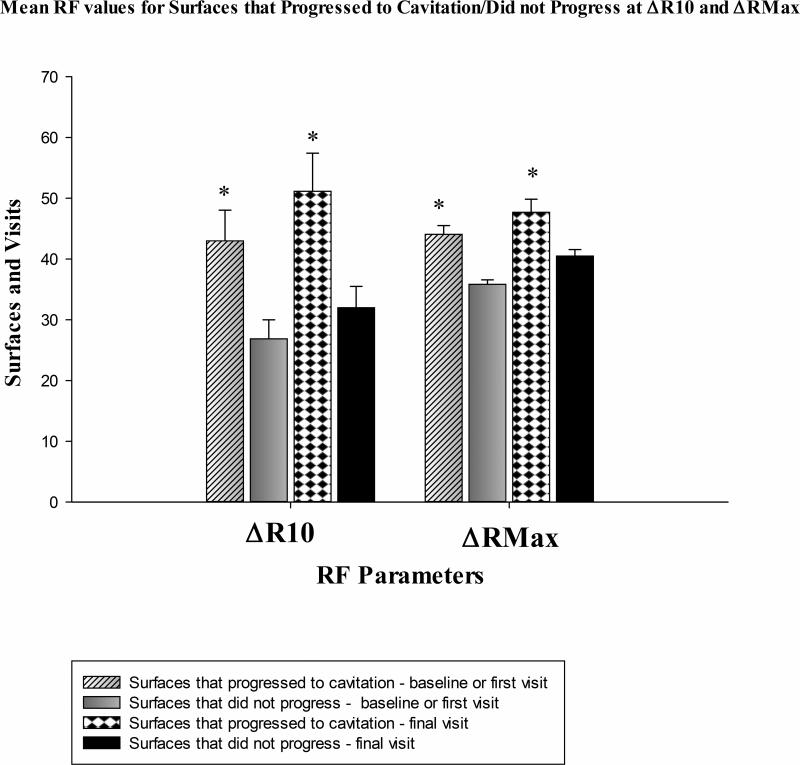
Comparisons of RF values at △RMax and △R10 during the first and final observations area are illustrated in Figure 1. For both first and final observations there was a significant difference for △RMax and △R10 for surfaces that progressed to cavitation compared to non-progressive surfaces. Carious surfaces that progressed to cavitation showed greater RF values during final observation at △R10. Surfaces that did not progress showed higher RF values at first observation at △RMax. Therefore the threshold kept at maximum level of cutoff was greater compared to RF value at △R10. RF is detected during first observation with the maximum level of cutoff. Also during final observation of non-progressive surfaces △RMax showed higher RF values.
Figure 1.
Comparison of Mean RF values at first and final observation for surfaces that progressed to cavitation and those that did not progress at △RMax and △R10.
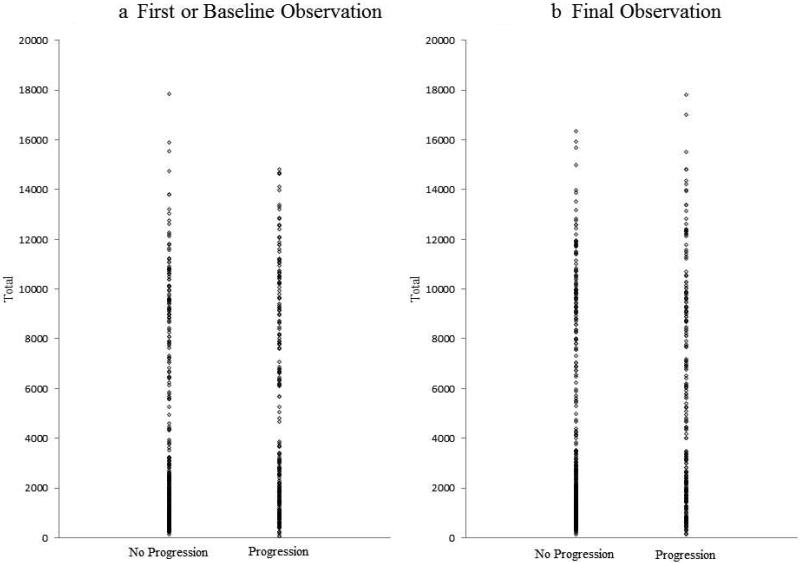
Dot plots are shown comparing surfaces that progressed to cavitation and non-progressive surfaces based on total values of RF during the baseline or the first observation (Figure 2a) and during the final observation (Figure 2b). The surfaces that progressed to cavitation and non-progressive surfaces were significantly different in both cases (p<0.0001).
