Abstract
Background
The HIV epidemic continues to expand among men who have sex with men (MSM) in China. The NIMH Project Accept/HPTN 043 trial suggested a borderline significant trend towards HIV incidence reduction among persons with higher testing rates.
Methods
We assessed HIV testing histories and infection status among a community-based Beijing MSM. HIV serostatus was lab-confirmed. We ascertained demographic/behavioral factors via questionnaire-based interviews. Associations of prior HIV testing with odds of current HIV infection were assessed, seeking improved like-with-like risk comparisons through multivariable logistic regression analysis with propensity score adjustment and restricted cubic spline modeling.
Results
Among 3,588 participants, 12.7% were HIV-infected; 70.8 % reported having ever tested for HIV. Compared to MSM who never tested, those ever testing had a 41% reduction in the odds of being HIV-positive (adjusted odds ratio [aOR], 0.59; 95% confidence interval [CI]: 0.48, 0.74). Higher HIV testing frequencies were associated with a decreasing trend in the odds of being infected with HIV vs. a referent group with no prior testing (>6 tests [aOR: 0.27; 95%CI: 0.18, 0.41]; 4–6 [aOR: 0.55; 95%CI: 0.39, 0.78]; 2–3 [aOR: 0.61; 95%CI: 0.45, 0.82]; P for trend <0.001). The multivariable adjusted model with restricted cubic spline of HIV testing frequency showed a higher frequency of prior HIV testing associated with lower odds of HIV infection, particularly among men with ≥10 lifetime male sexual partners.
Conclusions
Using risk probability adjustments to enable less biased comparisons, frequent HIV testing was associated with a lower HIV odds among Chinese MSM.
Keywords: HIV/AIDS, testing, prevention intervention, men who have sex with men, propensity score, China
Introduction
Modern antiretroviral therapy (ART) has led to a massive reduction in HIV-related morbidity and mortality.1–3 Achieving viral suppression among seropositive persons depends upon engagement and retention in stages of the care continuum, including HIV testing, linkage to care, and initiation of and adherence to ART.4–14 HIV testing is the entry point for health services, risk reduction counseling, and the benefits of early ART.2,15–17 Moreover, seropositive individuals who are aware of their HIV status are often less likely to engage in high-risk behaviors.18 Reduced risky behaviors, in combination with early and sustained ART adherence, can reduce the likelihood of HIV transmission.1,19–21
The HIV epidemic in China has evolved over the past three decades: first cases were most prevalent among persons who injected drugs, then plasma/blood donors, and now via sexual transmission. While HIV prevalence is <1% among female sex workers,22 it is >5% among men who have sex with men (MSM).23 If there is no change in prevention intervention coverage, models predict HIV prevalence to rise from 7.8% in 2010 to 21.4% by 2020 among MSM in Beijing.24
Despite the high HIV risk among Chinese MSM, their overall HIV testing rate is suboptimal. A recent meta-analysis of 54 independent studies showed that 47% of Chinese MSM ever tested for HIV and only 38% among the testers tested in the past 12 months.15 Both the Chinese government and community-based organizations (CBOs) have strengthened HIV testing by increasing free volunteer counseling and testing (VCT) and extending outreach. U.S. national guidelines recommend at least annual HIV testing among all MSM, every 3–6 months if at highest risk.25–27 In contrast, Chinese policies focus on expanding coverage without specific recommended testing frequencies.17 Previous studies also suggested some key barriers resulting in suboptimal HIV testing experience among Chinese MSM, such as low perception of HIV risk, inconvenient clinic time/location, HIV stigma/discrimination, name-based testing policy to potentially harm confidentiality, lack of professionalism and low quality in HIV testing service provision.28,29
Evidence has shown that frequent testing for HIV has been associated with knowing one’s last sexual partner’s HIV status, having fewer causal sex partners,30–33 and having a lower HIV risk.30 Whether this latter observation is due to lower risk characteristics of persons seeking testing or whether testing itself serves as a useful risk-reduction tool is unclear. The only evidence from a randomized controlled trial is the NIMH Project Accept/HPTN 043 study; increased HIV testing frequency was shown to have a marginal effect in reducing HIV incidence among presumed heterosexual populations in South Africa, Tanzania, Zimbabwe, and Thailand.34 Using the cross-sectional baseline HIV testing data from an NIH-funded randomized clinical trial among MSM in Beijing, China, we evaluated the association between prior HIV testing and HIV infection.
Methods
Study setting and population
The parent clinical trial (ClinicalTrials.gov Identifier: NCT01904877; Multi-component HIV Intervention Packages for Chinese MSM —Test, Link and Care) is known as the China-MP3 Project. The parent trial included two phases. In Phase I (recruitment phase), we cooperated with the Chaoyang AIDS Volunteer Group (CAVG) to expand our outreach to HIV-negative or status unknown MSM in Beijing for HIV testing and study participation. In Phase II, we conducted a randomized clinical trial to assess the efficacy of short message/peer counseling intervention versus standard-of-care in linkage to and engagement in HIV care among men who were diagnosed with HIV infection in Phase I. Data for the current analysis were from Phase I cross-sectional survey completed from March 2013 to March 2014, during which there was no intervention. Participants were recruited primarily via short message services, website advertisements, community outreach, and peer referrals. Interested MSM were invited for eligibility assessment, survey completion and blood drawn for HIV test in a private room at one of our four specified HIV clinics, including Beijing Municipal Center for Disease Control and Prevention (CDC), Chaoyang District CDC, Xicheng District CDC, and Jingcheng Dermatology Hospital. Inclusion criteria were: men, aged 18 years or older, self-reported sex with men (or transgender women) in the last 12 months, self-reported HIV-negative or status-unknown (the national HIV/AIDS database was used to validate these self-reported data), living in Beijing, having not previously participated in the current study, and able and willing to provide written informed consent. The protocol was approved by the institutional review boards of Vanderbilt University and the National Center for AIDS/STD Control and Prevention of the China Center for Disease Control and Prevention.
Data collection
Upon enrollment, a study nurse validated the potential participant’s eligibility by checking his name on the appointment list and dialing the cell phone that was used for scheduling the appointment. To estimate HIV prevalence among MSM in Beijing, we offered HIV testing to a specific subject at baseline in this study. To prevent duplicates, we informed the participants to only complete the survey and get HIV testing once, at the time of enrollment. Cellphone numbers, names and recorded dates were used to detect duplicates during the data cleaning phase. An interviewer-administered questionnaire was used to collect data on: (1) socio-demographics, including age, ethnicity, marital status, education, employment, income, legal residence, duration of living in the city, and health insurance coverage; (2) high risk behaviors, including recent (past 3 months) alcohol consumption, recent alcohol use before sex, recent illicit drug use, lifetime number of male or female sex partners, recent condomless insertive or receptive anal sex with men, recent condomless vaginal sex with women, recent commercial sex with men, recent sex with HIV-positive men, age of sex debut, years of homosexual activities (number of years since first sex with a male sex partner), and self-reported HIV risk perception; and (3) barriers and facilitators related to HIV testing uptake based on an option menu.
The primary exposure variable was prior HIV testing. Participants were asked how many times they had been tested for HIV prior to the HIV test received as a study participant. To measure the outcome (HIV infection status), staff drew blood for rapid HIV testing prior to completing the questionnaire. Participants who had negative results were informed immediately post-interview. For those with positive results, blood was sent for confirmatory testing, and participants were informed of results within two weeks. All results were entered into the database and linked to the completed questionnaire with a unique study number.
Laboratory tests
HIV rapid tests were performed using Alere Determine HIV1/2 (Colloidal Selenium Device; Alere Medical Co, Ltd., USA). Venous specimens were further screened for HIV antibody using ELISA (HIV ELISA testing kit 1, Zhuhai Livzon Diagnostics Inc., China). If positive by ELISA screening, the specimen was double tested using another ELISA kit (HIV ELISA testing kit 2, Beijing Wantai Biological Pharmacy Enterprise Co. Ltd., China). A specimen with a positive reaction in both or either ELISA tests was confirmed by Western blot test (HIV Blot 2.2 WB; MP Biomedicals Co, Ltd., China).
