Abstract
Parkinson’s disease (PD) is a progressive degenerative disease manifested by tremor, rigidity, bradykinesia and postural instability. Deficits in proprioceptive integration are prevalent in individuals with PD, even at early stages of the disease. These deficits have been demonstrated primarily during investigations of reaching. Here we investigated how PD affects sensory fusion of multiple modalities during upright standing. We simultaneously perturbed upright stance with visual, vestibular and proprioceptive stimulation, to understand how these modalities are re-weighted so that overall feedback remains suited to stabilizing upright stance in individuals with PD. Eight individuals with PD stood in a visual cave with a moving visual scene at 0.2 Hz while an 80 Hz vibratory stimulus was applied bilaterally to their Achilles tendons (stimulus turns on-off at 0.28 Hz) and a ±1mA bilateral monopolar galvanic stimulus was applied at 0.36 Hz. The visual stimulus was presented at different amplitudes (0.2, 0.8 deg rotation about ankle axis) to measure: the change in gain (weighting) to vision, an intra-modal effect; and a simultaneous change in gain to vibration and galvanic stimulation, both intermodal effects. Trunk/leg gain relative to vision decreased when visual amplitude was increased, reflecting an intramodal visual effect. In contrast, when vibration was turned on/off, leg gain relative to vision was equivalent in individuals with PD, indicating no reweighting of visual information when proprioception was disrupted through vibration (i.e., no intermodal effect). Trunk and leg angle gain relative to GVS also showed no reweighting in individuals with PD. These results are in contrast to previous results with healthy adults, who showed clear intermodal effects in the same paradigm, suggesting that individuals with PD not only have a proprioceptive deficit during standing, but also have a cross-modal sensory fusion deficit that is crucial for upright stance control.
Keywords: Parkinson’s Disease, sensory deficit, postural instability, sensory reweighting, cross-modal fusion, basal ganglia
1. Introduction
Parkinson’s Disease (PD) is a progressive degenerative disease manifested by tremor, rigidity, bradykinesia and postural instability. Gait in patients with PD is often described as stiff with limited arm swing and short hesitant steps that are sometimes quick (festinating). Postural instability is usually a relatively late symptom of the disease and one that is not amenable to current medical or surgical therapy (Bloem, 1992; Koller et al 1989), although some improvement in balance has been reported with bilateral subthalamic stimulators (Houeto et al., 2000). At the same time, PD patients exhibit poor orientation to vertical, often assuming a stooped posture with progression of the disease. Patients experiencing postural instability are at increased risk of falls resulting in traumatic injuries and are usually dependent on the use of assistive devices such as walkers. In addition, freezing and gait hesitation usually occur relatively late in the disease and can be quite debilitating even when the other symptoms of the disease, such as increased stiffness, are well-treated medically. PD is traditionally regarded as a primarily hypo-dopaminergic syndrome, with symptoms resulting mostly from loss of dopamine-producing neurons in the substantia nigra, which is the part of the basal ganglia. Postural instability is thought to be caused by disturbed motor programming in the basal ganglia. Balance impairment by postural instability is the most disabling feature of PD, increasing risk of falls which may result in traumatic injury and loss of independence (Grimbergen et al., 2009).
One of main factors that may contribute to poor balance control in individuals with PD is a proprioceptive deficit (Benatru et al., 2008; Horak et al., 2005; Schieppati et al., 1994; Mesure et al., 1999; Nieves et al., 2001; Keijsers et al., 2005; Rickards and Cody, 1997; Zia et al., 2002; Jacobs and Horak, 2006; Vaugoyeau et al., 2011). Proprioceptive deficits can be present even at early stages of the disease, but become more prevalent and worsen with disease progression. These deficits were originally observed during pointing or targeting tasks with arm movement in patients with PD. For example, Keijsers et al. showed that patients with PD undershoot targets with eyes closed when asked to reproduce an arm movement guided passively, suggesting that PD patients have deficits not only in motor performance, but also in the processing of proprioceptive information. Proprioceptive deficits are observed even with very mild symptoms of PD, and deficits in the use of visual feedback develop progressively in later stages of the disease (Keijsers et al., 2005). Rickards and Cody investigated the extent to which proprioceptive information is used to guide slow voluntary wrist movements in patients with PD using muscle vibration. They found that PD patients showed a reduced undershooting of the wrist movement by applied vibration, suggesting proprioceptive deficits which may contribute to motor performance deficits (Rickards and Cody, 1997). Zia et al. compared proprioceptive deficits of PD patients with those of control subjects in the perceptual task of identifying unilateral elbow-joint position under passive and active conditions. Their results showed that PD patients’ undershot the target under both active and passive conditions. They concluded that a quantitative impairment of joint position sense occurs in PD, suggesting that this deficit may contribute to the postural instability problems encountered in individuals with PD (Zia et al., 2002).
The relationship between a proprioceptive deficit and poor balance control was investigated by Jacobs and Horak (2006), who found that subjects with PD exhibited abnormally short compensatory steps in response to external postural perturbations. They argued that these short compensatory steps were due to abnormal proprioceptive-motor processing and showed that visual targets led to longer compensatory steps, suggesting that visual input can compensate for the proprioceptive deficit. Vaugoyeau et al. (2011) also investigated the relationship between proprioceptive impairment and postural control in PD during standing on a moving supporting platform with/without visual information. They compared the postural performances of their two groups of subjects with and without vision to determine whether the PD patients make greater use of visual information than normal subjects for postural control. The PD patients’ ability to respond to oscillations applied to the supporting platform improved when their eyes were open, without reaching the performance levels of the control subjects. These results showed that PD patients, unlike the control subjects, were unable to properly control their postural orientation on the basis of the available sensory information (i.e., proprioceptive and vestibular information) and PD patients’ increased visual dependence, reflecting an adaptive strategy to compensate for their proprioceptive deficit (Vaugoyeau et al, 2011). Despite these studies linking a proprioceptive deficit to poor balance control in patients with PD, their poor mobility may involve more than proprioception alone.
Postural control of human upright stance is heavily dependent upon the integration of information from multiple sensory modalities (i.e., sensor fusion) to detect center of gravity excursions and to generate appropriate muscle responses for upright stability. In order to understand how the nervous system changes the “emphasis” of a particular sensory input due to neurological injury/deficit or when environmental conditions change, numerous studies have demonstrated that sensor fusion appears to be dynamically regulated to adapt to changing environmental conditions, a process referred to as “sensory reweighting” (Horak and Macpherson, 1996; Peterka, 2002; Peterka and Loughlin, 2004; van der Kooij et al., 2001; Kiemel et al., 2002; Oie et al., 2001; 2002; Mahboobin et al, 2009). Most of these previous studies manipulated an individual sensory system or the combined influence of two sensory systems (e,g., Oie et al, 2002). Recently, we developed a novel “sensory fusion (or reweighting)” paradigm that investigated sensory reweighting between the three primary modalities for postural control in healthy young individuals (Hwang et al, 2014), While subjects stand within a “visual cave”, proprioceptive information was manipulated through vibration of the Achilles tendon and vestibular input was biased through galvanic stimulation. When the amplitude of visual input was increased, we not only found a clear decrease in the emphasis on visual input (an intramodal effect), but we also found simultaneous intermodal visual-proprioceptive and visual-vestibular reweighting effects, illustrating the dynamic interplay between the three primary modalities for control of upright posture. (Hwang et al, 2014). Here we expand this paradigm to individuals with PD, which may have implications for therapy directed at recovery of postural stability and functional mobility. We investigated how PD affects sensor fusion of multiple modalities through simultaneous stimulation of all three modalities while standing.