Figure 2.
Dot - plot comparison of surfaces that progressed to cavitation and those that did not progress with total values of RF during the first observation and during the final observation.
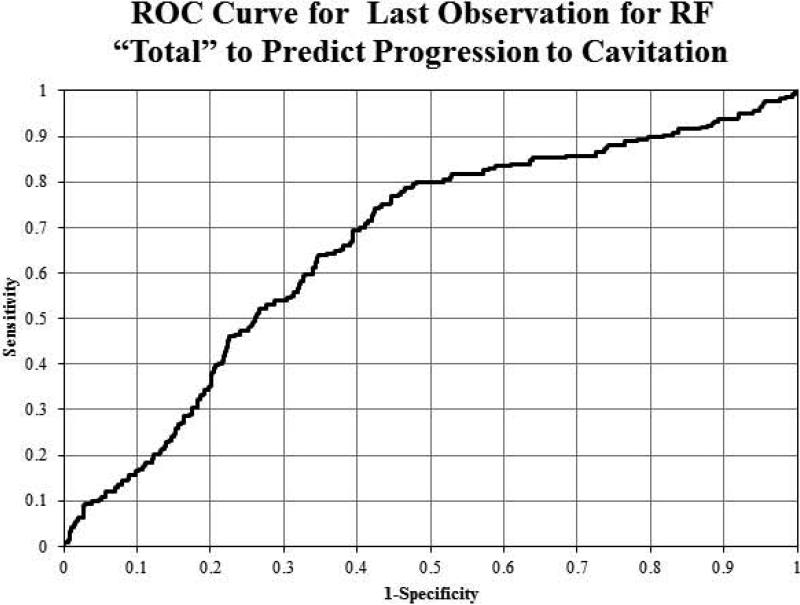
The area under the ROC curve was calculated based on the continuous variables in the RF analysis data. Sensitivity and specificity were calculated based on the ROC curve. ROC curve was plotted and was evaluated to determine the appropriate tradeoff between sensitivity and specificity. Figure 3 illustrates the ROC curve for the final observation (not progressed) or last observation before progression to cavitation for ‘Total’ to predict progression. We can see from the curve that the best combination was a sensitivity of 65% with a specificity of 63%.
Figure 3.
Receiver Operating Curve (ROC) predicting progression to cavitation from final Observation for RF Total
The intra class correlation coefficients (ICCs) indicated good repeatability for all RF measurements: 0.99 for △RMax, 0.97 for RF total, 0.97 for △R0, 0.87 for △R10, 0.88 for △R20, 0.89 for △R30, 0.94 for △R40 and △R50, 0.92 for △R60, 0.93 for △R70, 0.96 for △R80, and 0.78 for △R90.
Discussion
Predicting caries activity is considered the “holy grail” of cariology [Ismail, 2005]. To date only subjective methods are available to determine if a lesion will progress to cavitation or will arrest. It is known that not all early lesions progress to cavitation [Dirks, 1966]. Our study in this population also demonstrated that lesions with rapid changes in QLF parameters like area, depth and volume of the lesion progressed to cavitation [Ferreira Zandona et al., 2013]. Identifying lesions that are likely to progress to cavitation can have a great impact on how dentistry is practiced and how the caries paradigm shift towards the non-surgical management of dental caries can be implemented.
Most studies have focused on the red fluorescence of oral biofilm. The orange to red intrinsic fluorescence has been shown to be emanating from the oral biofilm (dental plaque) comprising of all the oral bacterial species. It does not come from single bacterial species [van der Veen et al., 2006]. Red excited fluorescence signal is likely derived from metabolic byproducts of oral bacteria in the dental biofilm [Koenig and Schneckenburger, 1994]. Red fluorescence emission seen on QLF images is proposed to be the result of excitation of endogenous porphyrins by the violet – blue light at a range of wavelength from 380 to 500nm. Fluorescing porphyrins in caries detected to some extent are protoporphyrin IX, coproporphyrin and uroporphyrin [Buchalla et al., 2008]. Red fluorescence from bacteria was shown to be an indicator of dentinal carious lesions [Lennon et al., 2006]. Higher levels of red to orange fluorescence can be an indicator for progression of lesions as demonstrated in our study. Early detection of orange to red fluorescence may serve as a caries indicator to predict caries activity.
