Abstract
Objective
Cochlear-implant manufacturers utilize different artifact-reduction methods to measure electrically evoked compound action potentials (ECAPs) in the clinical software. Two commercially available artifact-reduction techniques include forward masking (FwdMsk) and alternating polarity (AltPol). AltPol assumes that responses to the opposing polarities are equal, which is likely problematic. On the other hand, FwdMsk can yield inaccurate waveforms if the masker does not effectively render all neurons into a refractory state. The goal of this study was to compare ECAP thresholds, amplitudes, and slopes of the amplitude growth functions (AGFs) using FwdMsk and AltPol to determine whether the two methods yield similar results.
Design
ECAP AGFs were obtained from three electrode regions (basal, middle, and apical) across 24 ears in 20 Cochlear Ltd. recipients using both FwdMsk and AltPol methods. AltPol waveforms could not be resolved for recipients of devices with the older-generation chip (CI24R(CS); N=6).
Results
Results comparing FwdMsk and AltPol in the CI24RE- and CI512-generation devices showed significant differences in threshold, AGF slope, and amplitude between methods. FwdMsk resulted in lower visual-detection thresholds (p < 0.001), shallower slopes (p = 0.004), and larger amplitudes (p = 0.03) compared with AltPol.
Conclusions
Results from this study are consistent with recent findings showing differences in ECAP amplitude and latency between polarities for human CI recipients. When averaged, these differences likely result in a reduced ECAP response with AltPol. The next step will be to separate the effects of artifact-reduction method and stimulus polarity to determine the relative effects of each.
Keywords: cochlear implant, ECAP, electrically evoked compound action potential, forward masking, alternating polarity, artifact reduction
INTRODUCTION
All three of the major cochlear implant (CI) manufacturers offer devices with telemetry capabilities for measuring the electrically evoked compound action potential (ECAP) within the clinical programming software. The ECAP is characterized as an N1–P2 waveform, and because it is an early-latency response, it is generally obscured by stimulus artifact. Artifact reduction is therefore applied to resolve the ECAP waveform. Across CI manufacturers, there are two commonly used artifact-reduction methods: alternating polarity (AltPol) and a forward-masking subtraction paradigm (FwdMsk). AltPol is the only method currently available in Advanced Bionics’ (AB) SoundWave and MED-EL’s Maestro software. Both methods are available in Cochlear’s Custom Sound software (default is FwdMsk). Each artifact-reduction method has advantages and disadvantages; therefore, it is of interest to compare ECAP responses obtained with both methods in the same device to evaluate how each method affects the ECAP response. This was the goal of the present study.
Stimulus artifact results from a voltage decay following the biphasic current pulse. Although the artifact decays exponentially over several hundred microseconds, it overlaps with the neural response and is typically several orders of magnitude larger than the ECAP. The use of higher current levels will result in larger ECAPs; however, stimulus artifact also increases with level and may result in distortion or clipping of the ECAP negative peak (N1). The FwdMsk method exploits refractory properties of the cochlear nerve to isolate the ECAP from stimulus artifact. This method is illustrated in Fig. 1 (left panel). Four measurements are obtained using the following stimuli: (A) probe alone; (B) masker and probe, separated by a short inter-stimulus interval to induce a refractory state; (C) masker alone; and (D) switching of the recording amplifier. The subtracted trace, (A–B+C–D), yields the ECAP response to the probe (e.g., Brown et al. 1990; Brown and Abbas 1990; Brown et al. 1996; Abbas et al. 1999).
Fig. 1.
Schematic illustrating the two primary methods of artifact reduction used in today’s commercial software. Left: Forward-masking subtraction paradigm. Four frames are presented (A—D) to obtain various templates of artifact and neural response. The formula A–B+C–D is applied to resolve the neural response (see text). MPI: masker-probe interval. Right: Alternating polarity paradigm. Responses and artifacts are obtained for opposing polarities and then averaged together (C+A)/2 (see text). Subtraction of the switching artifact is not applied with alternating polarity in the commercial software.
One potential problem with FwdMsk is that unintended neural responses to the probe can be recorded in condition (B) if the masker is not completely effective. Insufficient masking can result if the masker-probe interval (MPI) is too long or too short, or if the masker level is not high enough (e.g., Cartee et al. 2000). If the MPI is too long (i.e., >500 µsec) or if the masker level does not provide sufficient masking, neurons could respond to the probe in (B). If the MPI is too short (i.e., <300 µs), neurons at the spatial edges of the excitation pattern that were partially depolarized by the masker can potentially integrate current from both the masker and probe, also leading to a partial response to the probe in (B). In either case, a partial response to the probe in (B) will be subtracted from the response in (A), leading to a falsely reduced ECAP amplitude.
AltPol overcomes some of the issues observed with FwdMsk by eliminating the need for a masker. AltPol averages the responses to both cathodic- and anodic-leading biphasic pulses (Fig. 1, right panel). When the pulse polarity is reversed, the artifact also reverses polarity, but the neural response does not. Upon averaging, the artifact cancels out, leaving the ECAP. The primary limitation with AltPol is the assumption that artifact amplitude, ECAP amplitude, and ECAP latencies are equivalent across the two polarities (Miller et al. 1998). If the responses do not have identical latencies or amplitudes, averaging responses will produce ECAPs with reduced amplitudes.
Two studies have compared ECAP amplitude growth functions (AGFs) using FwdMsk and AltPol in AB devices using non-commercial software (Frijns et al. 2002; Eisen & Franck 2004). Results from three subjects with the CII device showed that AltPol produced smaller amplitudes, similar thresholds, and shallower AGF slopes compared with FwdMsk (Frijns et al. 2002). Eisen and Franck (2004) reported a significant correlation between ECAP thresholds obtained with the two artifact-reduction methods for eight subjects (r = 0.76, p < 0.01); however, mean threshold values for each method (a measure of interest in the present study) were not reported. Visual inspection of their Figure 3 suggests that higher thresholds were obtained for FwdMsk than AltPol for many electrodes, which differs from the results of Frijns et al. (2002).
