Abstract
Background
Randomized controlled trials (RCT) suggest that the efficacy of tenofovir-based pre-exposure prophylaxis (PrEP) strongly depends on consistency of PrEP use. We explore how patterns of pill-taking and waning of PrEP protection may affect PrEP efficacy for HIV prevention.
Methods
A two-arm RCT was simulated by mathematical models assuming that prescribed daily doses were skipped periodically, randomly or in large blocks. Risk-driven adherence, in which PrEP was taken when sex was expected, was also investigated. Three temporal PrEP protection profiles were explored: long (5 days), intermediate (3 days) and short (24 hours). Modeling results were compared to the efficacy observed in completed RCTs.
Results
Expected PrEP efficacy was 60% with periodic, 50% with random and 34% with block adherence when PrEP had a long protection profile and pills were taken only 50% of the days. Risk-driven pill-taking resulted in 29% and 37% daily pills taken and efficacy of 43% and 51% for long protection. High PrEP efficacy comparable with that observed in Partners PrEP and CDC Botswana trials was simulated under long protection, high overall adherence and limited block pill-taking; the moderate efficacy observed in iPrEx and Bangkok trials was comparable with the 50% adherence scenarios under random pill-taking and long protection.
Conclusions
Pill-taking patterns may have a substantial impact on the protection provided by PrEP even when the same numbers of pills are taken. When PrEP retains protection for longer than a day, pill-taking patterns can explain a broad range of efficacies observed in PrEP RCTs.
Keywords: pre-exposure prophylaxis, mathematical modeling, HIV prevention, intervention efficacy, HIV acquisition risk
Introduction
Randomized controlled trials (RCT) have demonstrated that tenofovir-based pre-exposure prophylaxis (PrEP) significantly reduces risk of HIV acquisition. [1–7] Case-control analyses using biological evidence of tenofovir showed that in order for PrEP to be protective it is critically important it is taken consistently. A key conclusion of these studies is that the success of PrEP at a population level will strongly depend on high individual adherence.
Adherence to (or compliance with) a medication regimen is generally defined as the extent to which patients take medications as prescribed by their health care providers. One way to quantify adherence is to estimate the proportion of prescribed doses taken in a specific time period. Self-reported data collected in the concluded clinical trials indicates that overall adherence to PrEP was high. However, measures of drug detected in biological specimens showed much lower adherence than self-reported, likely contributing to the failure of two clinical trials to demonstrate PrEP efficacy. [8, 9] A subgroup analysis of data from studies among men who have sex with men (MSM) estimated PrEP efficacy at 73% when PrEP is taken on at least 90% of the days.[1] The same team projected that PrEP protection is 96% if taken every other day (50% adherence) and 76% if taken only 2 days per week. A study among serodiscordant couples reported that detectable drug was associated with 86% – 90% reduction in relative risk of acquiring HIV. [3]
A review of the prevalence of partial adherence or non-adherence to prescribed medications showed that suboptimal adherence that reduces the effectiveness of biomedical interventions can take different forms including delayed initiation, frequently missed doses, multi-week holidays and early discontinuation. [10] Given that the same number of doses may be distributed differently, we use mathematical models to explore the potential impact of different patterns of adherence on the efficacy of PrEP observed in clinical trials. We investigate pill-taking patterns in which PrEP doses are skipped randomly, periodically and in blocks under a range of assumptions of residual PrEP protection following dosing. We also simulate risk-driven pill-taking patterns in which PrEP is significantly more likely to be taken on days when sexual activity is expected. Our analysis provides plausible explanation for the discrepancies in the PrEP efficacy reported in concluded RCTs.
Methods
We used stochastic individual-based models to simulate clinical trials of daily oral PrEP. The models were designed to reproduce the sexual behavior of a cohort of sexually active HIV-uninfected women in high HIV prevalence settings and study their exposure to HIV through heterosexual contacts. [11] We assumed the cohort was enrolled over a 1 year period and randomized in a 1:1 ratio to active or placebo arms. We simulated an event-driven trial, i.e., the trial concluded when a specific number of infections had occurred. The sexual behavior of each woman, including partnership formation and dissolution as well as frequency and type of sex acts, was simulated for the duration of the trial in discrete time (units = days). Each day, a woman may acquire new partners, have sex (protected or unprotected) with one or more of her active partners, or terminate an active relationship. The male partners’ characteristics, baseline HIV status and risk of HIV acquisition were simulated according to data-derived parameters. Published research on sexual behavior patterns and studies on HIV transmission in South Africa informed behavioral and epidemiological assumptions in the model. [12–14]
Sexual Behavior
Each woman may be involved in two types of sexual partnerships: i) short-term partnerships with an average duration of 6 months and characterized by higher rates of protected sex; ii) long-term partnerships with an average duration of 10 years and a low rate of protected sex. All new partnerships start as short-term, converting into long-term after 9 months. Following the population structure described in Johnson et al. [13], we divided the women into low-risk and high-risk groups that define their simulated sexual activity. The high-risk women may have up to two concurrent partnerships, one of which may be long-term; while low-risk women are serially monogamous. This simplifying assumption allows us to reproduce the partnership distribution representative for South Africa [13, 14] where the majority of women are in stable partnerships and only a minority of women are involved in multiple partnerships with shorter duration.
