Abstract
Background
Posttraumatic stress disorder (PTSD) is associated with elevated risk for metabolic syndrome (MetS). However, the direction of this association is not yet established, as most prior studies employed cross-sectional designs. The primary goal of this study was to evaluate bidirectional associations between PTSD and MetS using a longitudinal design.
Methods
1,355 male and female veterans of the conflicts in Iraq and Afghanistan underwent PTSD diagnostic assessments and their biometric profiles pertaining to MetS were extracted from the electronic medical record at two time points (spanning ~2.5 years, n = 971 at time 2).
Results
The prevalence of MetS among veterans with PTSD was just under 40% at both time points and was significantly greater than that for veterans without PTSD; the prevalence of MetS among those with PTSD was also elevated relative to age-matched population estimates. Cross-lagged panel models revealed that PTSD severity predicted subsequent increases in MetS severity (β = .08, p = .002), after controlling for initial MetS severity, but MetS did not predict later PTSD symptoms. Logistic regression results suggested that for every 10 PTSD symptoms endorsed at time 1, the odds of a subsequent MetS diagnosis increased by 56%.
Conclusions
Results highlight the substantial cardiometabolic concerns of young veterans with PTSD and raise the possibility that PTSD may predispose individuals to accelerated aging, in part, manifested clinically as MetS. This demonstrates the need to identify those with PTSD at greatest risk for MetS and to develop interventions that improve both conditions.
Keywords: PTSD, metabolic syndrome, accelerated aging, cross-lagged design, veterans, longitudinal
Posttraumatic stress disorder (PTSD) is associated with substantial medical morbidity (Schnurr et al., 2000; Ahmadi et al., 2011; Bartoli et al., 2013; O'Donovan et al., 2015), with striking effects observed for obesity (Bartoli et al., 2015), and cardiometabolic and cardiovascular conditions (Ahmadi et al., 2011; Heppner et al., 2012; Bartoli et al., 2013; Wentworth et al., 2013; Roberts et al., 2015; Rosenbaum, Stubbs et al., 2015; Roy et al., 2015; Sumner et al., 2015). The co-occurrence of PTSD with metabolic syndrome (MetS), as defined by three or more of central obesity, hypertension, dyslipidemia, and elevated blood sugars (National Cholesterol Education Program, [NCEP], 2001; Grundy et al., 2005), is particularly high, with recent meta-analyses suggesting that MetS is prevalent in nearly 40% of those with PTSD (Bartoli et al., 2013; Rosenbaum, Stubbs et al., 2015). The association between PTSD and MetS is intriguing given that stress is implicated in the pathogenesis and course of MetS (Vitaliano et al., 2002; see also Epel, 2009) and that MetS may be part of the pathway linking PTSD to subsequent deleterious health conditions, such as cardiovascular disease (Roy et al., 2015; Sumner et al., 2015), type 2 diabetes (Roberts et al., 2015), decreased cortical thickness (Wolf et al., in press), cognitive impairment (Green et al., 2015), and premature mortality (Ahmadi et al., 2011).
Although nearly all of the studies linking PTSD to MetS employed a cross-sectional design, many investigators hypothesize that the stress of PTSD influences MetS risk (e.g., Bartoli et al., 2013). This could occur through biological pathways, such as increased autonomic reactivity, immune and hypothalamic-pituitary-adrenal (HPA) axis system dysregulation (Kibler et al., 2014; Levine et al., 2014), and/or oxidative stress processes (Grattagliano et al., 2008). Potential PTSD-related behavioral pathways include poor nutrition and sedentary lifestyle (Hall et al., 2015), cigarette and alcohol use (Dennis et al., 2014), and poor sleep (Talbot et al., 2015). It is also possible that MetS may negatively affect PTSD symptoms. For example, greater pre-deployment inflammation (C-reactive protein), which often co-occurs with MetS, was recently shown to predict subsequent post-deployment PTSD (Eraly et al., 2014). PTSD and MetS may also exert bidirectional effects on the severity of each other, particularly in trauma-exposed samples. In support of this, bidirectional effects have been reported for depression and MetS (Pulkki-Råback et al., 2009), and this may generalize to PTSD given that PTSD is highly comorbid with depression (Pietrzak et al., 2012) and both disorders may arise out of a shared underlying vulnerability towards internalizing psychopathology (Miller et al., 2008). Only longitudinal designs can address the question of directionality. To our knowledge, two such studies exist to date, and both had analytic concerns that limited the strength of the causal conclusions.
Specifically, Francis et al. (2015) followed 78 physically abused children and 349 non-abused children into middle age and found that childhood abuse was associated with PTSD symptoms during young adulthood, which, in turn, predicted obesity in middle-age. Baseline obesity was not controlled for analytically, making it difficult to draw conclusions about the direction of this association. Farr et al. (2015) also suggested that PTSD was associated with increasing metabolic risk by showing that, among 55 urban-area community adults, greater PTSD severity was associated with increased obesity and systolic blood pressure over the course of 2.5 years, controlling for baseline body mass index (BMI). Unfortunately, results were difficult to interpret because of the small sample size, control for only baseline BMI, and the fact that PTSD symptoms were split into quartiles based on sample distribution (i.e., not evaluated per the DSM diagnostic definition or total severity). Neither study tested whether MetS predicted subsequent PTSD.
In light of these concerns, our aim was to evaluate potential bidirectional influences between PTSD and MetS using a cross-lagged panel model (Rosenthal & Rosnow, 1991), which simultaneously evaluates the longitudinal effect of each variable on the other while controlling for baseline levels of both PTSD and MetS. We hypothesized that PTSD would be associated with increasing MetS risk over time, and that if there was evidence for MetS influencing subsequent PTSD, that this effect would be weaker in magnitude than that for PTSD predicting MetS. This aim was evaluated in a large national cohort of US military veterans who deployed to the wars in Iraq and/or Afghanistan and who completed two waves of assessments, separated by approximately 2.5 years (see Rosen et al., 2012). As women were over-sampled and represented just over 50% of the cohort, we were also able to evaluate potential sex differences in the relationship between PTSD and MetS.
Methods
Participants
Participants were U.S. Army or Marine Corps veterans enrolled between 2009 and 2012 in the baseline assessment of Project VALOR (Veterans’ After-Discharge Longitudinal Registry), a registry of VA mental health care users with and without PTSD who deployed in support of Operation Enduring Freedom or Operation Iraqi Freedom (see Supplementary Materials and Rosen et al., 2012 for details). To be included veterans must have undergone a mental health evaluation at a VA facility. Veterans with probable PTSD according to VA medical records (i.e., at least two instances of a PTSD diagnosis by a mental health professional associated with two separate visits) were oversampled at a 3:1 ratio, and female veterans were oversampled to comprise ~50% of the cohort.
The current study included the largest possible subsample of n = 1,355 participants from Project VALOR (out of 1,649 total) who had data pertaining to, at least, Time 1 (T1) PTSD severity and T1 MetS severity. Demographic characteristics of this sample are shown in Table 1. Time 2 (T2) PTSD severity was available for n = 1,124 (83%) of the T1 sample and T2 MetS severity data were available for n = 971 (72%) of the T1 participants, yielding the final T2 n of 971 (see Supplementary Materials for comparisons of those with versus without T2 data).
