Abstract
Objective
To investigate the concurrent relationships between human plasma erythropoietin concentrations and energy expenditure (EE), body composition, plasma leptin concentrations, and associations with weight change.
Methods
Plasma to measure erythropoietin and leptin and data for body composition; 24-hour EE measured in a whole-room calorimeter; and 75g oral glucose tolerance testing were available from 109 full-heritage Pima Indians (55% male) from a larger study designed to understand the causes of obesity. Seventy-nine subjects had data for weight at a later visit (mean follow-up = 4.3±1.9y) to calculate percent weight change per year.
Results
Erythropoietin, adjusted for covariates, correlated with 24h-EE (r=0.26, p=0.007), sleeping EE (r=0.29, p=0.003), fat free mass (r=0.19, p=0.05), and fat mass (r=0.27, p=0.005), but not insulin or glucose measures. The association of erythropoietin with 24h-EE was fully mediated by fat free mass. Erythropoietin associated with leptin in women (ρ=0.36, p=0.01), but not in men (p=0.9), independently from fat mass. The association of erythropoietin with percent weight change per year was in opposing directions (interaction: p=0.002) in males (r=−0.35, p=0.02) versus females (r=0.37, p=0.02).
Conclusions
Non-hematopoietic endogenous erythropoietin action may be involved in body weight regulation in opposing directions in men and women, i.e. weight loss in men and weight gain in women.
Keywords: Erythropoietin, weight loss, weight gain, 24hour - energy expenditure
Introduction
Erythropoietin (Epo), a protein primarily produced in the kidney, stimulates erythropoiesis (1) via a specific cell surface receptor (EpoR). The discovery of ubiquitous EpoR expression in non-erythroid cells led to greater understanding of the non-hematopoietic functions of Epo (2). Murine studies indicate Epo signaling may be important in body weight regulation as mice with EpoR expression limited to hematopoietic tissues develop obesity, in part due to decreased energy expenditure (EE) (3). Epo treatment of rodents, compared to saline, results in increased EE, decreased food intake and decreased fat mass at both supra-physiologic (3) and physiologic doses (4). In addition, Epo administration to numerous murine models of insulin resistance and type 2 diabetes mellitus (T2DM) improves both weight and glucose regulation (4). The effect of Epo on body weight in rodents is not a function of changes in hemoglobin as leptin deficient (ob/ob) mice treated with Epo had attenuation of weight gain, both with and without phlebotomy (3). Inducing Epo overexpression in skeletal muscle of obese mice leads to supraphysiologic increases in Epo serum concentrations, followed by a ~25% adipose tissue loss over 12 weeks (5).
In the mice with EpoR expression only in hematopoietic cells, females gain more weight (~60%) than males (~30%) (3). It is known that 17-β estradiol treatment decreases Epo synthesis and gene expression during hypoxia (6). In men, the increase in hemoglobin with testosterone administration is associated with significant increases in erythropoietin (7). It is unknown if non-hematopoietic Epo effects differ by sex in humans.
Humans living at high altitudes are both less likely to be obese and less likely to be diagnosed with new onset obesity (8). Chronic hypoxia, increased plasma leptin concentrations, and impaired intestinal function are among the hypotheses given to explain altitude-induced weight loss (9). At high altitude, hypoxic induction of Epo regulated in part by hypoxia inducible factor (HIF)-2 and prolyl hydroxylase domain-containing protein 2 offers a potential alternative explanation (10). In contrast, Tibetan highlanders, a people who have lived at high altitude for at least 25, 000 years, do not exhibit elevated Epo, which is associated in part to adaptive mutations in in the genes encoding these major regulators of hypoxic induction of Epo (10).
Pima Indians of Arizona have a high prevalence of obesity (11) and T2DM (12). Pima Indians living at a higher altitude (1400–1800m) in the Sierra Madre Mountains of Mexico are, on average, leaner and less likely to get T2DM than the Arizona Pima population (13). These two closely-related Pima Indian populations have differing lifestyles and food access, and after controlling for percent body fat, nondiabetic, Mexican Pima Indians have higher leptin concentrations than Arizona Pima Indians (14). These relatively higher levels of the appetite suppressing leptin (15) might protect against obesity. Whether Epo concentrations are different between these two populations or associate with leptin concentrations remains to be explored. Herein, we focused on the Arizona Pima population and hypothesized that human plasma Epo concentrations would be positively associated with fat free mass and 24h-EE, but negatively associated with fat mass, plasma leptin concentrations, and body weight changes, and that, sex specific differences might exist that modify these associations.
