Abstract
Introduction
Bileaflet mitral valve prolapse (MVP) can be associated with malignant ventricular arrhythmias. It is unknown whether surgical correction alone of this mitral valve pathology leads to a reduction in ventricular dysrhythmias.
Methods
We retrospectively analyzed 4,477 patients who underwent mitral valve surgery from 1993-2013 at the Mayo Clinic in Rochester, MN. Among these, eight patients with bileaflet MVP who had an internal cardioverter defibrillator (ICD) in place both pre and post-surgery were identified. ICD interrogation records were evaluated for episodes of ventricular tachycardia (VT), ventricular fibrillation (VF), and appropriate ICD shock therapy.
Results
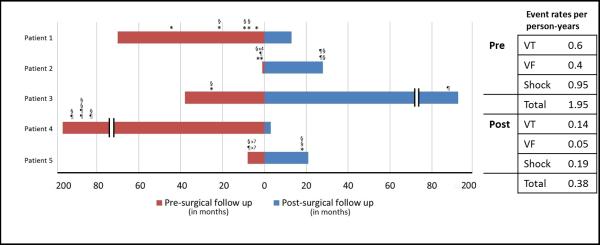
Of these eight patients, five had malignant ventricular arrhythmia prior to surgery. Data was available 4.6 ± 2.9 years before versus 6.6 ± 4.2 years following surgical intervention. Among these patients, there was a reduction in VF (0.6 versus 0.14 events per-person-year pre- and post-surgery, respectively), VT (0.4 versus 0.05 events per-person-year pre- and post-surgery, respectively), and ICD shocks (0.95 versus 0.19 events per-person-year pre- and post-surgery) following mitral valve surgery.
Conclusions
We report a series of cases where surgical correction of bileaflet MVP alone was associated with a reduction in malignant arrhythmia and appropriate shocks. These early observations merit further investigation involving larger cohorts to further evaluate the association between abnormal mechanical forces in degenerative mitral valve disease and ventricular dysrhythmias.
Keywords: Bileaflet mitral valve prolapse, mitral regurgitation, implantable cardioverter defibrillator, ventricular fibrillation
Introduction
Mitral valve prolapse (MVP) is common and is present in 2-3% of the population, approximately 7.8 million people in the United States and over 176 million people worldwide [1,2]. MVP is caused by myxomatous changes in the mitral valve leaflets which result in leaflet thickening and redundancy. MVP can involve either a single leaflet or both the anterior and posterior leaflets (bileaflet MVP). While MVP is benign generally, malignant arrhythmias such as ventricular tachycardia (VT), ventricular fibrillation (VF), out of hospital cardiac arrest (OHCA), and sudden cardiac death (SCD) can occur [3-8].
Recently, an arrhythmogenic phenotypic tetrad of MVP was described from a cohort of patients who had survived an OHCA. This subset was characterized by bileaflet MVP, female predominance, inferolateral T wave inversions, and complex ventricular ectopy on ambulatory monitoring [9]. The utility of implantable cardioverter defibrillator (ICD) for secondary prevention of malignant arrhythmias is well established. In patients with bileaflet mitral valve prolapse (biMVP) and malignant arrhythmia, it is unclear whether surgical repair of the mitral valve, electrophysiologic ablation or anti-arrhythmic medication is the optimal strategy for arrhythmia reduction. We describe rates of ventricular arrhythmias detected by ICD and appropriate ICD therapy before and after mitral valve surgery in patients with biMVP.