Statistical analyses
We used descriptive statistics, including proportions for categorical variables and the median with interquartile ranges (IQR) for continuous variables. All socio-demographic and behavioral characteristics were then compared between men who ever vs. never tested for HIV, and MSM with and without HIV infection. Pearson Chi-square tests were used to compare categorical variables. Wilcoxon rank-sum tests were used for continuous variables.
Bivariate and multivariable logistic regression models were performed to assess the association between prior HIV testing and HIV infection. Frequency of prior HIV testing was analyzed in three ways. Subjects were classified as ‘ever’ or ‘never’ tested for HIV. Bivariate categories (ever vs. never tested) were enhanced by dose-response categories based on the quintile of lifetime HIV testing frequency (0, 1, 2–3, 4–6, >6 [times]). Lastly, we analyzed the frequency of prior testing as a continuous variable. Restricted cubic spline with four knots was applied to fit the non-linear association between frequency of HIV testing and odds of HIV infection in the whole study sample.35,36 Three models were applied to identify and adjust for potential confounding variables. First, we used direct acyclic graphs (DAG) to evaluate and a priori select potential confounders to adjust in the multivariable model (Model A). We adjusted for age, education, marital status, health insurance coverage, drug use, alcohol use before sex, age of first sex, years of homosexual activities with men, condomless anal sex with men, lifetime number of male sexual partners, lifetime number of female partners, and HIV risk perception. Second, we assessed potential confounders and adjusted using propensity score adjustment method. The propensity score of a subject is defined as a subject’s conditional probability of being ever tested (or being tested with certain numbers in the quintile analysis) for HIV given a series of variables that summarized information across potential confounders and risk factors.37–39 In our study, we estimated the propensity score with socio-demographic and high-risk behavioral characteristics that were considered to be associated with HIV testing and HIV risk including age, education, marital status, employment, income, registered Beijing household registration (or “Hukou”), health insurance coverage, duration of living in Beijing, alcohol use before sex, recent (past three months) illicit drug use, year of sexual activities, age of first sex, lifetime number of male sex partners, recent condomless anal sex with men, recent sex with HIV-positive men, recent commercial sex with men, lifetime number of female sex partners, and HIV risk perception. The distribution of propensity score in ‘ever’ and ‘never’ testers (or in each quintile of testing frequencies) were evaluated and well overlapped based on common support assessment. Thus, we adjusted propensity score in the multivariable logistic regression as a continuous covariate variable with restricted cubic spline to allow non-linear regression of the propensity score (Model B). Lastly, our third model (Model C) combined above two models, including all variables identified using DAGs as well as subjects’ propensity for testing. Adjusting for both DAG identified variables and subjects’ propensity of being tested (being with certain testing frequency) allowed us to further minimize residual confounding and be robust in relative to model misclassification.40,41 All potential confounders and risk factors were assessed for collinearity prior to the multivariable model fitting and no statistically significant collinearity was present among the fitted covariates.
As our preliminary analysis suggested that the association between frequency of testing and HIV infection might differ by number of male sexual partners, we conducted subgroup analyses and assessed the association in two strata (<10 and ≥10) of lifetime male sexual partners. All statistical analyses were performed using Stata 12.0™ (StataCorp LP, College Station, Texas, USA).
Results
Population characteristics by HIV infection and prior testing status
Of the 3,760 recruited participants, we excluded 172 from analyses due to duplicate testing (126); confirmed prior HIV-positive (30); non-MSM (5); no blood sample (5); invalid identification numbers (4); and lack of questionnaire (2). Thus, 3,588 MSM (95.4%) were included in our analyses. Ages ranged from 16 to 75 years (median 28), 94% were Han ethnics, 15% married to women (none married to men), 72% had college and above education, 82% were employed, 25% had Beijing Hukou, and 61% had medical insurance.
HIV prevalence was 12.7%. Among HIV-positive MSM, the median CD4+ cell count was 395 cells/µL (interquartile range: 286–512). Compared with HIV-negative MSM, HIV-positive MSM were more likely to be unemployed or retired (7.5% vs. 4.7%; P<0.001), junior/middle school-educated (13.0% vs. 9.7%; P=0.04), drink alcohol before sex in past 3 months (25.9% vs. 19.8%; P=0.002), use illicit drugs in the past 3 months (34.7% vs. 26.5%; P<0.001), have ≥10 male partners in their lifetime (56.5% vs. 48.4%; P<0.001), have condomless receptive anal sex with men in the past 3 months (36.0% vs. 18.2%; P<0.001), have sex with HIV-positive men in the past 3 months (4.0% vs. 2.4%; P=0.04), and have high HIV risk perception (72.1% vs. 36.2%; P<0.001). HIV-positive men were less likely to be married to a woman (84.5% vs. 88.3%; P=0.03), have health insurance (51.0% vs. 62.6%; P<0.001), have Beijing Hukou (14.5% vs. 26.3%; P<0.001), have lived longer in Beijing (median years, 4 vs. 5; P<0.001), have condomless vaginal sex with a women in the past 3 months (3.7% vs. 7.0%; P=0.008) and have ever tested for HIV (63.5% vs. 71.7%; P<0.001; Table 1).
Table 1.
Demographic and behavioral characteristics of men who have sex with men in Beijing, China, 2013 (N=3,588)
| Characteristics | HIV testing history | HIV infection | |||||
|---|---|---|---|---|---|---|---|
| Total (N=3,588) n (%)* |
Ever tested (N=2,534) n (%) |
Never tested (N=1,054) n (%) |
P-value | HIV-positive (N=455) n (%) |
HIV-negative (N=3,133) n (%) |
P-value | |
| Age (year) | <0.001 | 0.37 | |||||
| Median, IQR | 28, (24–33) | 29, (25–34) | 26, (23–30) | 28, (24–32) | 28, (24–33) | ||
| Ethnicity | 0.34 | 0.28 | |||||
| Han | 3,361 (93.7) | 2,380 (93.9) | 981 (93.1) | 421 (92.5) | 2,940 (93.8) | ||
| Other | 227 (6.3) | 3,361 (93.7) | 73 (6.9) | 34 (7.5) | 193 (6.2) | ||
| Marital status | 0.005 | 0.03 | |||||
| Currently unmarried | 3,049 (85.0) | 2,126 (83.9) | 923 (87.6) | 402 (88.3) | 2,647 (84.5) | ||
| Currently married | 539 (15.0) | 408 (16.1) | 131 (12.4) | 53 (11.7) | 486 (15.5) | ||
| Education (year) | 0.23 | 0.04 | |||||
| College and above (>12) | 2,579 (71.9) | 1,799 (71.0) | 780 (74.0) | 330 (72.5) | 2,249 (71.8) | ||