To our knowledge, no study has empirically compared these two artifact-reduction methods using clinical software in a large subject group with recent-generation devices. If AGFs using AltPol or FwdMsk yield similar slopes, amplitudes, and thresholds, it would be ideal in many cases to utilize AltPol clinically. Unlike FwdMsk, AltPol does not require a high-level masker, which would allow for ECAPs to be measured at the upper range of loudness comfort, and would be more time efficient because it requires fewer stimulus frames. The aim of this study was to compare the ECAP AGF thresholds, slopes, and amplitudes using FwdMsk and AltPol in recipients of Cochlear devices using the commercially available software. It is unclear whether the differences observed between artifact-reduction methods for AB devices using non-commercial software (Frijns et al. 2002; Eisen & Franck 2004) would be observed for other manufacturers’ devices when using clinical software.
MATERIALS AND METHODS
Participants
Twenty participants (24 ears) were enrolled in this study. All participants were implanted with a Cochlear device (Cochlear Ltd., Macquarie, NSW, Australia). Six ears were implanted with the Nucleus CI24R(CS), 11 with the CI24RE(CA), and seven with the CI512 (all half-band Contour arrays). Thirteen participants were unilaterally implanted, and seven were bilaterally implanted. Of the seven bilaterally implanted participants, both ears were tested for four participants; these participants were given unique identifiers for their left and right ears, respectively (F10/F11, N1/R2, F1/R3, and F5/R4; see Table 1). Participants ranged in age from 13 to 83 years at the time of testing. Demographic information for these participants is listed in Table 1. Participants were included in this study if they were at least 11 years of age, had at least three months experience with their CI, and if they had measurable ECAP responses within comfortable-loudness limits. This study was approved by the Boys Town National Research Hospital Institutional Review Board (protocol 03-07-XP).
TABLE 1.
Subject demographic information. Age and duration values are reported in years, months.
| Subject | Device | Ear Tested |
Gender | Age at study |
Dur. of Deafness |
Age at CI | Dur. of CI use |
Etiology/Time Course of Hearing Loss |
Electrodes Utilized |
|---|---|---|---|---|---|---|---|---|---|
| N1b | CI512 | L | M | 60, 2 | 8, 11 | 58, 3 | 1, 10 | Familial & Noise Exposure/Progressive | 5,10,15 |
| N5 | CI512 | R | F | 51, 0 | 1, 8 | 50, 8 | 0, 3 | Unknown/Sudden SNHL | 2,10,20 |
| N6c | CI512 | R | M | 83, 11 | 4, 0 | 82, 0 | 1, 11 | Unknown/Progressive | 5,11,20 |
| N9 | CI512 | R | M | 80, 4 | Unknown | 79, 11 | 0, 4 | Unknown-Noise Exposure/Progressive | 5,11,17 |
| N10 | CI512 | R | M | 14, 10 | 5, 0 | 14, 7 | 0, 3 | Unknown/Progressive | 5,11,17 |
| N11 | CI512 | L | M | 67, 8 | 6, 0 | 67, 5 | 0, 3 | Familial & Noise Exposure/Progressive | 6,13,17 |
| N12 | CI512 | L | M | 18, 3 | 14, 0 | 17, 9 | 0, 6 | CHARGE/Progressive | 3,11,20 |
| F1b | 24RE(CA) | L | F | 65, 6 | 11, 0 | 60, 7 | 4, 10 | Unknown/Progressive | 3,11,17 |
| F2 | 24RE(CA) | R | F | 64, 2 | 10, 5 | 60, 3 | 3, 11 | Unknown/Progressive | 5,11,17 |
| F4a | 24RE(CA) | L | F | 21, 1 | 17, 6 | 17, 6 | 3, 7 | Ototoxicity/Unknown | 3,11,20 |
| F5b | 24RE(CA) | R | M | 50, 11 | 7, 6 | 48, 3 | 2, 8 | Unknown/Sudden from established hearing loss | 3,11,20 |
| F7 | 24RE(CA) | R | M | 42, 11 | 28, 1 | 39, 1 | 3, 9 | Trauma/Progressive | 5,10,15 |
| F9 | 24RE(CA) | R | M | 64, 0 | 1, 3 | 61, 1 | 2, 10 | Familial & Noise Exposure/Progressive | 3,11,20 |
| F10b, c | 24RE(CA) | R | F | 13, 6 | 8, 3 | 8, 3 | 5, 3 | Waardenburg Syndrome/Congenital | 5,10,19 |
| F11b, c | 24RE(CA) | L | F | 13, 6 | 1, 10 | 1, 10 | 11, 8 | Waardenburg Syndrome/Congenital | 5,10,20 |
| F13 | 24RE(CA) | L | F | 37, 10 | Unknown | 35, 2 | 2, 8 | Unknown/Unknown | 2,14,21 |
| F14 | 24RE(CA) | L | F | 18, 11 | 7, 0 | 14, 6 | 4, 5 | Unknown/Progressive | 5,11,20 |
| F15 a | 24RE(CA) | L* | M | 25, 0 | 22, 9 | 22, 9 | 2, 3 | Unknown/Congenital | 5,11,17 |
| R2b | 24R(CS) | R | M | 60, 6 | 3, 4 | 51, 8 | 8, 10 | Familial & Noise Exposure/Progressive | 3,11,20 |
| R3b | 24R(CS) | R | F | 65, 6 | 6, 0 | 56, 2 | 9, 4 | Unknown/Progressive | 5,11,17 |
| R4b | 24R(CS) | L | M | 50, 11 | 1, 2 | 41, 11 | 9, 0 | Unknown/Sudden from established hearing loss | 5,11,20 |
| R6a | 24R(CS) | R | F | 59, 8 | 6, 0 | 52, 5 | 7, 2 | Auto Immune Disease/Unknown | 7,11,20 |
| R7 | 24R(CS) | R | M | 68, 6 | 5, 10 | 62, 2 | 6, 4 | Unknown/Progressive | 5,11,20 |
| R10 | 24R(CS) | R | M | 69, 11 | 2, 0 | 61, 10 | 8, 1 | Unknown/Progressive | 3,11,19 |
Bilateral recipient;
bilateral recipient with both CIs enrolled in the study;
ear underwent reimplantation (duration of CI use is total time with electrical stimulation across devices);
yrs = years; mos = months; CI= cochlear implant.
