Abstract
Objective
Arteriovenous fistulas (AVF) are considered superior to arteriovenous grafts (AVG) because of longer secondary patency after successful cannulation for dialysis. We evaluated whether access interventions before successful cannulation impact the relative longevity of AVF and AVG after successful use.
Methods
This retrospective study of a prospective database identified patients who initiated dialysis with a catheter, and subsequently had a permanent access (289 AVF and 310 AVG) placed between 1/1/06-12/31/11 and successfully cannulated for dialysis at a large medical center. Patients were followed until 6/30/14, and we evaluated the clinical outcomes (secondary patency and frequency of interventions) of the vascular accesses.
Results
An intervention before successful cannulation was required more frequently with AVF than with AVG (50.5 vs 17.7%; OR 4.74; 95% CI 3.26 to 6.86, P < .0001). As compared to AVF that matured without interventions, those that required intervention had shorter secondary patency after successful cannulation (HR 1.84; 95% CI 1.30–2.60, P < .0001) and required more interventions per year after successful use (RR 1.81; 95% CI 1.49–2.20, P < .0001). Similarly, AVG that required intervention before successful cannulation had shorter secondary patency than those without prior intervention (OR 1.98, 95% CI 1.52 to 4.02, P < .0001) and required more interventions per year after successful use (RR 1.49; 95% CI 1.27–1.74, P < .0001). AVF requiring intervention before maturation had inferior secondary patency as compared to AVG that were cannulated without prior intervention (HR 1.45, 95% CI 1.08 to 2.01, P = 0.01), but required fewer annual interventions after successful use (RR 0.57; 95% CI 0.49–0.66, P < .0001).
Conclusions
The patency advantage of AVF over AVG is no longer evident in patients requiring an AVF intervention prior to successful cannulation, but the AVF require fewer interventions after successful use.
Introduction
Arteriovenous fistulas (AVF) are considered the preferred type of vascular access in hemodialysis patients1. Once they have matured sufficiently to be cannulated reproducibly for hemodialysis, AVF have secondary patency that is superior to that of arteriovenous grafts (AVG), and require less frequent interventions to maintain long-term patency for dialysis2. There has been a progressive increase in the use of AVF in hemodialysis patients during the past ten years, in response to national consensus guidelines3. Not surprisingly, concerted efforts to place AVF in most dialysis patients have led to an increase in non-maturating AVF, particularly in older patients and those with high co-morbidity4–6. These patients frequently require surgical or percutaneous interventions after the initial AVF surgery to convert a non-maturing AVF to a mature one7–9.
There is a paucity of literature evaluating the impact of procedures to promote AVF maturation on clinical AVF outcomes after maturation. A recent publication observed that AVF requiring interventions to achieve maturation have decreased secondary patency and require more frequent interventions to maintain long-term patency, as compared to AVF that mature without interventions9. These findings suggest that the patency advantage of AVF over AVG may no longer hold for the subset of patients with new AVF who require interventions to promote AVF maturation.
The primary goal of the present study was to compare the secondary patency of AVF and AVG that did or did not require intervention prior to their successful use for dialysis. A secondary aim was to compare the frequency of interventions to maintain long-term patency for dialysis in these patient groups. To answer these questions, we retrospectively queried a prospective vascular access database to identify patients who had an AVF or AVG placed after initiation of hemodialysis that was subsequently used successfully for dialysis. We then compared the secondary access patency for dialysis and the frequency of interventions to maintain such patency in patients with AVF or AVG that did or did not require interventions prior to successful use.
