Abstract
Objective
To determine if high intensity exercise (HIE) would improve endothelial function more than an isocaloric bout of moderate intensity exercise (MIE) following glucose ingestion in adults with prediabetes.
METHODS
Twelve subjects with prediabetes completed all 3 conditions: time-course matched control (CON), and isocaloric exercise (~200kcal) at moderate [MIE; at lactate threshold (LT)], and high-intensity (HIE; 75% of difference between LT and VO2peak). Brachial artery flow-mediated dilation (FMD) was measured before exercise (baseline), within 30 min post-exercise and 1 and 2 hr following a 75g OGTT. Plasma F2-isoprostanes were also assessed during the protocol (i.e. baseline to 2 hr OGTT) as a biomarker of oxidative stress.
RESULTS
MIE reduced post-exercise F2-isoprostanesAUC compared with CON and HIE. Although exercise had no statistical effect on FMD post-exercise or during the OGTT, elevations in FMDAUC after MIE and HIE was associated with reduced post-exercise F2-isoprostanesAUC.
CONCLUSION
Exercise at either intensity had no effect on FMD immediately post-exercise following glucose administration. However, individuals with reduced oxidative stress responses to exercise had greater exercise-induced improvement in FMD. Further work is required to identify the mechanism by which exercise alters oxidative stress to enhance endothelial function.
INTRODUCTION
Individuals with prediabetes have approximately 50% higher risk of cardiovascular disease (CVD) compared with healthy counterparts (1). Although obesity related skeletal muscle insulin resistance is a primary feature that contributes to hyperglycemia in people with prediabetes (2), associated impaired endothelial function is a leading candidate for macro- and micro-vascular disturbances (1). Indeed, attenuated endothelial function occurs as a result of postprandial hyperglycemia induced oxidative stress that promotes an imbalance of nitric oxide and endothelin-1 production in individuals who are lean healthy and in individuals with type 2 diabetic (3,4). Therefore, identifying interventions that can restore vascular function during periods of hyperglycemia is paramount to improving glucose regulation.
Habitual exercise lowers CVD and diabetes risk in part by increasing skeletal muscle glucose uptake (5,6) and limb blood flow (7,8). Despite reports indicating that a single bout or short-term exercise training at moderate intensity can restore/improve vasodilation following glucose ingestion in people with and without type 2 diabetes (9–12), the effect of exercise intensity on endothelial function during diet-induced hyperglycemia is unknown in adults with prediabetes. This is clinically relevant because post-prandial hyperglycemia is known to increase risk for CVD, and high intensity exercise (HIE) is thought to improve endothelial function more than low to moderate intensity exercise (MIE). We recently reported that acute HIE was more beneficial at lowering post-prandial blood glucose than an isocaloric bout of MIE in people with prediabetes and this result was attributed to improvement in insulin sensitivity (13). However, it is possible that enhanced endothelial function was in part responsible for the exercise-induced glycemic benefit (11). Therefore, in the present study we tested the hypothesis that HIE would increase endothelial function more than MIE following glucose ingestion in adults with prediabetes, and this change in vascular function would relate to glycemic benefit and lower post-exercise oxidative stress (i.e. F2-isoprostanes).
METHODS
Subjects
Participants were the same people who were included in our prior work on glucose tolerance (13), but only 12 subjects underwent vascular function assessment (Table 1). Prediabetes was defined as having either: impaired fasting plasma glucose (100–126 mg/dl) or impaired glucose tolerance (2 hr plasma glucose 140–200 mg/dl) after a 75g oral glucose tolerance test (OGTT), and/or elevated HbA1c (5.7–6.4%). Subjects were non-smoking and sedentary (exercise < 30 min/d, < 3 d/wk) and underwent physical examination that included a resting and exercise stress test with 12-lead electrocardiogram and blood and urine chemistry analysis to exclude people with type 2 diabetes, cardiac dysfunction, and renal/liver complications. All subjects provided signed and verbal informed consent and the study was approved by the University of Virginia Institutional Review Board.
Table 1.
Subject Characteristics.
| Control | |
|---|---|
| N, (M/F) | 12 (7M/5F) |
| Age (years) | 52.1 ± 3.3 |
| Body weight (kg) | 98.4 ± 5.9 |
| Body mass index (kg/m2) | 32.5 ± 2.0 |
| Fat mass (kg) | 41.0 ± 3.6 |
| Fat-free mass (kg) | 57.4 ± 2.9 |
| Body fat (%) | 41.0 ± 1.7 |
| VO2peak (L/min) | 2.1 ± 0.1 |
| VO2peak (ml/kg/min) | 22.3 ± 1.5 |
| OGTT Screening | |
| Fasting PG (mg/dl) | 106.8 ± 5.7 |
| 2 hour PG (mg/dl) | 170.1 ± 9.0 |
| HbA1c (%) | 5.8 ± 0.1 |
Data are expressed as mean ± SEM. PG = plasma glucose
Body Composition and Aerobic Fitness
Weight was assessed on a digital platform with minimal clothing, and height was recorded on a stadiometer. Body fat was measured using air displacement plethysmography (BodPod, Cosmed, Concord, CA) corrected for thoracic gas volume. Subjects completed a VO2peak/lactate threshold (LT) bicycle ergometer test using open-circuit spirometry (Viasys Vmax Encore, Yorba Linda, CA) as performed by our group (13).