ECAP Stimuli and Procedure
ECAPs were obtained using the Custom Sound EP commercial software (Cochlear Ltd., Macquarie, NSW, Australia). Each participant was tested with a laboratory Freedom speech processor and headpiece connected to the computer via the programming Pod. Prior to data collection, electrode impedance was measured for each participant. All participants’ impedances were within normal limits (565 Ohms to 30 kOhms for Contour arrays) with the exception of one known open circuit measured for F2 and N6 (E8 and E1, respectively). These electrodes were previously disabled in the recipients’ clinical maps and were not used as stimulating or recording electrodes in this study.
ECAP responses were obtained from three electrodes representing the basal, middle, and apical regions along the array. Typically these were electrodes 5, 11, and 17, unless AGFs could not be obtained for both methods (see Table 1). Stimulus presentation levels were determined using the “Stimulate Only” feature within Custom Sound EP (Advanced NRT module). Behavioral loudness judgments were obtained using a single ascending run in steps of five current-level (CL) units using 10 averages of the four-frame (forward masking) stimulus used to elicit the ECAP. Participants used a 10-point loudness scale to report loudness perceptions for first hearing (“1”), loud but comfortable (“7”), loud (“8”), and the upper-loudness limit (“9”) for each electrode.
AltPol and FwdMsk were both used to obtain AGFs. Both methods presented biphasic current pulses in monopolar mode (MP1) using a 25-µsec/phase pulse duration and a 7-µsec interphase gap (IPG; 25 µsec is the default IPG for CI24R(CS) devices). A 50-µsec/phase pulse duration was used for two subjects who exceeded voltage compliance limits with the 25-µsec/phase stimulus (F9 and R4). The recording electrode was offset apically by two electrode positions with MP2 as a reference. Recordings were typically obtained with a delay of 122 µs and 50 dB gain (60 dB for CI24R(CS) devices, which is the software default). Averaged recordings consisted of 50–100 sweeps with a 1600-µs window and a 20-kHz sample rate. Changes were made to the recording parameters on an individual basis, as needed. If the recording electrode, gain, delay, or number of averages were changed to optimize the ECAP, the same parameters were used across the two artifact-reduction methods within an electrode.
For the FwdMsk method, a 400-µsec MPI was used (software default). The masker and probe were presented to the same electrode with the masker level fixed at the loudness rating of “8”. For simplicity, “probe” level will refer to both the level of the probe in FwdMsk and the stimulus level for AltPol. The starting probe level for both methods was 10 CL below that of the masker1, and was subsequently decreased in 5-CL steps. The step size was reduced to 2- to 3-CL when approaching threshold. A visual-detection method was used to define threshold as the lowest stimulus that elicited a measurable response that was visually recognizable by the tester and larger than the residual noise within the response (Glassman & Hughes 2013). Test order was randomized across electrodes and artifact-reduction method. It should be noted that the ECAP response derived with FwdMsk is the response to the probe (not the masker). The masker level, limited by the subject’s subjective loudness rating or voltage compliance, was set to be higher than the probe to maximize the probability that no neural response was present in the masked-probe condition. FwdMsk and AltPol therefore utilized the same starting level for the probe (10 CL below an “8” rating) and the same step sizes so that responses could be compared across the same levels. For most recipients, 10 CL below an “8” rating typically fell within a “7” range (“loud but comfortable”). Six subjects (F4, F7, N1, N6, N10, and N12) reached voltage compliance limits before an “8” rating could be achieved. In these cases, the masker level was set 5 CL below the voltage compliance limit (the starting probe level was still 10 CL below the masker to ensure adequate masking). Amplitudes were calculated as the difference between N1 and P2 peaks selected by the software and adjusted as needed by the tester. The slope of the AGF was based on a linear regression fit to the data, applied automatically by the software.
Data Analysis
The mean threshold, slope, and amplitude values obtained with the two artifact-rejection methods across the basal, middle, and apical electrode regions were compared using repeated-measures analysis of variance (RM ANOVA). Data were analyzed using IBM SPSS Statistics (v. 22.0, IBM Corp., Armonk, NY).