| Senior high (10–12) | 593 (16.5) | 438 (17.3) | 155 (14.7) | 59 (13.0) | 534 (17.0) | ||
| Junior middle school (7–9) | 362 (10.1) | 260 (10.3) | 102 (9.7) | 59 (13.0) | 303 (9.7) | ||
| Primary school or lower(<=6) | 54 (1.5) | 37 (1.4) | 17 (1.6) | 7 (1.5) | 47 (1.5) | ||
| Employment | <0.001 | <0.001 | |||||
| Employed | 2,960 (82.5) | 2,170 (85.6) | 790 (74.9) | 377 (82.9) | 2,583 (82.4) | ||
| Unemployed/retired | 182 (5.1) | 123 (4.9) | 59 (5.6) | 34 (7.5) | 148 (4.7) | ||
| Student | 388 (10.8) | 193 (7.6) | 195 (18.5) | 29 (6.4) | 359 (11.5) | ||
| Other | 58 (1.6) | 48 (1.9) | 10 (1.0) | 15 (3.2) | 43 (1.4) | ||
| Monthly income (Chinese Yuan; k=1,000) | <0.001 | 0.91 | |||||
| Median, IQR | 5k, (3k–8k) | 5k (3k–9k) | 4k (2k–6k) | 5k (3k–8k) | 5k (3k–8k) | ||
| Health insurance | 0.36 | <0.001 | |||||
| No | 1,395 (38.9) | 973 (38.4) | 422 (40.0) | 223 (49.0) | 1,172 (37.4) | ||
| Yes | 2,193 (61.1) | 1,561 (61.6) | 632 (60.0) | 232 (51.0) | 1,961 (62.6) | ||
| Beijing Hukou | 0.60 | <0.001 | |||||
| No | 2,699 (75.2) | 1,900 (75.0) | 799 (75.8) | 389 (85.5) | 2,310 (73.7) | ||
| Yes | 889 (24.8) | 634 (25.0) | 255 (24.2) | 66 (14.5) | 823 (26.3) | ||
| Duration of living in Beijing (year) | <0.001 | <0.001 | |||||
| Median, IQR | 5, (2–10) | 6, (3–11) | 4, (2–8) | 4, (2–8) | 5, (3–10) | ||
| Alcohol use (past 3 months) | 0.002 | 0.12 | |||||
| Never | 1,574 (43.9) | 1,075 (42.4) | 599 (47.3) | 203 (44.6) | 1,371 (43.8) | ||
| Once a month | 1,108 (30.9) | 779 (30.7) | 329 (31.2) | 121 (26.6) | 987 (31.5) | ||
| 2–4 times a month | 594 (16.5) | 436 (17.2) | 158 (15.0) | 90 (19.1) | 507 (16.2) | ||
| ≥ twice a week | 312 (8.7) | 244 (9.7) | 68 (6.5) | 44 (9.7) | 268 (8.5) | ||
| Alcohol use before sex (past 3 months) | <0.001 | 0.002 | |||||
| No | 2,850 (79.4) | 1,972 (77.8) | 878 (83.3) | 337 (74.1) | 2,513 (80.2) | ||
| Yes | 738 (20.6) | 562 (22.2) | 176 (16.7) | 118 (25.9) | 620 (19.8) | ||
| Drug use (past 3 months) | 0.01 | <0.001 | |||||
| No | 2,600 (72.5) | 1,806 (71.3) | 794 (75.3) | 297 (65.3) | 2,303 (73.5) | ||
| Yes | 988 (27.5) | 728 (28.7) | 260 (24.7) | 158 (34.7) | 830 (26.5) | ||
| Age of sex debut | 0.33 | 0.06 | |||||
| Median, IQR | 20, (18–23) | 20, (18–23) | 20, (18–23) | 20, (18–22) | 20, (18–23) | ||
| Years of homosexual activities | <0.001 | 0.52 | |||||
| Median, IQR | 7, (4–12) | 8, (5–13) | 5, (2–9) | 8, (5–12) | 7, (4–12) | ||
| Lifetime number of male sexual partners | <0.001 | <0.001 | |||||
| <10 | 1,815 (50.6) | 1,075 (42.4) | 740 (70.2) | 198 (43.5) | 1,617 (51.6) | ||
| ≥10 | 1,773 (49.4) | 1,459 (57.6) | 314 (29.8) | 257 (56.5) | 1,516 (48.4) | ||
| Condomless insertive anal sex with men (past 3 months) | 0.85 | 0.22 | |||||
| No | 2,801 (78.1) | 1,976 (78.0) | 825 (78.3) | 345 (75.8) | 2,456 (78.4) | ||
| Yes | 787 (21.9) | 558 (22.0) | 229 (21.7) | 110 (24.2) | 677 (21.6) | ||
| Condomless receptive anal sex with men (past 3 months) | 0.02 | <0.001 | |||||
| No | 2,855 (79.6) | 2,043 (80.6) | 812 (77.0) | 291 (64.0) | 2,546 (81.8) | ||
| Yes | 733 (20.4) | 491 (19.4) | 242 (23.0) | 164 (36.0) | 569 (18.2) | ||
| Commercial sex with men (past 3 months) | 0.30 | 0.95 | |||||
| No | 3,487 (97.2) | 2,458 (97.0) | 1,029 (97.6) | 442 (97.1) | 3,045 (97.2) | ||
| Yes | 101 (2.8) | 76 (3.0) | 25 (2.3) | 13 (2.9) | 88 (2.8) | ||
| Sex with HIV-positive men (past 3 months) | 0.10 | 0.04 | |||||
| No | 3,496 (97.4) | 2,462 (97.2) | 1,034 (98.1) | 437 (96.0) | 3,059 (97.6) | ||
| Yes | 92 (2.6) | 72 (2.8) | 20 (1.9) | 18 (4.0) | 74 (2.4) | ||
| Condomless vaginal sex with women (past 3 months) | 0.95 | 0.008 | |||||
| No | 3,351 (93.4) | 2,367 (93.4) | 984 (93.4) | 438 (96.3) | 2,913 (93.0) | ||
| Yes | 237 (6.6) | 167 (6.6) | 70 (6.6) | 17 (3.7) | 220 (7.0) | ||
| Lifetime number of female sex partners | 0.10 | 0.84 | |||||
| 0 | 2,169 (60.5) | 1,510 (59.6) | 659 (62.5) | 277 (60.9) | 1,892 (60.4) | ||
| ≥1 | 1,419 (39.5) | 1,024 (40.4) | 395 (37.5) | 178 (39.1) | 1,241(39.6) | ||
| Perception of HIV risk | 0.11 | <0.001 | |||||
| Low or very low | 2,126 (59.3) | 1,523 (60.1) | 603 (57.2) | 127 (27.9) | 1,999 (63.8) | ||
| High or very high | 1,462 (40.7) | 1,011 (39.9) | 451 (42.8) | 328 (72.1) | 1,134 (36.2) | ||
| HIV infection in the current study | <0.001 | - | |||||
| No | 3,133 (87.3) | 2,245 (88.6) | 888 (84.3) | - | - | ||
| Yes | 455 (12.7) | 289 (11.4) | 166 (15.7) | - | - | ||
| Prior HIV testing | - | <0.001 | |||||
| Ever | 1,054 (29.4) | - | - | 166 (36.5) | 888 (28.3) | ||
| Never | 2,534 (70.6) | - | - | 289 (63.5) | 2,245 (71.7) | ||
Note: IQR, interquartile rage; Hukou, household registration in an area of residence; 1 Chinese yuan=0.15 U.S. dollar; drug use, intake of any of these drugs: methamphetamine, MDMA, rush, magu, ketamine, cannabis/marijuana, cocaine, opium, heroin, morphine; health insurance: self-reported absence of presence of health insurance received via the labor insurance schemes (LIS) or government employee insurance schemes (GIS);
Prior to study participation, 71% of our 3,588 participants reported ever testing for HIV in their lifetimes. Being an MSM who had ever tested for HIV was associated with older age (median age [years], 29 vs. 26; P<0.001), being married to a women (16.1% vs. 12.4%; P=0.005), being employed (85.6% vs. 74.9%; P<0.001), having higher monthly income (median, 5000 [US$750] vs. 4000 Chinese Yuan [US$600]; P<0.001), having lived longer in Beijing (median years, 6 vs. 4; P<0.001), drinking alcohol before sex in the past 3 months (22.2% vs. 16.7%; P<0.001), using illicit drugs in the past 3 months (28.7% vs. 24.7%; P=0.01), more years of homosexual activities (median years, 8 vs. 5; P<0.001), and having ≥10 lifetime male sexual partners (57.7% vs.30.0%; P<0.001). Notably, men who had condomless receptive anal sex in the past 3 months were less likely to test for HIV (19.4% vs. 23.0%; P=0.02; Table 1).
Association of prior HIV testing with HIV infection
Results from three multivariable models were similar in magnitude and consistent in directionality (Table 2). Compared to non-testers, those having ever tested for HIV was associated with 30%–40% lower odds of being newly diagnosed with HIV, with the model that adjusted for both a priori confounders and propensity score simultaneously suggesting the strongest associations (Model C, adjusted odds ratio [aOR]: 0.59; 95% confidence interval [CI]: 0.48, 0.74). The higher the testing frequency, the lower the risk of being tested positive for HIV. In the quintile-based HIV testing frequency, higher HIV testing frequency categories were associated with a decreasing trend in the odds of being infected with HIV, compared with no prior HIV testing as the reference (e.g., Model C, >6 tests [aOR: 0.27; 95%CI: 0.18, 0.41]; 4–6 [aOR: 0.55; 95%CI: 0.39, 0.78]; 2–3 [aOR: 0.61; 95%CI: 0.45, 0.82]; P for trend<0.001).