Metabolic Control
Subjects were instructed to consume approximately 200 g/d of carbohydrate and refrain from alcohol, caffeine, and vigorous physical activity for at least 72 hr prior to their OGTT. In addition, subjects were also instructed to avoid medications known to act on the vasculature (antihistamines, vitamin C, anti-hypertensive medications, etc.) for 5 days prior to each study visit. Women were studied during the early follicular phase (days 2–8) of the menstrual cycle to minimize the impact hormonal fluctuations may have on endothelial function. As a result, menstruating women (n=2) were studied approximately 1 month between conditions, and all other women were studied approximately 2–3 weeks between conditions.
Exercise/Control Conditions
Subjects reported to the Exercise Physiology Core Laboratory after a 10–12 hr overnight fast on 3 separate days and completed randomly assigned control (rest for 1h) and 200-kcal bouts of MIE (at LT, i.e. ~65% VO2peak) and HIE (75% of the difference between LT and VO2peak, i.e. ~90 VO2peak) as previously described (13).
Experimental Design
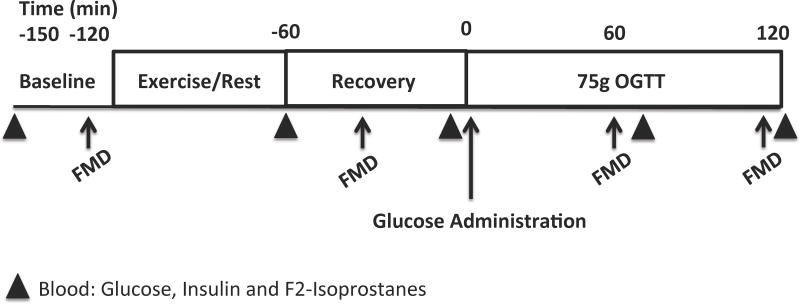
A study schematic is shown in Figure 1. Upon arrival to the laboratory, an intravenous catheter was placed in the dominant arm or hand, and baseline flow-mediated dilation (FMD) was performed to assess endothelial function (14). Subjects commenced exercise or continued rest for approximately 1 hr. FMD was measured within 30 min post-exercise or at the time-matched rest period to determine the acute effects of exercise intensity on fasting endothelial function prior to the OGTT. Thereafter, subjects received a 75g OGTT 1 hr post-exercise and FMD was repeated at 1 and 2 hr of the OGTT to provide assessment of post-prandial vascular function. Blood samples of F2-isoprostantes were collected at baseline, immediately post-exercise, pre-OGTT and at 1 and 2 hr during the OGTT to assess lipid peroxidation/oxidative stress. Plasma F2-isoprostane was measured using gas chromatographic/negative ion chemical ionization mass spectrometry (15).
Figure 1.
Study schematic. FMD = flow-mediated dilation. OGTT = oral glucose tolerance test.
FMD Assessment and Analysis
Images of the brachial artery were obtained by a single investigator (CAR) on the subject’s non-dominant arm in an extended position using high-resolution 2D and Doppler ultrasound (HDI 5000, ATL, Philips Ultrasound, Andover, MA) with a 12 MHz linear-array transducer. All images of the brachial artery were taken in the longitudinal plane, 2–10 cm proximal to the antecubital fold. The location of the probe was marked during the first measurement of each test day, and repeat measurements were performed in the same region. A manual blood pressure cuff was placed 2 cm distal to the antecubital fold. Before cuff inflation 1 minute of baseline images were captured every 5 seconds for determination of average brachial artery diameter. The cuff was inflated to 200 mmHg for 5 minutes in order to occlude the brachial artery. Upon cuff release digital images of the artery were captured every 5–10 seconds for 2 minutes post-occlusion to determine peak vessel dilation. Images were EKG-gated and captured at the onset of the R-wave. All brachial artery images were analyzed using custom edge-detection software (Brachial Analyzer, Iowa City, IA) by a single investigator (JYW) blinded to both time and condition. Arterial diameters (mm) were calculated as the mean distance between the anterior and posterior wall at the intima-lumen interface. Percent FMD was defined as the change in vessel diameter from pre-occlusion to peak dilation at each time point. We also expressed endothelial function as a single area under the curve (FMDAUC) value calculated with the percent FMD values from baseline to 2 hr post-OGTT.