2. Experimental Procedure
2.1. Experimental Methods
2.1.1. Subjects
Eight patients with PD (5 males, 3 females; weight 76±16kg, height 168.4±14.0cm, aged 66.3±8.6yr, PD stage 1.75±0.75) with all on-medication participated in the study. They all reported no musculoskeletal injuries or neurological disorders that might affect their ability to maintain balance. Individuals with PD were excluded from the study if they had a diagnosis of dementia, were being treated with an agent for dementia, or had a Mini-mental state exam (MMSE) < 25. Additional exclusion criteria were: 1) pregnancy; 2) electrical implants (e.g., pacemaker); 3) epilepsy; 4) actively bleeding tissue; 4) contraindication of electrical stimulation. All PD subjects were referred directly and clinically evaluated from Dr. Stephen Grill, Director of the Parkinson’s Disease and Movement Disorders Center in Elkridge, MD, U.S.A. Experimental procedures were approved by the Institutional Review Board at the University of Maryland and all the subjects gave written informed consent to participate as approved by the committee.
2.1.2. Visual, proprioceptive and vestibular sensory perturbations
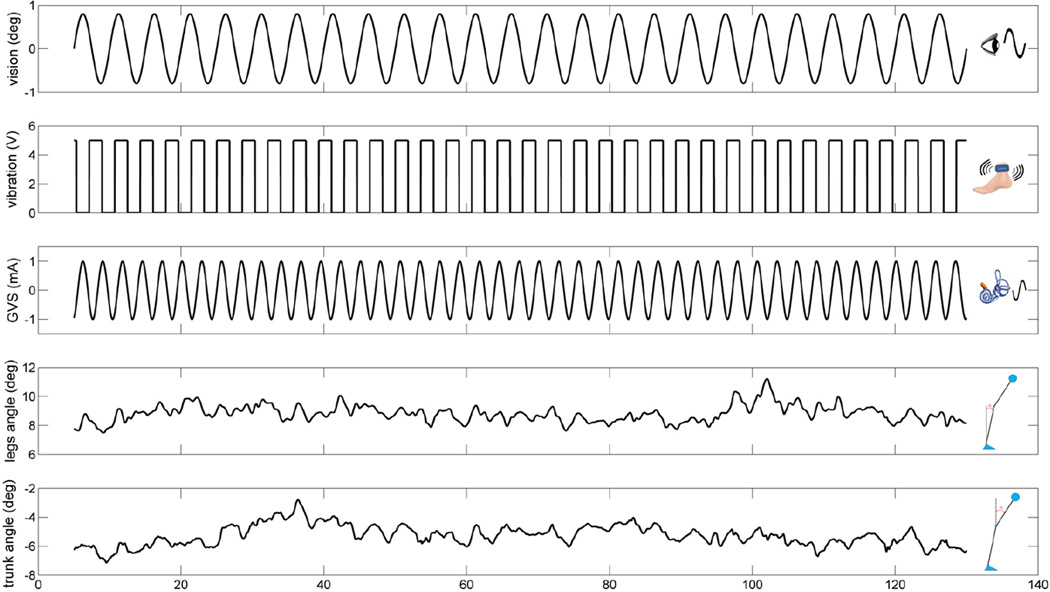
The visual perturbation was displayed with a visual cave, which consisted of three 2.67×3.33 m screens (Fakespace, Inc, Marshalltown, Iowa, USA) that were rear-projected (JVC DLA-M15U, Victor Company, Japan). The visual display consisted of 500 randomly distributed white triangles (3.4×3.4×3 cm) on a black background. To reduce aliasing effects in the foveal region, no triangles were displayed within a horizontal band of ±5 degree at eye height. The frame rate of the visual display was 60 Hz. Visual scene movement was displayed as a rotation around the ankle joint in the anterior-posterior direction (i.e., sagittal plane). Visual scene movement was presented at different amplitudes (0.2 deg and 0.8 deg rotation about ankle axis) at 0.2 Hz to measure: the change in response to vision, an intra-modal effect; and a change in response to vibration and galvanic stimulation, both intermodal effects. The response to each stimulus was measured by gain and phase, as described below in Section 2.2. For the proprioceptive perturbation, bilateral vibration of Achilles tendons was applied through two 20 mm vibrator motors, driven at 80 Hz and 1 mm amplitude displacement. While vibration is a common technique, it is typically used in an always-on or always-off manner (e.g., Capicikova et al, 2006). For this study, we designed the vibrator to turn on and off quickly to approximate a square-wave periodic stimulus of a specified frequency with equal on and off time durations. The vibrators were enclosed in a hollow rectangular PVC container (3.5 × 3.8 × 3.5 cm) with a flexible recessed surface mounted on the contact face for comfortable fitting around the Achilles tendon. The enclosure was held in place by an elastic strap. The proprioceptive sensory perturbation was applied to different conditions (standing with vibration or standing without vibration) at 0.28 Hz. For the vestibular perturbation, two linear isolated stimulators (Biopac Systems, Inc., Goleta, California, USA) were used as a binaural-monopolar galvanic vestibular stimulation (GVS). Independent stimuli were delivered to each side via a pair of circular electrodes secured over the mastoid process with an elastic headband and 2 cm ipsilateral to the T2 spinous process (Day et al. 2010). The electrodes were secured using adhesive tape and conductive electrode gel was applied at the electrode–skin interface to improve conductance. GVS consisted of a ±1 mA sinusoidal galvanic stimulus at 0.36 Hz and the polarity of stimulation was always the same for the two sides (binaural-monopolar GVS) to perturb subjects in the sagittal plane. GVS was applied to subjects in every trial of all conditions. Different frequencies for each stimulus were chosen so that we could measure a response to each modality independently and so that they did not share common low-order harmonics. Figure 1 shows an example of a trial showing the stimulus signals and segment angles used for signal processing.
Figure 1.
An example of a trial showing the stimulus signals and segments angles used for signal processing.