Methods
Vascular access management
Clinical nephrologists at the University of Alabama at Birmingham (UAB) oversaw the medical care for approximately 500 chronic hemodialysis patients in the metropolitan Birmingham area. Four experienced UAB transplant surgeons (241, 203, 129 and 105 vascular access procedures annually) performed the vast majority of access procedures in this patient population, and UAB radiologists performed preoperative vascular mapping and subsequent interventions required to maintain or restore access patency. Hemodialysis patients dialyzing with central vein catheters (CVC) routinely underwent standardized preoperative sonographic vascular mapping with a tourniquet, and the surgeons used the findings to plan their access surgery10,11. The minimal criteria for creation of a new AVF was an arterial diameter ≥2 mm, a venous diameter ≥2.5 mm, and no evidence of stenosis or thrombosis in the planned draining vein. The minimal criteria for placement of an AVG was an arterial diameter ≥2 mm, a venous diameter ≥4 mm, and no stenosis or thrombosis in the draining vein. Each surgeon created at least 25 vascular accesses annually. In accordance with the Fistula First guidelines, the surgeons generally preferred AVFs over AVGs, and radiocephalic over brachiocephalic AVFs. The choice between a transposed brachiobasilic AVF or AVG was at the discretion of the surgeon. Ultimately, the surgeons determined the type and location of vascular access in each patient after careful consideration of the vascular anatomy, the likelihood of AVF non-maturation, the expected patient survival, and the outcomes of prior vascular accesses in the same patient12. Venograms were not performed routinely to exclude central vein stenosis, but were obtained if there was a clinical suspicion.
Once the vascular access had been successfully used for dialysis, it was monitored for clinical evidence of dysfunction by the nurses and by the rounding nephrologists at the dialysis unit. Access surveillance was not performed Patients were referred for a diagnostic fistulogram if the physical examination suggested access dysfunction (e.g., absent thrill, abnormal auscultation or distal edema), if there were problems with the dialysis session (difficult cannulation, aspiration of clots, inability to achieve the target dialysis blood flow, or prolonged bleeding from the needle sites), or if there was an unexplained decrease in the delivered Kt/V13. Angioplasty was performed if the images demonstrated >50% stenosis. Clotted accesses underwent percutaneous mechanical thrombectomy in conjunction with angioplasty of the underlying stenotic lesion. Infected AVG were excised surgically. If an access could not be salvaged percutaneously or surgically, it was abandoned (permanent failure), and a new CVC placed for vascular access. Two full-time access coordinators scheduled all access procedures, and maintained a prospective, computerized database of all access procedures14.
Data collection
The Institutional Review Board approved analysis of existing computerized clinical and vascular access databases for this research project and waived the requirement for obtaining informed consent. The vascular access database was queried for a 6-year period (1/1/06 to 12/31/11) to identify a cohort of 599 patients who (1) initiated hemodialysis with a CVC; (2) received a new vascular access (AVF or AVG) after initiation of hemodialysis; and (3) whose access was subsequently used successfully for dialysis. In patients undergoing multiple access creations, only the first successful access was included in our analysis. If the initial AVF failed to mature and a subsequent AVG was placed, that AVG was used for the study analysis. HeRO grafts were not included in this analysis. The final cohort included 289 patients with a new AVF and 310 patients with a new AVG that were used successfully for dialysis. Access and patient events were recorded through 6/30/14. The access database was further queried to ascertain (1) when the CVC was removed due to successful access cannulation; (2) whether an access intervention was performed prior to successful use of the access for dialysis; (3) whether and when the access was permanently abandoned; and (4) the number of access interventions (angioplasty, thrombectomy, or surgical revision) performed after the access was used successfully for dialysis. Patient demographics and co-morbidities, including age, gender, race, diabetes mellitus, hypertension, congestive heart failure, cerebrovascular accident, symptomatic peripheral vascular disease, body mass index (BMI), co-morbidity score specifically validated for dialysis patients15, use of anti-platelet agents, and presence of an ipsilateral dialysis catheter were extracted from the electronic medical record.
Statistical analysis
Secondary functional access patency was calculated from first successful cannulation for dialysis until permanent access failure, regardless of the number of procedures required to maintain access patency for dialysis. For patients without permanent access failure, access patency was calculated until the censoring event (death, kidney transplant, transfer to a non-participating dialysis unit, or end of the follow-up period).