Our study focused on the teeth surfaces that were cleaned of visible biofilm before imaging. It showed that at specific thresholds or cutoff levels the orange /red fluorescence from lesions that progressed to cavitation compared to non-progressive carious lesions was significantly higher. At low thresholds of △R0, △R10 and △R20 at baseline or first available visit the orange/red fluorescence was significantly higher for the lesions that progressed to cavitation compared to lesions that did not progress. This indicates that the values of orange/red fluorescence have a potential to identify lesions that are likely to be active, that is, progress towards cavitation. This may be the first non-subjective means to determine caries activity at a single time point. This can have a significant impact on dental care ranging from caries risk to specific caries intervention.
Yet, there are several questions that remain to be answered. In our study at every visit prior to capturing QLF images the teeth were brushed and flossed by study personnel, however orange to red fluorescence was not only observable in the lesions, but also an apparent indicator of caries activity. What is the origin of this red fluorescence – are these metabolic products within the body of the lesion [Buchalla et al., 2008]? Could we just use the red fluorescence from the biofilm for caries prediction as some studies seem to indicate [Buchalla et al., 2010]?
Within limitations of the study showing moderate sensitivity, quantification of orange to red fluorescence was able to distinguish carious lesions that progressed to cavitation from lesions that did not progress at a single observation. Though there are several factors involved in the increased contribution of orange to red fluorescence, future studies identifying the microbiological factors causing the orange/red fluorescence and its implication in caries activity are indicated. Additionally, studies are needed to understand the role of porphyrins in active and arrested carious lesions.
Acknowledgements
The study was partially supported by NIH/NIDCR RO1DE017890. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. We thank the faculty and staff at Indiana University and University of Puerto Rico, who were part of the parent study, in particularly the examiners, Drs. Haftstein Eggertsson and Enrique Santiago. We would like to specially thank for the support given by Dr. Richard L. Gregory; Dr. Domenick Zero; Dr. Masatoshi Ando; Ms. Sue Kelly; Ms. Melissa Mau; Ms. Sharon Gwinn; Ms. Jennifer Tran, Dr. Mahmoud Jallad, and the supporting staff at Oral Health Research Institute, Indiana University School of Dentistry. We thank Dr. Elbert de Josselin Jong for the help related to the QLF software.
Footnotes
Declaration of Interests:
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
Role of authors
Grace F. Gomez, PhD student in Dental Sciences, Indiana University School of Dentistry, did image analysis and wrote the paper, also contributed to the conception. Mr. George J. Eckert, Biostatistician supervisor, Indiana University School of Medicine did statistical analysis, table and figures, critically revised the manuscript for accuracy and approved it upon changes. Dr. Andrea Ferreira Zandona was the mentor for this project and PI of the parent study. She supervised the collection of images and clinical assessment of the surfaces in the parent study. She facilitated the study design, input of extensive ideas from conceptualization, data interpretation, critically analyzed the paper and provided final approval upon changes.