Table 1.
Demographic and PTSD-Related Characteristics of the Sample
| Variable | M (SD) | Range | n | % |
|---|---|---|---|---|
| Age (years) | 37.86 (9.96) | 22 - 69 | ||
| Sex | ||||
| Male | 655 | 48.3 | ||
| Female | 700 | 51.7 | ||
| Race & Ethnicity | ||||
| White | 878 | 64.8 | ||
| Black | 224 | 16.5 | ||
| Hispanic | 174 | 12.8 | ||
| Other | 70 | 5.2 | ||
| Missing | 9 | 0.7 | ||
| Education Level | ||||
| High School Degree or Equivalent | 136 | 10.0 | ||
| Some Post-High School Education | 760 | 56.1 | ||
| College degree or Higher | 454 | 33.5 | ||
| PTSD | ||||
| Dx at T1 | 902 | 66.6 | ||
| Dx at T2 | 664 | 68.4 | ||
| Severity at T1 | 9.99 (4.79) | 0 - 17 | ||
| Severity at T2 | 11.87 (4.97) | 0 - 20 |
Note. Demographic characteristics are based on T1 data (n = 1,355). The sample size at T2 was 971 and T2 PTSD percentages are based on that total. Comparisons of demographic and other differences between those with and without T2 data are presented in the text. PTSD severity is based on a symptom count of the number of endorsed items on the SCID PTSD module. T1 = time 1; T2 = time 2; PTSD = posttraumatic stress disorder; Dx = diagnosis; M = mean; SD = standard deviation.
Measures
PTSD Module of the Structured Clinical Interview for DSM
Doctoral-level diagnosticians assessed current (past month) PTSD via telephone using the PTSD Module of the Structured Clinical Interview for DSM (SCID). The SCID for DSM-IV (First et al., 2000) was administered at T1 and for DSM-5 (First et al., 2015) at T2. Both have demonstrated excellent psychometric properties (Bovin & Weathers, 2012; Regier et al., 2013). The SCID was administered up to two times at each time point in relationship to two, distinct index traumatic experiences. PTSD symptom severity was operationalized as the maximum score (number of PTSD symptoms endorsed) from either of the two SCID administrations at each time point. PTSD diagnosis was operationalized as meeting the DSM-IV (at T1)/DSM-5 (at T2) diagnostic criteria based on either of the two concurrent SCID administrations. Interviews were digitally recorded and 100 were randomly chosen for secondary independent ratings at T1 and T2 yielding excellent interrater agreement at T1 (κ = .91) and T2 (κ = .82).
Life Events Checklist for DSM-IV (LEC)
The LEC (Gray et al., 2004) is a self-report questionnaire of trauma exposure that comprises the PTSD Criterion A1 assessment on the Clinician Administered PTSD Scale (Blake et al., 1995). Participants indicated if they experienced, witnessed, learned about, or were exposed to any of 16 potentially traumatic events.
Additional measures that were the focus of secondary analyses are described in the Supplementary Materials.
Procedure
At T1, participants provided informed consent verbally over the telephone in accordance with the research protocol approved by all institutional review boards and the Human Research Protection Office of the US Army Medical Research and Materiel Command. Study staff then invited participants to complete a self-administered survey either online or via mail. Once completed, participants underwent diagnostic interview by telephone and received $50 compensation. Approximately 2 to 4 years later, participants were re-contacted for the second phase of the study, which followed the same approach as T1. Participants were compensated $100 at T2.
Data pertaining to MetS features were extracted from the VA electronic medical record using lab values that were linked as closely as possible in time to the SCID-based PTSD assessment and were no more than +/− 6 months of the PTSD assessment. On average, there was well less than a month between the PTSD/biometric assessments (see Supplementary Materials). The two PTSD assessments occurred, on average, ~2.5 years apart (range: 18.80 to 56.50 months; see Supplementary Materials). Time difference variables were evaluated as covariates in preliminary analyses.
MetS was defined per the NCEP Adult Treatment Panel (ATP) III definition (NCEP, 2001; Grundy et al., 2006), as detailed in Table 2.1 MetS severity was defined as the number of MetS criteria present (0-5). MetS diagnosis was defined by meeting three or more of the MetS criteria (NCEP, 2001; Grundy et al., 2006).
Table 2.
Metabolic Syndrome Criteria Definitions
| Criterion | Definition | |
|---|---|---|
| Central Obesity | BMI ≥ 25a | |
| Dyslipidemia | ||
| HDL (mg/dL) | <40 (men) | Or taking cholesterol-lowering medication |
| < 50 (women) | ||
| Triglycerides (mg/dL) | ≥ 150 | Or taking medication for elevated triglycerides |
| Elevated Blood Sugars | ||
| Fasting glucose (mg/dL) | ≥ 100 | Or taking medication for diabetes or elevated glucose |
| Hypertension | ||
| Systolic blood pressure (mm Hg) | ≥ 130 | Or taking medication for hypertension |
| Diastolic blood pressure (mm Hg) | ≥ 85 | |
Note. 3 out of 5 criteria (central obesity, low HDL, high triglycerides elevated glucose, and elevated systolic or diastolic blood pressure) were required for the metabolic syndrome diagnosis. BMI = body mass index; HDL = high-density lipoprotein.
See Footnote #1.
Data Analysis
We first examined the prevalence2 of PTSD diagnosis (on the SCID) and MetS diagnosis (and each MetS criterion) at each time point in the sample overall and then conducted chi-square analyses to evaluate MetS-related differences as a function of PTSD diagnosis at each time point. We also compared a population-based estimate of MetS among 20-39 year olds (20.3%; Ervin, 2009) with the T1 MetS prevalence among veterans with PTSD in this same age group using a Z-test for two population proportions. We then examined each dimensional MetS variable (raw lab values and criteria count) as a function of PTSD diagnosis using t-tests for independent samples. We tested potential differences in metabolic profiles as a function of PTSD diagnosis and sex (and their interaction) using multivariate analysis of variance (MANOVA). Correlations between total lifetime trauma exposure, PTSD severity, and MetS severity at and across each time point and those between MetS, PTSD severity, and potential covariates were evaluated (see Supplementary Materials).
We then ran our primary cross-lagged panel model using the statistical modeling program Mplus 7.11 (Muthén & Muthén, 2012). In the path model, the autoregressive effects of each variable on itself over time (e.g., T1 PTSD to T2 PTSD) were modeled, as were the cross-lagged paths (e.g., T1 PTSD to T2 MetS). These models focused on PTSD severity (symptom count on the SCID) and MetS severity (number of MetS criteria met). The concurrent correlation between the two variables at T1 was modeled as was their residual correlation at T2. Total lifetime trauma exposure (on the LEC) was included as a predictor of T1 MetS and PTSD severity and the indirect effects of trauma exposure on T2 PTSD and MetS severity via T1 PTSD and MetS severity were estimated using the ‘model indirect’ command. Significant covariates, based on the results of initial bivariate correlations, were included as predictors of T1 PTSD and MetS severity. The model employed the robust maximum likelihood estimator (MLR), which accounts for non-normality in the data by adjusting the standard errors to reduce the likelihood of Type I error. This estimator includes all available data using full information likelihood estimation, conditional on the presence of at least one exogenous variable. Due to missing covariate data, the final n for the cross-lagged model was 1,341. Path models were evaluated using standard fit indices and guidelines (Hu & Bentler, 1999).
