Abstract
Background
The highest mortality rates after liver surgery are reported in patients who undergo resection for hilar cholangiocarcinoma (HCCA). In these patients, postoperative death usually follows the development of hepatic insufficiency. We sought to determine the factors associated with postoperative hepatic insufficiency and death due to liver failure in patients undergoing hepatectomy for HCCA.
Study Design
This study included all consecutive patients who underwent hepatectomy with curative intent for HCCA at two centers from 1996 through 2013. Preoperative clinical and operative data were analyzed to identify independent determinants of i) hepatic insufficiency and ii) liver failure–related death.
Results
The study included 133 patients with right or left major (n=67) or extended (n=66) hepatectomy. Preoperative biliary drainage was performed in 98 patients and was complicated by cholangitis in 40 cases. In all these patients, cholangitis was controlled before surgery. Major (Dindo III-IV) postoperative complications occurred in 73 patients (55%), with 29 suffering from hepatic insufficiency. Fifteen patients (11%) died within 90 days after surgery, 10 of them of liver failure. On multivariate analysis, predictors of postoperative hepatic insufficiency (all p<0.05) were preoperative cholangitis (odds ratio [OR]=3.2), future liver remnant (FLR) volume <30% (OR=3.5), preoperative total bilirubin level >3 mg/dl (OR=4), and albumin level <3.5 mg/dl (OR=3.3). Only preoperative cholangitis (OR=7.5, p=.016) and FLR volume <30% (OR=7.2, p=.019) predicted postoperative liver failure–related death.
Conclusions
Preoperative cholangitis and insufficient FLR volume are major determinants of hepatic insufficiency and postoperative liver failure–related death. Given the association between biliary drainage and cholangitis, the preoperative approach to patients with HCCA should be optimized to minimize the risk of cholangitis.
INTRODUCTION
Hilar cholangiocarcinoma (HCCA) is a relatively uncommon neoplasm originating from the malignant transformation of the epithelium of the proximal bile duct. While the tumor typically involves the biliary confluence, the tumor may extend proximally to second- and third-order biliary branches. The management of HCCA is difficult, mainly because, first, the tumor location close to the vascular hilar structures and liver parenchyma makes complex hepatic resection with bile duct excision necessary for a chance for long-term survival (1) and, second, because the lack of effective adjuvant treatment makes surgical resection the only potentially curative treatment for this tumor (2).
In the majority of patients with HCCA, the first symptom of HCCA is obstructive jaundice, which is associated with high postoperative morbidity and mortality rates, mainly because of infections and hepatic insufficiency.(3) Preoperative biliary drainage has become a routine procedure in the preoperative management of HCCA. The goal of this procedure is to prevent cholestasis-associated toxic effects (4) and to improve liver regeneration after both liver resection and portal vein embolization (PVE), when PVE is necessary.(5) Though several groups tried to demonstrate benefits of biliary drainage before liver resection for HCCA, no clear conclusion was reached. In contrast, preoperative biliary drainage has been reported to increase the incidence of postoperative cholangitis.(6)
A recent multicenter study of mortality after liver resection for HCCA preceded or not by biliary drainage revealed that preoperative biliary drainage may be beneficial for patients undergoing right hepatectomy but not left hepatectomy.(7) This divergent effect may be due to cholangitis after unnecessary biliary drainage before left hepatectomy, which is associated with a lower risk of hepatic insufficiency than right hepatectomy.(8)
The aim of this study was to determine the factors associated with postoperative hepatic insufficiency and liver failure-related death in patients undergoing major liver resection for HCCA, with a focus on the impact of preoperative biliary tract infections.
METHODS
All patients who had undergone hepatectomy for HCCA from 1996 through 2013 at two tertiary care institutions (Department of General Surgery and Surgical Oncology of Mauriziano Umberto I Hospital in Turin, Italy, and Department of Surgical Oncology of The University of Texas MD Anderson Cancer Center in Houston, Texas, USA) were retrospectively identified from institutional databases. Patients’ full medical records, including hospital charts, surgical records, and pathology reports, were retrieved. Patients were included in further analyses only if i) surgery had consisted of a macroscopically curative major or extended hepatectomy with common bile duct resection and Roux-en-Y biliary-enteric anastomosis (hepatopancreatoduodenectomy were excluded); ii) pathologic evaluation had confirmed HCCA; and iii) details were available on 1) serum bilirubin and albumin levels before and after surgery, 2) preoperative cholangitis associated or not with preoperative biliary drainage, 3) type of biliary drainage, 4) preoperative liver volumetry, and 5) postoperative complications. Data collection and analysis were performed according to institutional guidelines and conformed to the ethical standards of the Helsinki Declaration.
Definitions
HCCA extension was defined according to the Bismuth-Corlette classification.(9) HCCA was considered unresectable if a patient had locally advanced disease extensively involving the main portal vein proximally to its bifurcation or the common hepatic artery; encasement of the right or left portal branch with contralateral arterial involvement; or involvement of lymph nodes beyond the regional ones.
Major hepatectomy was defined as resection of three or more Couinaud segments. Extended hepatectomy was defined as resection of five or more Couinaud segments. Types of hepatectomy were classified according to the Brisbane 2000 terminology.(10) A resection was considered macroscopically curative when the margin was clear of tumor on microscopic examination (R0) or on macroscopic examination (R1). Operative mortality was defined as death before discharge from the hospital or within 90 days after surgery. Morbidity included any deviation from the normal postoperative course, and major morbidities were defined as any grade III or higher complication according to the classification scheme proposed by Dindo et al.(11)
Preoperative cholangitis was diagnosed when two or more of the following conditions existed: body temperature higher than 38.5°C, white blood cell count more than 12 × 109/l or less than 4 × 109/l, or upper right abdominal pain in the presence of a positive bile culture. (12)
Hepatic insufficiency was defined as a postoperative serum bilirubin level exceeding 7 mg/dl or, in patients with preoperative jaundice, as a higher serum bilirubin level than the preoperative level on postoperative day 5 or thereafter. (13, 14) Death from liver failure was defined as postoperative death directly related to progressive hepatic insufficiency. Postoperative bile leak, hemorrhage, and sepsis were defined according to internationally accepted criteria.(15-17)
Preoperative management
Computed tomography, magnetic resonance imaging, and in some patients, cholangiography were used to assess the longitudinal tumor extension along the bile duct; involvement of the portal vein and/or hepatic arteries; invasion of the liver parenchyma; and presence of hepatic or extrahepatic metastases. All patients included in the study underwent computed tomography volumetry of the future liver remnant (FLR). When the estimated FLR was considered insufficient, preoperative PVE of the contralateral liver was performed.(18-20) Liver resection was scheduled at least 4 weeks after PVE if sufficient hypertrophy was achieved.