2.1.3. Experiment setup
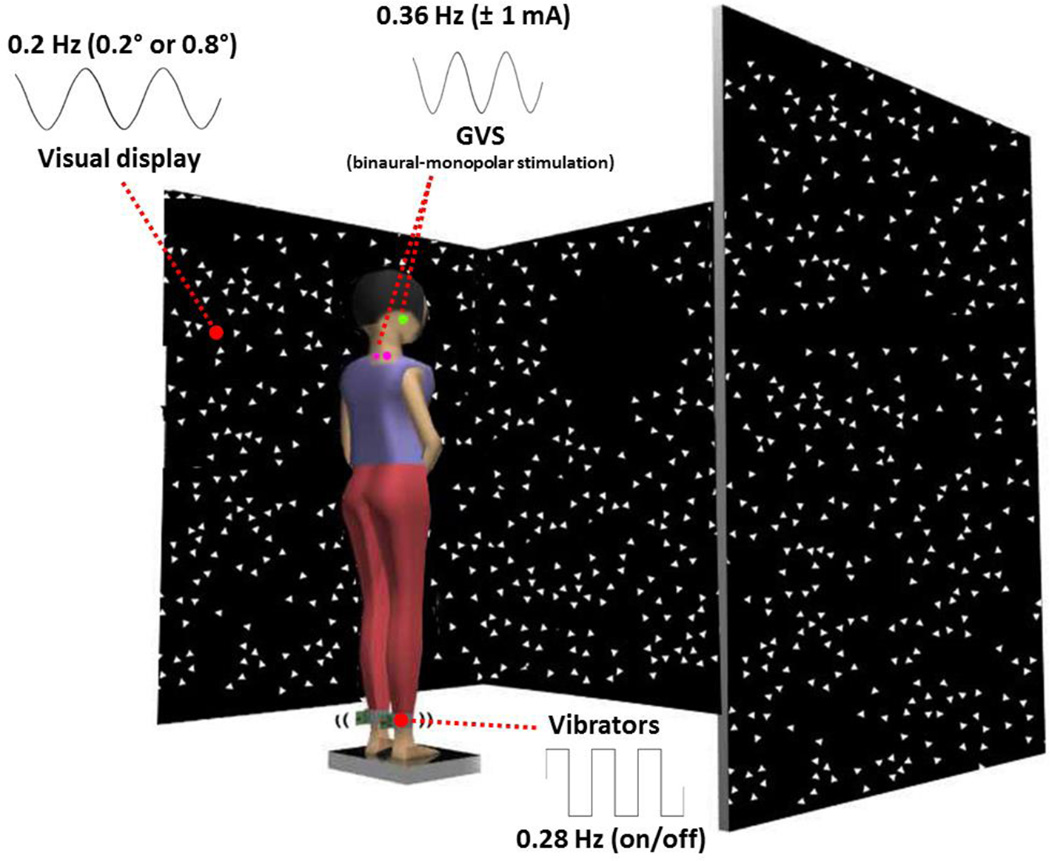
The experiment setup for visual, proprioceptive and vestibular sensory perturbations was identical to Hwang et al (2014) to allow comparison with young healthy adults. Subjects stood in the middle of a visual cave and faced the front wall, as shown in Figure 2. Subjects assumed a foot position with heels at a distance of ~11% of their heights and an angle of 14° between each foot and the midline (McIlroy & Maki, 2007). The instruction to the subjects was to look straight ahead at the front screen and stand upright comfortably. Foot position was marked in order to be consistent throughout the experiment. Subjects were tested in four conditions; standing with low amplitude visual scene movement - vibration - GVS (L-V-G); standing with low amplitude visual scene movement - GVS – no vibration (L-G); standing with high amplitude visual scene movement - vibration - GVS (H-V-G); standing with high amplitude visual scene movement - GVS - no vibration (H-G). Four trials, a trial from each condition, were run in randomized order, and repeated five times for a total of 20 trials for each subject. The length of each trial was 125 seconds with five seconds added at the beginning and end of each trial (total 135 seconds) to allow the visual and vestibular sensory perturbations to ramp up and ramp down; data during the ramp-up and ramp-down were not used for analysis. The ramp was not applied to the vibration signal because of the electronic functional limitation of the vibrator to turn on and off quickly to approximate a square-wave periodic stimulus.
Figure 2.
Experimental setup showing standing subject with simultaneous visual, vibration and galvanic vestibular perturbations. The visual stimulus at different amplitudes (0.2, 0.8 deg rotation about ankle axis) at 0.2 Hz, the 80 Hz vibratory stimulus to subject’s bilateral Achilles tendons (stimulus turns on-off at 0.28 Hz) and a ±1 mA bilateral monopolar galvanic vestibular stimulus at 0.36 Hz were simultaneously applied.
2.1.4. Kinematics
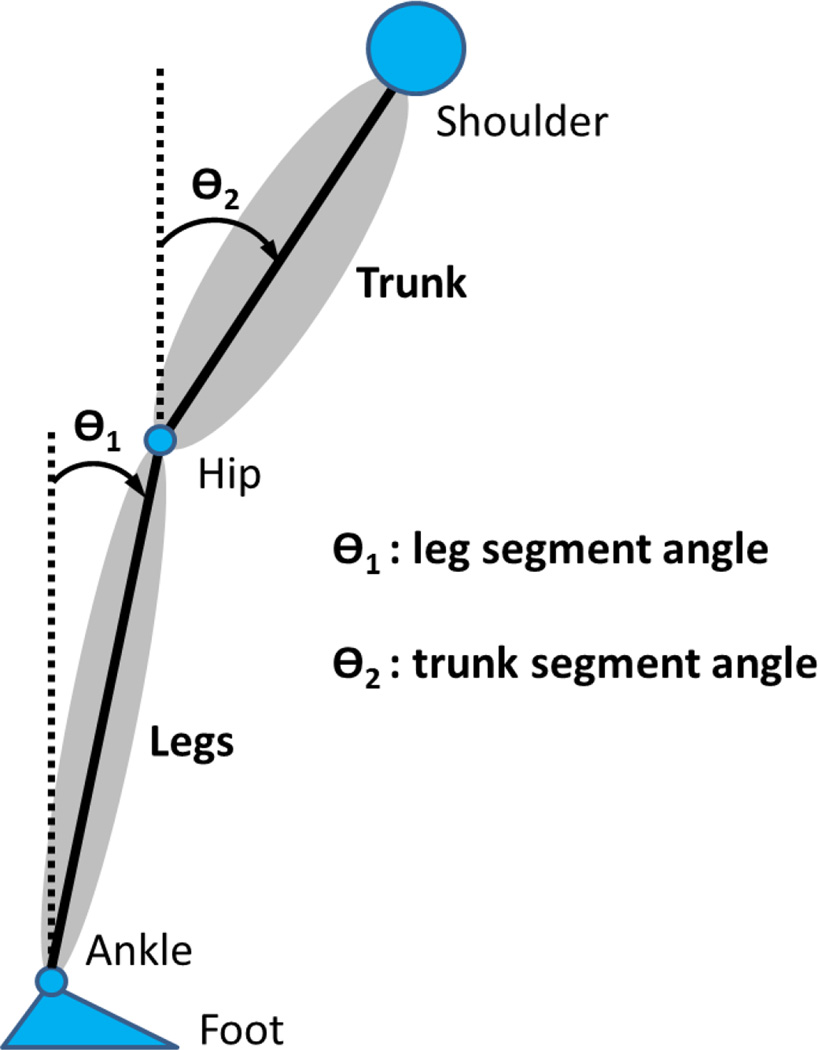
Kinematics were captured by Vicon MX digital optical motion capture system with six infrared cameras (Vicon, UK). The head (the temple), shoulder (the scapula), hip (the greater trochanter), knee (the lateral femoral condyle), ankle (the lateral malleolus) and foot (the first metatarsal head) were measured by attaching reflective markers on the right side of the subject to measure subject’s anterior-posterior movement in the sagittal plane. The leg angle ϴ1(t) and trunk angle ϴ2(t) with respect to vertical were determined by the anterior-posterior (AP) and vertical displacement of the shoulder, hip and ankle markers, as seen in Figure 3. Kinematics were sampled at 120 Hz.