Statistical Analysis
Data were analyzed using the statistical program R (Vienna, Austria 2013). Data were compared across conditions using repeated measures analysis of variance (ANOVA) and two-way repeated measures ANOVA (condition x time). In the event of a significant main effect or interaction, pairwise comparisons were used to identify the source of significance. Because blood glucose control was a primary outcome in our prior work (13), we examined herein the relationship of endothelial function, glucose tolerance, and insulin sensitivity to gain insight to the regulation of post-exercise glucose homeostasis. Pearson’s product moment correlation analysis was used to determine associations. Statistical significance was accepted as P≤0.05, and trends were interpreted as P<0.05x≤0.10. Data are reported as mean ± standard error of mean (SEM).
RESULTS
Demographics
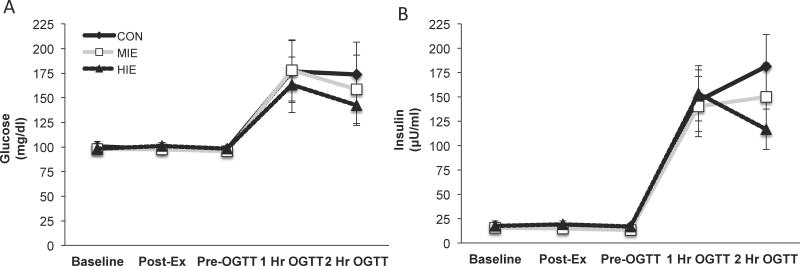
Subject characteristics and glucose as well as insulin concentrations were previously reported (13), but are highlighted here in this subgroup analysis for ease of interpretation (Table 1 and Figure 2). Subjects were middle-aged (52.1±3.3yr), obese (32.5±2.0kg/m2) and sedentary (VO2peak: 22.3±1.5 ml/kg/min). Exercise was performed for 40±2.6 min at 48.4±1.6% and 24±1.1 min at 82.6±2.7% of VO2peak for MIE and HIE respectively. Plasma glucose was improved during the late phase of the OGTT following HIE compared with MIE (13).
Figure 2.
Effect of exercise intensity on post-prandial metabolism. Plasma glucose (a) and insulin (b) concentrations. Data are expressed as mean ± SEM. Data were previously reported (13), but are highlighted here for ease of interpretation.
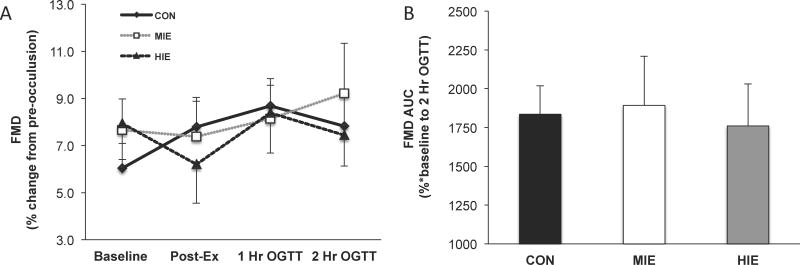
Endothelial Function
There was no statistical difference in pre-occlusion or post-occlusion (i.e. peak value) brachial artery diameters across conditions at any time-point during the OGTT (Table 2). FMD expressed as a percentage or AUC was not statistically different between MIE and HIE, compared with control (Figure 3a and 3b). Although the change in FMDAUC did not correlate with changes in early or late phase glucose tolerance following MIE (early: r=−0.09, P=0.76 and late: r=−38, P=0.21) or HIE (early: r=−0.21, P=0.51 or late: r=−0.38, P=0.21), individuals with low FMDAUC at control did have greater improvements in late phase glucose tolerance following HIE (r=0.61, P=0.02). There was no correlation between FMDAUC and late phase glucose tolerance after MIE (r=−0.02, P=0.94). In addition, the change in insulin sensitivity following MIE (r=0.37, P=0.23) or HIE (r=0.32, P=0.29) did not correlate with changes in FMDAUC.
Table 2.
Brachial artery diameter across conditions.