Assortative mixing is assumed in partnership formation with partnerships initiated more often between individuals from the same risk groups. In other words, women are more likely to partner with men who have similar risk (high or low). The degree of assortativity (the propensity to choose a partner with similar risk) is representative of the sexual mixing patterns in South Africa. [13] New partnerships are initiated at a fixed rate that is almost halved when women are in active short-term partnerships and reduced 7-fold if in long-term partnerships. The minimum duration of a partnership is fixed at 30 days. The long- and short-term partnerships dissolve at a different rate annually with an elevated dissolution rate if another partnership is active at the time. A low-risk woman has 2 months on average between the end of one and the beginning of a new relationship compared to 1 month for high-risk women. Values of all behavioral parameters are specified in Supplemental Digital Content, Table S1.
The frequency of sexual acts is assigned at the initiation of each partnership and remains constant for the duration of the relationship. Similar coital frequency is assumed for married (long-term) couples and unmarried (short-term) couples with sexual acts occurring at random. In the 20% of partnerships in which anal intercourse is practiced, an average of 40% of all sex acts were assumed to be anal based on data from Kalichman et al. [12] The proportion of sex acts protected by condom was assumed to be significantly higher in short-term partnerships (40%) compared to long-term partnerships (15%).
HIV transmission
All women are initially HIV-negative. The HIV status of their partners is randomly assigned based on assumed HIV prevalence in different risk groups (high and low) of the male partners. The HIV acquisition risk per vaginal intercourse was differentiated by the partner’s stage of infection with asymptomatic stage risk fixed at 0.24% (0.65%) for a long-term (short-term) partnership, with multiplicative factors representing elevated HIV risk during acute and late stages, taken from published meta-analyses. [15] Anal intercourse was assumed to be 10 times riskier than vaginal intercourse with respect to HIV transmission. [16] The protective efficacy of male condoms against HIV was fixed at 90%.
Cohort Characteristics
The number of short- and long-term partners currently active for each woman is assigned randomly based on demographic data representative of South Africa [13, 14] resulting in the vast majority (~85%) of low-risk individuals having a single partner, 11% not currently having a partner and less than 4% in concurrent partnerships. In contrast, high-risk individuals are less likely to have no partners (8%) or have one partner (74%) and more likely to report concurrent partnerships (18%). Partnership distribution of the female cohort at the start of each simulation prior to enrollment is presented in Supplemental Digital Content, Table S2. The HIV incidence among the control arm of the simulated cohort varies from 3.8% to 7.2% which is comparable to the HIV incidence observed in placebo arms of the VOICE trial.[9] This and other characteristics of the simulated female cohort are summarized in Supplemental Digital Content, Table S3. The influence of the behavior and epidemic assumptions employed in our analysis on the projected PrEP efficacy is investigated in multivariate sensitivity analysis included in the Supplemental Digital Content (Table S5 and Fig. S2).
PrEP regimen and protection
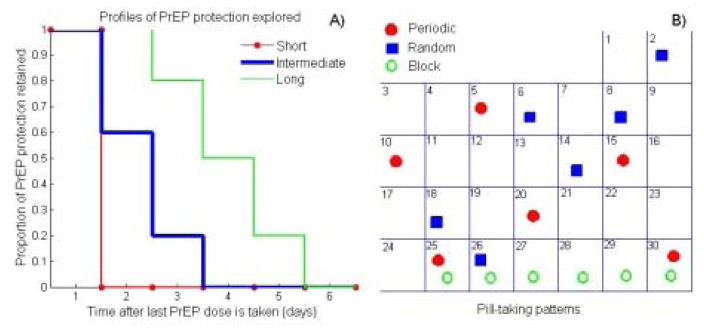
The efficacy of daily oral tenofovir disoproxil fumarate and emtricitabine (TDF–FTC) for preventing HIV in women ranged from −4% to 75% in RCTs, highly correlated with biological measures of adherence. [3, 4, 8, 9] Case-control analyses within PrEP RCTs suggest that if used consistently PrEP could reduce the HIV acquisition risk per sex act by at least 70% which we use as the estimate of “biological efficacy” in our main analysis (i.e. with perfect adherence, PrEP reduces the risk of HIV infection by 70%). An alternative scenario assuming 90% biological efficacy for PrEP in women is explored in the Supplemental Digital Content. To address the uncertainty in the duration and magnitude of residual PrEP protection following dosing we consider three protection profiles of PrEP over time (see Figure.1). The short profile assumes that PrEP protects only on the days it is taken. It is likely to represent the protection provided by topical PrEP. This profile is used in all modeling studies in which per-act PrEP efficacy is proportional to adherence. [17–20] The intermediate profile assumes that PrEP retains 100%, 60% and 20% of its biological efficacy during the three days after a pill is taken. It is likely to represent the protection in women with daily oral TDF which may be less forgiving of missing dosses compared to TDF-FTC.[21] Finally, the long profile assumes full biological efficacy for 48 hours and partial per act efficacy for the subsequent 3–5 days after the last dose is taken. This profile closely aligns with the relationship between adherence and efficacy reported by the iPrEx team for daily PrEP regimens in MSM. [22] No cumulative effect of multiple PrEP doses is assumed.