RESULTS
Figure 2 displays screen shots of AGFs obtained with the Custom Sound EP software using FwdMsk (Fig. 2A and 2C) and AltPol (Fig. 2B and 2D) for two subjects (each row). Within each screenshot, the left section displays the ECAP waveforms in order of descending probe level, the top right section displays a regression line fit to the ECAP AGF, and the bottom right section shows a single waveform obtained at a probe level near threshold. Figures 2A and 2B display AGFs obtained with FwdMsk and AltPol, respectively, from a recipient with a 24RE(CA) internal device (participant F5, e20). The single waveforms in the bottom right sections of Figure 2A and 2B are for a probe level of 155 CL (near threshold). Clear N1 and P2 peaks are observed with FwdMsk (Fig. 2A) but are absent for AltPol (Fig. 2B), resulting in a lower threshold for FwdMsk. Figures 2C and 2D display AGFs obtained with FwdMsk and AltPol, respectively, from a recipient with the older-generation CI24R(CS) device (participant R6, e11). The bottom right sections of Figs. 2C and 2D display the waveform obtained at a probe level of 180 CL for each method. There is a clear N1 and P2 seen at this level for FwdMsk (Fig. 2C), but the ECAP obtained with AltPol (Fig. 2D) does not have a clear N1 or P2. Unlike the results displayed in the left section of Fig. 2B, the waveforms in Fig. 2D exhibit an upward-sloping response as the latency increases, with indistinguishable peaks at lower levels that do not appear to decrease with the probe level. The poor ECAP morphology displayed in Fig. 2D is representative of the AltPol ECAPs observed across the six CI24R(CS) recipients enrolled in the study. Due to the poor morphology of the waveforms obtained from the CI24R(CS) devices, these data were not included in the group statistical analysis (described further in the Discussion). The CI24RE(CA) and CI512 devices are functionally equivalent (i.e., they have the same chip and amplifier), and differ only in physical design. Therefore, data from the 18 CI24RE(CA) and CI512 devices were combined for the statistical analyses.
Fig. 2.
Four screenshots from Cochlear’s Custom Sound EP software displaying AGFs obtained with FwdMsk (left panels, A and C) versus AltPol (right panels, B and D) from two participants with different internal devices. The top panels (A and B) show AGFs collected from a CI24RE internal device (participant F5, e20). The bottom panels (C and D) display AGFs obtained from a recipient with the older CI24R device (participant R6, e11).
Figure 3 displays six individual participants’ AGFs, with ECAP amplitude plotted as a function of CL. FwdMsk (gray symbols) and AltPol (white symbols) data from basal (circles), middle (triangles), and apical (squares) electrodes (left to right, respectively) are shown for participants F5, F11, N1, N6, N10, and N12. These examples illustrate various patterns observed across participants. For some, FwdMsk yielded larger ECAP amplitudes and lower thresholds than AltPol (all three electrodes for F11 and N1; e3 and e11 for F5). For others, AltPol produced larger amplitudes at higher CLs (N10) or at lower CLs (N12); in some cases, the functions crossed (N10, all three electrodes; N12, e11). Other participants showed similar amplitudes and thresholds across methods (N6, e5 and e11; F5, e20).
Fig. 3.
Individual AGFs from six participants (rows) displaying ECAP amplitude as a function of probe current level. Gray and white symbols represent FwdMsk and AltPol data, respectively. Data from the basal, middle, and apical electrodes are shown from left to right, respectively.
Figure 4 displays the group threshold data in a box-and-whisker plot (FwdMsk, gray boxes; AltPol, white) for the 18 ears. Electrode regions are labeled along the x-axis. The mean thresholds are indicated by the dashed lines and numerical values; solid lines represent medians. A two-way RM ANOVA was used to examine the effect of artifact-reduction method (FwdMsk, AltPol) and region (basal, middle, apical) on ECAP threshold. Results revealed a significant effect of method (F(1,17)=31.72, p < 0.001), where FwdMsk yielded lower average thresholds (mean = 163.1 CL) than AltPol (mean = 176.1 CL). A significant effect of region was also observed (F(2,34) = 12.33, p < 0.001), where average thresholds were highest for the middle region (178.4 CL), followed by the basal region (171.9 CL), with the lowest thresholds in the apical region (158.6 CL). Post-hoc comparisons (with Bonferroni correction) revealed significant differences in threshold between the basal and apical regions (p = 0.04), and the middle and apical (p = 0.001) regions. There was no significant difference between the basal and middle regions (p = 0.16). No significant interactions were observed between region and method (F(1.48, 25.12) = 2.75, p = 0.1; adjusted degrees of freedom using Greenhouse-Geisser Epsilon = 0.74; Mauchly’s W(2) = 0.65, p = 0.03).
Fig. 4.
ECAP threshold data for the two artifact-reduction methods (FwdMsk, gray; AltPol, white), separated by electrode region (basal, middle, apical). Box boundaries represent the 25th and 75th percentiles, whiskers represent the 10th and 90th percentiles, black circles represent outliers, and horizontal solid and dotted lines within the box represent medians and means, respectively. Mean threshold values are noted on each box.
Figure 5 shows group slope data for the two methods and three regions (data are plotted as in Fig. 4). Only 15 ears were included in this analysis because three subjects (F13, F14, and N11) had at least one electrode for which slope could not be calculated for AltPol. In these cases, there was only one non-zero data point on the AGF (i.e., threshold). A two-way RM ANOVA revealed a significant effect of artifact-reduction method on ECAP slope (F(1,14) = 11.79, p = 0.004), with AltPol yielding significantly steeper slopes on average (7.67 µV/CL) compared with FwdMsk (6.46 µV/CL). No significant effect of region was observed (F(1.26, 17.57) = 2.49, p = 0.13; adjusted degrees of freedom using Greenhouse-Geisser Epsilon = 0.63; Mauchly’s W(2) = 0.41, p = 0.003). Across methods, the mean slopes per region were 5.94 µV/CL for basal, 7.53 µV/CL for middle, and 7.73 µV/CL for apical. There was no significant interaction between method and region (F(2,28) = 3.23, p = 0.06). For both methods, the shallowest slopes occurred for the basal electrodes (5.63 µV/CL and 6.25 µV/CL for FwdMsk and AltPol, respectively; see Fig. 5). For FwdMsk, the steepest slopes occurred for apical electrodes (7.30 µV/CL; see Fig. 5, gray bars), whereas the steepest slopes for AltPol occurred for middle electrodes (8.59 µV/CL; see Fig. 5, white bars).