Methods
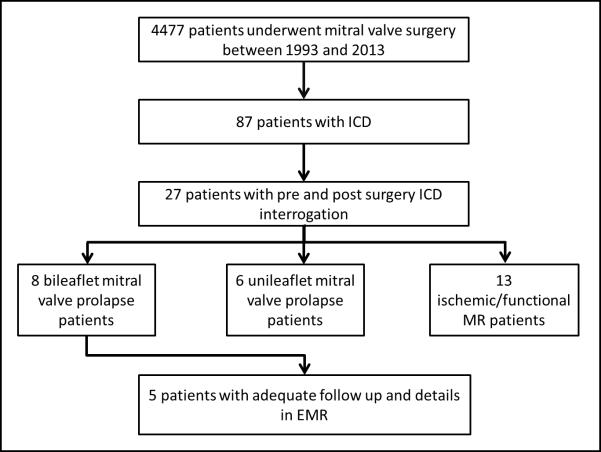
We retrospectively analyzed the mitral valve surgery database at Mayo Clinic, Rochester, MN which included 4,477 patients who underwent mitral valve surgery between 1993 and 2013. These patients were cross-referenced with our cardiac device database, and 87 patients with both mitral valve surgery and ICD placement prior to surgery were identified. Pre- and post-surgery ICD interrogation reports were present for 27 patients. BiMVP was defined on the basis of echocardiography or description by the surgeon at the time of surgery and was identified in 8 of these 27 patients. Five patients had pre-operative VT/VF and their clinical courses are described below (Figure 1).
Figure 1.
Derivation of the cohort
All demographic data, comorbidities, and medications were abstracted from the electronic medical record (EMR). Echocardiography reports were reviewed and pertinent measurements were recorded. The ICD database and the EMR were used to determine episodes of VT, VF, appropriate shocks and inappropriate shocks.
Results
Case #1
A 51-year-old male presented for evaluation of multiple premature ventricular complexes (PVCs, Table 1). An ICD was implanted eight years prior to presentation, due to inducible VF on electrophysiologic study (EPS) that was performed for syncope evaluation. Since then, he experienced five episodes of VF and received three appropriate shocks. Two months prior to presentation, he was placed on flecainide for control of multiform PVCs that were thought to be triggering VF (Table 2). Echocardiography revealed previously known biMVP and mitral valve regurgitation, which had now worsened to severe mitral regurgitation (Table 3). He underwent mitral valve repair along with prophylactic maze procedure due to severe left atrial enlargement. Post-surgery, he has not experienced any episodes of VF, VT, or ICD shocks (Table 4). He was continued on flecainide, with a plan to stop after one more year of therapy.
Table 1.
Demographic data (ACE: Angiotensin converting enzyme)
| Age | Sex | Coronary artery disease | Atrial fibrillation | Cardiomyopathy | Beta blocker | ACE inhibitor | Antiarrhythmic medications | Total follow up (years) | ICD indication | Age at OHCA/sentinel event | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 51 | M | N | N | N | Y | N | Flecainide | 1.2 | Secondary prevention: Syncope + inducible VF | 39 |
| 2 | 51 | F | N | N | N | N | N | Amiodarone, Sotalol postsurgery | 2.4 | Secondary prevention: Out of Hospital Cardiac Arrest, VF/VT | 44 |
| 3 | 57 | F | N | N | N | N | N | Propafenone | 19.0 | Secondary prevention: Out of Hospital Cardiac Arrest, VF | 38 |
| 4 | 55 | F | N | Y | N | Y | N | Sotalol, Amiodarone | 12.0 | Secondary prevention: Out of Hospital Cardiac Arrest, VF | 43 |
| 5 | 58 | M | N | Y | Y (Hypertrophic) | Y | Y | N | 2.3 | Primary prevention: HOCM with septum>30 mm + NSVT | NA |
Table 2.
Clinical and surgical data
| NYHA class | Other PMH | Number of PVC/24 hours - Holter | QTc (ms) | Type of surgery | Additional procedures | ||
|---|---|---|---|---|---|---|---|
| Pre | Post | ||||||
| 1 | I | NA | 1717 | 1184 | 422 | Repair | Maze + LAA closure |
| 2 | I | Marfan's syndrome | NA | NA | 436 | Repair | NA |
| 3 | III/IV | NA | NA | NA | NA | Repair | NA |
| 4 | II | NA | NA | 22718 | 450 | Repair | Maze + LAA ligation |
| 5 | III/IV | HOCM | 251 | 194 | 470 | Replacement | Tricuspid repair + Maze |
NYHA: New York Heart Association; PMH: Past medical history; PVC: Premature ventricular contraction; LAA: Left atrial appendage
Table 3.