Figure 1.
A) Profiles of PrEP protection explored in the analysis Each profile curve shows the proportion of biological efficacy retained over time after the last PrEP dose is taken. B) Pill-taking patterns explored in the analysis. The overall adherence in all patterns in this specific example is 20%.
Overall adherence and pill-taking patterns explored
We consider three PrEP adherence scenarios. First, all participants have a common adherence distribution at levels 20%, 50% and 80%. Second, mixture adherence distribution, in which half of the participants are high adherers (80% adherence) and half are poor adherers (20% adherence) is simulated. For each scenario with common and mixture adherence three patterns of adherence are considered: i) PrEP doses are skipped randomly (random pattern); ii) PrEP doses are skipped at regular intervals (periodic pattern); iii) PrEP doses are skipped in larger blocks (block pattern). An illustration of each pattern over one follow-up period (30 days) is presented in Figure 1B.
Finally, we simulate a risk-driven adherence in which the daily decision to take PrEP is based on the personal expectation to have sex. We assume that participants take PrEP with 80% probability in the days when sex is expected and 20% otherwise. We consider two scenarios with respect to the ability of the participants to predict their sexual activity: Scenario 1 in which the sexual expectation has 57% positive and 80% negative predictive value, based on surveys of sexual behavior and PrEP adherence in Kenya [23] and Scenario 2 (more optimistic) in which the sexual expectation has 60% positive and 90% negative predictive value (see Table S4 in the Supplemental Digital Content).
Outcomes of Interest
For each individual we compute the proportion of days fully covered (i.e. days with 100% of the biological efficacy of PrEP) and partially covered (i.e. days with some but less than100% biological efficacy) by PrEP over a 12-month period. Whether coverage is full or partial depends on the protection profile assumed (see Fig. 1) and the days since last dose. We also compute the overall PrEP coverage, which we define as the average daily % of biological efficacy provided by each pill-taking pattern, where 0% is assumed for days with no coverage. Similarly, we compute the proportion of sex acts fully and partially covered by PrEP as well as the overall coverage of sexual acts.
For each scenario, 100 event-driven RCTs are simulated to evaluate the efficacy of PrEP in reducing HIV acquisition risk. The relative risk of acquiring HIV when using PrEP is estimated as the ratio of HIV incidence observed in the active vs placebo arm. The observed PrEP efficacy is calculated as 1- relative risk.
Details on all simulation procedures are provided in the Supplemental Digital Content.
Results
Overall PrEP coverage and its impact on individual HIV risk
The comparison between the proportion of days and sex acts fully and partially covered by PrEP under different pill-taking patterns and protection profiles are presented in Table 1 and Table 2, respectively. If PrEP does not provide benefits outside the days it is taken (short-lasting profile), pill-taking patterns have no effect on the days covered. However, the sex act coverage may be affected if the pill-taking pattern is influenced by expected sexual activity. That is illustrated by the larger proportion of sex acts covered compared to days covered in our risk-driven scenarios even when short-lasting PrEP protection is assumed. With longer lasting protection, the proportion of days partially covered increases and overall PrEP coverage improves. Explicitly, the proportion of partially covered days under block, random and periodic patterns of 20% adherence is estimated at 6.1%, 28.6% and 39.4%, increasing overall PrEP coverage by 2.4%, 12% and 15.8%, respectively. The greatest effect of pill-taking patterns occurs with intermediate (50%) adherence where 79.8% PrEP coverage is provided by the periodic pattern compared to 52.4% by block adherence. The effect of pill-taking patterns is even more substantial if the protection of a single dose stretches over 5 days; in the case of low adherence (20%) the fraction of fully covered days varies from 23.1% to 39.7% while the overall PrEP coverage varies from 27.6% to 69.3% depending on the pill-taking pattern. The proportions of sexual acts covered fully, partially or overall are similar to the proportions of days covered for all three pill-taking patterns: periodic, random or block (see Table 2).