| CON | MIE | HIE | |
|---|---|---|---|
| Baseline FMD | |||
| Pre-occlusion diameter (mm) | 4.11 ± 0.24 | 4.02 ± 0.24 | 3.98 ± 0.24 |
| Post-occlusion Peak diameter (mm) | 4.35 ± 0.23 | 4.31 ± 0.23 | 4.28 ± 0.23 |
| Post-Exercise/Pre-OGTT | |||
| Pre-occlusion diameter (mm) | 4.05 ± 0.23 | 4.00 ± 0.25 | 4.15 ± 0.25 |
| Post-occlusion Peak diameter (mm) | 4.37 ± 0.25 | 4.26 ± 0.22 | 4.38 ± 0.24 |
| 1 hr OGTT | |||
| Pre-occlusion diameter (mm) | 3.94 ± 0.23 | 4.03 ± 0.27 | 4.03 ± 0.25 |
| Post-occlusion Peak diameter (mm) | 4.29 ± 0.25 | 4.33 ± 0.26 | 4.36 ± 0.26 |
| 2 hr OGTT | |||
| Pre-occlusion diameter (mm) | 4.13 ± 0.24 | 4.05 ± 0.26 | 4.05 ± 0.23 |
| Post-occlusion Peak diameter (mm) | 4.43 ± 0.24 | 4.38 ± 0.24 | 4.34 ± 0.24 |
Data are expressed as mean ± SEM. Conditions were compared by repeated measures analysis of variance (ANOVA). CON = control. MIE = moderate intensity exercise. HIE = high intensity exercise. OGTT = oral glucose tolerance test. No statistical differences within or between conditions were observed.
Figure 3.
Effect of exercise intensity on endothelial function. FMD time-course responses (a). Area under the curve (AUC) for FMD (b). FMD = flow-mediated dilation. FMD was calculated as the percent change from pre- to post-occulsion diameter. Data are expressed as mean ± SEM.
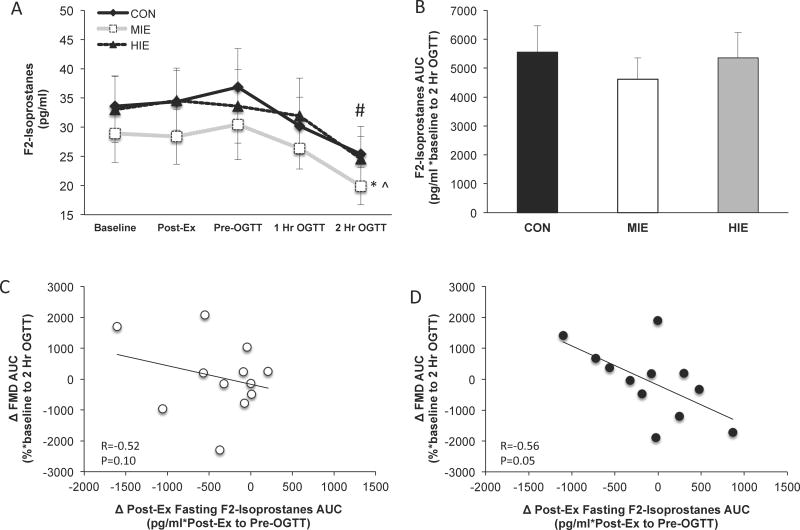
Oxidative stress
There was no statistical difference in baseline F2-isoprostane levels or AUC across conditions (Figure 4a and 4b). However, post-exercise plasma F2-isoprostane AUC (i.e. immediate post-exercise and pre-OGTT time points) was significantly lower following MIE (1765.1 ± 319.3 pg/ml) compared with HIE (2044.0 ± 334.7 pg/ml, P<0.05) and control (2136.0 ± 350.8 pg/ml, P<0.05). In addition, F2-isoprostanes levels during the OGTT were significantly lower following MIE compared with HIE and control (P<0.05, Figure 4a). Post-prandial F2-isoprostanes 2-hours following the OGTT were significantly lower compared with immediate post-exercise levels (time effect; P<0.05). Reduced post-exercise F2-isoprostaneAUC was associated with increased FMDAUC after MIE (r=−0.52, trend P=0.10; Figure 4c) and HIE (r=−0.56, P=0.05; Figure 4d).
Figure 4.
Effect of exercise intensity on oxidative stress and associations with endothelial function. F2-isoprostanes time-course responses (a). Area under the curve (AUC) for F2-isoprostanes (b). Correlation between isoprostanes and vascular function following MIE (moderate intensity exercise; (c) and HIE (high intensity exercise; (d)). Change (Δ) between exercise and control conditions. FMD = flow-mediated dilation and reflects endothelial function. *Compared to CON, P<0.05. ^Compared to MIE, P<0.05. #Condition effect compared with CON, P<0.05. Data are expressed as mean ± SEM.