We then conducted a logistic regression in SPSS to test whether T1 PTSD severity predicted T2 MetS diagnosis, controlling for T1 MetS severity and demographic covariates. Analyses evaluating potential moderators, covariates, and confounders (including combat exposure, depression, substance use, and psychotropic medication use) of our main associations are detailed in the Supplementary Materials.
Results
Prevalence and Severity of PTSD, MetS, and Their Co-Occurrence
Descriptive statistics pertaining to the presence and severity of current PTSD at T1 and T2 are listed in Table 1 and descriptive statistics for MetS are shown in Table 3. Among veterans with PTSD under age 40 at T1, the prevalence of MetS was 29.0%, which was significantly greater than the 20.3% previously reported (Ervin, 2009) in an age-matched epidemiological sample (Z = −3.19, p = .001). In contrast, the prevalence of MetS among veterans without current PTSD in this age group (20.2%) was nearly identical to that reported by Ervin (2009). In the full sample, the mean number of T1 MetS criteria was 2.00 (Table 3), with 89.4% meeting at least one MetS criterion and 70.0% meeting at least 2 MetS criteria. At T2, the mean number of MetS criteria was 2.10 (Table 3), with 90.5% above the threshold for at least one MetS criterion and 63.6% above the threshold for at least two criteria.3 At T1, 17.4% were taking cholesterol-lowering medication, 27% anti-hypertensive medication, and 2.4% were taking diabetes-related medication. At T2, 15.1% were taking cholesterol- lowering medication, 23.6% were taking anti-hypertensive, and 3.2% were taking diabetes-related medications. This medication use was factored into the MetS definition (Table 2).
Table 3.
Metabolic Syndrome Diagnosis and Features in the Overall Sample and as a Function of PTSD Diagnosis
| MetS Variable | Mean (SD) |
% Meeting MetS Criterion |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All | PTSD+ | PTSD- | p | All | PTSD+ | PTSD- | p | |||||||||
| T1 | T2 | T1 | T2 | T1 | T2 | T1 | T2 | T1 | T2 | T1 | T2 | T1 | T2 | T1 | T2 | |
|
Obesity (BMI; kg/m2) |
29.78 (5.41) |
30.22 (5.69) |
30.03 (5.29) |
30.13 (5.58) |
29.28 (5.60) |
30.40 (5.92) |
.018 | .509 | 79.9 | 81.8 | 82.5 | 80.8 | 74.7 | 83.9 | .001 | .254 |
|
Blood Pressure (mmHg) |
50.6 | 53.6 | 56.1 | 57.7 | 39.6 | 44.6 | .000 | .000 | ||||||||
| Diastolic | 76.22 (10.37) |
77.42 (10.07) |
76.83 (10.36) |
77.41 (10.03) |
75.02 (10.32) |
77.43 (10.16) |
.003 | .973 | ||||||||
| Systolic | 122.14 (12.77) |
123.26 (13.68) |
122.76 (12.94) |
123.44 (13.84) |
120.92 (12.37) |
122.87 (13.34) |
.013 | .555 | ||||||||
|
HDL Cholesterol (mg/dL) |
47.71 (15.08) |
49.99 (15.71) |
46.76 (15.08) |
49.91 (15.62) |
49.54 (14.93) |
50.16 (15.96) |
.009 | .849 | 46.1 | 41.6 | 47.3 | 43.2 | 43.8 | 38.0 | .316 | .208 |
|
Triglycerides (mg/dL) |
143.67 (107.16) |
147.29 (105.16) |
152.04 (115.51) |
151.20 (114.42) |
127.18 (86.28) |
138.59 (80.41) |
.001 | .155 | 48.0 | 51.8 | 50.6 | 51.8 | 43.0 | 51.9 | .029 | .985 |
| Glucose (mg/dl) | 95.61 (19.23) |
98.15 (28.13) |
97.38 (21.34) |
98.40 (25.53) |
91.91 (13.09) |
97.57 (33.46) |
.000 | .708 | 13.0 | 16.6 | 14.7 | 17.5 | 9.5 | 14.4 | .020 | .287 |
|
Total # MetS Features |
2.00 (1.26) |
2.10 (1.28) |
2.11 (1.27) |
2.16 (1.30) |
1.77 (1.21) |
1.96 (1.24) |
.000 | .026 | ||||||||
| MetS Dx | 33.1 | 35.6 | 36.6 | 37.8 | 26.3 | 30.9 | .000 | .038 | ||||||||
Note. The number of participants for each analysis varied due to missing data. Details are as follows: At Time 1: (a) BMI Total = 1303, PTSD+ = 864, PTSD- = 439; (b) Diastolic BP Total = 1325, PTSD+ = 881, PTSD- = 444; (c) Systolic BP Total = 1326, PTSD+ = 882, PTSD- = 444; (d) HDL Cholesterol Total = 900, PTSD+ = 594, PTSD- = 306; (e) Triglycerides Total = 888, PTSD+ = 589, PTSD- = 299; (f) Glucose Total = 1013, PTSD+ = 686, PTSD- = 327; (g) Total # of MetS Features Total = 1355, PTSD+ = 741, PTSD- = 407; (h) Mets Dx Total = 1355, PTSD+ = 741, PTSD- = 407. Sample sizes at Time 2: (a) Total = 927, PTSD+ = 635, PTSD- = 292; (b) Total = 944, PTSD+ = 649, PTSD- = 295; (c) Total = 946, PTSD+ = 649, PTSD- = 297; (d) Total = 669, PTSD+ = 461, PTSD- = 208; (e) Total = 658, PTSD+ = 454, PTSD- = 204; (f) Total = 760, PTSD+ = 532, PTSD- = 228; (g) Total = 971, PTSD+ = 664, PTSD- = 307; (h) Total = 971, PTSD+ = 664, PTSD- = 307. Using the higher-BMI cut-point of 30 in the diagnostic algorithm yielded a MetS prevalence of 25.7% at T1 and 28.5% at T2 (of those with T2 data). A greater percentage of individuals with PTSD were diagnosed with MetS using this more stringent BMI criterion at both T1 and T2, per chi-square analysis. Using the higher- BMI cut-point of 30 in the MetS criteria count revealed that 79% were above the threshold for at least 1 MetS criterion and 49% were above the threshold for at least 2 MetS criteria at T1. At T2, 80% were above the threshold on at least 1 MetS criterion and 52% were above the threshold on at least 2 MetS criteria. At both time points, t-tests revealed that individuals with PTSD met the threshold for a greater number of MetS criteria than did those without PTSD. MetS = metabolic syndrome; SD = standard deviation; PTSD+ = positive posttraumatic stress disorder diagnosis; PTSD- = negative posttraumatic stress disorder diagnosis; T1 = Time 1; T2 = Time 2; BMI = body mass index; HDL = high density lipoprotein; Dx = diagnosis. P-values for dimensional variables are based on independent t-tests as a function of current (T1 or T2) PTSD diagnosis. P-values for categorical variables are based on Pearson chi-square tests.