Methods
Study population
This study included 109 (55% male) full heritage Pima Indians recruited from the Gila River Indian Community in Arizona who did not smoke or take medications and were healthy as determined by history, physical examination and routine laboratory tests. Subjects included in this secondary analysis were admitted to our clinical research unit (CRU) between 2000 and 2008 as part of one of three larger studies designed to investigate 1) contributors to and consequences of obesity (16), 2) contributors to ad libitum eating behaviors (NCT00342732), and 3) the sympathetic nervous system's role in the regulation of metabolic rate and the development of obesity (NCT00341770). None of the women were post- or peri-menopausal at the initial visit. Inclusion criteria for this current analysis included available plasma stored for <10 years to reduce storage time effects, and complete data for body composition, a 75g oral glucose tolerance test (OGTT) and 24h-EE measured in a whole-room indirect calorimeter. Subjects were admitted to the CRU where they were fed a weight-maintaining diet (WMD) (50% carbohydrate, 30% fat, 20% protein) with caloric content determined from individual body weight and gender. Subjects were asked to abstain from exercise. OGTTs were used to exclude volunteers with T2DM according to American Diabetes Association guidelines (17). Measurements of height, weight and body composition (DXA, Lunar Prodigy, GE Healthcare) were performed. A subset of 79 subjects had data available for weight after their initial admission from either a return visit to the CRU (n=35) or a study visit as a participant of a longitudinal study (1965 – 2007) designed to understand the etiology of T2DM among the Gila River Indian Community, where most of the residents are Pima Indians (NCT00339482) (n=44) (mean follow-up time: 4.3±1.9; range 0.7–7.5 years). Participants in the latter study had examinations as frequently as every 2 years with measures of height and weight. All volunteers provided informed consent prior to participation in any study. All of these studies were approved by the Institutional Review Board of the NIDDK.
Measurements
24-hour energy expenditure
The assessment of 24h-EE was performed in a whole-room indirect calorimeter, as previously described (17). Energy intake during the assessment was 80% of the WMD to account for reduced activity during confinement to the calorimeter. Volunteers entered the calorimeter after an overnight fast and received meals at 08:00, 11:00, 16:30, and 19:00. Carbon dioxide production (VCO2) and oxygen consumption (VO2) were measured continuously for 23.25 h, averaged for each 15-min interval, and extrapolated to 24 hours. The 24-hour respiratory quotient (RQ) was calculated as the ratio of VCO2 to VO2. The 24h-EE was calculated from the VO2 and RQ as previously described (17). Carbohydrate and fat oxidation rates were calculated from the RQ after accounting for protein oxidation calculated from the measurement of 24-h urinary nitrogen excretion (18). Please see Supporting information for a detailed description of the plasma sample assays.
Statistical analysis
Normally distributed variables are described using means and standard deviations and skewed variables using medians and interquartile range. In multivariate models, insulin and adiponectin concentrations were log-transformed to meet the assumptions of linear regression as both variables had a positively skewed distribution. Weight changes are reported as percent weight change per year to account for differences in baseline weight and follow-up time. Correlations between continuous variables were determined using Pearson rank correlation coefficients (r) for normally distributed data and Spearman rank correlations (ρ) for skewed data. All significant correlations between variables and Epo were investigated further using multivariate linear regression in the whole group. Effect sizes of multivariate linear regression models are reported as parameter estimates with [95% confidence limits]. Because Epo may interact with sex hormones (6, 7, 19, 20), the interaction term between Epo and sex was considered in all multivariate models and, given the relatively small sample size for detecting interactions, models were also stratified by sex.
To assess for associations between Epo and metabolic variables independent from any differences in sample storage time, hemoglobin, and kidney function (represented by serum creatinine) between individuals, we adjusted Epo concentrations for these variables and used the remaining unexplained variance to determine relationships with anthropometric and metabolic variables. Similarly, leptin was adjusted for fat mass to assess for relationships between variables and differences in leptin separate from that expected due to differences in fat mass. All correlations between adjusted values and other variables were confirmed with multivariate linear regression models, which gave similar results (see Table S1). Adjusted Epo, leptin, and 24h-EE values were normally distributed. In sensitivity analyses, all models were repeated using Epo measured from neuraminidase-treated plasma and Epo measured from neuraminidase-treated plasma adjusted for covariates.
To investigate a possible mediating effect of FFM in the relationship between Epo and 24h-EE, mediation analysis using hierarchical regression models was used as preciously described (21). As the criteria for mediation were met, the Sobel test (22) was used to test whether the associations of Epo and 24h-EE are likely to be mediated by FFM. Statistical analysis was performed using SAS statistical software (SAS E-guide 4.2 and SAS version 9.2; SAS Institute, Cary, NC). Alpha was set at 0.05.
Results
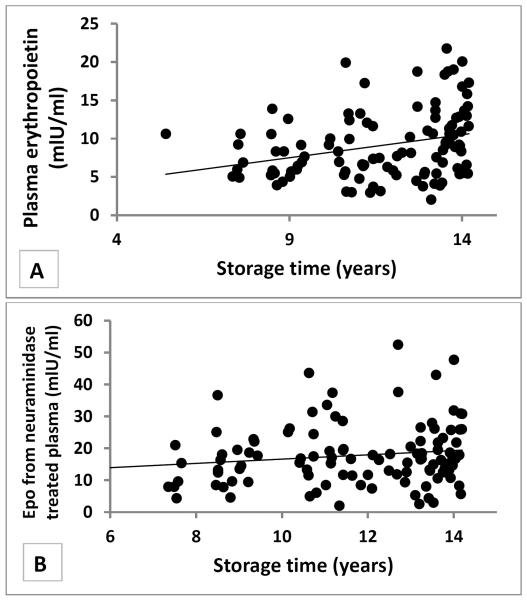
General, anthropometric, and EE characteristics of the study population can be found in Table 1. We found a positive association between storage time and Epo concentrations (ρ=0.33, p=0.0005, Figure 1), but no relationship between storage time and leptin or adiponectin concentrations (ρ=−0.01, p=0.92; ρ=0.003, p=0.97). Treatment of plasma with neuraminidase attenuated the storage time effect on Epo (ρ=0.15, p=0.1, Figure 1). Epo negatively associated with hemoglobin (ρ=−0.27, p=0.005) but did not associate with creatinine (r=0.001, p=0.92).