Multiple variable logistic regression analysis was used to determine which variables were independently associated with patient subgroups. Survival analysis techniques were used to generate Kaplan-Meier survival curves, and log rank tests used to analyze differences among the curves. Hazard ratios and their associated 95% confidence intervals were calculated. Multiple variable survival analysis was used to assess which variables independently predicted access failure. The frequency of access intervention was calculated by dividing the total number of procedures by the total years of access patency. The difference in access interventions between groups was calculated using Poisson regression with relative rates.
Results
Baseline patient characteristics
The study cohort consisted of 599 patients who initiated hemodialysis with a CVC, then underwent placement of a permanent access (289 AVFs and 310 AVGs), and whose access was subsequently used successfully for dialysis. The demographic data and clinical characteristics of the study population are summarized in Table I.
Table I.
Baseline characteristics of the study population
| Characteristic | N of patients (%) |
|---|---|
| Age >65 yr, N (%) | 131 (22%) |
| Female sex, N (%) | 271 (45%) |
| Black race, N (%) | 524 (87%) |
| Diabetes, N (%) | 307 (51%) |
| Hypertension, N (%) | 560 (93%) |
| Coronary artery disease, N (%) | 184 (31%) |
| Peripheral vascular disease, N (%) | 106 (18%) |
| Cerebrovascular disease, N (%) | 120 (20%) |
| Congestive heart failure, N (%) | 188 (31%) |
| Obese, N (%) | 202 (34%) |
| Ipsilateral CVC, N (%) | 177 (30%) |
| Comorbid score ≥4, N (%) | 76 (13%) |
| Antiplatelet drug, N (%) | 212 (35%) |
| Anticoagulation, N (%) | 23 (4%) |
| AVF type | |
| Radiocephalic | 102 (35%) |
| Brachiocephalic | 161 (56%) |
| Transposed brachiobasilic | 23 (8%) |
| Thigh | 3 (1%) |
| AVG type | |
| Forearm | 32 (10%) |
| Upper arm | 195 (63%) |
| Thigh | 83 (27%) |
Factors associated with intervention prior to successful access cannulation
An intervention prior to successful access use for dialysis was required in 50.5% of patients with AVF, but only 17.7% of those with an AVG (OR 4.74; 95% CI 3.26–6.86, P < .0001). As compared to patients with AVFs that matured without prior interventions, those with AVFs requiring an intervention prior to maturation were more likely to be female and diabetic (Table I). Using multiple variable logistic regression analysis, with age, gender, race, diabetes, hypertension, coronary artery disease, peripheral vascular disease, congestive heart failure, obesity, co-morbidity score, ipsilateral catheter, and anti-platelet drug in the model, only female gender predicted a higher likelihood of AVF requiring intervention prior to successful use (OR 2.98, 95% CI 1.78 to 2.98, P < .0001).
As compared to patients with AVGs that were usable without prior interventions, those patients with AVGs requiring an intervention prior to successful cannulation were less likely to have hypertension, and more likely to have coronary artery disease or peripheral vascular disease Table I). Using multiple variable logistic regression analysis, with age, gender, race, diabetes, hypertension, coronary artery disease, peripheral vascular disease, congestive heart failure, obesity, co-morbidity score, ipilateral catheter, and anti-platelet drug in the model, hypertension was negatively associated (OR 0.28, 95% CI 0.10 to 0.77, P = 0.01) and coronary artery disease positively associated (OR 2.27, 95% CI 1.22 to 4.18, P = 0.01) with an AVG requiring intervention prior to successful use.