As shown in Table 3, chi-square analyses revealed that the prevalence of T1 MetS diagnosis was greater among those with a concurrent PTSD diagnosis (36.6%) compared to those without (26.3%; p < .001). This held at T2, wherein the prevalence of T2 MetS diagnosis was 37.8% among those with a concurrent PTSD diagnosis and 30.9% among those without (p = .038). Individuals with PTSD at T1 also met criteria for a greater number of T1 MetS features compared with those without T1 PTSD (Table 3). Chi square analyses suggested that a greater percentage of individuals with T1 PTSD met the criteria for central obesity, hypertension, elevated blood sugars, and high triglycerides than those without PTSD (Table 3). Additionally, t-tests revealed higher mean raw lab values for each T1 MetS component among this group (Table 3). Those with PTSD at T2 also met criteria for a greater number of T2 MetS features compared with those without T2 PTSD (Table 3); however, no group differences emerged in the mean T2 raw metabolic values and the only T2 criterion difference was for hypertension (Table 3).
MANOVAs examined Sex, PTSD, and Sex X PTSD differences in raw metabolic lab values at each time point. At T1, the multivariate test yielded main effects for Sex (Pillai's Trace = .163; F [6, 800] = 25.96, p < .001) and PTSD (Pillai's Trace = .030, F [6, 800] = 4.14, p < .001), but no significant interaction between the two (Pillai's Trace = .013, F [6, 800] = 1.78, p = .10). All sex differences were in the direction of women evidencing less pathological lab values than the men (details available from first author). The main effect of Sex held at T2 (Pillai's Trace = .158, F [6, 619] = 19.35, p < .001), but there were no significant multivariate main effects of T2 PTSD or of PTSD X Sex. Based on this, sex was not included as a moderator in primary models, though it was included as a covariate and evaluated further in secondary analyses (see Supplementary Materials).
Cross-lagged Panel Models
Preliminary correlation-based analyses are detailed in the Supplementary Materials and Supplementary Table 1. We found that none of the time difference variables were correlated with their respective dependent variables, so they were excluded from path models. In contrast, race, sex, age, and education were associated with some or all of the PTSD and MetS variables (detailed in the Supplementary Materials) and were therefore included as covariates of T1 PTSD and MetS.4
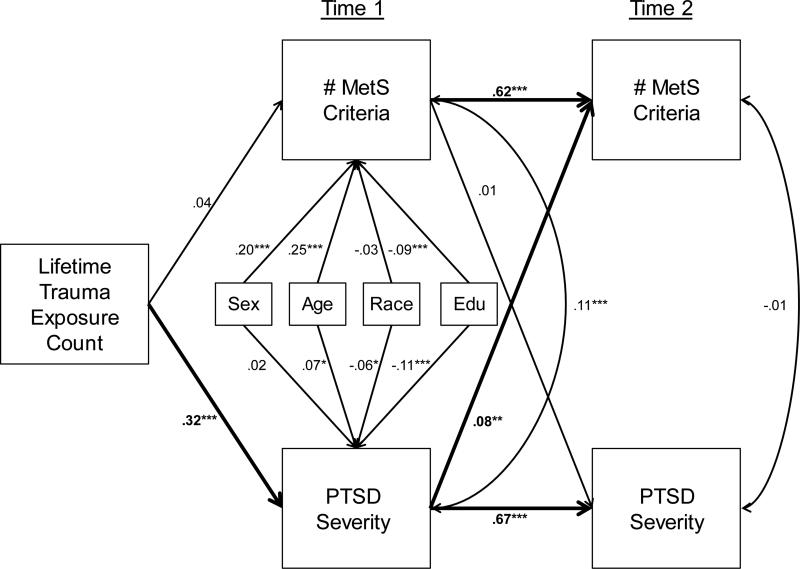
The cross-lagged panel model fit the data well: χ2 (10, n = 1341) = 59.10, p < .001, root mean square error of approximation (RMSEA) = .06, standardized root mean square residual (SRMR) = .02, confirmatory fit index (CFI) = .97, Tucker-Lewis index (TLI) = .91. As shown in Figure 1, T1 PTSD severity was a strong predictor of T2 PTSD severity (β = .67, p < .001), and T1 MetS severity was strongly related to T2 MetS severity (β = .62, p < .001). After controlling for these autoregressive effects, we found a significant cross-lagged effect, such that T1 PTSD severity predicted T2 MetS severity (β = .08, p = .002), but T1 MetS did not predict T2 PTSD severity (β = .005, p = .82). The association between PTSD and MetS severity at T1 was significant; however their residual correlation was not significant at T2 after controlling for the shared effects of T1 variables. Age, sex, and education were significant covariates of T1 MetS severity; age, race, and education were significant covariates of T1 PTSD severity (Figure 1). Total lifetime trauma exposure at T1 was significantly associated with T1 PTSD severity but not with T1 MetS. Results suggested indirect effects of trauma on T2 MetS severity via T1 PTSD severity (β = .03, p = .002) and on T2 PTSD severity via T1 PTSD severity (β = .21, p < .001). In total, the model explained 40% of the variance in T2 MetS severity and 44% of the variance in T2 PTSD severity. Analyses of potential confounds of key associations are reported in the Supplementary Materials; none altered the primary pattern of results.
Figure 1.
The figure shows the results of the cross-lagged panel model. Primary and significant paths of interest are bolded. Correlations are represented via double-headed arrows and regressive paths via single headed arrows. MetS = metabolic syndrome; PTSD = posttraumatic stress disorder; Edu = educational attainment. *p < .05. **p < .01. ***p < .001.
Effects of PTSD on Subsequent MetS Diagnosis
Finally, to evaluate the effects of PTSD severity on subsequent MetS diagnosis, we conducted a logistic regression with T1 PTSD severity and T1 MetS severity as predictors of T2 MetS diagnosis, controlling for age, race, sex, and education. We found that for every additional PTSD symptom at T1, the odds ratio for MetS diagnosis at T2 increased by 5.6% (95% CI: 1.9% to 9.4%; Wald χ2 [1, n = 961] = 9.09, p = .003). This means that for every 10 symptoms endorsed on the SCID at T1, the odds of a MetS diagnosis at T2 increased by 56%. Each increase in MetS criteria at T1 was associated with nearly three times the odds for a subsequent MetS diagnosis (OR = 2.86, 95% CI = 2.44 to 3.37, Wald χ2 [1, n = 961] = 124.31, p < .001). The Nagelkerke R2 for the overall model was .40 (p < .001).5
Discussion
This study adds to a growing chorus of concerns regarding substantial PTSD-related metabolic health decline among veterans of the conflicts in Iraq and Afghanistan. In contrast to prior epidemiological estimates of MetS in the US population (20.3% among 20-39 year olds; Ervin, 2009), we found substantially more veterans (29.0%) with PTSD in this age range with MetS. Our estimates of the prevalence of MetS (36.6% at T1 and 37.8% at T2) among those with PTSD are remarkably similar to the near 40% that has been reported in two recent meta-analyses (Bartoli et al., 2013; Rosenbaum, Stubbs et al., 2015). This study extended prior work by addressing a critical question that has, to date, gone unanswered regarding the temporal relationships between PTSD and MetS. Results indicated that PTSD increased MetS risk over the course of, on average, 2.5 years, after controlling for initial MetS features, but that MetS did not predict subsequent PTSD symptoms.