Table 1.
General, anthropometric, and energy expenditure characteristics of the study population
| All | Men | Women | |
|---|---|---|---|
| N | 109 | 60 | 49 |
| Age, (years) | 31.0 ± 7.5 (18.1–46.0) | 31.2 ± 7.5 (18.1–44.8) | 30.7 ± 7.6 (18.1–46.0) |
| Body weight, (kg) | 97.4 ± 21.6 (51.5–158.5) | 100.7 ± 19.6 (57.0–158.5) | 93.4 ± 23.4 (51.5–153.4) |
| Body weight at follow up, (kg) | 101.4 ± 25.6 (55.0–168) N=79 | 103.2 ± 23.6 (60.0–158) N=41 | 99.4 ± 27.8 (55.0–168) N=38 |
| Body weight follow up time, (years) | 4.3 ± 1.9 (0.7–7.5) N=79 | 4.0 ± 2.0 (0.7–7.5) N=41 | 4.5 ± 1.9 (1.0–7.5) N=38 |
| Body weight change per year, (kg) | 0.9 ± 4.0 (−16.2–14.5) N=79 | 0.71 ± 4.8 (−16.2–10.8) N=41 | 1.1 ± 3.0 (−3.4–14.5) N=38 |
| Body weight change per year, (%) | 1.0 ± 3.7 (−11.9–11.6) N=79 | 0.94 ± 4.4 (−11.9–11.6) N=41 | 1.0 ± 2.8 (−5.0–11.1) N=38 |
| BMI, (kg/m2) | 35.2 ± 7.5 (19.5–59.2) | 34.3 ± 6.2 (22.5–51.2) | 36.3 ± 8.8 (19.5–59.2) |
| Body fat, (%) | 41.8 ± 9.1 (17.0–61.2) | 36.2 ± 6.4 (17.0–49.6) | 48.8 ± 6.8 (31.3–61.2)*** |
| FM, (kg) | 41.6 ± 15.4 (10.9–93.0) | 37.3 ± 12.6 (10.9–71.6) | 46.9 ± 16.9 (16.2–93.0)** |
| FFM, (kg) | 55.8 ± 12.0 (33.3–91.3) | 63.5 ± 9.2 (46.1–91.3) | 46.5 ± 7.6 (33.3–61.8)*** |
| Fasting Glucose (mg/dl) | 89.9 ± 7.6 (73.0–116.0) | 88.7 ± 6.3 (73.0–103.0) | 91.3 ± 8.6 (78.0–116.0)* |
| 2h Glucose (mg/dl) | 127.8 ± 29.7 (65.0–189.0) | 121.7 ± 28.9 (65.0–189.0) | 135.2 ± 29.4 (67.0–187.0) |
| Fasting Insulin (μIU/mL) | 9.5 [6.5; 14.5] | 9.5 [6.0; 12.5] N=60 | 10.0 [8.0; 15.5] |
| 2h Insulin (μIU/mL) | 83.0 [43.0; 128.0] | 70.0 [31.0; 120.5] | 92.0 [58.0; 142.0] |
| 24h-EE (kcal/day) | 2456 ± 397 (1552–3548) | 2664 ± 314 (1933–3548) | 2200 ± 334 (1552–3014)*** |
| Sleeping EE (kcal) | 1759 ± 291 (1166–2588) | 1875 ± 274 (1302–2588) N=59 | 1614 ± 245 (1166–2138)*** N=47 |
| Respiratory Quotient | 0.85 ± 0.03 (0.78–0.94) | 0.85 ± 0.03 (0.78–0.94) | 0.85 ± 0.03 (0.79–0.9) |
| Carbohydrate oxidation (kcal) | 1104 ± 289 (479–2524) | 1216 ± 295 (665–2524) | 967 ± 217 (479–1465)*** |
| Lipid oxidation (kcal) | 1027 ± 331 (381–2006) | 1105 ± 334 (381–2006) | 931 ± 304 (424–1757)** |
| Erythropoietin (mlU/ml) | 8.3 [5.6; 11.6] | 7.4 [5.7; 10.6] | 9.2 [5.5; 13.0] |
| Epo after Neuraminidase treatment (mlU/ml) | 16.4 [11.6; 22.2] | 14.9 [11.5; 21.0] | 16.8 [12.5; 25.1] |
| Leptin (pg/ml) | 15876 [8541; 35258] | 9897 [6062; 15510] | 36642 [20951; 49148]*** |
| Adiponectin (ng/ml) | 1947 [1159; 2657] | 1865 [1073; 2893] | 2015 [1350; 2657] |
| Storage time, (years) | 11.7 ± 2.1 (5.4–14.2) | 11.5 ± 2.4 (5.4–14.2) | 12.0 ± 1.9 (7.4–12.5) |
Values are presented as mean ± SD and ranges in parentheses, except fasting and 2 hour Insulin, erythropoietin, leptin, and adiponectin, which are presented as median, and lower and upper quartiles in brackets. Differences between men and women were assessed by Student's t-test
= p < 0.05;
= p <0.01;
=p <0.0001.