Figure 3.
Schematic diagram of the body showing the leg segment angle ϴ1(t) and trunk segment angle ϴ2(t).
2.2. Analysis
2.2.1. Spectral analysis
For any two signals x(t) and y(t), the power spectral densities (PSDs) pxx(f) and pyy(f) and cross spectral density (CSD) pxy(f), where f is frequency, were computed using Welch’s method (Bendat and Piersol, 2000) with 50-s Hanning windows and 50% overlap and then averaged across trials. Note that 25 s is an integer multiple of the periods of all three perturbation signals, so the 50-s window contains an integer number of cycles of each perturbation signal. The frequency response function (FRF) is the CSD divided by the PSD of the input. Gain, the absolute value of the FRF, is the amplitude of the output divided by the amplitude of the input at each driving frequency. For example, if the amplitudes of a segment angle response and the visual perturbation at the driving frequency are the same, the unitless gain (deg/deg) will equal one. Phase of the FRF is a measure of the temporal relationship between the input and output; the output may lead the input (positive values) or lag behind it (negative values). Bootstrap standard errors were also computed for gains and phases using 1000 bootstrap resamples (Zoubir & Boashash, 1998).
2.2.2. Statistical analysis
In order to determine effects due to a change in visual amplitude and effect due to vibration on/off, values for gain/phase of leg angle ϴ1(t) and trunk angle ϴ2(t) relative to the visual stimulus and GVS were assessed through a Vision (low vs high amplitude) × Vibration (on-off) two way repeated-measures ANOVA. For the vibration perturbation, differences in gain/phase of leg and trunk angle relative to vibration were compared between low and high amplitude visual conditions (L-V-G vs H-V-G) by a paired t-test (gain/phase cannot be measured with vibration off).. Statistical significance was accepted at p<0.05.
3. RESULTS
3.1. Gain
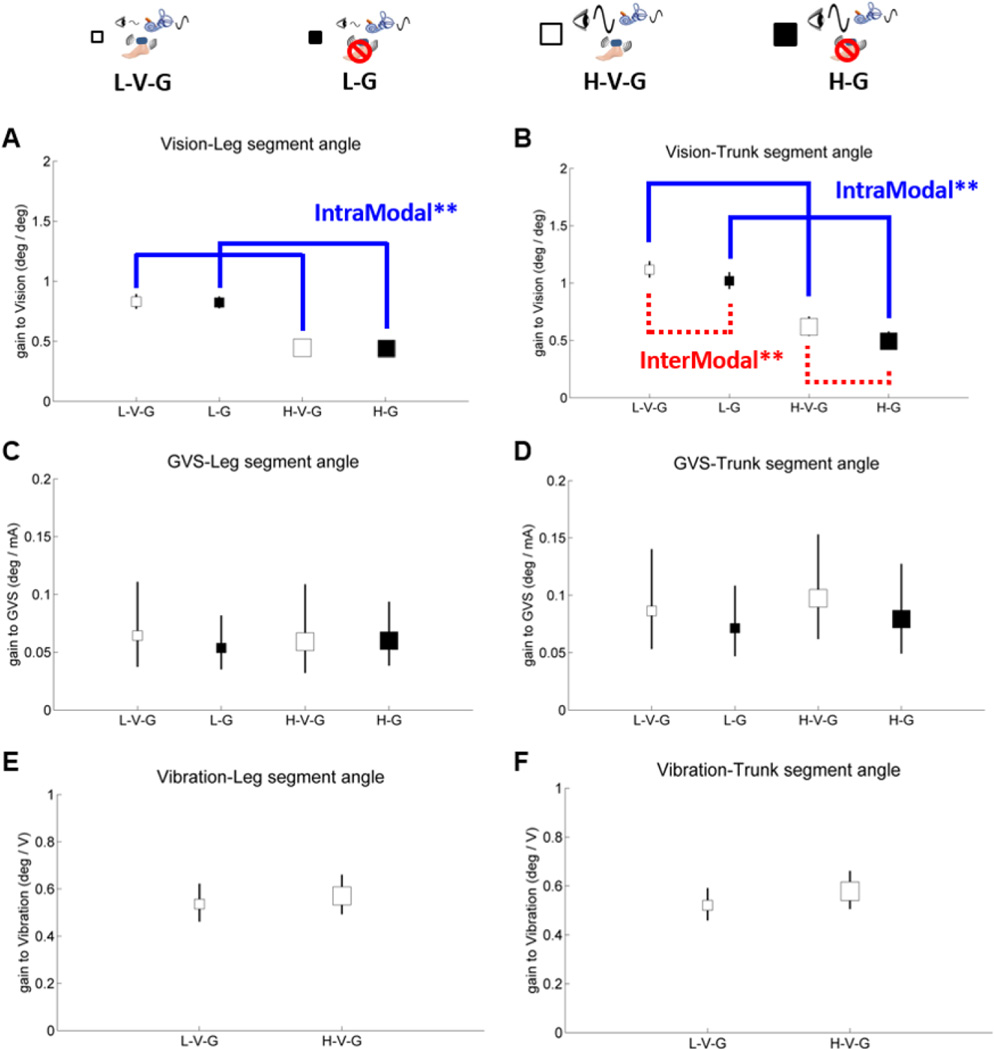
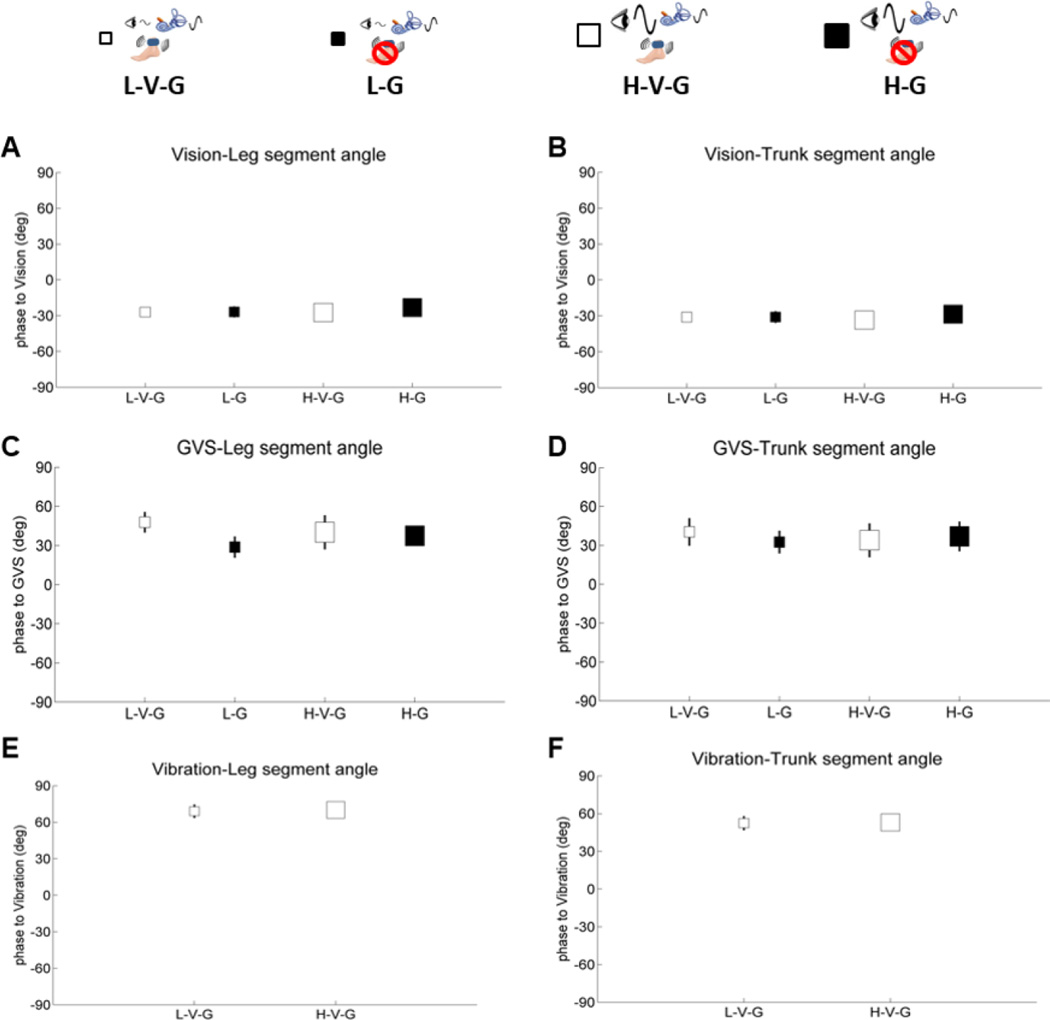
Leg/trunk gain and phase responses are shown on separate plots relative to each sensory perturbation in Figures 4–6. Small white squares represent standing with low amplitude visual scene movement – vibration – GVS (galvanic vestibular stimulation) (L-V-G); small black squares represent standing with low amplitude visual scene movement - GVS – no vibration (L-G); large white square represents standing with high amplitude visual scene movement - vibration - GVS (H-V-G); and large black squares represent standing with high amplitude visual scene movement - GVS - no vibration (H-G). It is most informative to digest how each sensory modality reacts to the same condition by comparing gains and phases across Figures 4–5.
Figure 4.
Gain of segment angles relative to vision, GVS and vibration, showing intramodal visual reweighting in both segments (blue solid line) and intermodal visual-proprioceptive reweighting only in the trunk segment (red dashed line). The blue line indicates significant gain responses relative to a change of visual amplitude (intramodal reweighting) and the red line indicates significant gain responses relative to vibration on-off (intermodal reweighting). Asterisks indicate significance levels (** for p<.01). A. gain of the leg segment angle relative to vision. B. gain of the trunk segment angle relative to vision. C. gain of the leg segment angle relative to GVS. D. gain of the trunk segment angle relative to GVS. E. gain of the leg segment angle relative to vibration. F. gain of the trunk segment angle relative to vibration. Error bars denote bootstrap standard errors.