That PTSD longitudinally predicted MetS carries implications for conceptualizing the course and treatment of both conditions. MetS is considered a syndrome (as opposed to a disease) in part because there is no obvious biological process that connects the individual MetS features. Traumatic stress may be one pathogenic environmental factor that, through biological and behavioral pathways, simultaneously intensifies the degeneration of multiple physiological processes and links them together. For example, PTSD may lead to both cardiovascular and HPA axis system dysregulation (Kibler et al., 2014; Brudey et al., 2015), which would be expected to increase blood pressure, circulating lipids, blood sugars, and inflammation (Epel, 2009); together, these alterations can increase central fat deposits (Epel, 2009). At the same time, PTSD-related increases in reactive oxygen species (Miller et al., 2014; Gautam et al., 2015; Atli et al., in press) may alter the expression of genes important for regulating metabolic processes, ultimately compounding metabolic dysregulation (Grattagliano et al., 2008). In addition, PTSD-related poor sleep (Gavrieli et al., 2015; Talbot et al., 2015), unhealthy diet (Hall et al., 2015), insufficient exercise (Georgiades et al., 2000; Hall et al., 2015), cigarette and alcohol use (Dennis et al., 2014), and psychotropic medication use (Vancampfort et al., 2015) may exert effects on metabolic health that additively and/or synergistically further contribute to the cascade of broad metabolic dysfunction.
We suspect that PTSD-related MetS may reflect an underlying process wherein the stress and chronicity of PTSD symptoms contribute to accelerated cellular aging and premature disease onset (Miller et al., 2014; Lohr et al., 2015; Wolf et al., 2016). The prevalence of MetS is strongly associated with age in the US population (Ervin et al., 2009); however, we found that PTSD was associated with MetS independent of age, with a prevalence that was greater than expected by age. Thus, MetS may occur prematurely among those with PTSD and may be a clinical manifestation of accelerated aging. Consistent with this, prior work suggests that: (a) PTSD is related to advanced cellular age compared to chronological age, as reflected in DNA methylation (Wolf et al., 2016) and telomere length (Tyrka et al., 2015); and (b) metabolic dysregulation is also associated with shortened telomere length (Epel, 2009) and contributes to biological aging (Belsky et al., 2015). Moreover, in our prior work in an independent sample of veterans from the wars in Iraq and Afghanistan, we found that PTSD-related MetS was cross-sectionally associated with substantial and widespread decreases in cortical thickness across temporal, parietal, and frontal brain regions (Wolf et al., in press). Together, these findings suggest that PTSD-related accelerated cellular aging may be reflected in premature genomic, physical health, and neurocognitive decline, highlighting the need to identify those at greatest risk and develop effective interventions.
It may be prudent to closely monitor the metabolic profiles of individuals with PTSD, even among young adults, so that early indications of problems can be discussed with the patient, careful consideration paid to the potential for weight gain side effects in prescribed medications, lifestyle changes recommended, and an appropriate treatment plan aimed at reducing metabolic pathologies enacted. Early screening for other age-dependent health conditions (e.g., cardiovascular disease, type 2 diabetes) may also be warranted. Although we found sex-related differences in MetS features, we found no evidence that PTSD was differentially related to MetS as a function of sex; thus early MetS screening among individuals with PTSD should be conducted with both men and women.
With respect to treatment implications, a recent, if small, meta-analysis found that physical activity was an effective intervention for PTSD (Rosenbaum, Vancampfort, et al., 2015) and may also improve physical health parameters among individuals with PTSD (Rosenbaum, Sherrington et al., 2015). No study to date has evaluated if exercise intervention for PTSD can reverse MetS, making this an important area for future research. It is also important for future trials of PTSD treatments to evaluate if psychological interventions for PTSD have indirect beneficial effects on MetS.
Results should be interpreted in light of study limitations including that metabolic profiles were not directly measured but instead were extracted from the medical record. This undoubtedly added methodological variance to the measurement of MetS (e.g., time between assessments, lab procedures), which would be expected to attenuate the magnitude of our associations. This medical record approach also led to missing data that we addressed via our analytic design, but which may alter results compared with complete data. There are also a number of other potentially important covariates and health indicators (e.g., insulin, inflammation, waist-to-hip-ratio, waist circumference) that we were unable to reliably assess via medical record review and that could have allowed us to test the International Diabetes Federation's ethnicity-based MetS criteria (Alberti et al., 2005). With respect to the longitudinal design of the study, we controlled for baseline PTSD symptoms, but the metabolic profiles of individuals prior to trauma exposure and PTSD onset were not available. We did not observe PTSD group differences in raw lab values at T2 and this may have been due to differences in sample characteristics (e.g., PTSD severity; see Supplementary Materials) among those who did versus did not complete T2. The DSM changed from version IV to 5 between T1 and T2, and this could have also lead to different patterns of results in group-based analyses at T2 compared to T1. However, this would not be expected to substantively alter our primary results, which were focused on PTSD severity evaluated via regression, as prior work comparing DSM-IV with DSM-5 PTSD severity suggests very strong correlations across the two definitions (Miller et al., 2013; Bovin et al., in press).
The strengths of this study include that it is the first longitudinal evaluation of potential bi-directional associations between PTSD and MetS that controls for baseline effects and does so parsimoniously in a single analysis. Additional study strengths include the large sample size, inclusion of Iraq/Afghanistan veterans from across the country, the ability to evaluate sex-specific effects, and our use of a structured diagnostic interview to assess PTSD.
In conclusion, we found that young veterans of the conflicts in Iraq and Afghanistan with PTSD exhibited signs of substantial premature health decline. This should be of grave concern to mental health and primary care clinicians alike and suggests the critical importance of developing interventions that reduce both psychiatric and metabolic pathology. MetS is hugely costly on its own (Sullivan et al., 2007), and the economic, personal, and societal costs can only balloon if the condition gives rise to other associated diseases such as premature cardiovascular disease (Lakka et al., 2002), type 2 diabetes (Wilson et al., 2005), cancer (Esposito et al., 2012), dementia (Yaffe et al., 2004), and death (Lakka et al., 2002). This is a major public health concern and addressing it in this population now has the potential to reduce preventable morbidity and mortality among the nation's newest cohort of veterans.
Supplementary Material
Acknowledgements
This work was supported by a Career Development Award (no grant #) to Erika J. Wolf from the United States (U.S.) Department of Veterans Affairs, Clinical Sciences Research and Development Program. Brian Marx and Terence Keane were supported by funding from the Department of Defense (W81XWH-08-2-0100, W81XWH-12-2-0117). Dr. Marx was additionally supported by funding from the National Institute of Mental Health (1R01MH095737-01A1), Defense Advanced Research Programs Agency (N66001-11-C-4006), and Department of Defense (W81XWH-10-2-0181), and the Department of Veterans Affairs (Cooperative Studies Program # 591). Dr. Keane was additionally supported by funding from the Consortium to Alleviate PTSD and National Institute of Mental Health (5T32MH019836-16). Raymond Rosen was supported by funding from the Department of Defense, (W81XWH-12-1-0532; W81XWH-14-2-0139; W81XWH-08-2-0102, and W81XWH-12-2-0121). Karen Mitchell's contribution was partly supported by funding from the National Institute of Mental Health (K01MH093750). Lewina Lee was supported by funding from the National Institute on Aging (K08AG048221) and the National Center for Advancing Translational Sciences (BU-CTSI Grant Number 1UL1TR001430). The contents of this article do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
We would like to thank Gayatri Rangananthan of NERI for her contribution to this work, which included data management and manuscript review.