Table 1.
Daily coverage provided by different pill-taking patterns under different PrEP protection profiles
| PrEP protection profile | Adherence pattern | Proportion of days covered by PrEP | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Days fully covered | Days partially covered | Overall PrEP coverage1 | ||||||||
| 20%adh | 50%adh | 80%adh | 20%adh | 50%adh | 80%adh | 20%adh | 50%adh | 80%adh | ||
| Short | Random | 20.0% | 50.1% | 80.0% | 0.0% | 0.0% | 0.0% | 20.0% | 50.1% | 80.0% |
| Periodic | 20.0% | 50.0% | 80.0% | 0.0% | 0.0% | 0.0% | 20.0% | 50.0% | 80.0% | |
| Block | 20.0% | 50.0% | 83.3% | 0.0% | 0.0% | 0.0% | 20.0% | 50.0% | 83.3% | |
| Risk driven2 Scenario 1 |
29.3% | 0.0% | 29.3% | |||||||
| Risk driven2 Scenario 2 |
37.1% | 0.0% | 37.1% | |||||||
| Intermediate | Random | 19.9% | 50.0% | 80.0% | 28.6% | 37.4% | 19.1% | 32.0% | 67.4% | 90.2% |
| Periodic | 20.0% | 50.0% | 80.0% | 39.4% | 49.7% | 19.7% | 35.8% | 79.8% | 91.8% | |
| Block | 20.0% | 50.0% | 83.3% | 6.1% | 6.1% | 6.1% | 22.4% | 52.4% | 85.8% | |
| Risk driven2 Scenario 1 |
29.5% | 36.9% | 45.4% | |||||||
| Risk driven2 Scenario 2 |
37.3% | 39.5% | 54.8% | |||||||
| Long | Random | 36.0% | 75.0% | 95.9% | 31.0% | 21.7% | 4.0% | 52.9% | 88.6% | 98.8% |
| Periodic | 39.7% | 99.7% | 99.7% | 59.2% | 0.0% | 0.0% | 69.3% | 99.7% | 99.7% | |
| Block | 23.1% | 53.1% | 86.4% | 9.2% | 9.2% | 9.2% | 27.6% | 57.6% | 91.0% | |
| Risk driven2 Scenario 1 |
29.4% | 55.8% | 70.3% | |||||||
| Risk driven2 Scenario 2 |
37.3% | 55.2% | 79.9% | |||||||
Overall coverage is calculated as average daily % of biological efficacy provided by each pill-taking pattern.
Risk-driven patterns assume that individuals take PrEP in 80% of the days they expect to have sex and 20% otherwise under the sexual expectation scenarios described in the text.
Table 2.
Coverage of sex acts provided by different pill-taking patterns under different PrEP protection profiles
| PrEP protection profile | Adherence pattern | Proportion of sex acts covered by PrEP | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Acts fully covered | Acts partially covered | Acts - overall coverage1 | ||||||||
| 20%adh | 50%adh | 80%adh | 20%adh | 50%adh | 80%adh | 20%adh | 50%adh | 80%adh | ||
| Short | Random | 19.9% | 50.3% | 80.0% | 0.0% | 0.0% | 0.1% | 19.9% | 50.3% | 80.0% |
| Periodic | 20.0% | 50.0% | 80.0% | 0.0% | 0.0% | 0.0% | 20.0% | 50.0% | 80.0% | |
| Block | 20.0% | 49.9% | 83.2% | 0.0% | 0.0% | 0.0% | 20.0% | 49.9% | 83.2% | |
| Risk driven2 Scenario 1 |
40.1% | 0.0% | 40.1% | |||||||
| Risk driven2 Scenario 2 |
61.2% | 0.0% | 61.2% | |||||||
| Intermediate | Random | 20.0% | 50.0% | 80.0% | 28.7% | 37.4% | 19.1% | 32.0% | 67.5% | 90.2% |
| Periodic | 20.1% | 50.1% | 80.1% | 39.6% | 49.7% | 19.7% | 35.9% | 79.9% | 91.9% | |
| Block | 20.1% | 50.1% | 83.5% | 6.1% | 6.1% | 6.0% | 22.5% | 52.5% | 85.9% | |
| Risk driven2 Scenario 1 |
40.4% | 30.9% | 53.8% | |||||||
| Risk driven2 Scenario 2 |
61.5% | 23.1% | 71.7% | |||||||
| Long | Random | 35.9% | 75.1% | 95.8% | 31.1% | 21.6% | 4.0% | 52.8% | 88.8% | 98.8% |
| Periodic | 39.9% | 99.7% | 99.7% | 59.1% | 0.0% | 0.0% | 69.3% | 99.7% | 99.7% | |
| Block | 23.2% | 53.1% | 86.3% | 9.0% | 9.1% | 9.1% | 27.8% | 57.7% | 90.8% | |
| Risk driven2 Scenario 1 |
40.2% | 46.9% | 74.6% | |||||||
| Risk driven2 Scenario 2 |
61.5% | 32.9% | 86.7% | |||||||
Overall coverage is calculated as the average % per act of biological efficacy provided by each pill-taking pattern.