Figure 5.
Phase of segment angles relative to vision, GVS and vibration. A. phase of the leg segment angle relative to vision. B. phase of the trunk segment angle relative to vision. C. phase of the leg segment angle relative to GVS. D. phase of the trunk segment angle relative to GVS. E. phase of the leg segment angle relative to vibration. F. phase of the trunk segment angle relative to vibration. Error bars denote bootstrap standard errors.
Gain responses to visual amplitude change
In Figure 4A, leg gain relative to vision decreases from the L-V-G condition to the H-V-G condition or the L-G condition to the H-G condition (F(1,7) = 26.2, p < .01) in individuals with PD, reflecting a clear intramodal downweighting of vision as visual scene amplitude increased. This result is consistent with previous studies conducted with healthy adults (cf., Kiemel et al., 2006; Peterka, 2002; Oie et al., 2002; Hwang et al., 2014). This intramodal downweighting of vision is also observed for trunk gain (F(1,7) = 40.0, p < .001) in Figure 4B.
There was no significant change in leg or trunk gain relative to the GVS stimulus in individuals with PD, as shown in Figures 4C–D. There were also no significant intermodal effects on vibration gain relative to a change in visual amplitude, as shown in Figures 4E–F.
Gain responses to vibration on/off
Intramodal effects are not observable with vibration because a gain response cannot be measured with vibration turned off. However, the effect of turning vibration on/off was clearly observed through intermodal effects on visual and GVS stimulation in healthy adults (Hwang et al., 2014). These intermodal effects in healthy adults suggest that vibration changes processing of proprioceptive information at the foot/ankle, forcing the nervous system to compensate by upweighting vision and vestibular information from L-G to L-V-G and H-G to H-V-G conditions (Hwang et al., 2014).
Figure 4A shows that leg gain relative to vision was equivalent regardless of whether vibration was on or off in individuals with PD, indicating no reweighting of visual information relative to the legs when proprioception was disrupted through vibration. Trunk gain relative to vision was upweighted when vibration was on as shown in Figure 4B (F(1,7) = 14.2, p < .01). We did a segment by condition ANOVA to see a distinction in visual reweighting across segments, and there was a significant distinction in visual reweighting across segments when vibration was on in individuals with PD (F(2,63) = 3.484, p < .05). This distinction suggests a proprioceptive deficit in the legs that is not observed in the trunk during standing. Additionally, Figure 4C–D show that leg and trunk gain relative to GVS showed no change in individuals with PD when vibration was turned off. These results suggest that individuals with PD not only have a proprioceptive deficit (particularly in the legs) during standing, but also have a cross-modal sensory fusion deficit that is crucial for upright stance control.
3.2 Phase
Phase of the leg/trunk segments relative to each of the sensory stimuli shown in Figure 5 indicated no differences across conditions. However, absolute differences in phase were observed relative to the mode of sensory stimulation. Both leg and trunk segment angles displayed similar phase lags of 25 deg ~ 30 deg relative to vision. Relative to the GVS stimulus, both leg and trunk phase of individuals with PD were advanced by 30 deg ~ 45 deg. In addition, both leg and trunk phase of individuals with PD were advanced by 60 deg ~ 70 deg relative to vibration.