FM = Fat mass; FFM = Fat free mass; BMI = Body mass index; 24h-EE = 24-hour energy expenditure. There were no differences between the whole study population and the subgroup with follow-up weight (N=79) (p>0.16 for all variables).
Figure 1.
Storage time effect on plasma Erythropoietin samples
Plasma Erythropoietin levels were increased with longer storage time (A, ρ=0.33, p=0.0005, N=109). This effect was partially reduced when Erythropoitin was measured from neuramindase treated plasma samples (B, ρ=0.15, p=0.1, N=108).
Associations between Erythropoietin and Body Composition
Epo positively associated with FM (ρ=0.32, p=0.0007), fasting insulin (ρ=0.22, p=0.03), and 2-hour insulin (ρ=0.25, p=0.008), but not with FFM (ρ=0.09, p=0.34) or glucose concentrations (p=0.9). Associations between Epo and insulin concentrations were no longer present after accounting for FM. Contrary to the unadjusted findings, Epo adjusted for covariates (including sex differences) associated with FFM (r=0.19, p=0.05) as well as FM (r=0.27, p=0.005). The associations between body composition variables and Epo or adjusted Epo were present only in men in the stratified analysis (FFM: ρ=0.28, p=0.03; r=0.32, p=0.01; FM: ρ=0.33, p=0.01; r=0.34, p=0.008), and not in women (FFM: ρ=0.25, p=0.09; r=0.2, p=0.18; FM: ρ=0.24, p=0.09; r=0.26, p=0.07). However, all correlations had a similar directionality and the interaction terms in the full model did not reach significance (all p>0.2).
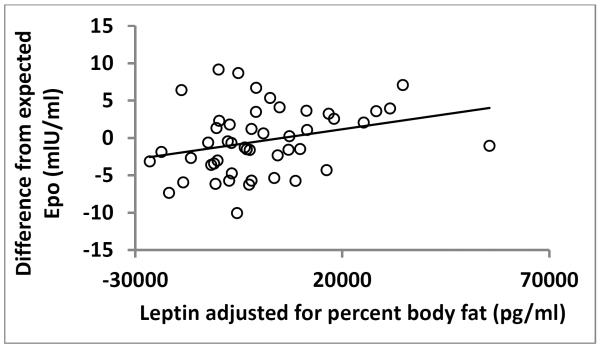
Plasma leptin correlated with percentage body fat (ρ=0.87, p<0.0001) and FM (ρ=0.73, p<0.0001). Leptin was not independently associated with Epo after adjusting leptin for percentage body fat (ρ=−0.04, p=0.63). However, in analyses stratified by sex, adjusted Epo associated with adjusted leptin in women (ρ=0.36, p=0.01, Figure 2), but not in men (r=0.02, p=0.9). There was no association between Epo and adiponectin (ρ=0.05, p=0.6). Adiponectin negatively associated with 2-hour glucose, fasting and 2-hour insulin (ρ=−0.19, p=0.03; ρ=−0.28, p=0.002; ρ=−0.32, p=0.0004, respectively), and the associations with insulin remained true after adjustment for percentage body fat (ρ=−0.28, p=0.004; ρ=−0.35, p=0.0002).
Figure 2.
Erythropoietin and Leptin in females
Erythropoietin (adjusted for creatinine, hemoglobin, and storage time) was positively associated with leptin (adjusted for percent body fat) in women (ρ =0.36, p=0.01, N=48).
Associations of Erythropoietin with Energy Expenditure and Substrate Oxidation
Unadjusted Epo was not associated with 24h-EE (ρ=0.1, p=0.3) nor sleeping EE (ρ=0.17, p=0.08). However, adjusted Epo associated with 24h-EE (r=0.26, p=0.007) and sleeping EE (r=0.29, p=0.003). In analyses stratified by sex, these associations between adjusted Epo and EE measures were only present in men (24h-EE: r =0.3, p=0.02; sleeping EE: r=0.33, p=0.01) but not in women (24h-EE: r=0.22, p=0.14; sleeping EE: r=0.23, p=0.12). In a multivariate model including FFM, FM and age, Epo was not an independent predictor of 24h-EE in either men (p=0.6) or women (p=0.9).
To further investigate the relationships between adjusted Epo, FFM and 24h-EE in men, we performed a mediation analysis to quantify a possible effect of Epo on 24h-EE mediated by FFM. In men only, all conditions for running a mediation analysis using FFM as the mediator variable were satisfied: Epo associated with 24h-EE (β=22.12±10.9 kcal [0.35, 43.9], p=0.04); Epo also associated with FFM (β=0.76±0.31 kg FFM [0.13, 1.39], p=0.02); and only FFM remained associated with 24h-EE in the final model including both Epo and FFM (β=25.3±3.1 kcal, [19.0, 31.6], p<0.0001). The indirect effect of Epo on 24h-EE exerted through FFM indicated almost complete mediation (Sobel test: p=0.023).