Risk-driven patterns assume that individuals take PrEP in 80% of the days they expect to have sex and 20% otherwise under the sexual expectation scenarios described in the text.
The two risk-driven pill taking scenarios considered in this study result in 29% and 37% overall adherence. Not surprisingly, the estimated PrEP coverage under these scenarios falls between the projections for 20% and 50% adherence with the periodic and random pill-taking. However, assuming a long PrEP profile, the risk-driven pattern provides better coverage than block adherence. More importantly, the sex act coverage with the risk-driven pattern is comparable with that of randomly taken PrEP with 50% adherence.
Potential impact of pill-taking patterns on the observed PrEP efficacy in RCT
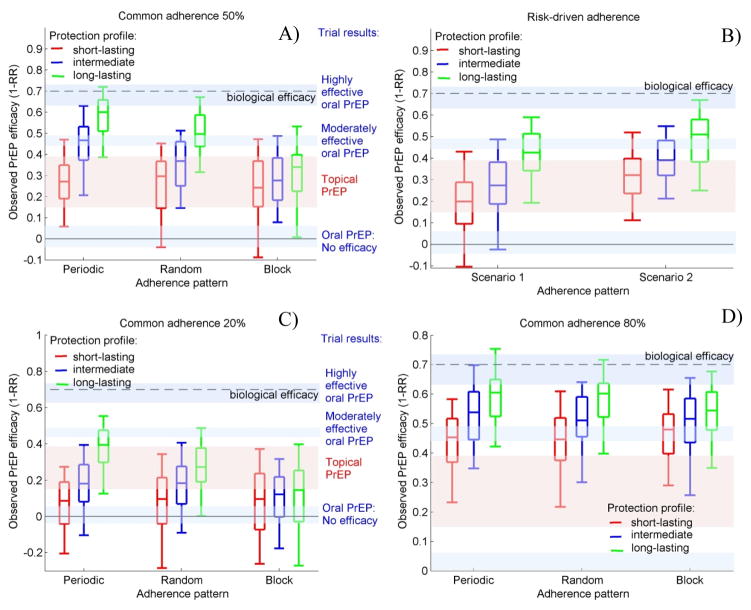
Scenarios with different pill-taking patterns and PrEP protection profiles are presented in Fig.2 and compared to results from concluded RCTs. The highly effective oral PrEP results of Partners PrEP and CDC Botswana suggest long protection combined with high overall adherence and very limited block pill-taking (Fig. 2D, green boxes). The moderate efficacy, observed in iPrEX and Bangkok trials, is comparable with 50% adherence scenarios with periodic pill-taking under intermediate protection or random pill-taking under long protection (Fig. 2A). However, it could also be obtained by the 80% adherence scenarios with short protection (Fig. 2D, red boxes) or risk-driven pill-taking under long protection (Fig. 2B). Trials in which oral PrEP was found not effective are best fitted by scenarios with low adherence and predominantly block pill-taking (Fig. 2C). Results from trials testing topical PrEP, for which we assume short protection, are comparable with the simulations with 50% adherence (Fig.2A, red boxes). The upper end of the efficacy range, observed in CAPRISA 004, could be also obtained by higher overall adherence (Fig.2D) or risk-driven pill-taking with good sex acts prediction (Fig.2B, Scenario 2). Possible explanations for the low efficacy observed in the gel arm of VOICE are low overall adherence (Fig.2C) or risk-driven pill-taking with poor sex acts prediction (Fig.2B, Scenario 1).
Figure 2. Observed PrEP efficacy under different PrEP protection profiles and pill-taking patterns.