4. DISCUSSION
In this study, we simultaneously perturbed upright stance with all three modalities, to understand how visual, vestibular, and proprioceptive feedback are re-weighted so that overall feedback remains suited to stabilizing upright stance in individuals with PD. We found evidence of a proprioceptive deficit during standing, consistent with previous studies (Benatru et al., 2008; Horak et al., 2005; Schieppati et al., 1994; Mesure et al., 1999; Nieves et al., 2001; Keijsers et al., 2005; Rickards and Cody, 1997; Zia et al., 2002; Jacobs and Horak, 2006; Vaugoyeau et al., 2011). In addition, we also found that PD affects sensory fusion of multiple modalities, indicating a problem with central processing of sensory information in individuals with PD.
Problem with Postural Control in PD is not the ability to generate movement but perception of movement
Postural control is defined as the process of regulating the body's position in space for the purpose of achieving an upright and stable stance (Shumway-Cook & Woollacott, 2001). As such, effective postural control requires both perception and action. 'Perception' refers to the detection and integration of sensory information to evaluate the position and motion of the body with respect to the environment. 'Action' refers to the body's ability to produce forces for controlling body position systems (Shumway-Cook & Woollacott, 2001). Thus, perception and action are dependent upon communication and interaction between the body's neural and musculoskeletal systems. Neural components of postural control include motor processes, sensory processes, and high-level integrative processes. Musculoskeletal components include joint range of motion, spinal flexibility, muscle properties, and the biomechanical relationships among body segments. Efficient communication between these systems is crucial if stability is to be maintained during the performance of daily activities. Thus, the body's systems for postural control communicate via feed-forward and feed-back inputs to ensure that the ultimate goal of postural control is achieved: to detect and correct disruptive movements that surpass the body's stability limits to prevent an injurious fall episode (Cooper, 2005). Typically, balance impairment by postural instability is one of the most disabling features of PD, which may increase risk of falls resulting in traumatic injuries and loss of independence. For individuals with PD, an important component of their inability to generate appropriate movement may be the detection and integration of sensory information to evaluate the position and motion of the body with respect to the environment as ‘perception’. Several studies have demonstrated that PD patients have a proprioceptive deficit, i.e. the perception problem, which can be present even at diagnosis or early stages of the disease, but becomes more prevalent with the progression of PD (Keijsers et al., 2005; Rickards and Cody, 1997; Zia et al., 2002; Jacobs & Horak, 2006; Vaugoyeau et al, 2011). Here we found that a sensory reweighting deficit might be a main factor contributing to this perception problem in individuals with PD.
Our results showed consistent intramodal reweighting of trunk/leg gain relative to a visual perturbation in individuals with PD. When visual amplitude increased from the L-V-G to the H-V-G conditions or from the L-G to the H-G conditions, trunk/leg segment gain relative to vision decreased, suggesting downweighting of vision that is consistent with previous studies (Peterka, 2002; Kiemel et al., 2006; Oie et al.; 2002). However, intermodal effects were sparse. The only evidence of intermodal reweighting was observed in trunk gain relative to vision, which showed a decrease with vibration OFF compared to the vibration ON conditions (L-V-G v L-G and H-V-G v H-G in Figure 4).
The limited evidence for intermodal reweighting effects in individuals with PD contrasts with that observed in healthy adults (Hwang et al, 2014). Under the same conditions of simultaneous 3-modality stimulation, healthy adults showed an increase in trunk/leg gain relative to the galvanic stimulus with the increase in visual amplitude, suggesting an intermodal upweighting of the vestibular information to compensate for visual downweighting. Individuals with PD showed no reweighting of vestibular information.
In addition, intermodal effects of vibration on trunk/leg gain relative to the visual and vestibular information were much weaker in individuals with PD compared to healthy adults. In Hwang et al. (2014), healthy adults showed an increase in leg and trunk gain relative to the visual and GVS stimuli from the L-G and H-G conditions to the L-V-G and H-V-G conditions, respectively, indicating that the visual and vestibular modalities are upweighted when vibration is turned on. This suggests an intermodal visual-proprioceptive reweighting effect and an intermodal vestibular-proprioceptive reweighting effect. These intermodal effects are observed because vibration changes proprioceptive inputs at the ankles thereby making these inputs a less reliable indicator of self-motion. Disruption of proprioception, even locally at the ankles, seems to affect visual and vestibular processing much more than a change in visual stimulus amplitude affects proprioception. This intermodal asymmetry emphasizes the delicate interplay between the modalities. The interplay of intramodal (vision) and intermodal (proprioceptive and vestibular) effects reflect a central “sensor fusion” process that continuously incorporates sensory input to generate the most reliable estimates of self-motion for the maintenance of upright equilibrium (Hwang et al., 2014). In contrast, leg gain relative to the visual and GVS stimuli was equivalent regardless of whether vibration was on or off in individuals with PD, indicating no reweighting of visual information and vestibular information in the legs when proprioception was disrupted through vibration. Trunk gain relative to vison was upweighted alike in healthy adults and individuals with PD when vibration was on. However, leg and trunk gain relative to GVS showed no reweighting in individuals with PD, unlike healthy adults (Hwang et al., 2014). These results suggest that individuals with PD not only have a proprioceptive deficit (particularly in the legs) during standing, but also have a cross-modal sensory fusion deficit that is crucial for upright stance control. In contrast, healthy adults reweight their sensory information (i.e. sensory reweighting) across multi-segments appropriately according to changing conditions of environment.
Intermodal upweighting of the proprioceptive channel was not observed in both groups. This may be due to the “local” nature of the vibratory stimulus. While the visual and vestibular modalities are anatomically organized so that a stimulus can bias the entire peripheral organ, muscle spindles are distributed throughout the musculature. A vibration localized to the Achilles tendon biases the sensory signals from an important muscle group related to posture, but there are many other muscles that play an active role in maintaining upright stance. Thus, intermodal effects of visual change on proprioception may be difficult to achieve, even in healthy adults, because the bias is distributed across many proprioceptive sensors and in different body segments (Hwang et al., 2014).
We also observed effects based upon body segment, as the trunk and leg segments respond differently to different types of stimuli in each group. Intramodal and intermodal effects on visual gain were almost equivalent across the leg and trunk segments in healthy adults (Hwang et al., 2014) whereas intramodal and intermodal effects on visual gain were shown in only trunk segments in individual with PD. This is consistent with the view for healthy adults that although the nervous system interprets visual information in the context of multi-segment dynamics, responses to visual information involves a single control signal that determines the activation of all muscles (Kiemel et al., 2008; Kiemel et al., 2011). Because the human postural control loop is consist of multiple components in closed feedback loop, it is difficult to answer why the sensory reweighting deficit in legs was shown particularly rather than the trunk without identifying each component of human postural control loop. It could be due to any combination of three component: different biomechanical properties (i.e., stiffness and rigidity) of body segments, the control strategy which is a process within the feedback component of the control loop and the sensory direct effect of the visual perturbation. Through the system identification of the human postural control loop, we might be able to evaluate the contribution of each component to the observed difference between legs and the trunk in PD (Hwang et al., 2016).