Table 2.
Logistic regression analyses of the association of prior HIV testing and current HIV infection among Chinese MSM (N=3,588)
| HIV testing | % of HIV+ (n/N) | Unadjusted OR (95% CI) | Adjusted OR(95% CI) | ||
|---|---|---|---|---|---|
| Model A a | Model B b | Model C c | |||
| Binary categories of the frequency of prior HIV tests | |||||
| Never tested | 15.8 (166/1,054) | 1.0 | 1.0 | 1.0 | 1.0 |
| Ever tested | 11.4 (289/2,534) | 0.69 (0.56,0.84) | 0.68 (0.55,0.85) | 0.62 (0.49,0.77) | 0.59 (0.48,0.74) |
| Quintile of the frequency of prior HIV tests | |||||
| 0 | 15.8 (166/1,054) | 1.0 | 1.0 | 1.0 | 1.0 |
| 1 | 15.4 (103/668) | 0.97 (0.75,1.27) | 0.94 (0.71,1.25) | 0.86(0.66,1.13) | 0.84 (0.63,1.11) |
| 2–3 | 12.4 (88/709) | 0.75 (0.57,1.00) | 0.71 (0.53,0.96) | 0.65 (0.49,0.86) | 0.61 (0.45,0.82) |
| 4–6 | 10.7 (60/558) | 0.64 (0.47,0.88) | 0.67 (0.48,0.94) | 0.54 (0.39,0.75) | 0.55 (0.39,0.78) |
| >6 | 6.3 (38/599) | 0.36 (0.25,0.52) | 0.33 (0.22,0.48) | 0.31 (0.21,0.46) | 0.27 (0.18,0.41) |
| P for trend | <0.001 | - | - | - | - |
NOTE: OR, odds ratio; CI, confidence interval
Adjusted for a priori confounders: age, education, marital status, health insurance, drug use, alcohol use before sex, age of sex debut, years of homosexual activities, condomless anal sex with men, lifetime male partners, lifetime female partners, HIV risk perception
Adjusted for the propensity score. The propensity score is derived using the following variables: age, education, marital status, employment, income, Beijing Hukou, health insurance, duration of living in Beijing, alcohol use before sex in the past 3 months, drug use in the past 3 months, years of homosexual activities, age of first sex, number of lifetime male sex partners, condomless sex with men in the past 3 months, sex with HIV-positive men in the past 3 months, commercial sex with male in the past 3 months, number of lifetime female sex partners and HIV risk perception
Adjusted for the a priori confounders and the propensity score
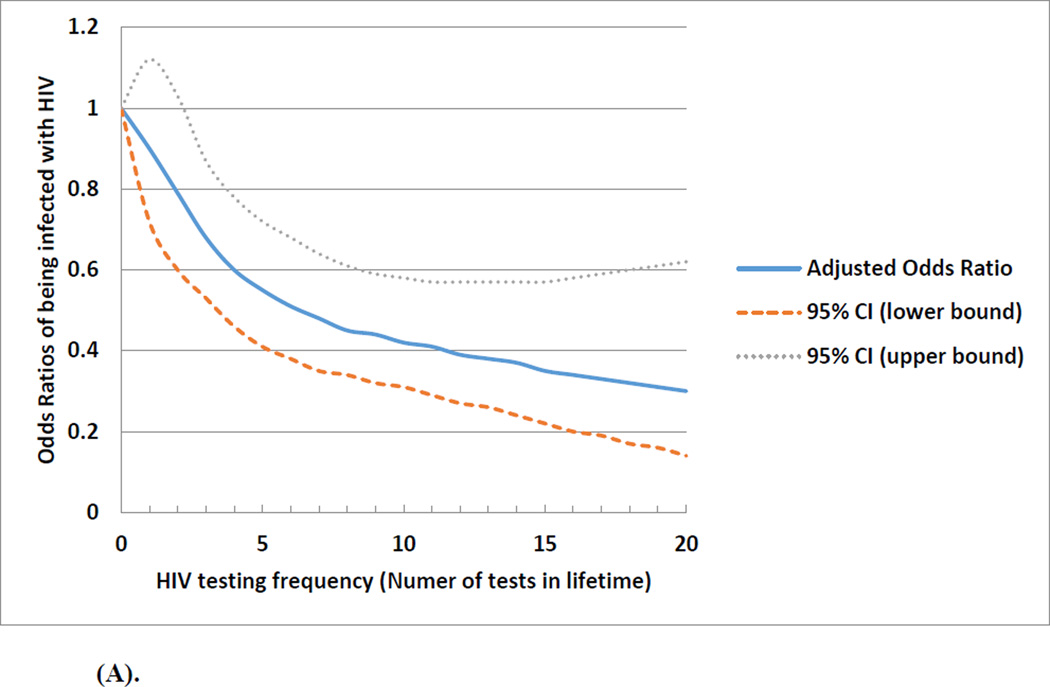
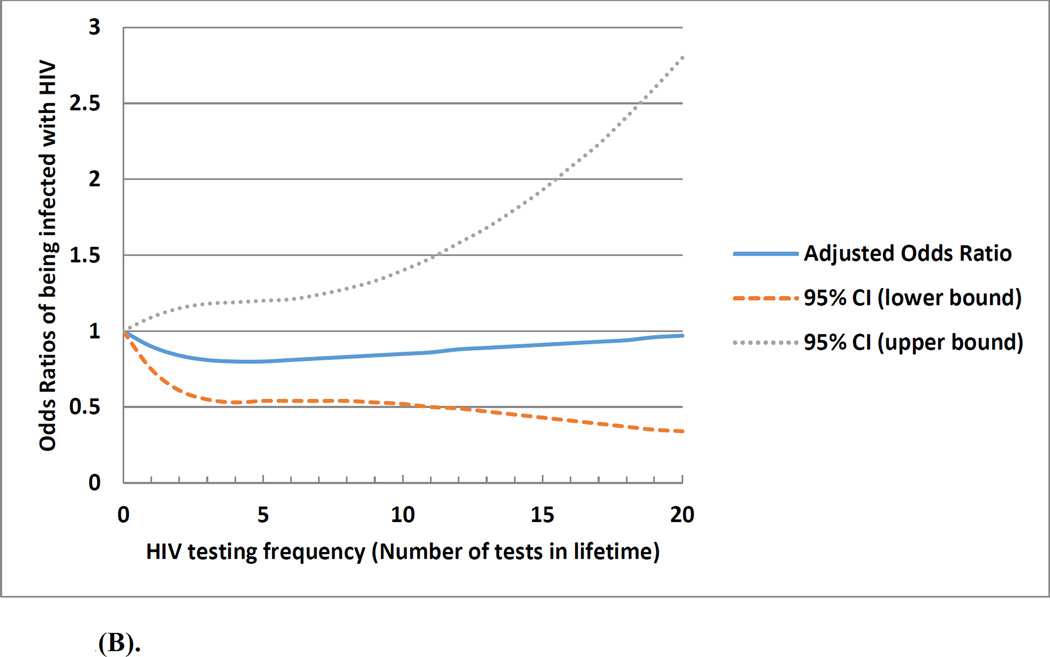
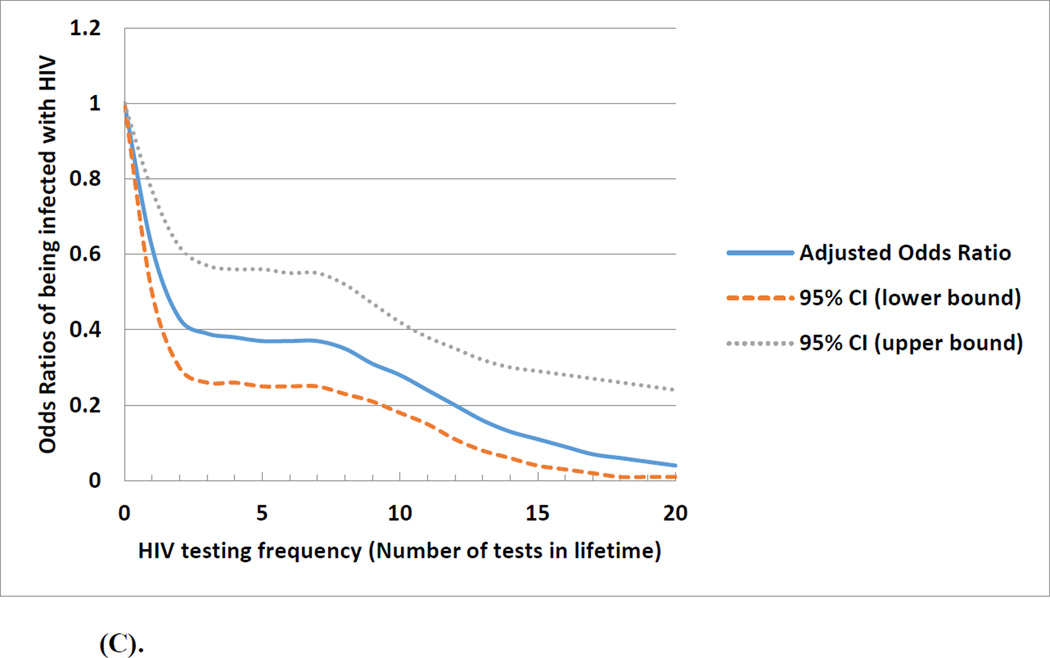
Figure 1(A) depicts the non-linear relationship of the frequency of prior HIV testing with the odds of being diagnosed positive with HIV. Although lifetime testing frequency in general was associated with a decreased odds of HIV infection, the effect was more dramatic when the testing frequency was less than 5 times. The association between frequency of prior HIV testing and HIV infection differed by the number of lifetime male sexual partners (median <10 vs. ≥10). Figure 1 (B) showed no association between prior HIV testing and HIV infection among men with <10 lifetime male sexual partners. Figure 1 (C) shows higher frequency of prior HIV testing was associated with a lower odds of HIV infection among men with ≥10 lifetime male sexual partners; in this subgroup, those with more prior tests were less likely to have engaged in condomless anal sex in the past 3 months (OR: 0.85; 95%CI: 0.66, 1.08).