Footnotes
Waist-to-hip ratio and waist circumference are superior metrics of central obesity but were not available in the medical record, so we used BMI instead. A BMI cut-off of 25 is classified as “overweight” and a BMI of 30 is the cut-point for “obese” (World Health Organization, 2000), with a wide range of optimal cut-points for metabolic syndrome reported in the literature (e.g., Zandieh et al., 2012; Liu et al., 2013). Given this, we ran our primary cross-lagged analyses using both cut-points and found no differences in results (i.e., same pattern of statistical significance and cross-lagged path coefficients within .01 of each other). Thus, we retained the lower cut-point to be as inclusive as possible.
We use the term “prevalence” throughout the manuscript with the following caveat: as the registry over-sampled veterans with probable PTSD and also over-sampled women, prevalence may be over-estimated and may not generalize to the broader population of veterans of the wars in Iraq and Afghanistan.
See note to Table 3 for discussion of results using the higher BMI cut-point.
In a separate model, we also included these demographic variables as covariates of T2 MetS and PTSD severity and found that doing so did not alter the primary pattern of results. Therefore, for the sake of simplicity, we present the results with these variables included as covariates of the T1 variables only.
Similar results were obtained when we substituted T1 PTSD diagnosis for T1 PTSD severity: the odds of a subsequent MetS diagnosis increased by 75% for veterans with T1 PTSD (95% CI: 1.24 to 2.46; p = .001).
Conflicts of Interest
All authors report no financial, professional, or personal conflicts of interest.
Ethical Standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
References
- Ahmadi N, Hajsadeghi F, Mirshkarlo HB, Budoff M, Yehuda R, Ebrahimi R. Post-traumatic stress disorders, coronary atherosclerosis, and mortality. American Journal of Cardiology. 2011;208:29–33. doi: 10.1016/j.amjcard.2011.02.340. [DOI] [PubMed] [Google Scholar]
- Alberti KG, Zimmet P, Shaw J, IDF Epidemiology Task Force Consensus Group The metabolic syndrome—a new world definition. Lancet. 2005;366:1059–1062. doi: 10.1016/S0140-6736(05)67402-8. [DOI] [PubMed] [Google Scholar]
- Atli A, Bulut M, Bez Y, Kaplan I, Özdemir PG, Uysal C, Selçuk H, Sir A. Altered lipid peroxidation markers are related to post-traumatic stress disorder (PTSD) and not trauma itself in earthquake survivors. European Archives of Psychiatry and Clinical Neuroscience. doi: 10.1007/s00406-015-0638-5. in press [epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Bartoli F, Carrà G, Crocamo C, Caretta D, Clerici M. Metabolic syndrome in people suffering from posttraumatic stress disorder: a systematic review and meta-analysis. Metabolic Syndrome and Related Disorders. 2013;11:301–308. doi: 10.1089/met.2013.0010. [DOI] [PubMed] [Google Scholar]
- Bartoli F, Crocamo C, Alamia A, Amidani F, Paggi E, Pini E, Clerici M, Carrà G. Posttraumatic stress disorder and risk of obesity: systematic review and meta-analysis. Journal of Clinical Psychiatry. 2015;76:e1253–e1261. doi: 10.4088/JCP.14r09199. [DOI] [PubMed] [Google Scholar]
- Belsky DW, Caspi A, Houts R, Cohen HJ, Corcoran DL, Danese A, Harrington H, Israel S, Levine ME, Schaefer JD, Sugden K, Williams B, Yashin AI, Poulton R, Moffitt TE. Quantification of biological aging in young adults. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:E4104–E4110. doi: 10.1073/pnas.1506264112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, Keane TM. The development of a clinician-administered PTSD scale. Journal of Traumatic Stress. 1995;8:75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- Bovin MJ, Marx BP, Weathers FW, Gallagher MW, Rodriguez P, Schnurr PP, Keane TM. Psychometric properties of the PTSD Checklist for DSM-5 (PCL-5) in Veterans. Psychological Assessment. doi: 10.1037/pas0000254. in press. [DOI] [PubMed] [Google Scholar]
- Bovin MJ, Weathers FW. Assessing PTSD Symptoms. In: Beck JG, Sloan DM, editors. The Oxford Handbook of Traumatic Stress Disorders. Oxford University Press; New York: 2012. pp. 235–249. [Google Scholar]
- Brudey C, Park J, Wiaderkiewicz J, Kobayashi I, Mellman T, Marvar P. Autonomic and inflammatory consequences of posttraumatic stress disorder and the link to cardiovascular disease. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology. 2015;309:R315–R321. doi: 10.1152/ajpregu.00343.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis PA, Watkins LL, Calhoun PS, Oddone A, Sherwood A, Dennis MF, Rissling MB, Beckham JC. Posttraumatic stress, heart rate variability, and the mediating role of behavioral health risks. Psychosomatic Medicine. 2014;76:629–637. doi: 10.1097/PSY.0000000000000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel ES. Psychological and metabolic stress: a recipe for accelerated cellular aging? Hormones (Athens) 2009;8:7–22. doi: 10.14310/horm.2002.1217. [DOI] [PubMed] [Google Scholar]
- Eraly SA, Nievergelt CM, Maihofer AX, Barkauskas DA, Biswas N, Agorastos A, O'Connor DT, Baker DG, Marine Resiliency Study Team Assessment of plasma C-reactive protein as a biomarker of posttraumatic stress disorder risk. JAMA Psychiatry. 2014;71:423–431. doi: 10.1001/jamapsychiatry.2013.4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ervin RB. Prevalence of metabolic syndrome among adults 20 years of age and over, by sex, age, race and ethnicity, and body mass index: United States, 2003-2006. National Health Statistics Report. 2009;(13):1–7. [PubMed] [Google Scholar]
- Esposito K, Chiodini P, Colao A, Lenzi A, Giugliano D. Metabolic syndrome and risk of cancer: a systematic review and meta-analysis. Diabetes Care. 2012;35:2402–2411. doi: 10.2337/dc12-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farr OM, Ko BJ, Joung KE, Zaichenko L, Usher N, Tsoukas M, Thakkar B, Davis CR, Crowell JA, Mantzoros CS. Posttraumatic stress disorder, alone or additively with early life adversity, is associated with obesity and cardiometabolic risk. Nutrition, Metabolism, and Cardiovascular Diseases. 2015;25:479–488. doi: 10.1016/j.numecd.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis MM, Nikulina V, Widom CS. A prospective examination of the mechanisms linking childhood physical abuse to body mass index in adulthood. Child Maltreatment. 2015;20:203–213. doi: 10.1177/1077559514568892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M, Spitzer R, Williams J, Gibbon M. Structured Clinical Interview for DSM-IV Axis 1 Disorders (SCID-I). In: Association AP, editor. Handbook of Psychiatric Measures. American Psychiatric Association; Washington DC.: 2000. pp. 49–53. [Google Scholar]