At MD Anderson Cancer Center, preoperative biliary drainage was performed in almost all patients presenting with jaundice. At Mauriziano Umberto I Hospital, indications for preoperative biliary drainage were cholangitis refractory to antibiotics, the need for PVE in a patient with serum bilirubin level higher than 10 mg/dl, hyperbilirubinemia-induced malnutrition, and anorexia causing a marginal performance status. Decompression of the biliary tree was achieved by percutaneous transhepatic biliary drainage of the FLR with internal-external drainage whenever possible, or by endoscopic retrograde biliary drainage. If the patient was referred to our centers with a malfunctioning biliary drainage catheter, this was replaced preoperatively with a new catheter placed by a percutaneous approach.
In cases of post-biliary drainage infection, resection was planned after the clinical resolution of cholangitis with an adequate medical treatment.
Surgical procedures
Beginning in 1997, laparoscopic exploration to rule out peritoneal carcinomatosis was performed routinely at Mauriziano Umberto I Hospital and selectively at MD Anderson Cancer Center. A right subcostal incision extended to the left of the midline or an inverted “L” incision was performed.(21) A midline split was sometimes necessary. Intraoperative liver ultrasonography was performed to assess resectability. Then the common bile duct was divided above the pancreas, and intraoperative frozen section evaluation was performed. The parenchymal transection technique was chosen by the surgeon. At MD Anderson Cancer Center, the “2-surgeon technique,” with both an ultrasonic dissector (employed by the primary surgeon) and a saline-linked cautery (employed by the second surgeon), was used in most cases.(22) At Mauriziano Umberto I Hospital, liver resections were performed using a similar technique with an ultrasonic dissector together with bipolar forceps with continuous irrigation and absorbable suture clips to ligate smaller vessels or bile ducts or suture ligature to ligate larger vessels or bile ducts. Total hepatic inflow occlusion (Pringle maneuver) for periods of up to 15 minutes, alternating with 5 minutes of restored inflow, was used in the majority of cases at MD Anderson Cancer Center and in the early cases at Mauriziano Umberto I Hospital. In the later cases at Mauriziano Umberto I Hospital, pedicle clamping was performed only when persistent or major bleeding occurred during parenchymal transection.(23) During liver resection, extrahepatic vascular inflow and outflow was routinely controlled when possible, with ligation and section of appropriate portal vein and hepatic artery branches and hepatic veins.
Statistical analysis
Statistical analysis was performed with SPSS (version 20.0, SPSS Inc.). Categorical data were expressed as frequency (percentage) and compared by chi-square or Fisher’s exact test as appropriate. Continuous data were expressed as median (range) and compared by the Mann-Whitney U test. Associations of perioperative variables with hepatic insufficiency and death from liver failure were first assessed at univariate analysis. Perioperative variables with a p value less than 0.2 at univariate analysis were entered into a multivariate logistic regression analysis in a backward stepwise manner. A p value less than 0.05 was considered statistically significant in all analyses.
RESULTS
Patient characteristics
A total of 133 patients, met the inclusion criteria and represented the study population; the majority of them (n=98, 74%) were treated after 2000. Patients’ characteristics are summarized in Table 1. HCCA was classified as type II in 19%, III in 71%, and IV in 10% of patients. Jaundice was the presenting symptom in 116 patients. Preoperative cholangitis before or after biliary drainage occurred in 42 patients. Preoperative biliary drainage was performed in 98 patients, and preoperative PVE was performed in 32 patients. Median (range) preoperative levels of albumin and total bilirubin were 3.6 mg/dl (2-4.9 mg/dl) and 1.9 mg/dl (0.2-32 mg/dl), respectively.
Table 1.
Characteristics of the Whole Study Cohort and Comparison of Patients with and without Preoperative Biliary Drainage
| Characteristic | Whole study cohort (n=133) |
Preoperative biliary drainage (n=98) |
No preoperative biliary drainage (n=35) |
p Value |
|---|---|---|---|---|
| Median age, y (range) | 66 (35-84) | 65 (40-84) | 67 (35-82) | 0.262 |
| Male sex, n (%) | 84 (63) | 62 (63) | 22 (63) | 0.996 |
| Jaundice at diagnosis, n (%) | 116 (87) | 91 (93) | 25 (71) | 0.002 |
| Cholangitis at diagnosis, n (%) | 7 (5) | 5 (5) | 2 (6) | 0.892 |
| Any preoperative cholangitis, n (%) | 42 (32) | 40 (41) | 2 (6) | <0.001 |
| Preoperative PVE, n (%) | 32 (24) | 31 (32) | 1 (3) | <0.001 |
| Tumor type, n (%)* | 0.544 | |||
| II | 25 (19) | 17 (17) | 8 (23) | |
| IIIa | 55 (41) | 43 (44) | 12 (34) | |
| IIIb | 40 (30) | 27 (28) | 13 (37) | |
| IV | 13 (10) | 11 (11) | 2 (6) | |
| FLR volume ≥ 30%, n (%) | 83 (62) | 59 (60) | 24 (69) | 0.380 |
| Median albumin level, mg/dL (range) | 3.6 (2-4.9) | 3.6 (2-44) | 3.7 (2-4.9) | 0.691 |
| Median total bilirubin level, mg/dL (range) |
1.9 (0.2-32) | 1.7 (0.2-21) | 5.5 (0.2-32) | 0.040 |
| Right hepatectomy, n (%) | 24 (18) | 18 (18) | 6 (17) | 0.871 |
| Extended right hepatectomy, n (%) | 56 (42) | 48 (49) | 8 (23) | 0.007 |
| Left hepatectomy, n (%) | 43 (32) | 26 (27) | 17 (49) | 0.017 |
| Extended left hepatectomy, n (%) | 10 (8) | 6 (6) | 4 (11) | 0.307 |
| Vascular resection, n (%) | 28 (21) | 21 (21) | 7 (20) | 0.859 |
| Median operation time, min (range) | 440 (190-1300) | 452 (190-1300) | 435 (135-590) | 0.558 |
| Median intraoperative blood loss, mL (range) |
577 (50-3500) | 578 (70-3500) | 550 (150-1500) | 0.620 |
| R0 resection, n (%) | 114 (86) | 82 (84) | 32 (91) | 0.260 |
| Overall complications, n (%) | 102 (77) | 78 (80) | 24 (69) | 0.186 |
| Major complications (Dindo III-V), n (%) |
73 (55) | 56 (57) | 17 (49) | 0.382 |
| Hepatic insufficiency | 29 (22) | 23 (24) | 6 (17) | 0.437 |
| Bile leak | 24 (18) | 18 (18) | 6 (17) | 0.872 |
| Sepsis | 17 (13) | 13 (13) | 4 (11) | 1 |
| Abdominal hemorrhage | 9 (7) | 7 (7) | 2 (6) | 1 |
| Death, n (%) | 15 (11) | 12 (12) | 3 (9) | 0.758 |
| Liver failure | 10 (8) | 8 (8) | 2 (6) | 0.989 |
| Sepsis | 2 (1.5) | 1 (1) | 1 (3) | 0.459 |
| Abdominal hemorrhage | 3 (2) | 2 (2) | 1 (3) | 1 |
Type according to Bismuth-Corlette classification.