DISCUSSION
The major finding from this study is that a single bout of MIE or HIE had no statistical effect on endothelial function following glucose ingestion in obese people with prediabetes. Prior studies in people at risk for type 2 diabetes report that exercise improves FMD (16–18), although the lack of effect of exercise intensity on FMD is not completely unexpected since HIE has been reported to impair (19) or have no effect (20) on fasting endothelial function. The reason we observed no change in FMD during the OGTT is somewhat surprising though giving that dietary or intravenous hyperglycemia impairs endothelial function (10,11,21). In fact, single bouts or short-term training at MIE can improve vascular function during periods of hyperglycemia (9–12). However, consistent with prior work (9–12) we report that FMD increased by ~1% (absolute difference from pre-OGTT; Figure 3a) during glucose ingestion of the control condition. This observation suggests that dietary-induced hyperglycemia per se from the OGTT did not impair FMD in these prediabetic individuals. Indeed, we did not detect statistical relationships between the change in endothelial function and glucose tolerance following MIE or HIE. The reason glucose ingestion did not exacerbate FMD as previously reported is unclear (10,11,21), but it may relate to the impact of pre-existing endothelial function on responsiveness to exercise. While the current study includes a modest sample size of people with prediabetes, it was not designed to test the effects of pre-existing endothelial dysfunction on responsiveness to exercise. However, prior work by our group suggests that people with obesity have blunted gains in fasting vascular function benefit following a single bout of MIE and HIE (24), and individuals with hemodynamic impairment are likely to improve FMD following training in post-menopausal women compared with those with normal FMD (22). Interestingly, individuals herein with low endothelial function had greater improvements in glucose tolerance following HIE but not MIE. This later finding is consistent with our prior report (13), and suggests that exercise intensity may contribute to improvements in glucose regulation via changes in blood flow. Therefore, further work is required understand the interaction of exercise intensity with pre-existing endothelial dysfunction on glucose regulation since inter-subject variability in response to exercise likely impacted our ability to statistically demonstrate an effect of exercise at either intensity on FMD (22,23).
There are several other reasons that may explain the overall lack of improvement in endothelial function immediately following exercise. First, the lack of increase in vascular function after exercise in the present study may relate to the observation that obese adults have larger arterial diameters than lean counterparts (24). This is consistent with recent work reporting that obese people do not improve basal endothelial function following low, moderate or high intensity exercise (24,25). However, each subject in our study served as their own control, and we observed no differences in baseline diameter among conditions. Thus, arterial diameter is unlikely to explain the overall lack of vascular function improvement post-exercise. Alternatively, oxidative stress impairs vasodilation via blunted nitric oxide bioavailability (26,27), and recent work has speculated that higher intensities of exercise transiently impair endothelial function through an oxidative stress related mechanism (20). Despite lower circulating F2-isoprostanes following MIE compared with HIE or control (Figure 4a), improvements in endothelial function following both exercise conditions were directly associated with lower post-exercise F2-isoprostanes (Figure 4c and 4d). This suggests that lower oxidative stress following exercise contributes to greater improvements in endothelial function independent of exercise intensity. The exact reason why some people generate more or less oxidative stress following exercise is beyond the scope of this study, but excessive oxidative stress does favor expression of endothelin-1 that in turn promotes vasoconstriction and impairs blood flow (3,4). Collectively, our findings highlight the need for additional investigations across different obese phenotypes to determine the exact mechanisms.
Another possible explanation for the lack of exercise-induced increases in FMD following glucose ingestion may relate to insulin action. Indeed, insulin is an important stimulus that contributes to increased blood flow in exercise-trained individuals (4,28–30). If insulin levels were reduced following exercise, then it would be reasonable to expect little to no improvement in FMD during the OGTT. We previously reported that plasma insulin levels were reduced following MIE and HIE (13), suggesting that less insulin in the circulation could have reduced a key stimulus for increasing endothelial function. When compared with our control condition, the exercise results observed herein are consistent with prior work reporting that hyperinsulinemia increases and protects endothelial function during periods of hyperglycemia compared with hyperglycemic conditions alone (21). Because insulin levels were lowered following both exercise conditions, it reasons that large conduit arteries may have become more sensitive to insulin, resulting overall in no change in FMD. However, we observed no correlation between increased insulin sensitivity and FMD following MIE or HIE, suggesting that changes in insulin action may not have impacted large conduit arteries post-exercise. While it would be more appropriate to test the effects of insulin on vascular function following exercise with the euglycemic hyperinsulinemic clamp to avoid confounding effects of pancreatic insulin secretion changes during an OGTT, it is worth noting that the microcirculation more closely aligns with insulin-stimulated glucose regulation than large conduit arteries (28). Thus, it remains possible that changes in skeletal muscle microcirculation were altered following exercise in our study and contributed to some extent to the decrease in plasma glucose following HIE (13). Further work is warranted to understand the effect of exercise intensity on insulin-mediated skeletal muscle vasculature.