Secondary vascular access patency
Decreased secondary functional AVF patency (from first successful cannulation to permanent failure) was associated with four variables on univariable patency analysis: intervention prior to AVF maturation (HR 1.84, 95% CI 1.29 to 2.63, P < .0001); peripheral vascular disease (HR 1.67, 95% CI 1.07 to 2.61, P = .03); age ≥65 years (HR 1.82, 95% CI 1.19 to 2.80, P = .006); and co-morbidity score (HR 1.51, 95% CI 1.10–2.20, P = .02). Using multiple variable survival analysis, with intervention prior to AVF maturation, age, gender, race, diabetes, hypertension, coronary artery disease, peripheral vascular disease, congestive heart failure, obesity, co-morbidity score, anti-platelet agent, and ipsilateral catheter in the model, only intervention prior to AVF maturation (HR 1.86, 95% CI 1.30 to 2.66, P < .0001) and older age (HR 1.84, 95% CI 1.20 to 2.80, P = .01) were associated with decreased secondary functional AVF patency.
Decreased secondary AVG patency was associated with three variables on univariable patency analysis: intervention prior to successful AVG cannulation (HR 2.04, 95% CI 1.37 to 3.03, P < .0001), ipsilateral catheter (HR 1.56, 95% CI 1.08 to 2.25, P = .02) and absence of hypertension (HR 0.48, 95% CI 0.26 to 0.87, P = .02). Using multiple variable survival analysis, with intervention prior to successful AVG use, age, gender, race, diabetes, hypertension, coronary artery disease, peripheral vascular disease, congestive heart failure, obesity, co-morbidity score, anti-platelet agent, and ipsilateral catheter in the model, only intervention prior to successful AVG use (HR 1.96, 95% CI 1.31 to 2.93, P < .0001) and ipsilateral catheter (HR 1.54, 95% CI 1.08 to 2.23, P = .02) were positively associated with secondary AVG patency.
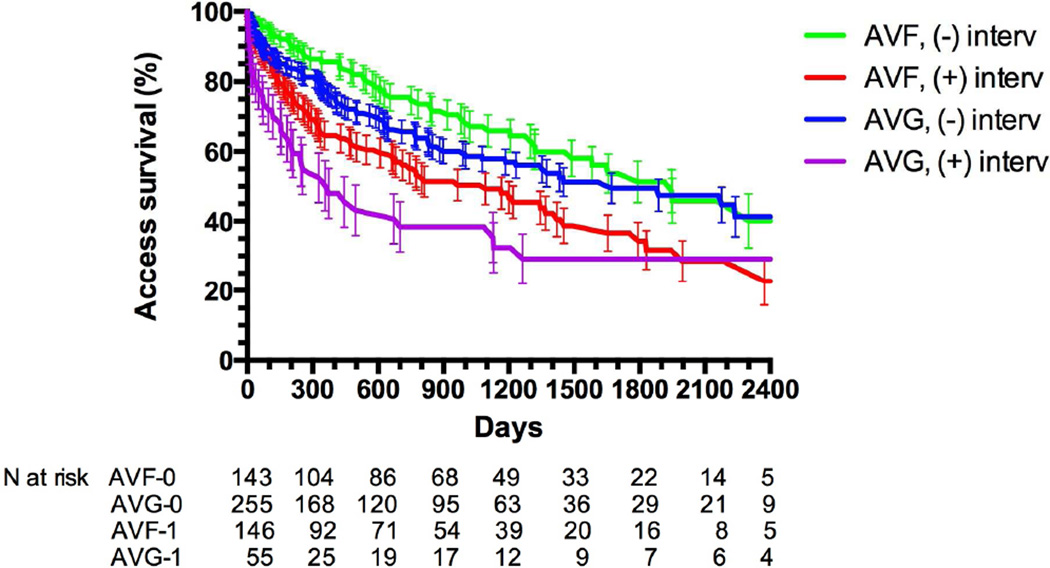
Overall, there was no difference in the functional secondary patency of AVG and AVF over up to 6.5 years of follow-up (HR 1.06 (0.83–1.35). The cumulative patencies of AVF and AVG with and without intervention prior to successful use are summarized in Figure 1 and Table II. AVGs underwent an average of 3.17 interventions after successful use (1.58 interventions per year of functional patency), whereas AVFs underwent an average of 0.63 interventions (0.63 interventions per year of functional patency). AVF that required intervention prior to maturation had shorter secondary patency than those that matured without an intervention. Similarly, AVG that required an intervention prior to successful use had a shorter secondary patency than those that were used without a prior intervention. Among accesses that were used successfully without a prior intervention, the secondary patency was similar for AVF and for AVG (mean followup 2.6 and 2.1 years, respectively). However, the secondary patency of the 50% of AVF that required an intervention prior to maturation was inferior to that of AVG that were cannulated successfully without prior intervention.