PVE, portal vein embolization; FLR, future liver remnant.
Resection consisted of excision of the entire extrahepatic bile duct combined with an en bloc major (n=67) or extended (n=66) hepatectomy, including in most patients (89.5%) a caudate lobectomy (caudate process, paracaval portion, and Spiegel lobe) (n=110) or resection of Spiegel lobe only (n=9). Portal vein resection and reconstruction was performed in 28 patients (21%). In one patient, the portal vein resection was combined with hepatic artery resection.
Overall, 102 patients (77%) suffered from postoperative complications and 73 (55%) from major complications. Among patients suffering from major complications, 29 had hepatic insufficiency, 24 bile leak, 17 sepsis, 9 abdominal hemorrhage, and 31 abdominal collections requiring drainage by an interventional radiologist. Postoperative death occurred in 15 patients and was due to liver failure in 10 patients. No significant changes in the mortality rate were observed over time, nor differences were found between centers. Vascular resection did not significantly increased the mortality rate (14.3% in patients undergoing versus 10.5% in those not undergoing vascular resection; p=0.519).
Comparison of patients with and without preoperative biliary drainage
Of the 98 patients who underwent preoperative biliary drainage, 50 underwent percutaneous transhepatic and 48 underwent endoscopic retrograde biliary drainage. Patients who did and did not undergo preoperative biliary drainage are compared in Table 1. The two groups did not differ significantly in terms of age, sex, tumor type, FLR volume, operation time, intraoperative blood loss, and postoperative characteristics (all p>.050). Extended right hepatectomy was more often performed after preoperative biliary drainage, unlike left hepatectomy. Patients who underwent preoperative biliary drainage were more likely to have jaundice at diagnosis (93% versus 71%; p<.001) and to undergo preoperative PVE (32% versus 3%; p<.001).
Although patients who did and did not undergo preoperative biliary drainage had similar rates of cholangitis at presentation, the overall rate of preoperative cholangitis was significantly higher in patients who underwent preoperative biliary drainage (41% versus 6%; p<.001). Among the 35 patients who did not undergo preoperative biliary drainage, preoperative cholangitis occurred in only 2 patients and was controlled preoperatively with antibiotics. In contrast, among the 98 patients who did undergo preoperative biliary drainage, 5 had cholangitis at diagnosis and an additional 35 (36%) developed cholangitis after undergoing biliary drainage. As a result of biliary drainage, the preoperative median bilirubin level was lower in the preoperative-biliary-drainage group (1.7 mg/dl versus 5.5 mg/dl; p=.040).
Risk factors for hepatic insufficiency and death from liver failure
Predictors of hepatic insufficiency and death from liver failure are shown in Tables 2 and 3. In univariate analysis, risk factors for hepatic insufficiency were preoperative cholangitis, FLR volume < 30%, and preoperative albumin level < 3.5 mg/dl. In multivariate analyses, these three factors and bilirubin level > 3 mg/dl independently predicted hepatic insufficiency. Specifically, the risk of developing hepatic insufficiency was about 3.5-fold higher in patients with FLR volume < 30%, preoperative cholangitis, and albumin level < 3.5 mg/dl, and about 4-fold higher in patients with bilirubin level > 3 mg/dl.
Table 2.