There was no correlation between Epo and respiratory quotient or lipid oxidation (ρ=0.12, p=0.2; ρ=0.03, p=0.8), but a correlation between Epo and carbohydrate oxidation was observed (ρ=0.2, p=0.04). The association with carbohydrate oxidation was present only in women (women: ρ=0.45, p=0.001; men: ρ=0.11, p=0.37). In multivariate models stratified by sex and adjusted for age, FM, and FFM, Epo was an independent predictor of carbohydrate and lipid oxidation in women (β=11.2 [0.35, 22.1] kcal, p=0.04; β=−13.3 [−26.8, −0.12] kcal, p=0.05), but not in men (β=−0.98 [−22.8, 20.9] kcal, p=0.92; β=−1.7 [−26.2, 22.8] kcal, p=0.89). None of the cross-sectional results differed if Epo measured from neuraminidase-treated plasma were used in the analyses instead.
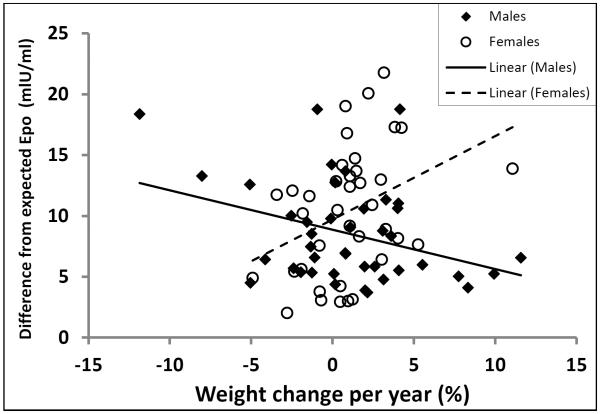
Associations between Erythropoietin and weight change
Epo was not associated with percent weight change per year (ρ=0.07, p=0.52) in the whole group. However, an interaction between Epo and sex was present in the multivariate model of percent weight change per year (sex*Epo interaction term: p=0.002). Exploration of this interaction revealed that the directionality of the association between Epo adjusted for differences in storage time, creatinine, and hemoglobin and percent weight change per year differed between men (r=−0.35, p=0.02) and women (r=0.37, p=0.02; Figure 3). This opposing directionality was essentially unchanged if unadjusted Epo was used in the model (men: ρ=−0.24, p=0.13; women: ρ=0.42, p=0.008), or if Epo measures from neuraminidase-treated plasma (men: ρ=−0.23, p=0.14; women: ρ=0.44, p=0.006), or neuraminidase treated plasma adjusted for covariates (men: ρ=−0.34, p=0.03; women: ρ=0.41, p=0.01) were used.
Figure 3.
Erythropoietin and weight change in males and females
The association of plasma erythropoietin concentrations (adjusted for creatinine, hemoglobin, and storage time) with weight change was found to be in opposing directions in men (closed squares, N=41) versus women (open circles, N=38) (males: r=−0.35, p= 0.02; females: r=0.37, p= 0.02). Exclusion of the visual outlier in the group of women with an annual weight gain of 11.1% did not substantially alter the results (r=0.36, p= 0.03).
Discussion
Although Epo is primarily recognized for its role in erythropoiesis, evidence from murine literature indicates that it may have a role in body weight regulation via an association with EE. We found that higher Epo concentrations associated with higher 24h-EE in men due to an indirect association of Epo on EE, mediated by FFM. Epo was also associated with FM at baseline. We observed opposing associations between Epo and body weight change in men and women. Higher Epo concentrations were associated with weight loss in men and weight gain in women indicating that non-hematopoetic Epo action may possibly be modulated by sex hormones. In women, there was an additional independent association of higher Epo concentrations with higher leptin concentrations, independent from the relationship with FM, raising the possibility that Epo may either mediate or be affected by leptin resistance.
Epo, mainly produced in the kidney and the key stimulant of erythropoiesis (1), has also been shown in murine studies to be produced by brain and osteoblasts, while EpoR is expressed in non-hematopoietic cells including white adipose tissue (23, 3). A recent study indicates that endogenous Epo-EpoR signaling is involved in white adipose tissue inflammation and might regulate obesity induced insulin resistance and glucose intolerance in mice (24). Epo may influence FM as we observed a positive association between Epo and FM. Our finding that Epo was positively associated with FFM, the main determinant of EE, is consistent with the fact that mice missing EpoR in non-hematopoietic cells have lower activity levels and oxygen consumption, and therefore, are more energy efficient then wild-type mice (3). Interestingly, we found a positive association between Epo and carbohydrate oxidation and a negative association with lipid oxidation in women (but not in men), which might partially explain the physiological underpinnings of the Epo-associated weight gain we observed in women. Carbohydrate oxidation predicts ad libitum food intake as well as short-term (25) and long-term changes in body weight (26). Studies of adipose tissue-specific disruption of EpoR in rodents have had variable results where adipocyte Epo signaling was found to be important for energy homeostasis only in models susceptible to diet induced obesity (27) but not in rodents on a mixed genetic background more resistant to obesity (28). This observation seems to support the differences we observed between men and women as, in general, women may have a greater susceptibility to ease of fat accumulation.
Leptin and erythropoietin act synergistically during erythroid development, and in our study, were positively associated with one another (29). Signaling of adequate fat stores by leptin may permit maximal stimulation of erythropoiesis by Epo. In women, the association of Epo and leptin was independent of covariates including FM. Because of the reported satiety effects of leptin (30), it might be expected that such an association would portend weight loss with higher Epo concentrations. However, we observed the opposite effect suggesting that Epo is related to leptin resistance in women. This may be because estrogen modifies any potential relationship between Epo and leptin. Alternatively, as Epo treatment is known to stimulate appetite (31), appetite-stimulating effects of Epo may be more important than Epo's effect on EE in women, compared to men. However, a recent murine study showed that increased food intake in transgenic mice overexpressing human Epo did not differ by sex (4). Epo was not independently associated with glucose or insulin concentrations, consistent with a prior study (32).