Central processing deficit with sensory reweighting deficit in PD – basal ganglia plays a role?
The central processing of sensory information ensures the production of a motor plan for task execution that is appropriate to the sensory environment (Allison & Jeka, 1994). Equilibrium control depends on the continual updating and prioritization of sensory information generated by the environment. The process of updating and prioritizing sensory information for postural control is referred to as sensory reweighing (Allison & Jeka, 1994). In spite of our finding about sensory reweighting deficit in PD in this study, the question as to whether this deficit is of central origin still remains to be answered.
Previous clinical studies have demonstrated that the basal ganglia play a prominent role in sensorimotor functions and action selection, both of which require the integration of multisensory information. For example,Nagy et al. (2006) provided evidence that caudate nucleus (CN) and substanitia nigra (SN) neurons integrate multisensory information through recordings of neuronal responses in CN and SN of anaesthetized cats to visual, auditory or somatosensory stimulation alone and in multisensory combinations. Recently, multisensory integration in the mouse striatum was also found through whole-cell recordings in mouse dorsal striatum during presentation of tactile and visual stimuli (Reig and Silberberg, 2014). All recorded neurons responded to bilateral whisker stimulation, and a subpopulation responded to visual stimulation, suggesting that striatum acts as a sensory “hub” with specialized functional roles for different types of neurons. Typically, postural instability is thought to be caused by disturbed motor programming within the basal ganglia. Automatic control of postural muscle tone and postural reflexes are modulated by the basal ganglia via their direct connections with the brainstem (Takakusaki et al., 2004). PD is traditionally regarded as a primarily hypo-dopaminergic syndrome, with symptoms resulting mostly from loss of dopamine-producing neurons in the substantia nigra, which is the part of the basal ganglia. Previous clinical evidence implicate the basal ganglia as being critical for central processing, particularly for the integration of sensory information, for postural control. Therefore, we suggest that the sensory reweighting deficit in PD is thought to be caused by a deficit in the basal ganglia which plays a role in integration of multiple sensory information and motor-programming.
Age effect in sensory fusion
The multisensory reweighting deficit of individuals with PD in this study is potentially an age effect. However, previous results have shown intact sensor fusion during standing in older adults. Allison et al. (2006) examined multisensory reweighting in healthy and fall-prone older adults using quantitative methods that have previously demonstrated sensory reweighting during standing in young adults. Both healthy and fall prone older adults demonstrated the same pattern of adaptive gain change as healthy young adults. Like the young adults, both elderly groups displayed clear evidence of intra- and inter-sensory reweighting to both vision and touch motion stimuli. They suggested that the central sensory reweighting adaptation process remains intact in healthy and fall-prone older adults with sufficiently intact peripheral sensation (Allison et al, 2006). Even though their study design was different from the current study, there is no current evidence that age for adult subjects is a factor in multisensory fusion during standing. Further investigation of multisensory reweighting in older adults using the current 3-modality paradigm to resolve the aging effect in multisensory reweighting more clearly.
5. CONCLUSION
In this study, we demonstrated intramodal and intermodal effects by simultaneous manipulation of the three sensory modalities that are critical for upright stance control in individuals with PD. Individuals with PD showed severe reweighting deficits, not only in the proprioceptive channel, as the literature suggests, but in the vestibular channel as well. More importantly, our paradigm is able to detect not just deficits in processing a single modality but is able to detect deficits in fusing the different modalities, through intermodal effects. We argue that in order to design better treatments for balance disorders, such knowledge is critical. For example, if PD patients are found to be deficient in reweighting a particular sensory input, rehabilitation could be focused on training modalities which they are able to upweight for better control of upright balance.
Acknowledgments
Support for this research provided by NIH grant RO1NS35070 (John Jeka, PI) at the University of Maryland.
Footnotes
The authors declare no competing financial interests.
References
- Allison L, Jeka JJ. The role of multisensory integration in balance disorders. In: Calvert G, Spence C, Stein BE, editors. Handbook of Multisensory Processes. Boston: MIT press; 1994. [Google Scholar]
- Allison L, Kiemel T, Jeka JJ. Multisensory reweighting of vision and touch is intact in healthy and fall-prone older adults. Exp Brain Res. 2006;175:342–352. doi: 10.1007/s00221-006-0559-7. [DOI] [PubMed] [Google Scholar]
- Benatru I, Vaugoyeau M, Azulay JP. Postural disorders in Parkinson’s disease. [Review] Clinical Neurophysiology. 2008;38:459–465. doi: 10.1016/j.neucli.2008.07.006. [DOI] [PubMed] [Google Scholar]
- Bendat JS, Piersol AG. Random data: Analysis and measurement procedures. 3rd. New York: Wiley; 2000. [Google Scholar]
- Bloem B. Postural instability in Parkinson’s disease. Clinical Neurology and Neurosurg. 1992;94:S41–S45. doi: 10.1016/0303-8467(92)90018-x. [DOI] [PubMed] [Google Scholar]
- Capíková N, Rocchi I, Hlava!ka F, Chiari I, Cappello A. Human postural response to lower leg muscle vibration of different duration. Physiol. Res. 2006;55(1):S129–S134. doi: 10.33549/physiolres.930000.55.S1.129. [DOI] [PubMed] [Google Scholar]
- Cooper SA. Master thesis. Canada: University of Lethbridge; 2005. Parkinsoni an sensory integration for balance control: time based postural effects of alterations in sensory information; pp. 9–10. [Google Scholar]
- Day BL, Marsden JF, Ramsay E, Mian OS, Fitzpatrick RC. Non-linear vector summation of left and right vestibular signals for human balance. J Physiol. 2010;508(4):671–682. doi: 10.1113/jphysiol.2009.181768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimbergen YA, Langston JW, Roos RA, Bloem BR. Postural instability in Parkinson’s disease: the adrenergic hypothesis and the locus coeruleus. Expert Rev Neurother. 2009;9(2):279–290. doi: 10.1586/14737175.9.2.279. [DOI] [PubMed] [Google Scholar]