Univariate and Multivariate Analysis of Factors Associated with Postoperative Hepatic Insufficiency
| Factor | n | Patients with hepatic insufficiency (n=29) |
Univariate analysis | Multivariate analysis* | |||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p Value | ||||
| n | % | ||||||
| Age ≥ 65 y | |||||||
| Yes | 67 | 13 | 19 | .752 (.329-1.720) | .500 | ||
| No | 66 | 16 | 24 | - | - | ||
| Male sex | |||||||
| Yes | 84 | 19 | 23 | 1.14 (.480-2.701) | .766 | . | |
| No | 49 | 10 | 20 | - | - | ||
| Jaundice at diagnosis | |||||||
| Yes | 116 | 25 | 22 | .893 (.268-2.979) | .854 | ||
| No | 17 | 4 | 23 | - | - | ||
| Preoperative biliary drainage |
|||||||
| Yes | 98 | 23 | 23 | 1.482 (.548- 4.011) |
.438 | ||
| No | 35 | 6 | 17 | - | - | ||
| Preoperative cholangitis |
|||||||
| Yes | 42 | 14 | 33 | 2.533 (1.086- 5.912) |
.029 | 3.170 (1.090- 9.221) |
0.034 |
| No | 91 | 15 | 16 | - | - | - | - |
| Preoperative PVE | |||||||
| Yes | 32 | 9 | 28 | 1.585 (.636- 3.948) |
.323 | ||
| No | 101 | 20 | 20 | - | - | ||
| Tumor type† | |||||||
| IV | 13 | 5 | 38 | 2.202 (.652- 7.444) |
.204 | ||
| III | 95 | 21 | 22 | 2.081 (.567- 7.636) |
.269 | ||
| II | 25 | 3 | 12 | - | - | ||
| FLR volume | |||||||
| < 30% | 50 | 16 | 32 | 2.53 (1.09-5.847) | .030 | 3.484 (1.259.708) |
0.017 |
| ≥ 30% | 83 | 13 | 16 | - | - | - | - |
| Albumin level < 3.5 mg/dL |
|||||||
| Yes | 49 | 18 | 37 | 2.965 (1.24- 7.094) |
.015 | 3.26 (1.232- 8.654) |
0.017 |
| No | 67 | 11 | 16 | - | - | - | - |
| Total bilirubin level > 3 mg/dL |
|||||||
| Yes | 54 | 15 | 28 | 1.882 (.771-4.59) | .165 | 4.028 (1.291- 12.565) |
0.017 |
| No | 79 | 14 | 18 | - | - | - | - |
| Vascular resection | |||||||
| Yes | 28 | 9 | 32 | 2.013 (.794- 5.106) |
.141 | 1.951 (.651- 5.842) |
0.232 |
| No | 105 | 20 | 19 | - | - | - | - |
All factors with a p value <0.2 in the univariate analysis were entered in the multivariate analysis. Body mass index was not included in the analysis because of missing data for 33 patients.
According to Bismuth-Corlette classification.
OR, odds ratio; CI, confidence interval; PVE, portal vein embolization; FLR, future liver remnant.
Table 3.
Univariate and Multivariate Analysis of Factors Associated with Death from Postoperative Liver Failure
| Factor | n | Liver failure death (n=10), n (%) |
Univariate analysis | Multivariate analysis* | |||
|---|---|---|---|---|---|---|---|
| n | % | OR (95% CI) | p Value |
OR (95% CI) | p Value | ||
| Age ≥ 65 y | |||||||
| Yes | 67 | 5 | 7 | .984 (.271-3.571) | 0.98 | ||
| No | 66 | 5 | 7 | - | - | ||
| Male sex | |||||||
| Yes | 84 | 8 | 9 | 2.474 (.504-12.15) | 0.265 | ||
| No | 49 | 2 | 4 | - | - | ||
| Jaundice at diagnosis | |||||||
| Yes | 116 | 10 | 9 | 1.5 *108 (.00-∞) | 0.998 | ||
| No | 17 | 0 | 0 | - | - | ||
| Preoperative biliary drainage |
|||||||
| Yes | 98 | 8 | 8 | 1.467 (.296-7.26) | 0.639 | ||
| No | 35 | 2 | 6 | - | - | ||
| Preoperative cholangitis | |||||||
| Yes | 42 | 8 | 19 | 10.471 (2.12-51.81) | 0.001 | 7.544 (1.46-38.99) | 0.016 |
| No | 91 | 2 | 2 | - | - | - | - |
| Preoperative PVE | |||||||
| Yes | 32 | 3 | 9 | 1.389 (.337-5.72) | 0.649 | ||
| No | 101 | 7 | 7 | - | - | ||
| Tumor type† | |||||||
| IV | 13 | 1 | 8 | .955 (.108-8.447) | 0.967 | ||
| III | 95 | 7 | 7 | 1.043 (.086-12.71) | 0.973 | ||
| II | 25 | 2 | 8 | - | - | ||
| FLR volume | |||||||
| < 30% | 50 | 8 | 16 | 7.69 (1.56-38.46) | 0.012 | 7.19 (1.39-37.037) | 0.019 |
| ≥ 30% | 83 | 2 | 2 | - | - | - | - |
| Albumin level < 3.5 mg/dL |
|||||||
| Yes | 49 | 6 | 12 | 2.114 (.563-.793) | 0.267 | ||
| No | 67 | 4 | 6 | - | - | ||
| Total bilirubin level > 3 mg/dL |
|||||||
| Yes | 54 | 3 | 6 | .617 (.152-2.502) | 0.499 | ||
| No | 79 | 7 | 9 | - | - | ||
| Vascular resection | |||||||
| Yes | 28 | 2 | 7 | .933 (.187-4.661) | 0.932 | ||
| No | 105 | 8 | 8 | - | - | ||
All factors with a p value <0.2 in the univariate analysis were entered in the multivariate analysis. Body mass index was not included in the analysis because of lacking data for 33 patients.
According to Bismuth-Corlette classification.
OR, odds ratio; CI, confidence interval; PVE, portal vein embolization; FLR, future liver remnant.
Preoperative cholangitis and FLR volume < 30% were the only predictors of death from liver failure, both in univariate and in multivariate analysis, and independently predicted a risk of death from liver failure about 7.5-fold higher, compared to patients who did not have preoperative cholangitis or who had an FLR volume ≥30%.
Comparison of patients with and without preoperative cholangitis
Patients who developed and did not develop preoperative cholangitis are compared in Table 4. There was a trend toward higher median age and higher rate of preoperative PVE in patients with cholangitis, but these differences were not significant. A FLR volume < 30% was significantly more common among patients with preoperative cholangitis. Among the 98 patients who underwent preoperative biliary drainage, cholangitis was more common with endoscopic retrograde biliary drainage than with percutaneous transhepatic biliary drainage (p=.002).
Table 4.