This study has limitations that may affect our interpretation. We recognize that FMD was not corrected for shear stress in the current study as proposed by some, but not all (31,32), and this may contribute to the lack of FMD change post-exercise and/or during the OGTT. However, recent work observed no correlation between endothelial function and methods of shear stress adjustment in older populations, suggesting that shear stress is not necessary for identification of CVD risk (31). In addition, FMD uncorrected for shear stress is an independent predictor of CVD risk and mortality (33). Thus, expressing FMD as a percentage of pre-occlusion diameter is a valid approach for assessing vascular function (14). We cannot rule out the possibility that fluctuations in blood glucose per se and/or sympathetic activation of counter regulatory hormones could have blunted the endothelium to dilate in the post-exercise period (14). However, use of the OGTT provides a more physiologic assessment of FMD than use of a hyperglycemic and/or hyperinsulinemic clamp technique. We also acknowledge that the mode of exercise (i.e. cycling vs. running vs. weight lifting) might contribute to the different responses seen between studies (34). In fact, is possible that lower body muscle contraction (i.e. cycling) in our study could have affected local responses and minimized the stimulus to increase upper body blood flow. Lastly, while less oxidative stress was associated with higher endothelial responses following acute MIE and HIE in the current study, it remains possible that elevated oxidative stress responses from exercise promotes “supra-normalization” of endothelial function over a longer time-course. In fact, greater oxidative stress produced during repeated bouts of exercise may be an important stimulus for vascular function adaptation and preservation of the training effect (19,35,36). Therefore, additional work is needed to understand the time-course effects of training at different exercise intensities on skeletal muscle vascular adaptations in relation to cardiometabolic risk factors (e.g. glycemic control or blood pressure) in obese individuals with prediabetes.
In conclusion, endothelial function was not improved following a single bout of MIE or HIE in people with prediabetes after glucose ingestion. However, individuals with low endothelial function had greater improvements in glucose tolerance following HIE. Improvements in vascular function was also linked to reduced oxidative stress. Taken together, our findings suggest that exercise is an effective therapy for people with endothelial dysfunction to lower cardiometabolic disease risk, and improved blood flow may at least partially contribute to improved glucose tolerance following high intensity exercise. Further work is warranted to understand how exercise impacts vascular function across the pan-arterial tree in order to maximize lifestyle prescriptions for the prevention and/or treatment of type 2 diabetes and CVD.
What is already known about this subject?
Exercise improves fasting endothelial function.
Oxidative stress is associated with attenuated flow-mediated dilation.
Improved vascular function contributes to exercise-induced glycemic benefit.
What this study adds
Acute exercise does not improve endothelial function following glucose ingestion regardless of intensity.
Post-exercise oxidative stress is associated with reduced endothelial function in people with prediabetes.
Acknowledgments
We thank the nursing staff of the Clinical Research Unit for technical assistance, and the dedicated research assistants and participants for their effort. The authors report no conflict of interest. This research was supported by the Curry School of Education Foundation (SKM) and Virginia Commonwealth Health Research Board and NIH-RR00847 (AW).
Footnotes
Financial Disclosures: Curry School of Education Foundation (SKM) and Virginia Commonwealth Health Research Board and NIH- RR00847 (AW)
Conflict of Interest: The authors declare no conflict of interest.
References
- 1.DeFronzo RA, Abdul Ghani MA. Assessment and treatment of cardiovascular risk in prediabetes: impaired glucose tolerance and impaired fasting glucose. Am J Cardiol. 2011;108:3–24B. doi: 10.1016/j.amjcard.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 2.Malin SK, Haus JM, Solomon TPJ, Blaszczak A, Kashyap SR, Kirwan JP. Insulin sensitivity and metabolic flexibility following exercise training among different obese insulin resistant phenotypes. Am J Physiol Endocrinol Metab. 2013;305:E1292–8. doi: 10.1152/ajpendo.00441.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baron AD, Laakso M, Brechtel G, Hoit B, Watt C, Edelman SV. Reduced postprandial skeletal muscle blood flow contributes to glucose intolerance in human obesity. J Clin Endocrinol Metab. 1990;70:1525–33. doi: 10.1210/jcem-70-6-1525. [DOI] [PubMed] [Google Scholar]