Echocardiography data
| Mitral anatomy | MR degree |
Flail | Ejection fraction (%) |
ERO (cm2) | Regurgitant Volume (mL) | LVESD (mm) |
||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | |||||
| 1 | Barlow | Severe | Mild | N | 65 | 66 | 0.82 | 190 | 40 | 34 |
| 2 | Non-Barlow | Severe | Trivial | N | 65 | 60 | 0.38 | 80 | 34 | 32 |
| 3 | Non-Barlow | Severe | Mild | N | 60 | 65 | NA | NA | NA | 26 |
| 4 | Barlow | Severe | Trivial | N | 60 | 50 | 0.53 | 67 | 38 | 43 |
| 5 | Non-Barlow | Moderate-severe | None | N | 65 | 53 | 0.37 | 58 | 43 | 39 |
MR: Mitral regurgitation; ERO: Effective regurgitant orifice, LVESD: Left ventricular end systolic diameter
Table 4.
ICD outcomes
| Preoperative | Postoperative | Mortality | |||||||
|---|---|---|---|---|---|---|---|---|---|
| VT | VF | Appropriate shock | Inappropriate shock | VT | VF | Appropriate shock | Inappropriate shock | ||
| 1 | 0 | 5 | 3 | 0 | 0 | 0 | 0 | 0 | Alive |
| 2 | 1 | 2 | 4 | 0 | 2 | 0 | 2 | 1 | Alive |
| 3 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | Alive |
| 4 | 4 (VT/VF) | 4 | 1 | 0 | 0 | 0 | 0 | Alive | |
| 5 | 7 | 0 | 7 | 0 | 0 | 1 | 2 | 1 | Alive |
VT: Ventricular tachycardia; VF: Ventricular fibrillation; ATP: Anti-tachycardia pacing
Case #2
A 51-year-old female was admitted for recurrent VF. Her history was significant for Marfan syndrome and biMVP. She suffered an OHCA due to VF and subsequently had ICD implantation for secondary prevention one month prior to current admission. In the intervening period, she suffered two episodes of VF, and one episode of polymorphic VT, leading to a total of four appropriate shocks. She was placed on amiodarone for treatment. Echocardiography at admission revealed severe mitral regurgitation and biMVP. She underwent mitral valve repair surgery. Amiodarone was discontinued at the time of discharge after surgery, and she was later maintained on sotalol for prevention of dysrhythmias, and has not experienced any recurrence of VF. She suffered an inappropriate shock for paroxysmal atrial tachycardia and two appropriate shocks for ventricular tachycardia two years after initial surgery.
Case #3
A 57-year-old female presented for repair of severe mitral regurgitation secondary to biMVP. She experienced an OHCA secondary to VF three years prior to current presentation. She underwent ICD implantation for secondary prevention of VF. She experienced a single episode of VF with one appropriate shock. She underwent repair of biMVP. Propafenone was continued for 6 years after surgery. She has not had recurrent VF, but had one episode of VT terminated by anti-tachycardia pacing 12 years after surgery.
Case #4
A 55-year-old female presented with dyspnea on exertion. Her history was significant for an OHCA 11 years prior to presentation, with subsequent ICD implantation for secondary prevention. She experienced four episodes of VT/VF and received four appropriate shocks. She underwent mitral valve repair and biatrial maze procedure along with left atrial ligation. She was started on dofetilide post-operatively for recurrent atrial fibrillation. Dofetilide was thought to be contributing to ventricular ectopy and discontinued at 3 months post-surgery. She has not experienced recurrence of VT/VF.
Case #5
A 58-year-old male with hypertrophic cardiomyopathy and massive septal hypertrophy presented with severe dyspnea on exertion He had a history of biMVP. An ICD was placed two years prior to presentation for non-sustained ventricular tachycardia. One year prior to presentation, he developed seven episodes of VT, with seven appropriate shocks. He underwent mitral valve replacement, tricuspid valve repair, maze procedure, left atrial appendage closure and left atrial reduction atrioplasty. He was never on anti-arrhythmic drugs. In follow-up, he had one episode of VT during severe exertion, which was terminated by two shocks.