Figure 1.
(A). Overall association and projected odds ratios between frequency of prior HIV testing and being infected with HIV among a cohort of men who have sex with men in Beijing, China, 2013 March-2014 March (n=3,588)
(B). Association and projected odds ratios between frequency of prior HIV testing and being infected with HIV among a cohort of men who have sex with men who had less than 10 lifetime male sexual partners in Beijing, China, 2013 March-2014 March (n=1,815)
(C). Association and projected odds ratios between frequency of prior HIV testing and being infected with HIV among a cohort of men who have sex with men who had 10 or more lifetime male sexual partners in Beijing, China, 2013 March-2014 March (n=1,773)
Perceived barriers and facilitators for HIV testing
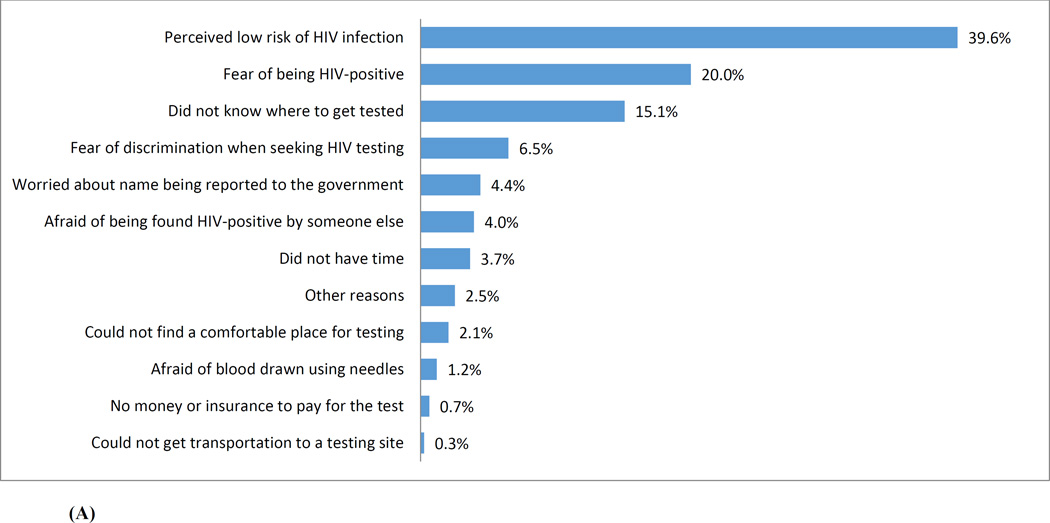
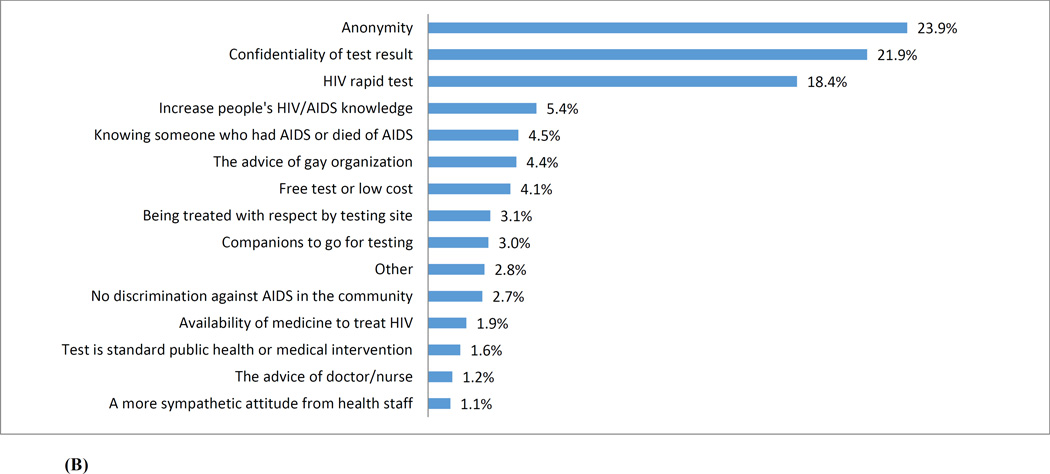
Figure 2 (A) presents the reasons reported by participants for not getting tested for HIV. Among 1,054 MSM who never tested for HIV, the top three barriers to HIV testing were: “perceived low risk of HIV infection” (39.6%), “fear of being HIV-positive” (20.0%), and “not knowing where to get tested” (15.1%). Factors that were considered to have the potential to mobilize HIV testing were illustrated in Figure 2(B). Among all 3,588 participants, the three most commonly perceived facilitators were: “anonymity in taking HIV testing” (23.9%), “confidentiality of test results” (21.9%), and using a “rapid test” (18.4%).
Figure 2.