Evidence for the role of erythropoietin in human body weight regulation prior to our study is limited to reports in patients with cancer or undergoing hemodialysis. Relative body weight and subcutaneous fat stores increased with recombinant Epo treatment of anemic hemodialysis patients (33), and Epo-treated cancer patients have better nutritional resources and less wasting compared to a control group (34). We observed that higher baseline Epo concentrations were associated with weight loss in men and weight gain in women. 17-β estradiol is known to attenuate hypoxic induction of Epo in ovariectomized rats (6). Epo also plays a critical role in the estrogen-dependent cyclical angiogenesis that occurs in the murine uterus (6), and a contribution of Epo to the cyclic proliferation and differentiation of human endometrial epithelial cells has been reported (35). The authors suggest that ovarian steroids may stimulate Epo production in human endometrial glandular epithelial cells (35). In addition, Epo is known to stimulate testosterone production (36) in men. On the other hand, the haematopoietic effect of testosterone does not appear to be mediated by stimulation of erythropoietin production (37). Higher concentrations of testosterone are associated with increased lean mass and muscle strength as well as decreased FM (38). Thus, we would hypothesize that sex hormones may modify any effects of Epo on body weight regulation or that Epo may interact with sex hormones to direct nutritional resources to meet sex-specific needs, i.e. increased lean mass via testosterone versus reproductive functions in females. In men, Epo concentrations were positively associated with 24h-EE, a relationship completely mediated by FFM, the main determinant of EE. We did not have data to determine if Epo was associated with hunger or food intake; however, we would hypothesize that blood loss in fertile women with regular menses requires a signal to increase energy stores in anticipation of future pregnancy.
One limitation of our study was that longer storage time was associated with higher measures of elevated plasma Epo levels. After reviewing prior literature (39), this was thought to be due to differences in the amount of sialic acid moieties that attach to the Epo molecule over time leading to increased ELISA sensitivity. We addressed this concern by treating the plasma with neuraminidase to remove sialic acid (19), before redoing the ELISA assay to measure Epo. Although the neuraminidase treatment reduced the association between Epo and storage time, all of our results were essentially unchanged whether the variable used was Epo measured in untreated or treated plasma. We also chose to address this limitation statistically by adjusting Epo measurements for differences in individual storage time to minimize this potential confounder.
The associations between Epo and body composition were significant in men but did not cross the statistical significance threshold in women which may, in part, be due to the smaller sample size for women. Our study population included only full heritage Pima Indians as this was a secondary analysis of data from a study of the cause and consequences of obesity in a primarily Pima Indian population. Members of the Pima Indian community in Arizona are, on average, more obese and have a higher prevalence of T2DM (12) than other ethnicities in the US population. However, findings within the Pima population are usually prototypic of those found in other groups.
The FFM-dependent association between Epo and 24h-EE plus the positive association between Epo and weight loss in males raise the possibility that endogenous Epo action in non-hematopoetic tissue may contribute to protection against male obesity. The opposite association between Epo and weight gain in females, and the association between Epo and greater carbohydrate oxidation in women indicate the potential for an estrogen-modulated inhibitory effect on non-hematopetic Epo response, possibly to help improve energy stores in preparation for reproduction. In women, higher Epo concentrations were also associated with higher leptin concentrations independent from FM. These data indicate that non-hematopoetic Epo action may be modulated by sex hormones, and, in women, Epo action may either mediate or be affected by leptin resistance. Prospective clinical studies applying phlebotomy or administration of recombinant erythropoietin in a sex-specific manner, for instance, might help to further elucidate a potential role for Epo in body weight regulation.
Supplementary Material
What is already known about this subject?
Erythropoietin signaling may be important in body weight regulation as mice without erythropoietin receptor expression in non-hematopoietic tissues develop obesity, in part, due to decreased energy expenditure.
What does this study add?
We report findings in healthy individuals whereas prior evidence for the role of erythropoietin in human body weight regulation is limited to patients with severe illnesses.
We found that higher erythropoietin concentrations were associated with higher 24 hour energy expenditure in men due to an indirect effect of erythropoietin on energy expenditure mediated by fat free mass.
We observed opposing associations between plasma erythropoietin and body weight change in men and women, i.e. weight loss in men and weight gain in women.
Acknowledgments
The authors thank the dietary, nursing, and technical staff of the NIH Clinical Unit in Phoenix, AZ for their assistance. Most of all, we thank the volunteers for their participation in the study.
Funding: This research was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases.