Comparison of Patients who Did and Did Not Develop Preoperative Cholangitis
| Characteristic | Preoperative cholangitis (n=42) |
No preoperative cholangitis (n=91) |
p Value |
|---|---|---|---|
| Median age, y (range) | 69 (46-84) | 64 (35-82) | 0.086 |
| Male sex, n (%) | 29 (69) | 55 (60) | 0.339 |
| Jaundice at diagnosis, n (%) | 37 (88) | 79 (87) | 0.837 |
| Preoperative biliary drainage, n (%) |
40 (95) | 58 (64) | <0.001 |
| Endoscopic retrograde biliary drainage |
27 (64) | 21 (36) | 0.002 |
| Percutaneous transhepatic biliary drainage |
13 (33) | 37 (64) | |
| Preoperative PVE, n (%) | 14 (33) | 18 (20) | 0.089 |
| Tumor type, n (%)* | |||
| II | 11 (26) | 14 (15) | 0.299 |
| III | 28 (67) | 67 (74) | |
| IV | 3 (7) | 10 (11) | |
| FLR volume < 30% | 21 (50) | 29 (32) | 0.045 |
| Median albumin level, mg/dL (range) |
3.7 (2.0-4.4) | 3.55 (2.0-4.9) | 0.308 |
| Median total bilirubin level, mg/dL (range) |
1 (0.3-20.9) | 3 (0.1-30.2) | 0.001 |
| Overall complications, n (%) | 40 (95) | 62 (68) | <0.001 |
| Major complications, n (%) | 31 (74) | 42 (46) | 0.003 |
| Hepatic insufficiency | 14 (33) | 15 (16) | 0.029 |
| Bile leak | 13 (31) | 20 (22) | 0.265 |
| Sepsis | 5 (12) | 12 (13) | 0.837 |
| Abdominal hemorrhage | … | … | … |
| Death, n (%) | 10 (24) | 5 (5) | 0.002 |
| Liver failure | 8 (19) | 2 (2) | 0.001 |
| Sepsis | 1 (2) | 1 (1) | 0.572 |
| Abdominal hemorrhage | 2 (5) | 1 (1) | 0.186 |
Type according to Bismuth-Corlette classification.
PVE, portal vein embolization; FLR, future liver remnant.
Compared to patients without preoperative cholangitis, patients with preoperative cholangitis had a higher risk of overall (95% versus 65%; p<.001) and major complications (74% versus 46%; p=.003), mainly due to a higher risk of hepatic insufficiency (33% versus 16%; p=.029), and a higher risk of death (24% versus 5%; p=.002), mainly due to a higher rate of death from liver failure (19% versus 2%; p=.001).
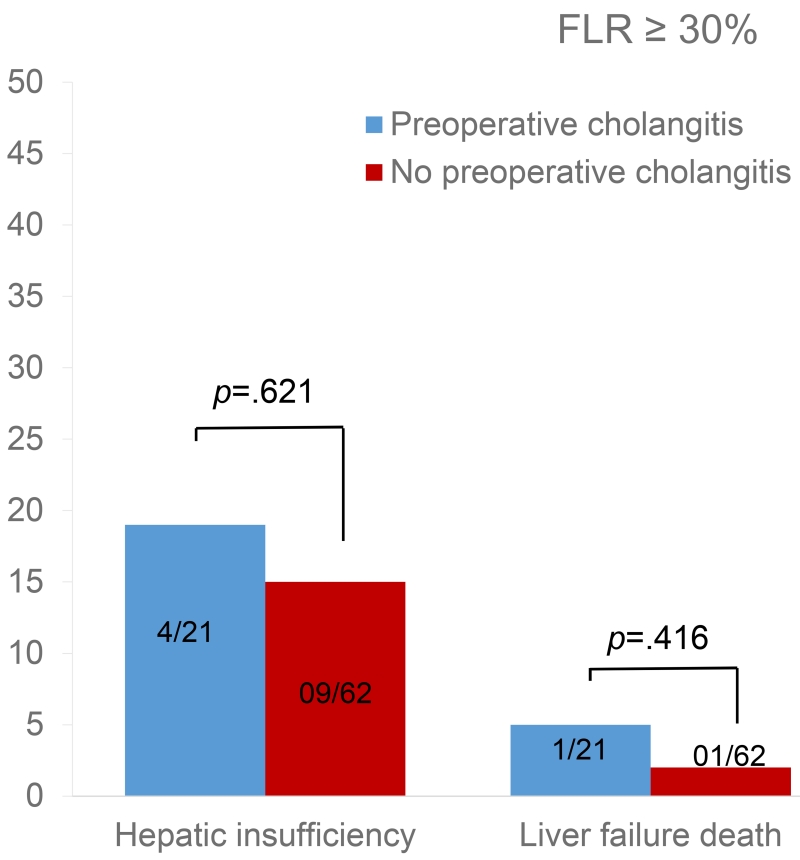
The effect of cholangitis on early outcomes differed according to the FLR volume. When the FLR volume was < 30%, rates of hepatic insufficiency and death from liver failure were significantly higher among patients with preoperative cholangitis than among patients without (p=0.040 and p=0.004, respectively) (Figure 1). Conversely, when the FLR volume was ≥ 30%, cholangitis did not affect rates of hepatic insufficiency or death from liver failure (p=0.621 and p=0.416, respectively) (Figure 2).
Figure 1.
Effect of cholangitis on early postoperative outcomes in patients with future liver remnant (FLR) volume < 30%.
Figure 2.
Effect of cholangitis on early postoperative outcomes in patients with future liver remnant (FLR) volume ≥30%.
DISCUSSION
Consistent with previous reports,(3, 24-26) results from our study confirmed high rates of major complications and death following liver resection for HCCA, with hepatic insufficiency emerging as the most common major complication and liver failure emerging as the most common cause of death. By investigating predictors of hepatic insufficiency and death from liver failure, we confirmed the importance of an adequate FLR volume in improving short-term results of surgery, and we showed for the first time an independent and strong association of preoperative cholangitis with an increased risk of both hepatic insufficiency and death from liver failure.
The association between an insufficient FLR volume and higher risks of hepatic insufficiency and death from liver failure after major hepatectomy was an expected result and is consistent with results from previous studies.(27-29) In this context, preoperative PVE to increase the FLR volume and thereby improve results of surgery was important: among 32 patients whose FLR was initially considered insufficient and who consequently underwent PVE before operation, 15 (47%) achieved an FLR volume higher than 30%, and none of these patients developed postoperative hepatic insufficiency or died of liver failure.