Discussion
The observation of MVP as an isolated cardiac entity on autopsies for SCD led to its implication in the pathogenesis of malignant ventricular arrhythmia and SCD [3,5]. A subset of MVP patients characterized by biMVP, female sex, abnormal repolarization, and complex ventricular ectopy were described from a cohort of idiopathic OHCA patients [9]. Optimal management strategies for patients with malignant arrhythmia and biMVP are unknown. We report five cases of biMVP who underwent mitral valve surgery followed by a marked reduction in malignant arrhythmias and appropriate shocks post-operatively.
Ventricular arrhythmias among patients with MVP
While PVCs and complex ventricular ectopy are common findings, malignant arrhythmias and SCD are rare in MVP. Duren et al reported three cases of SCD among 300 patients over 6.1 years of mean follow up [4]. SCD occurred in 2.5% MVP patients in a series from our own institution [10]. VF has been noted as the most likely cause of SCD among MVP patients [8]. BiMVP was associated with VF recurrences requiring ICD therapy in our own experience [9]. In the current series, VF was the most frequent malignant arrhythmia and was present in all but one patient; VT was also common.
Mechanisms of ventricular arrhythmia in patients with arrhythmogenic biMVP
The precise mechanism of ventricular arrhythmia among patients with MVP remains unknown, although several mechanisms have been proposed. Endocardial fibrotic lesions, presumably as a result of friction between the chordae and myocardium, were common in patients with MVP and SCD [11]. MVP results in abnormal stretching of the papillary muscles during systole, which could be arrhythmogenic. Myocardial stretch can generate premature ventricular contractions via early and delayed afterdepolarizations [12,13]. The observation of myocardial fibrosis involving the papillary muscles on MRI imaging in patients with MVP lends further credence to the mechanical theory of arrhythmogenesis [14]. Complex ventricular arrhythmias were more common among patients with late gadolinium enhancement in the papillary muscles. In our previous series, PVCs originating from the papillary muscles were present among patients with BiMVP and history of OHCA [9].
A recent paper by Basso et al further supports myocardial fibrosis in the papillary muscles as the myocardial substrate for malignant arrhythmia in bileaflet MVP patients. Among 43 patients with MVP and sudden death, bileaflet MVP was present in 70% patients and papillary muscle fibrosis was present in all cases at autopsy. In addition, MVP patients with complex ventricular arrhythmias were significantly more likely to have late gadolinium enhancement in papillary muscles (83%) compared to controls (14%) [15]. Genesis of malignant arrhythmia in bileaflet MVP patients probably involves a combination of scarred myocardial substrate (either due to mechanical papillary muscle stretch or contact lesions due to prolapsing leaflets) and PVCs triggered by mechanical stretch [16].
Mitral valve repair would theoretically relieve stretch on the papillary muscles, and could lead to reduction in ventricular arrhythmias due to mechanical stretch. Mitral valve repair can also result in improvement in heart failure and ventricular remodeling, but such improvement was not consistently seen among our patients (Table 3).
Reduction in ventricular arrhythmia after surgical repair of biMVP
There are conflicting reports on the efficacy of surgical repair in reducing ventricular arrhythmia in MVP patients. Improvement in malignant arrhythmias has been reported in isolated cases with mitral valve replacement or repair [17-19]. In contrast, Vohra et al reported two patients with MVP operated solely for arrhythmia suppression, and both had VT inducible three months after surgery [6]. It must be noted that clinically apparent spontaneous arrhythmia was not reported in these two patients after surgery.
In our current report, all five patients with biMVP had a reduction in malignant arrhythmia and appropriate shocks after mitral valve surgery. Although speculative, mitral valve repair or replacement may be a useful option for arrhythmia reduction in selected cases with biMVP. However, further prospective studies are required before surgery can be considered primarily for malignant arrhythmia reduction in patients with biMVP without a surgical indication based on its degree of regurgitation. It is unclear whether similar arrhythmia reduction occurs in unileaflet mitral valve prolapse patients. In the 6 patients with unileaflet mitral valve prolapse reviewed as part of this study, there was no clear reduction in arrhythmic events.