(A) Barriers to test for HIV identified by a cohort of men who have sex with men who never tested for HIV in Beijing, China (N=1,054)
(B) Perceived facilitators for enhancing HIV testing among a cohort of men who have sex with men in Beijing, China (N=3,588)
Discussion
Our study assessed the association between prior HIV testing and HIV infection among Chinese MSM. We found that frequent HIV testing was associated with lower odds of being HIV-positive. Such result is supportive of findings from the NIMH Project Accept/HPTN 043 community randomized clinical trial showing that community-based voluntary counselling and testing led to borderline significant reduction on HIV-incidence among all participants (RR, 0.86; 95%CI, 0.73–1.02; p=0.08).34 Our study is also consistent with findings from previous studies among Chinese MSM that “ever-testers” had a lower likelihood of being HIV-infected than non-testers.17,42–44 We observed a strong signal that higher lifetime HIV testing frequency was associated with a decreased odds of HIV infection among Chinese MSM. In our study, the benefit of HIV testing for reducing HIV risk was most notable among men who had ≥10 lifetime male sexual partners. It may be that men with more testing did not necessarily reduce sexual partners, but rather reduced condomless anal sex. As indicated by our hypothesized pathway, the observed association of frequent testing and reduced HIV odds among Chinese MSM is plausible, given the counseling that accompanies testing in China might convey more knowledge or inspire motivation for HIV prevention and enhance HIV awareness,31 thus frequent testers might be more attentive to taking precautions by reducing high-risk behaviors to lower their HIV risk.45 Although our statistical adjustments, particularly the use of propensity scores, may help mitigating confounding by indication, we cannot rule out the alternative explanation that frequent testers represented by “worried well” MSM are better informed to begin with, and therefore have a greater likelihood to get regular testing and/or benefit from the concomitant counseling or knowledge. In our sub-analyses (data not shown), we found among ever-testers, those tested more were associated with lower odds of practicing condomless receptive anal sex (Compared to men tested only once, OR: 1.05, 95% CI: 0.31, 4.41[2–3 tests]; OR: 0.29, 95%CI: 0.03, 2.67 [4–6 tests]; OR: 0.28, 95%CI: 0.03, 2.49 [≥6 tests]; P for trend: 0.03). However, only prospective data from longitudinal studies or RCTs could further justify our hypothesized pathway.
The robustness of the HIV epidemic in global MSM populations remains a huge public health concern.46,47 China has an estimated 1.4 billion population in 2015, over 19% of the global populace. If 2–5% of adult men have male-to-male sexual activity, about 10–25 million Chinese men (out of the total 514 million men aged 15–64 years) may be at higher risk of HIV due to condomless receptive anal intercourse. In our Beijing urban study, we observed an HIV prevalence of 12.7%, substantially higher than the latest national estimate (7.3%),48 and similar to the findings documented in recent studies among Chinese MSM in other metropolitan areas (12.4%–14.5%).43,49–51 MSM had a high HIV prevalence in Beijing, confirming our mathematical projection that the HIV epidemic continues to grow in this key population.24 Consistent with other findings,42,51–54 we found familiar risk factors among HIV-positive MSM, including younger age of sexual debut, lower educational level, never married, transient residence, more lifetime male sexual partners, ever engaging in condomless anal sex, drug use, and alcohol use before sex.
Higher HIV prevalence among MSM in large Chinese cities is to be expected. Large cities are less vulnerable to traditional taboos that suppress male-to-male sexual activity, urban sexual networks are more complex and anonymous, and more venues exist for men to seek sexual partners. These factors likely contribute to higher HIV risk and the likelihood of transmitting the virus. Large Chinese cities attract internal migrants who seek employment; some migrants are MSM. Large numbers of single men increase the possibility of HIV spread due to male-male sexual activity or sexual contact with sex workers.52
In China, HIV testing is mainly sponsored by the government through self-initiated free VCT and provider-initiated testing and counseling. However, many MSM do not use this service or fail to return for periodic testing due to a perceived lack of confidentiality in identity and results, long wait-times, and stigma associated with HIV testing.28,55 Most of our participants thought “anonymity”, “confidentiality of the test results” or “rapid HIV testing” would facilitate their testing activities, while “not knowing where to test” and “perceived low risk of HIV infection” were two of the top three barriers to testing. Successful experiences have demonstrated the benefits of self-testing, such as time/location convenience, protection against privacy disclosure, and increased HIV testing frequency. 28,29,56,57Innovative testing provisions, such as the community-initiated home-based counseling and self-testing, should be expanded to see whether they can succeed in involving more Chinese MSM to periodically test for HIV.
About 71% of our studied participants ever tested for HIV in their lifetimes prior to the survey, which is comparable to previous studies conducted in Beijing, somewhat higher than elsewhere in China.17,58 This relatively high testing rate masks huge challenges. Among these ever-testers, nearly 71% of them had been sexually active with men for >5 years, but only 21% tested ≥5 times, which signaled a relatively low repeated testing rate. By study design, all HIV-infected MSM were unaware of their HIV seropositive status prior to our study. Within the sexual dynamics of Beijing MSM, undiagnosed seropositive men are more likely to be infectious to partners than those who knew their status and were taking preventive precautions and/or ART. Our study suggests the urgent need for expanding HIV testing coverage and facilitating periodic testing.
We found MSM who were married were more likely to test for HIV, also noted by Han et al.55 It is possible that the responsibility for their wives or family members motivates men to take HIV tests and to take precautions if they are infected. Some studies have found that younger age was associated with higher HIV testing rates.43,44 A possible explanation is that younger men visit MSM venues (e.g., gay bars, bathhouses) more frequently and would be more likely to expose to venue-based VCT.43 However, consistent with other studies,59 we found younger MSM were less likely to ever test for HIV. Student MSM seemed to be more represented among non-testers than in testers (7.6% vs. 18.5%; P<0.001), perhaps due to stigma, peer discrimination, and low HIV risk perception.60,61 The duration of living in a city is also an indicator of HIV testing uptake among Chinese MSM.44 We found those who lived for fewer years in Beijing tended to have no prior testing experience. MSM with less income were also less likely to ever test. This subgroup may overlap with those without Beijing Hukou and consist of migrants. Studies in Beijing showed that migrant population were low in socio-economic status and were limited in knowledge of HIV and benefit of timely HIV diagnosis.58,62 Interventions to increase frequency of HIV testing should focus on transient residents.
There are several limitations to our study. First, by design of the parent HIV project, known HIV seropositive MSM were not tested/included, so this selection bias may explain some of the magnitude of the relationship between more testing and lower HIV odds. Second, we only assessed recent risk behaviors in the past 3 months prior to the HIV diagnosis, which might not represent the risk profile by the time of actual HIV acquisition; and we were limited to elucidate the temporal relationship among testing behavior, HIV awareness/perception and the change of high-risk behaviors, which lessened our confidence in drawing any causal inference regarding the effect of testing on HIV risk based on our interpretation. Third, participants were analyzed cross-sectionally; those who self-selected to test or not test for HIV were likely to vary substantially in baseline characteristics, decreasing the comparability between testers and non-testers on HIV risk. Fourth, questions related to sexual behaviors were culturally sensitive and may subject the self-reported data to social desirability bias.
Despite of the limitations of potential selection bias and the cross-sectional study design, our study has numerous strengths including large sample size, rigorous/explorative multivariable modeling, and the use of propensity score adjustments to control for the variance in baseline characteristics in terms of prior HIV testing. We found frequent HIV testing was associated with reduced odds of HIV. Although we cannot confirm any causal inference, strengthening the benefit and practice of frequent HIV testing among Chinese MSM must be a continuing effort to identify men in need of care, to initiate and sustain lower HIV risk behavior, and to reduce HIV transmission.63,64 The HIV testing and linkage-to-care (TLC) model is a viable strategy for increasing detection of HIV-infected MSM and linking them to ART-based care, a step towards ‘treatment as prevention’ in China.
Acknowledgments
This work was sponsored by the grants from U.S. National Institutes of Health/National Institute of Allergy and Infectious Diseases (R01AI094562 and R34AI091446).We thank the staff at Chinese Center for Disease Control and Prevention (CDC), Beijing Municipal CDC, Chaoyang District CDC, and Jingcheng Dermatology Hospital, and Xicheng District CDC for recruiting study participants and conducting study activities. We also thank all study participants.