- First MB, Williams JBW, Karg RS, Spitzer RL. Structured Clinical Interview for DSM-5—Research Version (SCID-5 for DSM-5, Research Version; SCID-5-RV) American Psychiatric Association; Arlington, VA: 2015. [Google Scholar]
- Gautam A, D'Arpa P, Donohue DE, Muhie S, Chakraborty N, Luke BT, Grapov D, Carroll EE, Meyerhoff JL, Hammamieh R, Jett M. Acute and chronic plasma metabolomics and liver transcriptomic stress effects in a mouse model with features of post-traumatic stress disorder. PLoS One. 2015;10:e0117092. doi: 10.1371/journal.pone.0117092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrieli A, Farr OM, Davis CR, Crowell JA, Mantzoros CS. Early life adversity and/or posttraumatic stress disorder severity are associated with poor diet quality, including consumption of trans fatty acids, and fewer hours of resting or sleeping in a US middle-aged population: A cross-sectional and prospective study. Metabolism. 2015;64:1597–1610. doi: 10.1016/j.metabol.2015.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiades A, Sherwood A, Gullette EC, Babyak MA, Hinderliter A, Waugh R, Tweedy D, Craighead L, Bloomer R, Blumenthal JA. Effects of exercise and weight loss on mental stress-induced cardiovascular responses in individuals with high blood pressure. Hypertension. 2000;36:171–176. doi: 10.1161/01.hyp.36.2.171. [DOI] [PubMed] [Google Scholar]
- Grattagliano I, Palmieri VO, Portincasa P, Moschetta A, Palasciano G. Oxidative stress-induced risk factors associated with metabolic syndrome: a unifying hypothesis. Journal of Nutritional Biochemistry. 2008;19:491–504. doi: 10.1016/j.jnutbio.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Gray MJ, Litz BT, Hsu JL, Lombardo TW. Psychometric Properties of the Life Events Checklist. Assessment. 2004;11:330–341. doi: 10.1177/1073191104269954. [DOI] [PubMed] [Google Scholar]
- Green E, Fairchild JK, Kinoshita LM, Noda A, Yesavage J. Effects of posttraumatic stress disorder and metabolic syndrome on cognitive aging in veterans. Gerontologist. 2015 doi: 10.1093/geront/gnv040. pii: gnv040. [DOI] [PubMed] [Google Scholar]
- Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, Jr, Spertus JA, Costa F. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement: Executive Summary. Critical Pathways in Cardiology. 2005;4:198–203. doi: 10.1097/00132577-200512000-00018. [DOI] [PubMed] [Google Scholar]
- Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, Jr, Spertus JA, Costa F. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Current Opinion in Cardiology. 2006;21:1–6. doi: 10.1097/01.hco.0000200416.65370.a0. [DOI] [PubMed] [Google Scholar]
- Hall KS, Hoerster KD, Yancy WS., Jr Post-traumatic stress disorder, physical activity, and eating behaviors. Epidemiologic Review. 2015;37:103–115. doi: 10.1093/epirev/mxu011. [DOI] [PubMed] [Google Scholar]
- Heppner PS, Lohr JB, Kash TP, Jin H, Wang H, Baker DG. Metabolic syndrome: relative risk associated with post-traumatic stress disorder (PTSD) severity and antipsychotic medication use. Psychosomatics. 2012;53:550–558. doi: 10.1016/j.psym.2012.05.005. [DOI] [PubMed] [Google Scholar]
- Hu L, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling. 1999;6:1–55. [Google Scholar]
- Kibler JL, Tursich M, Ma M, Malcolm L, Greenbarg R. Metabolic, autonomic and immune markers for cardiovascular disease in posttraumatic stress disorder. World Journal of Cardiology. 2014;6:455–461. doi: 10.4330/wjc.v6.i6.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakka HM, Laaksonen DE, Lakka TA, Niskanen LK, Kumpusalo E, Tuomilehto J, Salonen JT. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA. 2002;288:2709–2716. doi: 10.1001/jama.288.21.2709. [DOI] [PubMed] [Google Scholar]
- Levine AB, Levine LM, Levine TB. Posttraumatic stress disorder and cardiometabolic disease. Cardiology. 2014;127:1–19. doi: 10.1159/000354910. [DOI] [PubMed] [Google Scholar]
- Liu P, Ma F, Lou H, Liu Y. The utility of fat mass index vs. body mass index and percentage of body fat in the screening of metabolic syndrome. BMC Public Health. 2013;13:629. doi: 10.1186/1471-2458-13-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohr JB, Palmer BW, Eidt CA, Aailaboyina S, Mausbach BT, Wolkowitz OM, Thorp SR, Jeste DV. Is post-traumatic stress disorder associated with premature senescence? A review of the literature. American Journal of Geriatric Psychiatry. 2015;23:709–725. doi: 10.1016/j.jagp.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MW, Fogler JM, Wolf EJ, Kaloupek DG, Keane TM. The internalizing and externalizing structure of psychiatric comorbidity in combat veterans. Journal of Traumatic Stress. 2008;21:58–65. doi: 10.1002/jts.20303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MW, Sadeh N. Traumatic stress, oxidative stress and post-traumatic stress disorder: neurodegeneration and the accelerated-aging hypothesis. Molecular Psychiatry. 2014;19:1156–1162. doi: 10.1038/mp.2014.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MW, Wolf EJ, Kilpatrick D, Resnick H, Marx BP, Holowka DW, Keane TM, Rosen RC, Friedman MJ. The prevalence and latent structure of proposed DSM-5 posttraumatic stress disorder symptoms in US national and veteran samples. Psychological Trauma. 2013;5:501–512. [Google Scholar]
- Muthén LK, Muthén BO. Mplus user's guide. Muthèn & Muthèn; Los Angeles, CA: 2012. [Google Scholar]
- National Cholesterol Education Program [NCEP] Executive summary of the third report of the National Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- O'Donovan A, Cohen BE, Seal KH, Bertenthal D, Margaretten M, Nishimi K, Neylan TC. Elevated risk for autoimmune disorders in Iraq and Afghanistan veterans with posttraumatic stress disorder. Biological Psychiatry. 2015;77:365–374. doi: 10.1016/j.biopsych.2014.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrzak RH, Goldstein RB, Southwick SM, Grant BF. Psychiatric comorbidity of full and partial posttraumatic stress disorder among older adults in the United States: results from wave 2 of the National Epidemiologic Survey of Alcohol and Related Conditions. American Journal of Geriatric Psychiatry. 2012;20:380–390. doi: 10.1097/JGP.0b013e31820d92e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulkki-Råback L, Elovainio M, Kivimäki M, Mattsson N, Raitakari OT, Puttonen S, Marniemi J, Viikari JS, Keltikangas-Järvinen L. Depressive symptoms and the metabolic syndrome in childhood and adulthood: a prospective cohort study. Health Psychology. 2009;28:108–116. doi: 10.1037/a0012646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regier DA, Narrow WE, Clarke DE, Kraemer HC, Kuramoto SJ, Kuhl EA, Kupfer DJ. DSM-5 field trials in the United States and Canada, part II: Test-retest reliability of selected categorical diagnoses. The American Journal of Psychiatry. 2013;170:59–70. doi: 10.1176/appi.ajp.2012.12070999. [DOI] [PubMed] [Google Scholar]