References
- Alammari MR, Smith PW, de Josselin de Jong E, Higham SM. Development of caries indices using quantitative light-induced fluorescence (QLF) in vitro.. ICQ (3rd International Conference on Quantitative light induced flourescence, QLF); Liverpool, UK. 2010. [Google Scholar]
- Alfano RR, Yao SS. Human teeth with and without dental caries studied by visible luminescent spectroscopy. J Dent Res. 1981;60:120–122. doi: 10.1177/00220345810600020401. [DOI] [PubMed] [Google Scholar]
- Benedict HC. A note on the fluorescence of teeth in ultra-violet rays. Science. 1928;67:442. doi: 10.1126/science.67.1739.442. [DOI] [PubMed] [Google Scholar]
- Bittar DG, Pontes LRA, Calvo AFB, Novaes TF, Braga MM, Freitas PM, Tabchoury CPM, Mendes FM. Is the red fluorescence of dental plaque related to its cariogenicity? J Biomed Opt. 2014;19:065004–065004. doi: 10.1117/1.JBO.19.6.065004. [DOI] [PubMed] [Google Scholar]
- Bjelkhagen H, Sundstrom F, Angmar-Mansson B, Ryden H. Early detection of enamel caries by the luminescence excited by visible laser light. Swed Dent J. 1982;6:1–7. [PubMed] [Google Scholar]
- Buchalla W. Comparative fluorescence spectroscopy shows differences in noncavitated enamel lesions. Caries Res. 2005;39:150–156. doi: 10.1159/000083162. [DOI] [PubMed] [Google Scholar]
- Buchalla W Attin T, Niedmann Y, Niedmann PD, Lennon AM. Porphyrins are the cause of red fluorescence of carious dentine: Verified by gradient reversed-phase HPLC. Caries Res. 2008;42:223. [Google Scholar]
- Buchalla W, Lennon AM, Attin T. Fluorescence spectroscopy of dental calculus. J Periodontal Res. 2004a;39:327–332. doi: 10.1111/j.1600-0765.2004.00747.x. [DOI] [PubMed] [Google Scholar]
- Buchalla W, Lennon AM, Attin T. Comparative fluorescence spectroscopy of root caries lesions. Eur J Oral Sci. 2004b;112:490–496. doi: 10.1111/j.1600-0722.2004.00173.x. [DOI] [PubMed] [Google Scholar]
- Buchalla W, Lennon A, Techert S, Krause J, Becker K, Attin T. Dental biofilm fluorescence may indicate caries risk. Caries Res. 2010;44:230. [Google Scholar]
- Coulthwaite L, Pretty IA, Smith PW, Higham SM, Verran J. The microbiological origin of fluorescence observed in plaque on dentures during qlf analysis. Caries Res. 2006;40:112–116. doi: 10.1159/000091056. [DOI] [PubMed] [Google Scholar]
- de Josselin de Jong E, Sundstrom F, Westerling H, Tranaeus S, ten Bosch JJ, Angmar-Mansson B. A new method for in vivo quantification of changes in initial enamel caries with laser fluorescence. Caries Res. 1995;29:2–7. doi: 10.1159/000262032. [DOI] [PubMed] [Google Scholar]
- Dirks OB. Posteruptive changes in dental enamel. J Dent Res. 1966;45:503–511. [Google Scholar]
- Waller E, Van. Daelen CJ, van der Veen MH. Application of QLF™ for Diagnosis and Quality Assessment in Clinical Practice. [August 31, 2015];Inspektor Research Systems. 2012 from http://www.inspektor.nl/download/WhitepaperQLF11.pdf.
- Ferreira Zandona A, Ando M, Gomez GF, Garcia-Corretjer M, Eckert GJ, Santiago E, Katz BP, Zero DT. Longitudinal analyses of early lesions by fluorescence: An observational study. J Dent Res. 2013;92:84s–89s. doi: 10.1177/0022034513490167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira Zandona A, Santiago E, Eckert G, Fontana M, Ando M, Zero DT. Use of ICDAS combined with quantitative light-induced fluorescence as a caries detection method. Caries Res. 2010;44:317–322. doi: 10.1159/000317294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira Zandona A, Santiago E, Eckert GJ, Katz BP, Pereira de Oliveira S, Capin OR, Mau M, Zero DT. The natural history of dental caries lesions: A 4-year observational study. J Dent Res. 2012;91:841–846. doi: 10.1177/0022034512455030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira Zandoná A, Eggertson H, Wefel J, Barry K, Ofner S, Eckert G. Clinical validation study of qlf at indiana.. In: Stookey GK, editor. Early Detection of Dental Caries III: Proceedings of the 6th Indiana Conference. Indiana University School of Dentistry; Indianapolis, IN. 2003.pp. 363–373. [Google Scholar]