Footnotes
The authors declare no conflicts of interest
References
- 1.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011 Aug 11;365(6):493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Layer EH, Kennedy CE, Beckham SW, et al. Multi-level factors affecting entry into and engagement in the HIV continuum of care in Iringa, Tanzania. PLoS One. 2014;9(8):e104961. doi: 10.1371/journal.pone.0104961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.MacCarthy S, Hoffmann M, Ferguson L, et al. The HIV care cascade: models, measures and moving forward. J Int AIDS Soc. 2015;18(1):19395. doi: 10.7448/IAS.18.1.19395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gardner EM, McLees MP, Steiner JF, Del Rio C, Burman WJ. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis. 2011 Mar 15;52(6):793–800. doi: 10.1093/cid/ciq243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burns DN, DeGruttola V, Pilcher CD, et al. Toward an endgame: finding and engaging people unaware of their HIV-1 infection in treatment and prevention. AIDS Res Hum Retroviruses. 2014 Mar;30(3):217–224. doi: 10.1089/aid.2013.0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hull MW, Wu Z, Montaner JS. Optimizing the engagement of care cascade: a critical step to maximize the impact of HIV treatment as prevention. Curr Opin HIV AIDS. 2012 Nov;7(6):579–586. doi: 10.1097/COH.0b013e3283590617. [DOI] [PubMed] [Google Scholar]
- 7.Forsyth AD, Valdiserri RO. Reaping the prevention benefits of highly active antiretroviral treatment: policy implications of HIV Prevention Trials Network 052. Current opinion in HIV and AIDS. 2012 Mar;7(2):111–116. doi: 10.1097/COH.0b013e32834fcff6. [DOI] [PubMed] [Google Scholar]
- 8.Kurth AE, Celum C, Baeten JM, Vermund SH, Wasserheit JN. Combination HIV prevention: significance, challenges, and opportunities. Curr HIV/AIDS Rep. 2011 Mar;8(1):62–72. doi: 10.1007/s11904-010-0063-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vermund SH, Tique JA, Cassell HM, Pask ME, Ciampa PJ, Audet CM. Translation of biomedical prevention strategies for HIV: prospects and pitfalls. Journal of acquired immune deficiency syndromes. 2013 Jun 1;63(Suppl 1):S12–S25. doi: 10.1097/QAI.0b013e31829202a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burns DN, Dieffenbach CW, Vermund SH. Rethinking prevention of HIV type 1 infection. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2010 Sep 15;51(6):725–731. doi: 10.1086/655889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nachega JB, Uthman OA, del Rio C, Mugavero MJ, Rees H, Mills EJ. Addressing the Achilles' heel in the HIV care continuum for the success of a test-and-treat strategy to achieve an AIDS-free generation. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2014 Jul;59(Suppl 1):S21–S27. doi: 10.1093/cid/ciu299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vermund SH, Hayes RJ. Combination prevention: new hope for stopping the epidemic. Curr HIV/AIDS Rep. 2013 Jun;10(2):169–186. doi: 10.1007/s11904-013-0155-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McNairy ML, Cohen M, El-Sadr WM. Antiretroviral therapy for prevention is a combination strategy. Curr HIV/AIDS Rep. 2013 Jun;10(2):152–158. doi: 10.1007/s11904-013-0152-1. [DOI] [PubMed] [Google Scholar]
- 14.Chang LW, Serwadda D, Quinn TC, Wawer MJ, Gray RH, Reynolds SJ. Combination implementation for HIV prevention: moving from clinical trial evidence to population-level effects. Lancet Infect Dis. 2013 Jan;13(1):65–76. doi: 10.1016/S1473-3099(12)70273-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zou H, Hu N, Xin Q, Beck J. HIV testing among men who have sex with men in China: a systematic review and meta-analysis. AIDS Behav. 2012 Oct;16(7):1717–1728. doi: 10.1007/s10461-012-0225-y. [DOI] [PubMed] [Google Scholar]
- 16.South A, Wringe A, Kumogola Y, et al. Do accurate HIV and antiretroviral therapy knowledge, and previous testing experiences increase the uptake of HIV voluntary counselling and testing? Results from a cohort study in rural Tanzania. BMC Public Health. 2013;13:802. doi: 10.1186/1471-2458-13-802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao Y, Zhang L, Zhang H, et al. HIV Testing and Preventive Services Accessibility Among Men Who Have Sex With Men at High Risk of HIV Infection in Beijing, China. Medicine (Baltimore) 2015 Feb;94(6):e534. doi: 10.1097/MD.0000000000000534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fonner VA, Denison J, Kennedy CE, O'Reilly K, Sweat M. Voluntary counseling and testing (VCT) for changing HIV-related risk behavior in developing countries. Cochrane Database Syst Rev. 2012;9:CD001224. doi: 10.1002/14651858.CD001224.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marks G, Crepaz N, Senterfitt JW, Janssen RS. Meta-analysis of high-risk sexual behavior in persons aware and unaware they are infected with HIV in the United States: implications for HIV prevention programs. J Acquir Immune Defic Syndr. 2005 Aug 1;39(4):446–453. doi: 10.1097/01.qai.0000151079.33935.79. [DOI] [PubMed] [Google Scholar]
- 20.Marks G, Crepaz N, Janssen RS. Estimating sexual transmission of HIV from persons aware and unaware that they are infected with the virus in the USA. AIDS. 2006 Jun 26;20(10):1447–1450. doi: 10.1097/01.aids.0000233579.79714.8d. [DOI] [PubMed] [Google Scholar]
- 21.Cooley LA, Oster AM, Rose CE, Wejnert C, Le BC, Paz-Bailey G. Increases in HIV testing among men who have sex with men--National HIV Behavioral Surveillance System, 20 U.S. Metropolitan Statistical Areas, 2008 and 2011. PLoS One. 2014;9(9):e104162. doi: 10.1371/journal.pone.0104162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang Z, Su J, Peng X, Wu N. A decline in HIV and syphilis epidemics in Chinese female sex workers (2000–2011): a systematic review and meta-analysis. PloS one. 2013;8(12):e82451. doi: 10.1371/journal.pone.0082451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu Z, Xu J, Liu E, et al. HIV and syphilis prevalence among men who have sex with men: a cross-sectional survey of 61 cities in China. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2013 Jul;57(2):298–309. doi: 10.1093/cid/cit210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lou J, Blevins M, Ruan Y, et al. Modeling the impact on HIV incidence of combination prevention strategies among men who have sex with men in Beijing, China. PloS one. 2014;9(3):e90985. doi: 10.1371/journal.pone.0090985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Department of Health and Human Services; Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines 2010. MMWR Recomm Rep. 2010;59:1–116. [Google Scholar]
- 26.Centers for Disease Control and Prevention. HIV testing and risk behaviors among gay, bisexual, and other men who have sex with men—United States. MMWR Morb Mortal Wkly Rep. 2013;62:958–962. [PMC free article] [PubMed] [Google Scholar]
- 27.Centers for Disease Control and Prevention. HIV risk, prevention, and testing behaviors among men who have sex with men—National HIV Behavioral Surveillance System, 21 U.S. cities, United States, 2008. MMWR Surveill Summ. 2011;60:1–34. [PubMed] [Google Scholar]
- 28.Wei C, Yan H, Yang C, et al. Accessing HIV testing and treatment among men who have sex with men in China: a qualitative study. AIDS Care. 2014;26(3):372–378. doi: 10.1080/09540121.2013.824538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu Y, Sun X, Qian HZ, et al. Qualitative Assessment of Barriers and Facilitators of Access to HIV Testing Among Men Who Have Sex with Men in China. AIDS patient care and STDs. 2015 Sep;29(9):481–489. doi: 10.1089/apc.2015.0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Phillips G, 2nd, Magnus M, Kuo I, et al. Correlates of frequency of HIV testing among men who have sex with men in Washington, DC. AIDS Care. 2013;25(12):1481–1484. doi: 10.1080/09540121.2013.774314. [DOI] [PubMed] [Google Scholar]
- 31.Straub DM, Arrington-Sanders R, Harris DR, et al. Correlates of HIV testing history among urban youth recruited through venue-based testing in 15 US cities. Sex Transm Dis. 2011 Aug;38(8):691–696. doi: 10.1097/OLQ.0b013e318214bb70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilson DP, Hoare A, Regan DG, Law MG. Importance of promoting HIV testing for preventing secondary transmissions: modelling the Australian HIV epidemic among men who have sex with men. Sex Health. 2009 Mar;6(1):19–33. doi: 10.1071/sh08081. [DOI] [PubMed] [Google Scholar]