Common A) 50% adherence; B) risk-driven C) 20% adherence; and D) 80% adherence of each participant is assumed. Box plots (5th, 25th, 50th, 75th, and 95th percentiles) reflect estimated variation over 100 trials simulated. Risk-driven scenarios, as described in the Methods, result in 29% and 37% actual adherence. Shaded regions illustrate the efficacy estimates obtained in concluded RTCs
This analysis shows that PrEP protection profile has a critical influence on the observed efficacy inducing differences of 33%, 20% and 10% between scenarios when periodic, random or block adherence is assumed, respectively (Fig.2A). The importance of the pill-taking pattern increases when long PrEP protection is assumed. No influence of the pattern of adherence is expected if PrEP protects only in the days when taken as it is likely the case with topical PrEP (red boxes). The long protection profile is associated with 26% difference in the observed efficacy across different protection profiles; we found this mostly benefits periodic pill-taking because of the residual PrEP protection on days when doses are skipped. The differences in the observed efficacy due to the pill-taking pattern decreases if the overall adherence level is high (see Fig. 2D).
Clinical trials with risk-driven pill-taking in which 37% of the daily doses are taken are likely to show better efficacy than trials with random or block pill-taking in which 50% of the daily doses are taken provided that participants are relatively accurate in predicting their sexual activity (Fig.2B, Scenario 2). In populations with less reliable predictions of sex, the projected PrEP efficacy is expected to be 10%–12% lower (Fig.2B, Scenario 1). However, risk-driven pill-taking is still more effective than 50% block adherence if PrEP has long protection (Fig.2A vs. Fig.2B).
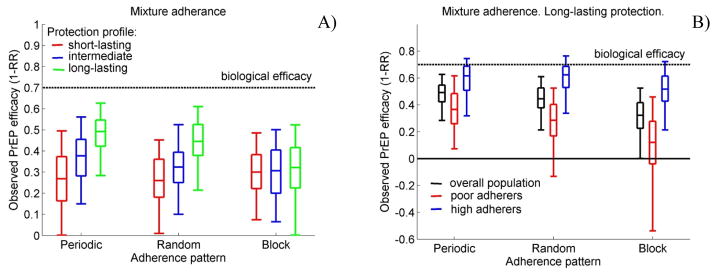
Simulations with a mixture adherence distribution in which all participants follow the same pill-taking pattern show that the differences in PrEP efficacy induced by the pill-taking pattern are primarily driven by the low adherers (Fig.3).
Figure 3. Simulations of populations consisting of equal numbers of high (80% adherence) and poor (20% adherence) adherers.
Observed PrEP efficacy A) in the overall population and B) by adherence levels under different PrEP protection profiles and pill-taking patterns. Box plots (5th, 25th, 50th, 75th, and 95th percentiles) reflect estimated variation over 100 trials simulated.
Results from simulating scenarios assuming 90% biological efficacy of PrEP in reducing the HIV acquisition risk per act are presented in the Supplemental Digital Content (see Fig. S3). They demonstrate that the most optimistic estimates of PrEP efficacy can be matched and even exceeded with 50% actual adherence if PrEP is highly effective and has long-lasting protection. Notable, if block pill-taking is prevalent then low to intermediate efficacy will be observed.
Discussion
Variation in the efficacy observed in PrEP clinical trials has been largely attributed to poor adherence. In this study we demonstrated that pill-taking patterns, in addition to the PrEP protection, profile affect the individual risk of HIV acquisition in PrEP users and may have had a significant influence on the efficacy observed in RCTs that test daily regimens of oral PrEP. The importance of the adherence pattern increases if PrEP provides protection beyond the day in which a dose is taken. Such residual protection was suggested for MSM by the iPrEx team which estimated that even two PrEP doses per week reduced HIV risk by 76%. However, the PrEP protection profile may be different for women due to differences between PrEP accumulation levels in vaginal and rectal mucosa. [24, 25]
We found that the pill-taking pattern mostly affects PrEP efficacy under intermediate and low adherence when PrEP retains protective power for longer than a day. In this case periodic pill-taking pattern provides the greatest PrEP coverage and therefore the highest PrEP efficacy. Although unlikely to be prevalent, such behavior could result from pill sharing with friends or relatives. On the other hand, block adherence had the lowest PrEP protection because the residual efficacy during the days after a dose is taken is barely utilized. However, longer periods of non-use could occur because of separation from a regular sex partner. In that case, the projected disadvantage of this pill-taking pattern could be overestimated.
Risk-driven pill-taking may provide better sex act coverage than random pill-taking with significantly fewer doses but the effectiveness of risk-driven pill-taking strongly depends on the ability of the PrEP users to predict their sexual activity. A study among Kenyan men and women estimated that 20% of the sexual acts are unexpected [23]. However, a coitally-dependent regimen of oral PrEP was evaluated in the recently concluded trial conducted among MSM in France and demonstrated high efficacy [7]. Our analysis suggests that participants with less reliable sexual expectations may expect limited benefit from PrEP use.