Fig. 5.
Mean slope data compared across artifact-reduction method and region. Data are plotted similar to Fig. 4.
Because thresholds and slopes differed across methods, amplitudes were compared using only the same range of current levels that yielded non-zero values for both methods; this is illustrated in Figure 6. AGFs are shown for both methods for subject F7, electrode 5. The vertical dashed line indicates the lowest CL that yielded non-zero amplitudes for both artifact-reduction methods (200 CL in this example). The range of CLs used for amplitude comparisons is indicated by the horizontal arrows.
Fig. 6.
Example AGFs for both polarities from subject F7, electrode 5, illustrating how amplitude comparisons were made. The vertical dashed line represents the lowest current level that yielded non-zero amplitudes for both artifact-reduction methods. Amplitude values to the right of the dashed line (indicated with arrows) represents the current-level range at which amplitudes were compared between methods.
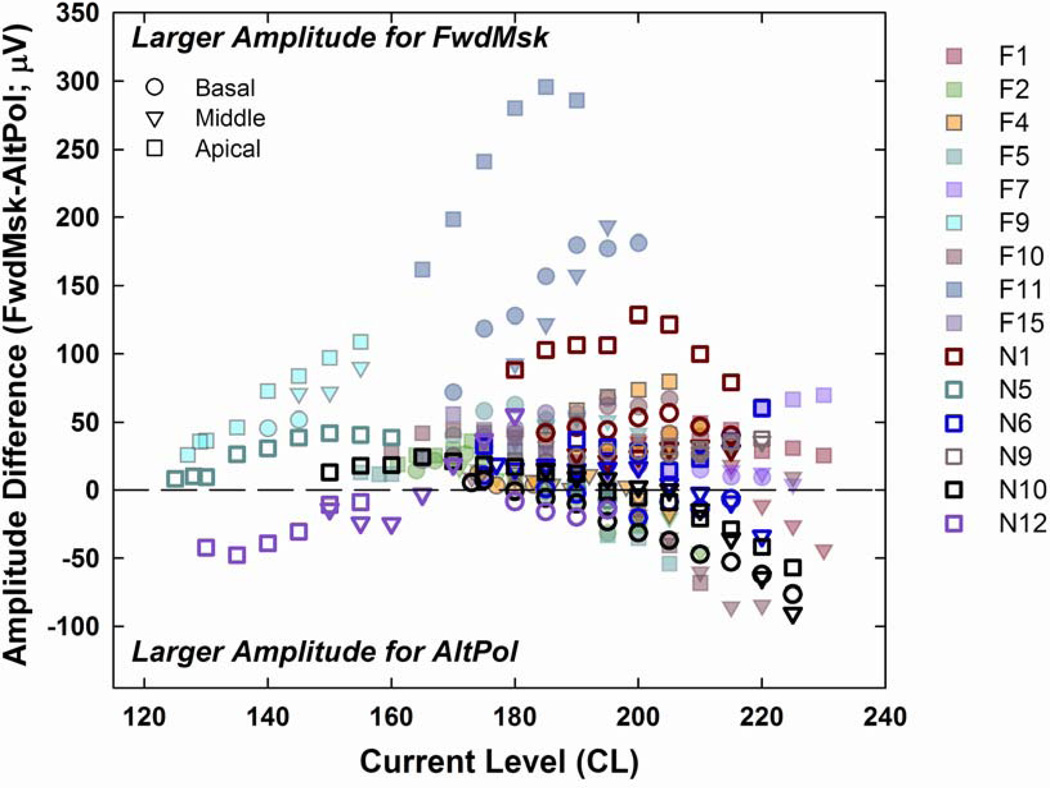
Figure 7 displays amplitude differences between methods (FwdMsk minus AltPol) across the AGF stimulus levels, as defined in Fig. 6, for the same 15 participants shown in Figure 5. The horizontal dashed line at zero microvolts represents no difference in amplitude; positive numbers indicate larger amplitudes for FwdMsk, and negative numbers indicate larger amplitudes for AltPol. Basal, middle, and apical electrode regions are distinguished by circles, triangles, and squares, respectively. The majority of data points fell above the dashed line, indicating a greater incidence of larger amplitudes for FwdMsk at equal current levels.
Fig. 7.
Amplitude differences (FwdMsk minus AltPol) plotted across stimulus levels (as illustrated in Fig. 6) for all AGFs in all subjects (identified by color in the legend). Positive numbers indicate larger amplitudes with FwdMsk and negative numbers indicate larger amplitudes with AltPol. The horizontal dashed line represents no difference. Basal, middle, and apical electrode regions are differentiated by circles, triangles, and squares, respectively.
Figure 8 shows the averaged amplitudes across artifact-reduction method and electrode region. For each subject, the mean amplitude across each AGF (as defined in Figure 6) was calculated, so that each subject contributed a single data point for each condition. Data are from the same 15 participants shown in Figures 5 and 7. A two-way RM ANOVA was used to examine the effects of artifact-reduction method and region on mean ECAP amplitude. Results showed a significant effect of method (F(1,14) = 6.17, p = 0.03), with FwdMsk yielding larger average amplitudes (148.0 µV) than AltPol (113.7 µV). There was also a significant effect of region (F(2,28) = 3.41, p = 0.047), where mean amplitudes were smallest for the basal region (104.8 µV), followed by the middle region (121.5 µV), with the largest amplitudes occurring for the apical region (166.1 µV). Post-hoc comparisons (with Bonferroni correction), however, revealed no significant differences between any of the pairwise comparisons. There was no significant interaction between method and region (F(2,28) = 2.32, p = 0.12). In summary, FwdMsk resulted in significantly lower average visual-detection thresholds, shallower slopes, and larger amplitudes compared with AltPol.