- 33.Mannheimer SB, Wang L, Wilton L, et al. Infrequent HIV testing and late HIV diagnosis are common among a cohort of black men who have sex with men in 6 US cities. J Acquir Immune Defic Syndr. 2014 Dec 1;67(4):438–445. doi: 10.1097/QAI.0000000000000334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coates TJ, Kulich M, Celentano DD, et al. Effect of community-based voluntary counselling and testing on HIV incidence and social and behavioural outcomes (NIMH Project Accept; HPTN 043): a cluster-randomised trial. Lancet Glob Health. 2014 May;2(5):e267–e277. doi: 10.1016/S2214-109X(14)70032-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harrell FE. Regression modeling strategies : with applications to linear models, logistic regression, and survival analysis. New York: Springer; 2001. [Google Scholar]
- 36.Alvarez-Uria G, Pakam R, Midde M, Naik PK. Incidence and mortality of tuberculosis before and after initiation of antiretroviral therapy: an HIV cohort study in India. J Int AIDS Soc. 2014;17:19251. doi: 10.7448/IAS.17.1.19251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causaleffects. Biometrika. 1983;70:41–55. [Google Scholar]
- 38.Lu B. Propensity score matching with time-dependent covariates. Biometrics. 2005 Sep;61(3):721–728. doi: 10.1111/j.1541-0420.2005.00356.x. [DOI] [PubMed] [Google Scholar]
- 39.Tran BX, Nguyen LT, Do CD, Nguyen QL, Maher RM. Associations between alcohol use disorders and adherence to antiretroviral treatment and quality of life amongst people living with HIV/AIDS. BMC Public Health. 2014;14:27. doi: 10.1186/1471-2458-14-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vansteelandt S, Daniel RM. On regression adjustment for the propensity score. Stat Med. 2014 Oct 15;33(23):4053–4072. doi: 10.1002/sim.6207. [DOI] [PubMed] [Google Scholar]
- 41.Connors AF, Jr, Speroff T, Dawson NV, et al. The effectiveness of right heart catheterization in the initial care of critically ill patients. SUPPORT Investigators. Jama. 1996 Sep 18;276(11):889–897. doi: 10.1001/jama.276.11.889. [DOI] [PubMed] [Google Scholar]
- 42.Wang X, Lan G, Shen Z, et al. HIV and syphilis prevalence trends among men who have sex with men in Guangxi, China: yearly cross-sectional surveys, 2008–2012. BMC Infect Dis. 2014;14:367. doi: 10.1186/1471-2334-14-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bai X, Xu J, Yang J, et al. HIV prevalence and high-risk sexual behaviours among MSM repeat and first-time testers in China: implications for HIV prevention. J Int AIDS Soc. 2014;17:18848. doi: 10.7448/IAS.17.1.18848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li X, Wu G, Lu R, et al. HIV-testing behavior and associated factors among MSM in Chongqing, China: results of 2 consecutive cross-sectional surveys from 2009 to 2010. Medicine (Baltimore) 2014 Dec;93(27):e124. doi: 10.1097/MD.0000000000000124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fan W, Yin L, Qian HZ, et al. HIV risk perception among HIV negative or status-unknown men who have sex with men in China. BioMed research international. 2014;2014:232451. doi: 10.1155/2014/232451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Beyrer C, Sullivan P, Sanchez J, et al. The increase in global HIV epidemics in MSM. AIDS. 2013 Nov 13;27(17):2665–2678. doi: 10.1097/01.aids.0000432449.30239.fe. [DOI] [PubMed] [Google Scholar]
- 47.Beyrer C, Sullivan PS, Sanchez J, et al. A call to action for comprehensive HIV services for men who have sex with men. Lancet. 2012 Jul 28;380(9839):424–438. doi: 10.1016/S0140-6736(12)61022-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.State Council AIDS Working Committee Office (SCAWCO):China 2014 UNGASS Country Progress Report. Beijing, China: Ministry of Health of the People’s Republic of China; 2014. [Google Scholar]
- 49.Long Q, Lin P, Li Y, et al. [Prevalence of human immunodeficiency virus and associated risk factors among men who have sex with men in the major regions of Pearl River Delta, from 2009 to 2013] Zhonghua Liu Xing Bing Xue Za Zhi. 2014 Nov;35(11):1227–1230. [PubMed] [Google Scholar]
- 50.Wang QQ, Chen XS, Yin YP, et al. HIV prevalence, incidence and risk behaviours among men who have sex with men in Yangzhou and Guangzhou, China: a cohort study. J Int AIDS Soc. 2014;17:18849. doi: 10.7448/IAS.17.1.18849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zeng Y, Zhang L, Li T, et al. Risk factors for HIV/syphilis infection and male circumcision practices and preferences among men who have sex with men in China. Biomed Res Int. 2014;2014:498987. doi: 10.1155/2014/498987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mao H, Ma W, Lu H, et al. High incidence of HIV and syphilis among migrant men who have sex with men in Beijing, China: a prospective cohort study. BMJ Open. 2014;4(9):e005351. doi: 10.1136/bmjopen-2014-005351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang X, Yu J, Li M, et al. Prevalence and related risk behaviors of HIV, syphilis, and anal HPV infection among men who have sex with men from Beijing, China. AIDS Behav. 2013 Mar;17(3):1129–1136. doi: 10.1007/s10461-011-0085-x. [DOI] [PubMed] [Google Scholar]
- 54.Li D, Jia Y, Ruan Y, et al. Correlates of incident infections for HIV, syphilis, and hepatitis B virus in a cohort of men who have sex with men in Beijing. AIDS Patient Care STDS. 2010 Sep;24(9):595–602. doi: 10.1089/apc.2010.0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Han L, Bien CH, Wei C, et al. HIV self-testing among online MSM in China: implications for expanding HIV testing among key populations. J Acquir Immune Defic Syndr. 2014 Oct 1;67(2):216–221. doi: 10.1097/QAI.0000000000000278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Han L, Bien CH, Wei C, et al. HIV self-testing among online MSM in China: implications for expanding HIV testing among key populations. Journal of acquired immune deficiency syndromes. 2014 Oct 1;67(2):216–221. doi: 10.1097/QAI.0000000000000278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jamil MS, Prestage G, Fairley CK, et al. Rationale and design of FORTH: a randomised controlled trial assessing the effectiveness of HIV self-testing in increasing HIV testing frequency among gay and bisexual men. BMC infectious diseases. 2015;15(1):561. doi: 10.1186/s12879-015-1300-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Song Y, Li X, Zhang L, et al. HIV-testing behavior among young migrant men who have sex with men (MSM) in Beijing, China. AIDS Care. 2011 Feb;23(2):179–186. doi: 10.1080/09540121.2010.487088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chow EP, Jing J, Feng Y, et al. Pattern of HIV testing and multiple sexual partnerships among men who have sex with men in China. BMC Infect Dis. 2013;13:549. doi: 10.1186/1471-2334-13-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang L, Ding X, Lu R, et al. Predictors of HIV and syphilis among men who have sex with men in a Chinese metropolitan city: comparison of risks among students and non-students. PLoS One. 2012;7(5):e37211. doi: 10.1371/journal.pone.0037211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang Z, Jin M, Dong Z, Zhang S, Qiu Z. [Prevalence of HIV among men who have sex with men of inland high school and college students in China: a meta-analysis] Wei Sheng Yan Jiu. 2013 Jul;42(4):689–692. [PubMed] [Google Scholar]
- 62.Wang B, Li X, Stanton B, Liu Y, Jiang S. Socio-demographic and behavioral correlates for HIV and syphilis infections among migrant men who have sex with men in Beijing, China. AIDS Care. 2013;25(2):249–257. doi: 10.1080/09540121.2012.701714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Weinhardt LS, Carey MP, Johnson BT, Bickham NL. Effects of HIV counseling and testing on sexual risk behavior: a meta-analytic review of published research, 1985–1997. Am J Public Health. 1999 Sep;89(9):1397–1405. doi: 10.2105/ajph.89.9.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Williams-Roberts H, Chang Y, Losina E, Freedberg KA, Walensky RP. Frequent HIV testing among participants of a routine HIV testing program. Virulence. 2010 Mar-Apr;1(2):68–71. doi: 10.4161/viru.1.2.10570. [DOI] [PMC free article] [PubMed] [Google Scholar]