Our study has some limitations. When simulating the sexual activity of the female cohort, we do not explicitly account for use of ARV drugs by HIV positive male partners and assume the same risk of HIV acquisition per act with partners in the same HIV stage. The extremely high incidence of HIV infections among women in PrEP trials in South Africa suggests that few of their partners are virally suppressed. Also, we do not account for the disclosure of HIV status by male partners and its influence on the sexual behavior, condom use and adherence to PrEP. The difference in adherence between the RCTs that test oral PrEP in sero-discordant couples and in individually enrolled women suggests that the knowledge of the partner’s HIV status could be a strong incentive to take PrEP consistently. We illustrated the potential effects of pill-taking patterns on the reduction in HIV incidence observed in RCTs by following a cohort of women in high-prevalence settings. Although quantitative results in this study are not directly transferable to other populations and settings we believe that the qualitative conclusions remain valid.
To our knowledge, this is the first modeling study investigating the impact of pill-taking patterns on the efficacy of PrEP. Our analysis provides insights into the consequences of different patterns of poor adherence and the comparative effectiveness of random, block and risk-driven adherence. In addition, we have demonstrated how different patterns of adherence could have resulted in different efficacies, as observed in the PrEP RCTs. We expect that data from ongoing RCTs using Electronic Drug Monitoring devices to track pill-taking patterns of the participants will help us better understand the most prevalent pill-taking practices.[26] Our results suggest that this data should be accounted for when the efficacy of the PrEP is evaluated.
Supplementary Material
Acknowledgments
The authors are grateful to Thea Swanson, Executive Assistant at the Vaccine and Infectious Disease Division of Fred Hutchinson Cancer Research Center for proofreading and editing the final version of the manuscript.
Source of support: DTD and DD are supported by NIH grant (UM1 AI068617).
Footnotes
D.T.D., B.R.M, and D.D. designed the study, interpreted the data, and drafted the article. D.T.D. collected data and carried out the modeling analysis.
All authors reviewed the final draft of the article and approved it for submission.
Data will be presented at: National HIV Prevention Conference, Atlanta, GA, December 6–9, 2015
Contributor Information
Dobromir T. DIMITROV, Vaccine and Infectious Disease Division, Fred Hutchinson Cancer Research Center and Department of Applied Mathematics, University of Washington, Seattle, Washington, USA
Benoît R. MÂSSE, CHU Sainte-Justine Research Centre, University of Montreal, Montreal, Quebec, Canada
Deborah DONNELL, Vaccine and Infectious Disease Division, Fred Hutchinson Cancer Research Center and Department of Global Health, University of Washington, Seattle, Washington, USA.
References
- 1.Grant RM, et al. Preexposure Chemoprophylaxis for HIV Prevention in Men Who Have Sex with Men. New England Journal of Medicine. 2010;363(27):2587–2599. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abdool Karim Q, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329(5996):1168–1174. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baeten JM, et al. Antiretroviral Prophylaxis for HIV Prevention in Heterosexual Men and Women. New England Journal of Medicine. 2012;367(5):399–410. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thigpen MC, et al. Antiretroviral Preexposure Prophylaxis for Heterosexual HIV Transmission in Botswana. New England Journal of Medicine. 2012;367(5):423–434. doi: 10.1056/NEJMoa1110711. [DOI] [PubMed] [Google Scholar]
- 5.Choopanya K, et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2013;381(9883):2083–2090. doi: 10.1016/S0140-6736(13)61127-7. [DOI] [PubMed] [Google Scholar]
- 6.McCormack SD, David . CROI 2015. Seattle, WA: 2015. Pragmatic Open-Label Randomised Trial of Preexposure Prophylaxis: The PROUD Study. [Google Scholar]
- 7.Jean-Michel Molina CC, Charreau Isabelle, Meyer Laurence, Spire Bruno, Pialoux Gilles, Chidiac Christian, Delfraissy Jean-Francois, Tremblay Cecile. CROI 2015. Seattle, WA: 2015. On Demand PrEP With Oral TDF-FTC in MSM: Results of the ANRS Ipergay Trial. [Google Scholar]