Fig. 8.
Mean amplitude data compared across artifact-reduction method and regions. Data are plotted similar to Figs. 4 and 5.
To assess whether the artifact-reduction method differentially affected ECAP amplitude as a function of level, ECAP amplitudes obtained at the highest probe level and at the lowest common probe level that yielded a non-zero ECAP were compared. For example, in Fig. 6, amplitudes obtained for probe levels of 220 CL (highest level) and 200 CL (lowest level yielding non-zero amplitudes for both methods) were examined. A three-way RM ANOVA showed significant effects of method (F(1,14) = 5.79, p = 0.03), region (F(2,28) = 4.46, p = 0.02), and level (F(1,14) = 26.33, p < 0.001). For method and region, results were similar to those reported above for the averaged amplitudes, where ECAPs were larger for FwdMsk than for AltPol, and ECAPs were smallest for the basal region and largest for the apical region. As expected, amplitudes were larger for the higher level and smaller for the lower level. Importantly, there were no significant interactions between any of the factors (region*level, p = 0.09; method*level, p = 0.75; region*method*level, p = 0.68). Average amplitudes for FwdMsk were higher than AltPol at both the high (FwdMsk, 277.9 µV; AltPol, 250.7 µV) and low (FwdMsk, 47.6 µV; AltPol, 16.9 µV) levels. These results suggest that the artifact-reduction method did not differentially affect ECAP amplitude as a function of level.
DISCUSSION
Poor morphology with 24R devices
For the six CI24R(CS) participants, typical growth functions could not be obtained when using the AltPol method due to poor ECAP morphology. Improved morphology in the AltPol growth functions with CI24RE and CI512 devices (both have the fourth-generation internal electronics) is the result of an improved amplifier that was implemented when the CI24RE was released. The newer amplifier has a lower noise floor, recovers more quickly from saturation, and the response is more symmetrical than the amplifier in the CI24R(CS) (third-generation internal electronics; Battmer et al. 2004; Patrick et al. 2006). Because symmetry is essential when using AltPol, the resulting ECAP waveform is free of artifact only if the artifact responses to the opposing stimulus polarities are equal in shape. If the saturation level of the amplifier is not the same for the anodic- and cathodic-leading pulses, the saturation will begin at different time points, violating the assumption of equal and opposite artifacts.
Threshold differences
Results from the present study revealed significantly higher thresholds for AltPol than for FwdMsk. Recent studies using pseudomonophasic pulses for either a masker or probe have shown evidence that anodic-leading polarity is more effective at exciting the human auditory pathway than cathodic-leading polarity (Macherey et al. 2008; Undurraga et al. 2010, 2012). Specifically, larger ECAP amplitudes (Undurraga et al. 2010) and shorter latencies (Macherey et al. 2008) have been found for anodic stimulation. Using a cathodic-leading, symmetrical, biphasic masker and symmetrical biphasic probes that were either anodic- or cathodic-leading, Macherey et al. (2008) found shorter latencies for the anodic-leading probes, suggesting that the neural response to the biphasic probe was primarily due to the anodic phase. These results were only obtained at supra-threshold levels, so it is not clear whether polarity-dependent latency differences remain near threshold. If the latency differences truly are caused by the timing differences between when the anodic phase is presented within the biphasic pulse, then we would expect polarity-dependent latency differences to remain near threshold. By averaging responses with different latencies, as with AltPol, the resulting ECAP amplitude will likely be smaller than with either polarity alone, which would subsequently yield higher thresholds.
When comparing AGFs using FwdMsk and AltPol in three subjects with AB CII devices, Frijns and colleagues (2002) found that extrapolated thresholds were similar across the two methods. Eisen and Franck (2004) found a moderate correlation (r = 0.76) between FwdMsk and AltPol thresholds obtained for 86 electrodes in eight AB CII recipients, with lower thresholds for AltPol occurring for many electrodes (mean thresholds for each method were not reported). These findings differ from the present data obtained with Cochlear devices, which revealed significantly higher thresholds for AltPol. Differences between the present and previous findings may be a combination of methodological differences and differences in amplifier noise floor across manufacturers. Both Frijns et al. (2002) and Eisen and Franck (2004) calculated thresholds based on extrapolation or linear regression applied to the AGF, whereas the present study used visual detection to identify threshold. The benefit of linear regression is that it can estimate threshold for systems with a high noise floor. Both of the aforementioned studies used AB devices, which have a higher noise floor compared to that of the newer-generation Cochlear devices used in this study (20–50 µV versus 2–5 µV, respectively; Botros et al. 2007; van Dijk et al. 2007; Glassman & Hughes 2013). The disadvantage of linear regression, however, is that it can over- or under-estimate threshold if the AGF is nonlinear (Glassman & Hughes 2013). In several cases in the present study, AGFs obtained with FwdMsk demonstrated nonlinear functions, where amplitude growth was shallower at low levels and steeper at higher levels (e.g., F11, N1, and N10 in Fig. 2). ECAPs forming the shallow portion of the growth function would not be seen in devices with a high noise floor, and would thereby yield higher thresholds. In contrast, the AltPol functions generally did not exhibit a slow-growth portion at lower levels. These differences are more likely to be resolved when using visual detection in devices with a lower noise floor, as was the case in the present study. When linear regression is utilized, threshold values are affected by the slope of the regression line. These limitations are discussed further in the next section.