- Horak FB, Macpherson JM Postural orientation and equilibrium. Handbook of Physiology, Exercise: Regulation and Integration of Multiple Systems. Vol. 12. New York: Oxford; 1996. pp. 255–292. [Google Scholar]
- Horak FB, Dimitrova D, Nutt JG. Direction-specific postural instability in subjects with Parkinson’s disease. Experimental Neurology. 2005;193:504–521. doi: 10.1016/j.expneurol.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Houeto JL, Damier P, Bejjani PB, Staedler C, Bonnet AM, Arnulf I, et al. Subthalamic stimulation in. Parkinson's disease: a multidisciplinary approach. Arch Neurol. 2000;57:461–465. doi: 10.1001/archneur.57.4.461. [DOI] [PubMed] [Google Scholar]
- Hwang S, Agada P, Kiemel T, Jeka JJ. Dynamic Reweighting of Three Modalities for Sensor Fusion. PLoS ONE. 2014;(1):e88132. doi: 10.1371/journal.pone.0088132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang S, Agada P, Kiemel T, Jeka JJ. Identification of the unstable human postural control system. Front. Syst. Neurosci. 2016 doi: 10.3389/fnsys.2016.00022. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs JV, Horak FB. Abnormal proprioceptive-motor integration contributes to hypometric postural responses of subjects with Parkinson’s disease. Neuroscience. 2006;141:999–1009. doi: 10.1016/j.neuroscience.2006.04.014. [DOI] [PubMed] [Google Scholar]
- Keijsers NL, Admiraal MA, Cools AR, Bloem BR, Gielen CC. Differential progression of proprioceptive and visual information processing deficits in Parkinson’s disease. European Journal of Neuroscience. 2005;21:239–248. doi: 10.1111/j.1460-9568.2004.03840.x. [DOI] [PubMed] [Google Scholar]
- Kiemel T, Oie KS, Jeka JJ. Multisensory fusion and the stochastic structure of postural sway. Biological Cybernetics. 2002;87:262–277. doi: 10.1007/s00422-002-0333-2. [DOI] [PubMed] [Google Scholar]
- Kiemel T, Oie KS, Jeka JJ. Slow dynamics of postural sway are in the feedback loop. Journal of Neurophysiology. 2006;95:1410–1418. doi: 10.1152/jn.01144.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiemel T, Elahi AJ, Jeka JJ. Identification of the plant for upright stance in humans: multiple movement patterns from a single neural strategy. Journal of Neurophysiology. 2008;100:3394–3406. doi: 10.1152/jn.01272.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiemel T, Zhang Y, Jeka JJ. Visual flow is interpreted relative to multisegment postural control. Journal of Motor Behavior. 2011;43(3):237–246. doi: 10.1080/00222895.2011.568991. [DOI] [PubMed] [Google Scholar]
- Koller WC, Glatt S, Vetere-Overfield B, Hassanein R. Falls and Parkinson’s Disease. Clin Neurophrmacol. 1989;12:98–105. doi: 10.1097/00002826-198904000-00003. [DOI] [PubMed] [Google Scholar]
- Mahboobin A, Loughlin P, Atkeson C, Redfern M. A mechanism for sensory re-weighting in postural control. Med Biol Eng Comput. 2009;47:921–929. doi: 10.1007/s11517-009-0477-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIlroy WE, Maki BE. Preferred placement of the feet during quiet stance: Development of a standardized foot placement for balance testing. Clinical Biomechanics. 2007;12:66–70. doi: 10.1016/s0268-0033(96)00040-x. [DOI] [PubMed] [Google Scholar]
- Mesure S, Azulay JP, Pouget J, Amblard B. Strategies of segmental stabilization during gait in Parkinson’s disease. Experimental Brain Research. 1999;129:573–581. doi: 10.1007/s002210050927. [DOI] [PubMed] [Google Scholar]
- Nagy A, Eordegh G, Paroczy Z, Markus Z, Benedek G. Multisensory integration in the basal ganglia. European Journal of Neuroscience. 2006;24:917–924. doi: 10.1111/j.1460-9568.2006.04942.x. [DOI] [PubMed] [Google Scholar]
- Nieves AV, Miyasaki JM, Lang AE. Acute onset dystonic camptocormia caused by lenticular lesions. Movement Disorders. 2001;16:177–180. doi: 10.1002/1531-8257(200101)16:1<177::aid-mds1035>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Oie KS, Kiemel T, Jeka JJ. Human multisensory fusion of vision and touch: detecting nonlinearity with small changes in the sensory environment. Neuroscience Letters. 2001;315:113–116. doi: 10.1016/s0304-3940(01)02348-5. [DOI] [PubMed] [Google Scholar]
- Oie KS, Kiemel T, Jeka JJ. Multisensory fusion: Simultaneous re-weighting of vision and touch for the control of human posture. Cognitive Brain Research. 2002;14:164–176. doi: 10.1016/s0926-6410(02)00071-x. [DOI] [PubMed] [Google Scholar]
- Peterka RJ. Sensorimotor integration in human postural control. J Neurophysiol. 88(3):1097–1118. doi: 10.1152/jn.2002.88.3.1097. 200. [DOI] [PubMed] [Google Scholar]
- Peterka RJ, Loughlin PJ. Dynamic regulation of sensorimotor integration in human postural control. Journal of Neurophysiology. 2004;91:410–423. doi: 10.1152/jn.00516.2003. [DOI] [PubMed] [Google Scholar]
- Reig R, Silberberg G. Multisensory Integration in the mouse striatum. Neuron. 2014;83:1200–1212. doi: 10.1016/j.neuron.2014.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickards C, Cody FW. Proprioceptive control of wrist movements in Parkinson’s disease. Reduced muscle vibration-induced errors. Brain. 1997;120:977–990. doi: 10.1093/brain/120.6.977. [DOI] [PubMed] [Google Scholar]
- Schieppati M, Hugon M, Grasso M, Nardone A, Galante M. The limits of equilibrium in young and elderly normal subjects and in parkinsonians. Electroencephalography and Clinical Neurophysiology. 1994;93:286–298. doi: 10.1016/0168-5597(94)90031-0. [DOI] [PubMed] [Google Scholar]
- Shumway-Cook A, Woollacott MH. Motor Control: Theory and practical applications. Baltimore, MA: Lippincott Williams Wilkins; 2001. [Google Scholar]
- Takakusaki K, Oohinata-Sugimoto J, Saitoh K, Habaguchi T. Role of basal ganglia-brainstem systems in the control of postural muscle tone and locomotion. Progress in Brain Research. 2004;143:231–237. doi: 10.1016/S0079-6123(03)43023-9. [DOI] [PubMed] [Google Scholar]
- van der Kooij H, Jacobs R, Koopman B, van der Helm F. An adaptive model of sensory integration in a dynamic environment applied to human stance control. Biol Cybern. 2001;84(2):103–115. doi: 10.1007/s004220000196. [DOI] [PubMed] [Google Scholar]
- Vaugoyeau M, Hakam H, Azulay JP. Proprioceptive impairment and postural orientation control in Parkinson’s disease. Hum Mov Sci. 2011;30(2):405–414. doi: 10.1016/j.humov.2010.10.006. [DOI] [PubMed] [Google Scholar]
- Zia S, Cody FW, O’Boyle DJ. Identification of unilateral elbow-joint position is impaired by Parkinson’s disease. Clinical Anatomy. 2002;15:23–31. doi: 10.1002/ca.1087. [DOI] [PubMed] [Google Scholar]
- Zoubir AM, Boashash B. The bootstrap and its application in signal processing. IEEE Signal Processing Magazine. 1998;15:56–76. [Google Scholar]