We also found an independent association between preoperative serum bilirubin level higher than 3 mg/dl and a higher risk of hepatic insufficiency. High preoperative serum bilirubin level was previously reported as an independent predictor of death after liver resection for HCCA.(7) Several studies have shown that hepatic resection in jaundiced patients can be associated with higher mortality and morbidity rates due to hemorrhages, subphrenic abscesses from biliary fistulae, sepsis, and liver failure.(7, 30-33) Experimental studies shed light on the mechanisms underlying these associations, showing that cholestasis makes the liver parenchyma more susceptible to ischemia/reperfusion damage and inflammation, likely because of a reduction of antioxidant activity and an increase in the inflammatory response.(34, 35) In our study, of 98 patients who underwent preoperative biliary drainage, 65 (66%) had their jaundice relieved preoperatively and had a serum bilirubin level lower than 3 mg/dl at the time of surgery. The median interval between preoperative biliary drainage and surgery was significantly longer among patients whose jaundice was relieved (56 days) than among patients operated on with persistent jaundice (33 days; p<.001), suggesting that, among patients undergoing preoperative biliary drainage, delay of the operation to obtain complete relief of jaundice may be beneficial.
Serum albumin is a marker of the synthetic capacity of the liver and has traditionally been used to assess liver function in the context of the Child-Pugh classification. Consistent with previous reports, we found that low preoperative albumin level was associated with an increased risk of hepatic insufficiency. Although hypoalbuminemia in patients with sepsis may be simply a result of the infection, low albumin levels in other patients might identify patients with impaired nutritional status according to the Nutritional Risk Index, which can be used to identify patients who require a preoperative nutritional intervention to reduce the risks of surgery.(36) We speculate that in such patients, optimizing the nutritional status, especially with immunonutrition, might reduce the incidence of complications. While we await the results of the NCT02041871 trial (registered at clinicaltrials.gov) evaluating the interest of preoperative immunonutrition in unselected patients undergoing liver resection for cancer, we believe that malnourished patients should receive oral nutritional support.
The most interesting finding of the current study was the strong association between preoperative cholangitis and poor outcomes after liver resection. Several authors (6, 27, 37, 38) have reported that biliary drainage before liver resection for HCCA is associated with higher rates of postoperative infectious complications, suggesting a correlation between biliary contamination due to the biliary drainage procedure and the development of infections after liver resection. However, none of these prior studies found a direct association between preoperative biliary drainage and an increased risk of major complications or death, suggesting that while preoperative biliary drainage contributes to adverse short-term outcomes in one group of patients, another group of patients may benefit from this procedure. In this context, our study is important since it is the first western study to show an association between preoperative cholangitis and postoperative hepatic insufficiency and death from liver failure. Our findings are consistent with those of a previous study (39) that showed that the incidence of hepatic insufficiency following major hepatectomy was 71% in patients without and 88% in patients with preoperative cholangitis. However, the rates of hepatic insufficiency in this previous study were extremely high as a consequence of the criteria adopted to define this complication. Therefore, based on this previous evidence, the exact impact of cholangitis on post resection outcomes was poorly defined and difficult to substantiate in discussions with patients about surgical risk. The data from the current study, in which we used an internationally recognized and validated definition of hepatic insufficiency and the modern practice of reporting of mortality through 90 days, offers a clear and novel insight into the problem of preoperative biliary drainage–related cholangitis. Even when controlled preoperatively with antibiotics, cholangitis may be associated with persistent subclinical biliary tract infection, which predisposes to the development of postoperative infections and impairs the regenerative capacity of the liver. In fact, experimental data obtained in a rat model indicate that segmental cholangitis reduces significantly the liver regeneration rate following partial hepatectomy.(40) Consistent with those experimental findings, in patients with preoperative biliary drainage–related cholangitis, the increase in the FLR volume per day after PVE is lower than in patients without cholangitis.(39) We also found that, among the 98 patients who underwent preoperative biliary drainage, preoperative cholangitis was significantly more common after endoscopic retrograde biliary drainage (67.5%) than after percutaneous transhepatic biliary drainage (32.5%; p=.002). This is similar to the findings of previous studies reporting higher rates of cholangitis and lower rates of technical and therapeutic success after endoscopic retrograde than after percutaneous transhepatic biliary drainage.(41, 42) Interestingly, a subgroup analysis also showed that the detrimental effect of preoperative cholangitis on surgical outcomes might be attenuated by large FLR volumes. Therefore, in patients experiencing preoperative biliary drainage–related cholangitis, surgery should not be performed, even if cholangitis has been controlled, until the FLR has reached a safe volume of 30%.
This study has some limitations. First, the retrospective nature of the study introduces selection biases. Second, given the long study period, there may have been heterogeneity in the preoperative use of both preoperative biliary drainage and PVE. However, the study reflects the use of PVE and preoperative biliary drainage over time that was based on the clinical evaluation of individual patients. In this context, we offer the following recommendations: 1) In jaundiced patients with insufficient FLR volume, preoperative biliary drainage should be performed before PVE to induce FLR volume increase. 2) Only the FLR should be drained, if possible; bilateral preoperative biliary drainage should be limited to patients with segmental cholangitis or with uncertain longitudinal tumor extension.
In conclusion, preoperative biliary drainage remains an important strategy to allow FLR volume increase in patients needing PVE, to treat jaundice-induced liver or renal failure, and to correct severe undernourishment or hypoalbuminemia. However, preoperative biliary drainage is frequently complicated by cholangitis, which is associated with an increased risk of hepatic insufficiency and death from liver failure following liver resection. Strategies to reduce the risk of preoperative biliary drainage–induced cholangitis, such as the preemptive prolonged use of antibiotics before and after the procedure, frequent checks and changes of the catheter, and use of external drains whenever possible, should be further investigated in order to optimize the outcome of patients with HCCA.
Precis.
Preoperative cholangitis and insufficient future liver remnant volume are major determinants of hepatic insufficiency and postoperative liver failure-related death. Given the association between biliary drainage and cholangitis, the preoperative approach to patients with hilar cholangiocarcinoma should be optimized to minimize the risk of cholangitis.