Footnotes
Disclosure: The authors declare no conflict of interest
Authors Contributions: M.R. wrote the manuscript. M.R. and M.S.T. analyzed the data. M.S.T., and M.R. designed the study. S.D. and C.T.N. analysed the plasma samples. M.R., S.D., C.T.N., Y.Z., J.K., and M.S.T. contributed to the interpretations of findings and commented on and edited the drafts. M.S.T. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Kapitsinou PP, Liu Q, Unger TL, et al. Hepatic HIF-2 regulates erythropoietic responses to hypoxia in renal anemia. Blood. 2010;116:3039–3048. doi: 10.1182/blood-2010-02-270322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arcasoy MO. The non-haematopoietic biological effects of erythropoietin. Br J Haematol. 2008;141:14–31. doi: 10.1111/j.1365-2141.2008.07014.x. [DOI] [PubMed] [Google Scholar]
- 3.Teng R, Gavrilova O, Suzuki N, et al. Disrupted erythropoietin signalling promotes obesity and alters hypothalamus proopiomelanocortin production. Nat Commun. 2011;2:520. doi: 10.1038/ncomms1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Katz O, Stuible M, Golishevski N, et al. Erythropoietin treatment leads to reduced blood glucose levels and body mass: insights from murine models. J Endocrinol. 2010;205:87–95. doi: 10.1677/JOE-09-0425. [DOI] [PubMed] [Google Scholar]
- 5.Hojman P, Brolin C, Gissel H, et al. Erythropoietin Over-Expression Protects against Diet-Induced Obesity in Mice through Increased Fat Oxidation in Muscles. PLoS ONE. 2009;4 doi: 10.1371/journal.pone.0005894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mukundan H, Resta TC, Kanagy NL. 17Beta-estradiol decreases hypoxic induction of erythropoietin gene expression. Am J Physiol Regul Integr Comp Physiol. 2002;283:R496–504. doi: 10.1152/ajpregu.00573.2001. [DOI] [PubMed] [Google Scholar]
- 7.Bachman E, Travison TG, Basaria S, et al. Testosterone induces erythrocytosis via increased erythropoietin and suppressed hepcidin: evidence for a new erythropoietin/hemoglobin set point. J Gerontol A Biol Sci Med Sci. 2014;69:725–735. doi: 10.1093/gerona/glt154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Voss JD, Allison DB, Webber BJ, Otto JL, Clark LL. Lower Obesity Rate during Residence at High Altitude among a Military Population with Frequent Migration: A Quasi Experimental Model for Investigating Spatial Causation. PLoS ONE. 2014;9:e93493. doi: 10.1371/journal.pone.0093493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamad N, Travis SPL. Weight loss at high altitude: pathophysiology and practical implications. Eur J Gastroenterol Hepatol. 2006;18:5–10. doi: 10.1097/00042737-200601000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Petousi N, Croft QP, Cavalleri GL, Cheng HY, Formenti F, Ishida K. Tibetans living at sea level have a hyporesponsive hypoxia inducible factor system and blunted physiological response to hypoxia. J Appl Physiol (1985) 2014 Apr 1;116(7):893–904. doi: 10.1152/japplphysiol.00535.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith CJ, Nelson RG, Hardy SA, Manahan EM, Bennett PH, Knowler WC. Survey of the Diet of Pima Indians Using Quantitative Food Frequency Assessment and 24-Hour Recall. J Am Diet Assoc. 1996;96:778–784. doi: 10.1016/s0002-8223(96)00216-7. [DOI] [PubMed] [Google Scholar]
- 12.Pavkov ME, Hanson RL, Knowler WC, Bennett PH, Krakoff J, Nelson RG. Changing Patterns of Type 2 Diabetes Incidence Among Pima Indians. Diabetes Care. 2007;30:1758–1763. doi: 10.2337/dc06-2010. [DOI] [PubMed] [Google Scholar]
- 13.Ravussin E, Valencia ME, Esparza J, Bennett PH, Schulz LO. Effects of a Traditional Lifestyle on Obesity in Pima Indians. Diabetes Care. 1994;17:1067–1074. doi: 10.2337/diacare.17.9.1067. [DOI] [PubMed] [Google Scholar]
- 14.Fox C, Esparza J, Nicolson M, et al. Plasma leptin concentrations in Pima Indians living in drastically different environments. Diabetes Care. 1999;22:413–417. doi: 10.2337/diacare.22.3.413. [DOI] [PubMed] [Google Scholar]
- 15.Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–770. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- 16.Lillioja S, Mott DM, Spraul M, et al. Insulin Resistance and Insulin Secretory Dysfunction as Precursors of Non-Insulin-Dependent Diabetes Mellitus: Prospective Studies of Pima Indians. N Engl J Med. 1993;329:1988–1992. doi: 10.1056/NEJM199312303292703. [DOI] [PubMed] [Google Scholar]
- 17.Ravussin E, Lillioja S, Anderson TE, Christin L, Bogardus C. Determinants of 24-hour energy expenditure in man. Methods and results using a respiratory chamber. J Clin Invest. 1986;78:1568–1578. doi: 10.1172/JCI112749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jequier E, Acheson K, Schutz Y. Assessment of Energy Expenditure and Fuel Utilization in Man. Annu Rev Nutr. 1987;7:187–208. doi: 10.1146/annurev.nu.07.070187.001155. [DOI] [PubMed] [Google Scholar]