Limitations
The current report is a description of only five cases encountered in our institution, and statistical comparisons with other types of mitral regurgitation were not possible due to small sample size. Systematic studies are required to further advise appropriate management of malignant ventricular arrhythmia among patients with biMVP. Tracings of PVCs or ventricular arrhythmia were not available pre and post-surgery for all patients. We were therefore unable to deduce the location of origin of ventricular arrhythmia. Other potential factors that could have led to arrhythmia reduction include medication changes and changes in device settings. Case 1 was placed on flecainide prior to surgery, and sotalol was initiated in case 2 after surgery. It is possible that these anti-arrhythmics contributed to arrhythmia reduction in these cases, but there were no new anti-arrhythmics in the other 3 cases. Device settings were unchanged in three patients; VT detection was discontinued in one patient eleven years after surgery, and device setting changes were unknown in another patient.
Conclusion
We report that surgical correction of biMVP is associated with a reduction in malignant arrhythmia and appropriate shock therapy in a small cohort of patients. These early observations merit further investigation in larger cohorts to evaluate the association between abnormal mechanical forces in degenerative mitral valve disease and ventricular arrhythmogenesis.
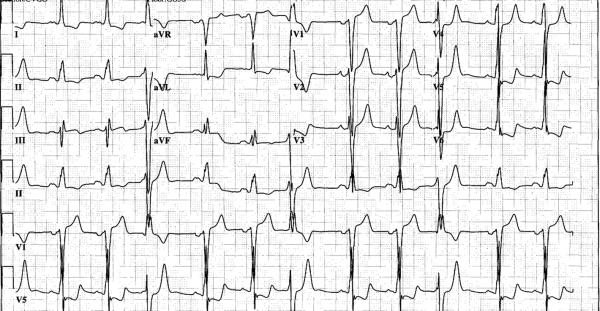
Figure 2.
Pre-operative ECG from patient 5 showing superior axis PVCs with right bundle morphology, characteristic of papillary muscle origin PVCs
Figure 3.
Bar chart representing ventricular fibrillation, ventricular tachycardia and appropriate shock therapy in 5 patients with bileaflet mitral valve prolapse
Acknowledgments
Financial support: CVD is supported by NIH T32 Training grant #HL007111
Abbreviations
- biMVP
bileaflet mitral valve prolapse
- EMR
electronic medical record
- ICD
implantable cardioverter defibrillator
- OHCA
out of hospital cardiac arrest
- MVP
mitral valve prolapse
- PVC
premature ventricular contraction
- SCD
sudden cardiac death
- VF
ventricular fibrillation
- VT
ventricular tachycardia
Footnotes
Conflict of interest: None
References
- 1.Delling FN, Vasan RS. Epidemiology and pathophysiology of mitral valve prolapse: new insights into disease progression, genetics, and molecular basis. Circulation. 2014;129(21):2158–2170. doi: 10.1161/CIRCULATIONAHA.113.006702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Freed LA, Levy D, Levine RA, Larson MG, Evans JC, Fuller DL, et al. Prevalence and clinical outcome of mitral-valve prolapse. N Engl J Med. 1999;341(1):1–7. doi: 10.1056/NEJM199907013410101. [DOI] [PubMed] [Google Scholar]
- 3.Anders S, Said S, Schulz F, Puschel K. Mitral valve prolapse syndrome as cause of sudden death in young adults. Forensic Sci Int. 2007;171(2-3):127–130. doi: 10.1016/j.forsciint.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 4.Duren DR, Becker AE, Dunning AJ. Long-term follow-up of idiopathic mitral valve prolapse in 300 patients: a prospective study. J Am Coll Cardiol. 1988;11(1):42–47. doi: 10.1016/0735-1097(88)90164-7. [DOI] [PubMed] [Google Scholar]
- 5.Knackstedt C, Mischke K, Schimpf T, Neef P, Schauerte P. Ventricular fibrillation due to severe mitral valve prolapse. Int J Cardiol. 2007;116(3):e101–102. doi: 10.1016/j.ijcard.2006.08.073. [DOI] [PubMed] [Google Scholar]