ACKNOWLEDGMENT
The authors dedicate this study to Dr Lorenzo Capussotti, former Chairman of Surgery at Mauriziano Umberto I Hospital, who passed away prematurely. Dr Capussotti assumed leadership of the care of the patients operated on at Mauriziano Umberto I Hospital during the study period. The impact of Dr Capussotti’s excellent mentorship and contributions to hepatobiliary and pancreatic surgery will be long lasting.
Support: This research was supported in part by the National Institutes of Health through MD Anderson’s Cancer Center Support Grant, CA016672.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Information: Nothing to disclose.
Presented at the Western Surgical Association 123rd Scientific Session, Napa Valley, CA, November 2015.
REFERENCES
- 1.Tsao JI, Nimura Y, Kamiya J, et al. Management of hilar cholangiocarcinoma: comparison of an American and a Japanese experience. Ann Surg. 2000;232:166–174. doi: 10.1097/00000658-200008000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vauthey JN, Blumgart LH. Recent advances in the management of cholangiocarcinomas. Semin Liver Dis. 1994;14:109–114. doi: 10.1055/s-2007-1007302. [DOI] [PubMed] [Google Scholar]
- 3.Nagino M, Kamiya J, Uesaka K, et al. Complications of hepatectomy for hilar cholangiocarcinoma. World J Surg. 2001;25:1277–1283. doi: 10.1007/s00268-001-0110-8. [DOI] [PubMed] [Google Scholar]
- 4.Smith RC, Pooley M, George CR, Faithful GR. Preoperative percutaneous transhepatic internal drainage in obstructive jaundice: a randomized, controlled trial examining renal function. Surgery. 1985;97:641–648. [PubMed] [Google Scholar]
- 5.Imamura H, Shimada R, Kubota M, et al. Preoperative portal vein embolization: an audit of 84 patients. Hepatology. 1999;29:1099–1105. doi: 10.1002/hep.510290415. [DOI] [PubMed] [Google Scholar]
- 6.Ferrero A, Lo Tesoriere R, Vigano L, et al. Preoperative biliary drainage increases infectious complications after hepatectomy for proximal bile duct tumor obstruction. World J Surg. 2009;33:318–325. doi: 10.1007/s00268-008-9830-3. [DOI] [PubMed] [Google Scholar]
- 7.Farges O, Regimbeau JM, Fuks D, et al. Multicentre European study of preoperative biliary drainage for hilar cholangiocarcinoma. Br J Surg. 2013;100:274–283. doi: 10.1002/bjs.8950. [DOI] [PubMed] [Google Scholar]
- 8.Abdalla EK, Denys A, Chevalier P, et al. Total and segmental liver volume variations: implications for liver surgery. Surgery. 2004;135:404–410. doi: 10.1016/j.surg.2003.08.024. [DOI] [PubMed] [Google Scholar]
- 9.Bismuth H, Nakache R, Diamond T. Management strategies in resection for hilar cholangiocarcinoma. Ann Surg. 1992;215:31–38. doi: 10.1097/00000658-199201000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Strasberg SM, For the International Hepato-Pancreato-Biliary Association Terminology Committee Survey The Brisbane 2000 Terminology of Liver Anatomy and Resections. HPB. 2000;2:333–339. [Google Scholar]
- 11.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kiriyama S, Takada T, Strasberg SM, et al. TG13 guidelines for diagnosis and severity grading of acute cholangitis (with videos) J Hepatobiliary Pancreat Sci. 2013;20:24–34. doi: 10.1007/s00534-012-0561-3. [DOI] [PubMed] [Google Scholar]
- 13.Skrzypczyk C, Truant S, Duhamel A, et al. Relevance of the ISGLS definition of posthepatectomy liver failure in early prediction of poor outcome after liver resection: study on 680 hepatectomies. Ann Surg. 2014;260:865–870. doi: 10.1097/SLA.0000000000000944. [DOI] [PubMed] [Google Scholar]
- 14.Mullen JT, Ribero D, Reddy SK, et al. Hepatic insufficiency and mortality in 1,059 noncirrhotic patients undergoing major hepatectomy. J Am Coll Surg. 2007;204:854–862. doi: 10.1016/j.jamcollsurg.2006.12.032. [DOI] [PubMed] [Google Scholar]
- 15.Koch M, Garden OJ, Padbury R, et al. Bile leakage after hepatobiliary and pancreatic surgery: a definition and grading of severity by the International Study Group of Liver Surgery. Surgery. 2011;149:680–688. doi: 10.1016/j.surg.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 16.Rahbari NN, Garden OJ, Padbury R, et al. Post-hepatectomy haemorrhage: a definition and grading by the International Study Group of Liver Surgery (ISGLS) HPB (Oxford) 2011;13:528–535. doi: 10.1111/j.1477-2574.2011.00319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20:864–874. [PubMed] [Google Scholar]
- 18.Madoff DC, Abdalla EK, Vauthey JN. Portal vein embolization in preparation for major hepatic resection: evolution of a new standard of care. J Vasc Interv Radiol. 2005;16:779–790. doi: 10.1097/01.RVI.0000159543.28222.73. [DOI] [PubMed] [Google Scholar]
- 19.Ribero D, Amisano M, Lo Tesoriere R, et al. Additional resection of an intraoperative margin-positive proximal bile duct improves survival in patients with hilar cholangiocarcinoma. Ann Surg. 2011;254:776–781. doi: 10.1097/SLA.0b013e3182368f85. [DOI] [PubMed] [Google Scholar]
- 20.Palavecino M, Abdalla EK, Madoff DC, Vauthey JN. Portal vein embolization in hilar cholangiocarcinoma. Surg Oncol Clin N Am. 2009;18:257–267. viii. doi: 10.1016/j.soc.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 21.Chang SB, Palavecino M, Wray CJ, et al. Modified Makuuchi incision for foregut procedures. Arch Surg. 2010;145:281–284. doi: 10.1001/archsurg.2010.7. [DOI] [PubMed] [Google Scholar]