- 19.Yasuda Y, Masuda S, Chikuma M, Inoue K, Nagao M, Sasaki R. Estrogen-dependent production of erythropoietin in uterus and its implication in uterine angiogenesis. J Biol Chem. 1998;273:25381–25387. doi: 10.1074/jbc.273.39.25381. [DOI] [PubMed] [Google Scholar]
- 20.Masuda S, Kobayashi T, Chikuma M, Nagao M, Sasaki R. The oviduct produces erythropoietin in an estrogen- and oxygen-dependent manner. Am J Physiol Endocrinol Metab. 2000;278:E1038–1044. doi: 10.1152/ajpendo.2000.278.6.E1038. [DOI] [PubMed] [Google Scholar]
- 21.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 22.SOBEL M. Asymptotic Confidence Intervals for Indirect Effects in Strucutural Equation Models. Sociol Methodol 1982. 1982 [Google Scholar]
- 23.Rankin EB, Wu C, Khatri R, et al. The HIF Signaling Pathway in Osteoblasts Directly Modulates Erythropoiesis through the Production of EPO. Cell. 2012;149:63–74. doi: 10.1016/j.cell.2012.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alnaeeli M, Raaka BM, Gavrilova O, Teng R, Chanturiya T, Noguchi CT. Erythropoietin Signaling: A Novel Regulator of White Adipose Tissue Inflammation During Diet-Induced Obesity. Diabetes. 2014;63:2415–2431. doi: 10.2337/db13-0883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pannacciulli N, Salbe AD, Ortega E, Venti CA, Bogardus C, Krakoff J. The 24-h carbohydrate oxidation rate in a human respiratory chamber predicts ad libitum food intake. Am J Clin Nutr. 2007;86:625–632. doi: 10.1093/ajcn/86.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zurlo F, Lillioja S, Puente AE-D, et al. Low ratio of fat to carbohydrate oxidation as predictor of weight gain: study of 24-h RQ. Am J Physiol - Endocrinol Metab. 1990;259:E650–E657. doi: 10.1152/ajpendo.1990.259.5.E650. [DOI] [PubMed] [Google Scholar]
- 27.Wang L, Di L, Noguchi CT. Erythropoietin, a Novel Versatile Player Regulating Energy Metabolism beyond the Erythroid System. Int J Biol Sci. 2014;10:921–939. doi: 10.7150/ijbs.9518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luk CT, Shi SY, Choi D, Cai EP, Schroer SA, Woo M. In Vivo Knockdown of Adipocyte Erythropoietin Receptor Does Not Alter Glucose or Energy Homeostasis. Endocrinology. 2013;154:3652–3659. doi: 10.1210/en.2013-1113. [DOI] [PubMed] [Google Scholar]
- 29.Mikhail AA, Beck EX, Shafer A, et al. Leptin stimulates fetal and adult erythroid and myeloid development. Blood. 1997;89:1507–1512. [PubMed] [Google Scholar]
- 30.Auwerx J, Staels B. Leptin. The Lancet. 1998;351:737–742. doi: 10.1016/S0140-6736(97)06348-4. [DOI] [PubMed] [Google Scholar]
- 31.Levin NW. Quality of life and hematocrit level. Am J Kidney Dis Off J Natl Kidney Found. 1992;20:16–20. [PubMed] [Google Scholar]
- 32.Shah R, Ye C, Woo M, et al. Erythropoietin and glucose homeostasis in women at varying degrees of future diabetic risk. J Diabetes Complications. 2015;29:26–31. doi: 10.1016/j.jdiacomp.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 33.Bárány P, Pettersson E, Ahlberg M, Hultman E, Bergström J. Nutritional assessment in anemic hemodialysis patients treated with recombinant human erythropoietin. Clin Nephrol. 1991;35:270–279. [PubMed] [Google Scholar]
- 34.Daneryd P, Svanberg E, Körner U, et al. Protection of Metabolic and Exercise Capacity in Unselected Weight-losing Cancer Patients following Treatment with Recombinant Erythropoietin: A Randomized Prospective Study. Cancer Res. 1998;58:5374–5379. [PubMed] [Google Scholar]
- 35.Yokomizo R, Matsuzaki S, Uehara S, Murakami T, Yaegashi N, Okamura K. Erythropoietin and erythropoietin receptor expression in human endometrium throughout the menstrual cycle. Mol Hum Reprod. 2002;8:441–446. doi: 10.1093/molehr/8.5.441. [DOI] [PubMed] [Google Scholar]
- 36.Foresta C, Mioni R, Bordon P, Miotto D, Montini G, Varotto A. Erythropoietin stimulates testosterone production in man. J Clin Endocrinol Metab. 1994;78:753–756. doi: 10.1210/jcem.78.3.8126153. [DOI] [PubMed] [Google Scholar]
- 37.Maggio M, Snyder PJ, Ceda GP, et al. Is the haematopoietic effect of testosterone mediated by erythropoietin? The results of a clinical trial in older men. Andrology. 2013;1:24–28. doi: 10.1111/j.2047-2927.2012.00009.x. [DOI] [PubMed] [Google Scholar]
- 38.van den Beld AW, de Jong FH, Grobbee DE, Pols HA, Lamberts SW. Measures of bioavailable serum testosterone and estradiol and their relationships with muscle strength, bone density, and body composition in elderly men. J Clin Endocrinol Metab. 2000;85:3276–3282. doi: 10.1210/jcem.85.9.6825. [DOI] [PubMed] [Google Scholar]
- 39.Karkas JD, Chargaff E. Studies on the Stability of Simple Derivatives of Sialic Acid. J Biol Chem. 1964;239:949–957. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.