Slope differences
Mean slope data calculated in this study showed a significant effect of artifact-reduction method, with AltPol yielding steeper slopes on average compared to FwdMsk. These results differ from those reported by Frijns et al. (2002), who found shallower slopes with AltPol versus FwdMsk in AB devices (Eisen & Franck did not report slope data for their comparisons). This inconsistent finding is likely due to the aforementioned device and methodological differences between studies. First, as noted above, FwdMsk produced a shallower rate of growth near threshold (see Fig. 2, F11, N1, and N10), which would likely not be visible in systems with a higher noise floor (i.e., AB devices). If the slow-growth portion of the function is obscured by the noise floor, the resulting slope for the visible portion of the function will be steeper than if the slow-growth portion is included. Second, Frijns et al. (2002) reported that the AltPol functions in their dataset saturated more quickly at higher levels than the FwdMsk functions, thus producing shallower AGF slopes. Shallower AGF slopes with AltPol would also underestimate extrapolated thresholds. Thus, the thresholds reported by Frijns et al. might have actually been higher than what was estimated by extrapolation, and could explain the similar thresholds they reported for both methods (see previous section). It should also be noted that the high stimulus levels in their study reportedly exceeded voltage compliance limits, which likely caused the saturated AGFs. In the present study, only current levels within voltage compliance were used, and saturation of the AGF was not observed.
Amplitude differences
For the AB CII device, Frijns et al. (2002) found that the peak-to-peak amplitudes obtained with AltPol were generally smaller than those using FwdMsk, which was consistent with the present study. Smaller amplitudes with AltPol could be due to an inaccurate assumption that the responses obtained from the two polarities are equivalent. Animal studies have shown latency and amplitude differences when comparing ECAP responses obtained with anodic versus cathodic stimulation (Miller et al. 1998; Klop et al. 2004), and these response differences across polarities have been shown to vary across species (Miller et al. 1998). As noted above, anodic-leading pulses have been shown to yield larger ECAP amplitudes (Undurraga et al. 2010) and shorter latencies (Macherey et al. 2008) in human CI recipients. While the mechanisms that underlie these different response characteristics are still under investigation, it is clear that changes in polarity produce different response amplitudes and latencies within and across species, which conflict with the assumptions underlying the AltPol method. Averaging responses that have different latencies and amplitudes will result in a temporally smeared recording, which is consistent with the smaller amplitude responses typically observed using AltPol.
While the majority of data showed larger amplitudes with FwdMsk, there were cases where AltPol yielded larger amplitudes, consistent with findings reported by Bahmer et al. (2010) for a single MED-EL recipient. This occurred at one or more CLs in 17 of 54 electrodes in the present study. Examples are illustrated in Fig. 3 (F5, e20; N6, e11; N10, all three electrodes; and N12, all three electrodes). As displayed in Figures 3 and 7, these cases typically occurred at relatively high stimulation levels. Westen et al. (2011) reported a plateau in FwdMsk AGFs at high stimulation levels in guinea pigs, and concluded that the relation between the number of stimulated nerve fibers and the ECAP amplitude should not be assumed as linear, and nerve fibers stimulated at high levels may have different response properties than those at lower levels. However, this conclusion might also apply to ECAPs obtained with AltPol (AltPol was not utilized in Westen et al.). A plateau in the AGF can occur with FwdMsk if the masker does not sufficiently force all fibers into a refractory period to ensure that only artifact is recorded in response to the probe in the forward-masked condition. Plateaus can also occur with either FwdMsk or AltPol if stimulus levels exceed voltage compliance limits (e.g., Fig. 6 in Frijns et al. 2002, using AltPol), or if the probe level is sufficiently high enough to recruit all cochlear fibers (i.e., neural saturation). The latter case is more probable in experiments with anesthetized animals than in awake humans who can communicate loudness discomfort. Regardless, there were no plateaus in the data set for the present study, with the exception of a single AGF (N12, e11, AltPol) in which the highest CL yielded a response that was slightly smaller than that obtained with the second-highest CL, but larger than that obtained with the third-highest CL (180 CL = 66.76 µV, 175 CL = 71.97 µV, and 170 CL = 56.43 µV).
CONCLUSIONS
AltPol is advantageous because it does not require a higher-level masker, which allows for ECAP measures to be obtained at higher probe levels than allowed with FwdMsk; this is particularly advantageous for individuals with very small ECAP dynamic ranges. Additionally, fewer stimulus frames are required, which may be more time efficient. However, emerging evidence suggests that the human auditory system responds differently to anodic versus cathodic phases for electrical stimulation. The result is likely a temporally smeared ECAP response with AltPol, which was supported by the present dataset. FwdMsk produced larger ECAP peak-to-peak amplitudes, lower visual-detection thresholds, and shallower slopes compared with AltPol. The next step will be to separate the effects of artifact-reduction method and stimulus polarity to determine the relative effects of each.
Acknowledgments
Source of Funding: This research was supported by the National Institutes of Health (NIH), the National Institute on Deafness and Other Communication Disorders (NIDCD), grants R01 DC009595 and P30 DC04662. The content of this project is solely the responsibility of the authors and does not necessarily represent the official views of the NIDCD or the NIH. The authors thank Gina Diaz for assistance with data collection.
Footnotes
Conflicts of Interest; Michelle Hughes is a member of the Ear and Hearing editorial board. No other conflicts of interest are declared for any of the authors.
Data from Hughes et al. (2001) showed that for a given probe level, increasing the masker by more than 10 CL above the probe results in no appreciable change in ECAP amplitude.
Portions of this study were presented at the 8th International Symposium on Objective Measures in Auditory Implants meeting in Toronto, Canada, October 15–18, 2014.