- 22.Palavecino M, Kishi Y, Chun YS, et al. Two-surgeon technique of parenchymal transection contributes to reduced transfusion rate in patients undergoing major hepatectomy: analysis of 1,557 consecutive liver resections. Surgery. 2010;147:40–48. doi: 10.1016/j.surg.2009.06.027. [DOI] [PubMed] [Google Scholar]
- 23.Vigano L, Jaffary SA, Ferrero A, et al. Liver resection without pedicle clamping: feasibility and need for “salvage clamping”. Looking for the right clamping policy. Analysis of 512 consecutive resections. J Gastrointest Surg. 2011;15:1820–1828. doi: 10.1007/s11605-011-1625-4. [DOI] [PubMed] [Google Scholar]
- 24.Nuzzo G, Giuliante F, Ardito F, et al. Improvement in perioperative and long-term outcome after surgical treatment of hilar cholangiocarcinoma: results of an Italian multicenter analysis of 440 patients. Arch Surg. 2012;147:26–34. doi: 10.1001/archsurg.2011.771. [DOI] [PubMed] [Google Scholar]
- 25.Liu CL, Fan ST, Lo CM, et al. Improved operative and survival outcomes of surgical treatment for hilar cholangiocarcinoma. Br J Surg. 2006;93:1488–1494. doi: 10.1002/bjs.5482. [DOI] [PubMed] [Google Scholar]
- 26.van Gulik TM, Kloek JJ, Ruys AT, et al. Multidisciplinary management of hilar cholangiocarcinoma (Klatskin tumor): extended resection is associated with improved survival. Eur J Surg Oncol. 2011;37:65–71. doi: 10.1016/j.ejso.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 27.Laurent A, Tayar C, Cherqui D. Cholangiocarcinoma: preoperative biliary drainage (Con) HPB (Oxford) 2008;10:126–129. doi: 10.1080/13651820802007472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rocha FG, Matsuo K, Blumgart LH, Jarnagin WR. Hilar cholangiocarcinoma: the Memorial Sloan-Kettering Cancer Center experience. J Hepatobiliary Pancreat Sci. 2010;17:490–496. doi: 10.1007/s00534-009-0205-4. [DOI] [PubMed] [Google Scholar]
- 29.Ribero D, Amisano M, Bertuzzo F, et al. Measured versus estimated total liver volume to preoperatively assess the adequacy of the future liver remnant: which method should we use? Ann Surg. 2013;258:801–806. doi: 10.1097/SLA.0000000000000213. [DOI] [PubMed] [Google Scholar]
- 30.Cherqui D, Benoist S, Malassagne B, et al. Major liver resection for carcinoma in jaundiced patients without preoperative biliary drainage. Arch Surg. 2000;135:302–308. doi: 10.1001/archsurg.135.3.302. [DOI] [PubMed] [Google Scholar]
- 31.Nagino M, Takada T, Miyazaki M, et al. Preoperative biliary drainage for biliary tract and ampullary carcinomas. J Hepatobiliary Pancreat Surg. 2008;15:25–30. doi: 10.1007/s00534-007-1277-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Belghiti J, Ogata S. Preoperative optimization of the liver for resection in patients with hilar cholangiocarcinoma. HPB (Oxford) 2005;7:252–253. doi: 10.1080/13651820500372335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seyama Y, Makuuchi M. Current surgical treatment for bile duct cancer. World J Gastroenterol. 2007;13:1505–1515. doi: 10.3748/wjg.v13.i10.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kloek JJ, Marsman HA, van Vliet AK, et al. Biliary drainage attenuates postischemic reperfusion injury in the cholestatic rat liver. Surgery. 2008;144:22–31. doi: 10.1016/j.surg.2008.03.030. [DOI] [PubMed] [Google Scholar]
- 35.Kloek JJ, Levi M, Heger M, et al. Cholestasis enhances liver ischemia/reperfusion-induced coagulation activation in rats. Hepatol Res. 2010;40:204–215. doi: 10.1111/j.1872-034X.2009.00579.x. [DOI] [PubMed] [Google Scholar]
- 36.The Veterans Affairs Total Parenteral Nutrition Cooperative Study Group Perioperative total parenteral nutrition in surgical patients. N Engl J Med. 1991;325:525–532. doi: 10.1056/NEJM199108223250801. [DOI] [PubMed] [Google Scholar]
- 37.Hochwald SN, Burke EC, Jarnigan WR, et al. Association of preoperative biliary stenting with increased postoperative infectious complications in proximal cholangiocarcinoma. Arch Surg. 1999;134:261–266. doi: 10.1001/archsurg.134.3.261. [DOI] [PubMed] [Google Scholar]
- 38.Liu F, Li Y, Wei Y, Li B. Preoperative biliary drainage before resection for hilar cholangiocarcinoma: whether or not? A systematic review. Dig Dis Sci. 2011;56:663–672. doi: 10.1007/s10620-010-1338-7. [DOI] [PubMed] [Google Scholar]
- 39.Yokoyama Y, Ebata T, Igami T, et al. The adverse effects of preoperative cholangitis on the outcome of portal vein embolization and subsequent major hepatectomies. Surgery. 2014;156:1190–1196. doi: 10.1016/j.surg.2014.04.036. [DOI] [PubMed] [Google Scholar]
- 40.Watanabe K, Yokoyama Y, Kokuryo T, et al. Segmental cholangitis impairs hepatic regeneration capacity after partial hepatectomy in rats. HPB (Oxford) 2010;12:664–673. doi: 10.1111/j.1477-2574.2010.00229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kloek JJ, van der Gaag NA, Aziz Y, et al. Endoscopic and percutaneous preoperative biliary drainage in patients with suspected hilar cholangiocarcinoma. J Gastrointest Surg. 2010;14:119–125. doi: 10.1007/s11605-009-1009-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Walter T, Ho CS, Horgan AM, et al. Endoscopic or percutaneous biliary drainage for Klatskin tumors? J Vasc Interv Radiol. 2013;24:113–121. doi: 10.1016/j.jvir.2012.09.019. [DOI] [PubMed] [Google Scholar]