- 6.Vohra J, Sathe S, Warren R, Tatoulis J, Hunt D. Malignant ventricular arrhythmias in patients with mitral valve prolapse and mild mitral regurgitation. Pacing Clin Electrophysiol. 1993;16(3 Pt 1):387–393. doi: 10.1111/j.1540-8159.1993.tb01599.x. [DOI] [PubMed] [Google Scholar]
- 7.Dollar AL, Roberts WC. Morphologic comparison of patients with mitral valve prolapse who died suddenly with patients who died from severe valvular dysfunction or other conditions. J Am Coll Cardiol. 1991;17(4):921–931. doi: 10.1016/0735-1097(91)90875-a. [DOI] [PubMed] [Google Scholar]
- 8.Boudoulas H, Schaal SF, Stang JM, Fontana ME, Kolibash AJ, Wooley CF. Mitral valve prolapse: cardiac arrest with long-term survival. Int J Cardiol. 1990;26(1):37–44. doi: 10.1016/0167-5273(90)90244-y. [DOI] [PubMed] [Google Scholar]
- 9.Sriram CS, Syed FF, Ferguson ME, Johnson JN, Enriquez-Sarano M, Cetta F, et al. Malignant bileaflet mitral valve prolapse syndrome in patients with otherwise idiopathic out-of-hospital cardiac arrest. J Am Coll Cardiol. 2013;62(3):222–230. doi: 10.1016/j.jacc.2013.02.060. [DOI] [PubMed] [Google Scholar]
- 10.Nishimura RA, McGoon MD, Shub C, Miller FA, Jr., Ilstrup DM, Tajik AJ. Echocardiographically documented mitral-valve prolapse. Long-term follow-up of 237 patients. N Engl J Med. 1985;313(21):1305–1309. doi: 10.1056/NEJM198511213132101. [DOI] [PubMed] [Google Scholar]
- 11.Chesler E, King RA, Edwards JE. The myxomatous mitral valve and sudden death. Circulation. 1983;67(3):632–639. doi: 10.1161/01.cir.67.3.632. [DOI] [PubMed] [Google Scholar]
- 12.Franz MR. Mechano-electrical feedback in ventricular myocardium. Cardiovasc Res. 1996;32(1):15–24. [PubMed] [Google Scholar]
- 13.Wang Z, Taylor LK, Denney WD, Hansen DE. Initiation of ventricular extrasystoles by myocardial stretch in chronically dilated and failing canine left ventricle. Circulation. 1994;90(4):2022–2031. doi: 10.1161/01.cir.90.4.2022. [DOI] [PubMed] [Google Scholar]
- 14.Han Y, Peters DC, Salton CJ, Bzymek D, Nezafat R, Goddu B, et al. Cardiovascular magnetic resonance characterization of mitral valve prolapse. JACC Cardiovasc Imaging. 2008;1(3):294–303. doi: 10.1016/j.jcmg.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 15.Basso C, Perazzolo Marra M, Rizzo S, De Lazzari M, Giorgi B, Cipriani A, et al. Arrhythmic Mitral Valve Prolapse and Sudden Cardiac Death. Circulation. 2015 doi: 10.1161/CIRCULATIONAHA.115.016291. [DOI] [PubMed] [Google Scholar]
- 16.Noseworthy PA, Asirvatham SJ. The Knot That Binds Mitral Valve Prolapse and Sudden Cardiac Death. Circulation. 2015 doi: 10.1161/CIRCULATIONAHA.115.017979. [DOI] [PubMed] [Google Scholar]
- 17.Reece IJ, Cooley DA, Painvin GA, Okereke OU, Powers PL, Pechacek LW, et al. Surgical treatment of mitral systolic click syndrome: results in 37 patients. Ann Thorac Surg. 1985;39(2):155–158. doi: 10.1016/s0003-4975(10)62556-8. [DOI] [PubMed] [Google Scholar]
- 18.Ross A, DeWeese JA, Yu PN. Refractory ventricular arrhythmias in a patient with mitral valve prolapse. Successful control with mitral valve replacement. J Electrocardiol. 1978;11(3):289–295. doi: 10.1016/s0022-0736(78)80131-9. [DOI] [PubMed] [Google Scholar]
- 19.Pocock WA, Barlow JB, Marcus RH, Barlow CW. Mitral valvuloplasty for life-threatening ventricular arrhythmias in mitral valve prolapse. Am Heart J. 1991;121(1 Pt 1):199–202. doi: 10.1016/0002-8703(91)90976-o. [DOI] [PubMed] [Google Scholar]