- Roberts AL, Agnew-Blais JC, Spiegelman D, Kubzansky LD, Mason SM, Galea S, Hu FB, Rich-Edwards JW, Koenen KC. Posttraumatic stress disorder and incidence of type 2 diabetes mellitus in a sample of women: a 22-year longitudinal study. JAMA Psychiatry. 2015;72:203–210. doi: 10.1001/jamapsychiatry.2014.2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen RC, Marx BP, Maserejian NN, Holowka DW, Gates MA, Sleeper LA, Vasterling JJ, Kang HK, Keane TM. Project VALOR: design and methods of a longitudinal registry of post-traumatic stress disorder (PTSD) in combat-exposed veterans in the Afghanistan and Iraqi military theaters of operations. International Journal of Methods in Psychiatric Research. 2012;21:5–16. doi: 10.1002/mpr.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum S, Sherrington C, Tiedemann A. Exercise augmentation compared with usual care for post-traumatic stress disorder: a randomized controlled trial. Acta Psychiatrica Scandinavica. 2015;131:350–359. doi: 10.1111/acps.12371. [DOI] [PubMed] [Google Scholar]
- Rosenbaum S, Stubbs B, Ward PB, Steel Z, Lederman O, Vancampfort D. The prevalence and risk of metabolic syndrome and its components among people with posttraumatic stress disorder: a systematic review and meta-analysis. Metabolism. 2015;64:926–933. doi: 10.1016/j.metabol.2015.04.009. [DOI] [PubMed] [Google Scholar]
- Rosenbaum S, Vancampfort D, Steel Z, Newby J, Ward PB, Stubbs B. Physical activity in the treatment of post-traumatic stress disorder: a systematic review and meta-analysis. Psychiatry Research. 2015;230:130–136. doi: 10.1016/j.psychres.2015.10.017. [DOI] [PubMed] [Google Scholar]
- Rosenthal R, Rosnow RL. Essentials of Behavioral Research: Methods and Data Analysis. Second Edition McGraw-Hill; Boston, MA: 1991. [Google Scholar]
- Roy SS, Foraker RE, Girton RA, Mansfield AJ. Posttraumatic stress disorder and incident heart failure among a community-based sample of US veterans. American Journal of Public Health. 2015;105:757–763. doi: 10.2105/AJPH.2014.302342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnurr PP, Spiro A, 3rd, Paris AH. Physician-diagnosed medical disorders in relations to PTSD symptoms in older male military veterans. Health Psychology. 2000;19:91–97. doi: 10.1037//0278-6133.19.1.91. [DOI] [PubMed] [Google Scholar]
- Sullivan PW, Ghushchyan V, Wyatt HR, Hill JO. The medical cost of cardiometabolic risk factor clusters in the United States. Obesity (Silver Spring) 2007;15:3150–3158. doi: 10.1038/oby.2007.375. [DOI] [PubMed] [Google Scholar]
- Sumner JA, Kubzansky LD, Elkind MS, Roberts AL, Agnew-Blais J, Chen Q, Cerdá M, Rexrode KM, Rich-Edwards JW, Spiegelman D, Suglia SF, Rimm EB, Koenen KC. Trauma exposure and posttraumatic stress disorder symptoms predict onset of cardiovascular events in women. Circulation. 2015;132:251–259. doi: 10.1161/CIRCULATIONAHA.114.014492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot LS, Rao MN, Cohen BE, Richards A, Inslicht SS, O'Donovan A, Maguen S, Metzler TJ, Neylan TC. Metabolic risk factors and posttraumatic stress disorder: the role of sleep in young, healthy adults. Psychosomatic Medicine. 2015;77:383–391. doi: 10.1097/PSY.0000000000000176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrka AR, Parade SH, Price LH, Kao HT, Porton B, Philip NS, Welch ES, Carpenter LL. Alterations of mitochondrial DNA copy number and telomere length with early adversity and psychopathology. Biological Psychiatry. 2015 doi: 10.1016/j.biopsych.2014.12.025. [epub ahead of print], pii: S0006-3223(15)00041-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vancampfort D, Stubbs B, Mitchell AJ, De Hert M, Wampers M, Ward PB, Rosenbaum S, Correll CU. Risk of metabolic syndrome and its components in people with schizophrenia and related psychotic disorders, bipolar disorder and major depressive disorder: a systematic review and meta-analysis. World Psychiatry. 2015;14:339–347. doi: 10.1002/wps.20252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitaliano PP, Scanlan JM, Zhang J, Savage MV, Hirsch IB, Siegler IC. A path model of chronic stress, the metabolic syndrome, and coronary heart disease. Psychosomatic Medicine. 2002;64:418–435. doi: 10.1097/00006842-200205000-00006. [DOI] [PubMed] [Google Scholar]
- Wentworth BA, Stein MB, Redwine LS, Xue Y, Taub PR, Clopton P, Nayak KR, Maisel AS. Post-traumatic stress disorder: a fast track to premature cardiovascular disease? Cardiology in Review. 2013;21:16–22. doi: 10.1097/CRD.0b013e318265343b. [DOI] [PubMed] [Google Scholar]
- Wilson PW, D'Agostino RB, Parise H, Sullivan L, Meigs JB. Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation. 2005;112:3066–3072. doi: 10.1161/CIRCULATIONAHA.105.539528. [DOI] [PubMed] [Google Scholar]
- Wolf EJ, Logue MW, Hayes JP, Sadeh N, Schichman SA, Stone A, Salat DH, Milberg W, McGlinchey R, Miller MW. Accelerated DNA methylation age: Associations with PTSD and neural integrity. Psychoneuroendocrinology. 2016;63:155–162. doi: 10.1016/j.psyneuen.2015.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf EJ, Sadeh N, Leritz EC, Logue MW, Stoop T, McGlinchey R, Milberg W, Miller MW. PTSD as a catalyst for the association between Metabolic Syndrome and reduced cortical thickness. Biological Psychiatry. doi: 10.1016/j.biopsych.2015.11.023. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . Obesity: preventing and managing the global epidemic; Report of a WHO Consultation (WHO Technical Report Series) World Health Organization; Geneva: 2000. [PubMed] [Google Scholar]
- Yaffe K, Kanaya A, Lindquist K, Simonsick EM, Harris T, Shorr RI, Tylavsky FA, Newman AB. The metabolic syndrome, inflammation, and risk of cognitive decline. JAMA. 2004;292:2237–2242. doi: 10.1001/jama.292.18.2237. [DOI] [PubMed] [Google Scholar]
- Zandieh A, Esteghamati A, Morteza A, Noshad S, Khalilzadeh O, Gouya MM, Nakhiavani M. Appropriate BMI cut-off values for identification of metabolic risk factors: third national surveillance of risk factors of non-communicable diseases in Iran (SuRFNCD-2007). Annals of Human Biology. 2012;39:484–489. doi: 10.3109/03014460.2012.716860. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.