- 4.Clark MG. Impaired microvascular perfusion: a consequence of vascular dysfunction and a potential cause of insulin resistance in muscle. Am J Physiol Endocrinol Metab. 2008;295:E732–50. doi: 10.1152/ajpendo.90477.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Utriainen T, Mäkimattila S, Virkamäki A, Lindholm H, Sovijärvi A, Yki-Järvinen H. Physical fitness and endothelial function (nitric oxide synthesis) are independent determinants of insulin-stimulated blood flow in normal subjects. J Clin Endocrinol Metab. 1996;81:4258–63. doi: 10.1210/jcem.81.12.8954024. [DOI] [PubMed] [Google Scholar]
- 6.Malin SK, Niemi N, Solomon TPJ, Haus JM, Kelly KR, Filion J, et al. Exercise Training with Weight Loss and either a High- or Low-Glycemic Index Diet Reduces Metabolic Syndrome Severity in Older Adults. Ann Nutr Metab. 2012;61:135–141. doi: 10.1159/000342084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ebeling P, Bourey R, Koranyi L, Tuominen JA, Groop LC, Henriksson J, et al. Mechanism of enhanced insulin sensitivity in athletes. Increased blood flow, muscle glucose transport protein (GLUT-4) concentration, and glycogen synthase activity. J Clin Invest. 1993;92:1623–31. doi: 10.1172/JCI116747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hardin DS, Azzarelli B, Edwards J, Wigglesworth J, Maianu L, Brechtel G, et al. Mechanisms of enhanced insulin sensitivity in endurance-trained athletes: effects on blood flow and differential expression of GLUT 4 in skeletal muscles. J Clin Endocrinol Metab. 1995;80:2437–46. doi: 10.1210/jcem.80.8.7629239. [DOI] [PubMed] [Google Scholar]
- 9.Fujita S, Rasmussen BB, Cadenas JG, Drummond MJ, Glynn EL, Sattler FR, et al. Aerobic exercise overcomes the age-related insulin resistance of muscle protein metabolism by improving endothelial function and Akt/mammalian target of rapamycin signaling. Diabetes. 2007;56:1615–22. doi: 10.2337/db06-1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu W, Zhong C, Yu Y, Li K. Acute effects of hyperglycaemia with and without exercise on endothelial function in healthy young men. Eur J Appl Physiol. 2007;99:585–91. doi: 10.1007/s00421-006-0378-3. [DOI] [PubMed] [Google Scholar]
- 11.Weiss E, Arif H, Villareal D, Marzetti E, Holloszy J. Endothelial function after high-sugar-food ingestion improves with endurance exercise performed on the previous day. Am J Clin Nutr. 2008;88:51–7. doi: 10.1093/ajcn/88.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mikus CR, Fairfax ST, Libla JL, Boyle LJ, Vianna LC, Oberlin DJ, et al. Seven days of aerobic exercise training improves conduit artery blood flow following glucose ingestion in patients with type 2 diabetes. J Appl Physiol. 2011;111:657–664. doi: 10.1152/japplphysiol.00489.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rynders CA, Weltman JY, Jiang B, Breton M, Patrie J, Barrett EJ, et al. Effects of exercise intensity on postprandial improvement in glucose disposal and insulin sensitivity in prediabetic adults. J Clin Endocrinol Metab. 2014;99:220–8. doi: 10.1210/jc.2013-2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thijssen DHJ, Black MA, Pyke KE, Padilla J, Atkinson G, Harris RA, et al. Assessment of flow-mediated dilation in humans: a methodological and physiological guideline. Am J Physiol Heart Circ Physiol. 2011;300:H2–12. doi: 10.1152/ajpheart.00471.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Milne GL, Sanchez SC, Musiek ES, Morrow JD. Quantification of F2-isoprostanes as a biomarker of oxidative stress. Nat Protoc. 2007;2:221–6. doi: 10.1038/nprot.2006.375. [DOI] [PubMed] [Google Scholar]
- 16.Currie KD, McKelvie RS, Macdonald MJ. Flow-mediated dilation is acutely improved after high-intensity interval exercise. Med Sci Sports Exerc. 2012;44:2057–64. doi: 10.1249/MSS.0b013e318260ff92. [DOI] [PubMed] [Google Scholar]
- 17.Harvey PJ, Picton PE, Su WS, Morris BL, Notarius CF, Floras JS. Exercise as an alternative to oral estrogen for amelioration of endothelial dysfunction in postmenopausal women. Am Heart J. 2005;149:291–7. doi: 10.1016/j.ahj.2004.08.036. [DOI] [PubMed] [Google Scholar]
- 18.Padilla J, Harris RA, Fly A, Rink LD, Wallace JP. The effect of acute exercise on endothelial function following a high-fat meal. Eur J Appl Physiol. 2006;98:256–62. doi: 10.1007/s00421-006-0272-z. [DOI] [PubMed] [Google Scholar]
- 19.Rognmo O, Bjørnstad TH, Kahrs C, Tjønna AE, Bye A, Haram PM, et al. Endothelial function in highly endurance-trained men: effects of acute exercise. J Strength Cond Res. 2008;22:535–42. doi: 10.1519/JSC.0b013e31816354b1. [DOI] [PubMed] [Google Scholar]