REFERENCES
- Abbas P, Brown C, Shallop J, et al. Summary of results using the Nucleus CI24M implant to record the electrically evoked compound action potential. Ear Hear. 1999;20:45–59. doi: 10.1097/00003446-199902000-00005. [DOI] [PubMed] [Google Scholar]
- Bahmer A, Peter O, Baumann U. Recording and analysis of electrically evoked compound action potentials (ECAPs) with MED-EL cochlear implants and different artifact reduction strategies in Matlab. J Neurosci Meth. 2010;191:66–74. doi: 10.1016/j.jneumeth.2010.06.008. [DOI] [PubMed] [Google Scholar]
- Battmer R-D, Dillier N, Lai WK, et al. Evaluation of the Neural Response Telemetry (NRT) capabilities of the Nucleus Research Platform 8: Initial results from the NRT trial. Int J Audiol. 2004;43:S10–S15. [PubMed] [Google Scholar]
- Botros A, van Dijk B, Killian M. AutoNRT: an automated system that measures ECAP thresholds with the Nucleus Freedom cochlear implant via machine intelligence. Artif Intell Med. 2007;40:15–28. doi: 10.1016/j.artmed.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Brown C, Abbas P. Electrically evoked whole-nerve action potentials: Parametric data from the cat. J Acoust Soc Am. 1990;88:2205–2210. doi: 10.1121/1.400117. [DOI] [PubMed] [Google Scholar]
- Brown C, Abbas P, Gantz B. Electrically evoked whole-nerve action potentials: Data from human cochlear implant users. J Acoust Soc Am. 1990;88:1385–1391. doi: 10.1121/1.399716. [DOI] [PubMed] [Google Scholar]
- Brown CJ, Abbas PJ, Borland J, et al. Electrically evoked whole nerve action potentials in Ineraid cochlear implant users: Responses to different stimulating electrodes configurations and comparison to psychophysical responses. J Sp Hear Res. 1996;39:453–467. doi: 10.1044/jshr.3903.453. [DOI] [PubMed] [Google Scholar]
- Cartee LA, van den Honert C, Finley CC, et al. Evaluation of a model of the cochlear neural membrane. I. Physiological measurement of membrane characteristics in repsonse to intrameatal electrical stimulation. Hear Res. 2000;146:143–152. doi: 10.1016/s0378-5955(00)00109-x. [DOI] [PubMed] [Google Scholar]
- Eisen MD, Franck KH. Electrically evoked compound action potential amplitude growth functions and HiResolution programming levels in pediatric CII implant subjects. Ear Hear. 2004;25:528–538. doi: 10.1097/00003446-200412000-00002. [DOI] [PubMed] [Google Scholar]
- Frijns JHM, Briaire JJ, de Laat JAPM, et al. Initial evaluation of the Clarion CII cochlear implant: Speech perception and Neural Response Imaging. Ear Hear. 2002;23:184–197. doi: 10.1097/00003446-200206000-00003. [DOI] [PubMed] [Google Scholar]
- Glassman EK, Hughes ML. Determining electrically evoked compound action potential thresholds: A comparison of computer versus human analysis methods. Ear Hear. 2013;34:96–109. doi: 10.1097/AUD.0b013e3182650abd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes ML, Vander Werff KR, Brown CJ, et al. A longitudinal study of electrode impedance, the electrically evoked compound action potential, and behavioral measures in Nucleus 24 cochlear implant users. Ear Hear. 2001;22:471–486. doi: 10.1097/00003446-200112000-00004. [DOI] [PubMed] [Google Scholar]
- Klop WMC, Hartlooper A, Briare JJ, et al. A new method for dealing with the stimulus artefact in electrically evoked compound action potential measurements. Acta Otolaryngol. 2004;124:137–143. doi: 10.1080/00016480310016901. [DOI] [PubMed] [Google Scholar]
- Macherey O, Carlyon RP, van Wieringen A, et al. Higher sensitivity of human auditory nerve fibers to positive electrical currents. J Assoc Res Otolaryngol. 2008;9:241–251. doi: 10.1007/s10162-008-0112-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CA, Abbas PJ, Rubinstein JT, et al. Electrically evoked compound action potentials of guinea pig and cat: Responses to monopolar, monophasic stimulation. Hear Res. 1998;119:142–154. doi: 10.1016/s0378-5955(98)00046-x. [DOI] [PubMed] [Google Scholar]
- Patrick JF, Busby PA, Gibson PJ. The development of the Nucleus Freedom cochlear implant system. Trends Amplif. 2006;10(4):175–200. doi: 10.1177/1084713806296386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Undurraga JA, Carlyon RP, Macherey O, et al. Spread of excitation varies for different electrical pulse shapes and stimulation modes in cochlear implants. Hear Res. 2012;290:21–36. doi: 10.1016/j.heares.2012.05.003. [DOI] [PubMed] [Google Scholar]
- Undurraga JA, van Wieringen A, Carlyon RP, et al. Polarity effects on neural responses of the electrically stimulated auditory nerve at different cochlear sites. Hear Res. 2010;269:146–161. doi: 10.1016/j.heares.2010.06.017. [DOI] [PubMed] [Google Scholar]
- van Dijk B, Botros AM, Battmer R-D, et al. Clinical results of AutoNRT, a completely automatic ECAP recording system for cochlear implants. Ear Hear. 2007;28:558–570. doi: 10.1097/AUD.0b013e31806dc1d1. [DOI] [PubMed] [Google Scholar]
- Westen AA, Dekker DMT, Briaire JJ, et al. Stimulus level effects on neural excitation and eCAP amplitude. Hear Res. 2011;280:166–176. doi: 10.1016/j.heares.2011.05.014. [DOI] [PubMed] [Google Scholar]