- 8.Van Damme L, et al. Preexposure Prophylaxis for HIV Infection among African Women. New England Journal of Medicine. 2012;367(5):411–422. doi: 10.1056/NEJMoa1202614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marrazzo JM, et al. Tenofovir-Based Preexposure Prophylaxis for HIV Infection among African Women. New England Journal of Medicine. 2015;372(6):509–518. doi: 10.1056/NEJMoa1402269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blaschke TF, et al. Adherence to Medications: Insights Arising from Studies on the Unreliable Link Between Prescribed and Actual Drug Dosing Histories. Annual Review of Pharmacology and Toxicology. 2012;52:275-+. doi: 10.1146/annurev-pharmtox-011711-113247. [DOI] [PubMed] [Google Scholar]
- 11.Dimitrov D, Donnell D, Brown ER. High Incidence Is Not High Exposure: What Proportion of Prevention Trial Participants Are Exposed to HIV? PLoS ONE. 2015;10(1):e0115528. doi: 10.1371/journal.pone.0115528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kalichman SC, et al. Heterosexual anal intercourse among community and clinical settings in Cape Town, South Africa. Sexually Transmitted Infections. 2009;85(6):411–415. doi: 10.1136/sti.2008.035287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson L, et al. Sexual behaviour patterns in South Africa and their association with the spread of HIV: insights from a mathematical model. Demographic Research. 2009;21(11):289–340. [Google Scholar]
- 14.Shisana O, TR, Simbayi LC, Zuma K, Jooste S, Mbelle N, Van Zyl J, Pezi S, Parker W, Zungu NP, Pillay-Van Wyk V. South African national HIV prevalence, incidence, behaviour and communication survey, 2008: a turning tide among teenagers? HSRC Press; 2009. p. 120. [Google Scholar]
- 15.Boily MC, et al. Heterosexual risk of HIV-1 infection per sexual act: systematic review and meta-analysis of observational studies. Lancet Infectious Diseases. 2009;9(2):118–129. doi: 10.1016/S1473-3099(09)70021-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baggaley RF, White RG, Boily MC. HIV transmission risk through anal intercourse: systematic review, meta-analysis and implications for HIV prevention. International Journal of Epidemiology. 2010;39(4):1048–1063. doi: 10.1093/ije/dyq057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hallett TB, et al. Optimal Uses of Antiretrovirals for Prevention in HIV-1 Serodiscordant Heterosexual Couples in South Africa: A Modelling Study. Plos Medicine. 2011;8(11):e1001123. doi: 10.1371/journal.pmed.1001123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gomez GB, et al. The Potential Impact of Pre-Exposure Prophylaxis for HIV Prevention among Men Who Have Sex with Men and Transwomen in Lima, Peru: A Mathematical Modelling Study. Plos Medicine. 2012;9(10):e1001323. doi: 10.1371/journal.pmed.1001323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abbas UL, et al. Factors Influencing the Emergence and Spread of HIV Drug Resistance Arising from Rollout of Antiretroviral Pre-Exposure Prophylaxis (PrEP) Plos One. 2011;6(4):e18165. doi: 10.1371/journal.pone.0018165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dimitrov DT, Masse BR, Boily MC. Beating the Placebo in HIV Prevention Efficacy Trials: The Role of the Minimal Efficacy Bound. Jaids-journal of Acquired Immune Deficiency Syndromes. 2013;62(1):95–101. doi: 10.1097/QAI.0b013e3182785638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Donnell D, et al. HIV Protective Efficacy and Correlates of Tenofovir Blood Concentrations in a Clinical Trial of PrEP for HIV Prevention. Jaids-journal of Acquired Immune Deficiency Syndromes. 2014;66(3):340–348. doi: 10.1097/QAI.0000000000000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anderson PL, et al. Emtricitabine-Tenofovir Concentrations and Pre-Exposure Prophylaxis Efficacy in Men Who Have Sex with Men. Science Translational Medicine. 2012;4(151):151ra125. doi: 10.1126/scitranslmed.3004006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Curran K, et al. Daily short message service surveys to measure sexual behavior and pre-exposure prophylaxis use among Kenyan men and women. AIDS and behavior. 2013;17(9):2977–85. doi: 10.1007/s10461-013-0510-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Louissaint NA, et al. Single Dose Pharmacokinetics of Oral Tenofovir in Plasma, Peripheral Blood Mononuclear Cells, Colonic Tissue, and Vaginal Tissue. Aids Research and Human Retroviruses. 2013;29(11):1443–1450. doi: 10.1089/aid.2013.0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patterson KB, et al. Penetration of Tenofovir and Emtricitabine in Mucosal Tissues: Implications for Prevention of HIV-1 Transmission. Science Translational Medicine. 2011;3(112):112re4. doi: 10.1126/scitranslmed.3003174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.HIV Prevention Trials Network. HPTN 067 The ADAPT study: A Phase II, Randomized, Open-Label, Pharmacokinetic and Behavioral Study of the Use of Intermittent Oral Emtricitabine/Tenofovir Disoproxil Fumarate Pre-Exposure Prophylaxis (PrEP) 2013 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.