- 20.Birk GK, Dawson EA, Batterham AM, Atkinson G, Cable T, Thijssen DHJ, et al. Effects of exercise intensity on flow mediated dilation in healthy humans. Int J Sports Med. 2013;34:409–14. doi: 10.1055/s-0032-1323829. [DOI] [PubMed] [Google Scholar]
- 21.Perkins JM, Joy NG, Tate DB, Davis SN. Acute effects of hyperinsulinemia and hyperglycemia on vascular inflammatory biomarkers and endothelial function in overweight and obese humans. Am J Physiol Endocrinol Metab. 2015;309:E168–76. doi: 10.1152/ajpendo.00064.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Swift D, Weltman J, Patrie J, Saliba S, Gaesser G, Barrett EJ, et al. Predictors of improvement in endothelial function after exercise training in a diverse sample of postmenopausal women. J Womens Health. 2014;23:260–6. doi: 10.1089/jwh.2013.4420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Green D, Eijsvogels T, Bouts Y, Maiorana A, Naylor L, Scholten R, et al. Exercise training and artery function in humans: nonresponse and its relationship to cardiovascular risk factors. J Appl Physiol. 2014;117:345–52. doi: 10.1152/japplphysiol.00354.2014. [DOI] [PubMed] [Google Scholar]
- 24.Hallmark R, Patrie J, Liu Z, Gaesser GA, Barrett EJ, Weltman A. The effect of exercise intensity on endothelial function in physically inactive lean and obese adults. PLoS ONE. 2014;9:e85450. doi: 10.1371/journal.pone.0085450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harris RA, Padilla J, Hanlon K, Rink L, Wallace J. The flow-mediated dilation response to acute exercise in overweight active and inactive men. Obesity. 2008;16:578–84. doi: 10.1038/oby.2007.87. [DOI] [PubMed] [Google Scholar]
- 26.Vincent HK, Morgan JW. Obesity exacerbates oxidative stress levels after acute exercise. Med Sci Sports Exerc. 2004;36:772–9. doi: 10.1249/01.mss.0000126576.53038.e9. [DOI] [PubMed] [Google Scholar]
- 27.Bergholm R, Mäkimattila S, Valkonen M, Liu ML, Lahdenperä S, Taskinen MR, et al. Intense physical training decreases circulating antioxidants and endothelium-dependent vasodilatation in vivo. Atherosclerosis. 1999;145:341–9. doi: 10.1016/s0021-9150(99)00089-1. [DOI] [PubMed] [Google Scholar]
- 28.Barrett EJ, Eggleston EM, Inyard AC, Wang H, Li G, Chai W, et al. The vascular actions of insulin control its delivery to muscle and regulate the rate-limiting step in skeletal muscle insulin action. Diabetologia. 2009;52:752–64. doi: 10.1007/s00125-009-1313-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dela F, Larsen JJ, Mikines KJ, Ploug T, Petersen LN, Galbo H. Insulin-stimulated muscle glucose clearance in patients with NIDDM. Effects of one-legged physical training. Diabetes. 1995;44:1010–1020. doi: 10.2337/diab.44.9.1010. [DOI] [PubMed] [Google Scholar]
- 30.De Filippis E, Cusi K, Ocampo G, Berria R, Buck S, Consoli A, et al. Exercise-induced improvement in vasodilatory function accompanies increased insulin sensitivity in obesity and type 2 diabetes mellitus. J Clin Endocrinol Metab. 2006;91:4903–10. doi: 10.1210/jc.2006-1142. [DOI] [PubMed] [Google Scholar]
- 31.Thijssen DHJ, Bullens LM, van Bemmel MM, Dawson EA, Hopkins N, Tinken TM, et al. Does arterial shear explain the magnitude of flow-mediated dilation?: a comparison between young and older humans. Am J Physiol Heart Circ Physiol. 2009;296:H57–64. doi: 10.1152/ajpheart.00980.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pyke KE, Tschakovsky ME. Peak vs. total reactive hyperemia: which determines the magnitude of flow-mediated dilation? J Appl Physiol. 2007;102:1510–9. doi: 10.1152/japplphysiol.01024.2006. [DOI] [PubMed] [Google Scholar]
- 33.Yeboah J, Crouse JR, Hsu F, Burke GL, Herrington DM. Brachial flow-mediated dilation predicts incident cardiovascular events in older adults: the Cardiovascular Health Study. Circulation. 2007;115:2390–7. doi: 10.1161/CIRCULATIONAHA.106.678276. [DOI] [PubMed] [Google Scholar]
- 34.Dawson E, Green D, Cable NT, Thijssen DHJ. Effects of acute exercise on flow-mediated dilatation in healthy humans. J Appl Physiol. 2013;115:1589–98. doi: 10.1152/japplphysiol.00450.2013. [DOI] [PubMed] [Google Scholar]
- 35.Cocks M, Shaw C, Shepherd S, Fisher J, Ranasinghe A, Barker T, et al. Sprint interval and endurance training are equally effective in increasing muscle microvascular density and eNOS content in sedentary males. J Physiol (Lond) 2013;591:641–56. doi: 10.1113/jphysiol.2012.239566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prior SJ, Goldberg AP, Ortmeyer HK, Chin ER, Chen D, Blumenthal JB, et al. Increased skeletal muscle capillarization independently enhances insulin sensitivity in older adults after exercise training and detraining. Diabetes. 2015;64:3386–95. doi: 10.2337/db14-1771. [DOI] [PMC free article] [PubMed] [Google Scholar]