- Fontana M, Santiago E, Eckert GJ, Ferreira-Zandona AG. Risk factors of caries progression in a hispanic school-aged population. J Dent Res. 2011;90:1189–1196. doi: 10.1177/0022034511413927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail AI, Banting D, Eggertsson H, Ekstrand K, Ferreira-Zandona A, Longbottom C, Pitts NB, Reich E, Ricketts D, Selwitz R, Topping Sohn S, Doughlas GVA, Zero D. Rationale and evidence for the international caries detection and assessment system (ICDAS II). In: GK S, editor. Proceedings of the 7th Indiana Conference; Indianapolis. 2005; Indiana University; pp. 161–221. [Google Scholar]
- Kim YS, Lee ES, Kwon HK, Kim BI. Monitoring the maturation process of a dental microcosm biofilm using the quantitative light-induced fluorescence-digital (QLF-D). J Dent. 2014;42:691–696. doi: 10.1016/j.jdent.2014.03.006. [DOI] [PubMed] [Google Scholar]
- Koenig K, Schneckenburger H. Laser-induced autofluorescence for medical diagnosis. J Fluoresc. 1994;4:17–40. doi: 10.1007/BF01876650. [DOI] [PubMed] [Google Scholar]
- Konig K, Flemming G, Hibst R. Laser-induced autofluorescence spectroscopy of dental caries. Cell Mol Biol (Noisy-le-Grand, France) 1998;44:1293–1300. [PubMed] [Google Scholar]
- Konig K, Schneckenburger H, Hibst R. Time-gated in vivo autofluorescence imaging of dental caries. Cell Mol Biol (Noisy-le-Grand, France) 1999;45:233–239. [PubMed] [Google Scholar]
- Lee ES, Kang SM, Ko HY, Kwon HK, Kim BI. Association between the cariogenicity of a dental microcosm biofilm and its red fluorescence detected by quantitative light-induced fluorescence-digital (qlf-d). Journal of dentistry. 2013;41:1264–1270. doi: 10.1016/j.jdent.2013.08.021. [DOI] [PubMed] [Google Scholar]
- Lennon AM, Buchalla W, Brune L, Zimmermann O, Gross U, Attin T. The ability of selected oral microorganisms to emit red fluorescence. Caries Res. 2006;40:2–5. doi: 10.1159/000088898. [DOI] [PubMed] [Google Scholar]
- Pitts N. “ICDAS”- an international system for caries detection andassessment being developed to facilitate caries epidemiology, research and appropriate clinical management. Community Dent Health. 2004;21:193–198. [PubMed] [Google Scholar]
- Slimani A, Panayotov I, Levallois B, Cloitre T, Gergely C, Bec N, Larroque C, Tassery H, Cuisinier F. Porphyrin involvement in redshift fluorescence in dentin decay. Proc. SPIE 9129, Biophotonics: Photonic Solutions for Better Health Care IV. 2014;9129:91291C. [Google Scholar]
- Stubel H. Die fluoreszenz tierischer gewebe in ultraviolettem licht. Pflugers Arch Physio. 1911;142:1–14. [Google Scholar]
- Sundstrom F, Fredriksson K, Montan S, Hafstrom-Bjorkman U, Strom J. Laser-induced fluorescence from sound and carious tooth substance: Spectroscopic studies. Swed Dent J. 1985;9:71–80. [PubMed] [Google Scholar]
- van der Veen MH, Thomas RZ, Huysmans MC, de Soet JJ. Red autofluorescence of dental plaque bacteria. Caries Res. 2006;40:542–545. doi: 10.1159/000095655. [DOI] [PubMed] [Google Scholar]
- van der Veen MH, Buchalla W, de Josselin de, Jong E. QLF™ technologies : Recent advances. In: Stookey GK, editor. Proceedings of the 6th Indiana Conference; Indianapolis. 2003; Indiana University School of Dentistry; pp. 291–304. [Google Scholar]
- Volgenant CM, van der Veen MH, de Soet JJ, ten Cate JM. Effect of metalloporphyrins on red autofluorescence from oral bacteria. Eur J Oral Sci. 2013;121:156–161. doi: 10.1111/eos.12045. [DOI] [PubMed] [Google Scholar]
- Zandona AF, Zero DT. Diagnostic tools for early caries detection. J Am Dent Assoc. 2006;137:1675–1684. doi: 10.14219/jada.archive.2006.0113. quiz 1730. [DOI] [PubMed] [Google Scholar]
- Zezell DM, Ribeiro AC, Bachmann L, Gomes AS, Rousseau C, Girkin J. Characterization of natural carious lesions by fluorescence spectroscopy at 405-nm excitation wavelength. doi: 10.1117/1.2821192. [DOI] [PubMed] [Google Scholar]