Figure 1. Cumulative access patency of AVFs that matured with or without prior interventions and AVGs that did or did not require intervention prior to successful cannulation.
Access patency was shorter for AVF with prior intervention than AVF without interventions (P < .0001). Access patency was shorter for AVG with prior interventions than AVG without intervention (P < .0001). Access patency was similar for AVF and AVG without prior interventions (P =.16). Cumulative access patency was worse for AVF with prior interventions than for AVG without prior interventions (P = .01).
Table II.
Associations of baseline patient characteristics with vascular access subgroups
| All AVF | All AVG | |||||
|---|---|---|---|---|---|---|
| Parameter | AVF w/ interv |
AVF w/o interv |
Odds ratio (95% CI) |
AVG w/ interv |
AVG w/o interv |
Odds ratio (95% CI) |
| N patients | 146 | 143 | 55 | 255 | ||
| Age ≥65 yr, N (%) | 28 (19%) | 26 (18%) | 1.07 (0.59–1.93) | 14 (25%) | 62 (24%) | 1.06 (0.54–2.08) |
| Female, N (%) | 67 (46%) | 32 (22%) | 2.94 b (1.75–5.00) | 31 (56%) | 141 (55%) | 1.04 (0.58–1.88) |
| Black race, N (%) | 125 (86%) | 120 (84%) | 1.14 (0.60–2.17) | 50 (91%) | 229 (90%) | 1.14 (0.42–3.10) |
| DM, N (%) | 77 (53%) | 55 (38%) | 1.74 a (1.09–2.79) | 26 (47%) | 149 (58%) | 0.66 (0.37–1.19) |
| HTN, N (%) | 138 (94%) | 132 (92%) | 1.18 (0.44–3.14) | 47 (85%) | 243 (95%) | 0.33 a (0.12–0.89) |
| CAD, N (%) | 46 (32%) | 42 (29%) | 1.08 (0.66–1.79) | 24 (44%) | 72 (28%) | 2.03 a (1.11–3.71) |
| PVD, N (%) | 26 (18%) | 19 (13%) | 1.39 (0.73–2.65) | 16 (29%) | 45 (18%) | 1.96 (1.01–3.83) |
| CVD, N (%) | 25 (17%) | 27 (19%) | 0.88 (0.48–1.60) | 14 (25%) | 54 (21%) | 1.30 (0.66–2.57) |
| CHF, N (%) | 45 (31%) | 44 (31%) | 0.99 (0.60–1.64) | 16 (29%) | 83 (32%) | 0.87 (0.46–1.66) |
| Obese, N (%)c | 58 (41%) | 41 (31%) | 1.55 (0.94–2.56) | 18 (38%) | 85 (37%) | 1.01 (0.53–1.92) |
| Ipsilateral CVC, N (%) | 45 (31%) | 47 (33%) | 0.91 (0.55–1.49) | 14 (25%) | 71 (28%) | 0.81 (0.57–1.15) |
| Comorbid score ≥4, N (%) | 53 (36%) | 40 (28%) | 1.47 (0.89–2.41) | 23 (42%) | 102 (40%) | 1.08 (0.60–1.95) |
| Antiplatelet drug, N (%) | 58 (40%) | 44 (31%) | 1.45 (0.89–2.37) | 19 (34%) | 91 (36%) | 0.97(0.52–1.80) |
AVF, arteriovenous fistula; AVG, arteriovenous graft; Interv, intervention; DM, diabetes; HTN, hypertension; CAD, coronary artery disease; PVD, peripheral vascular disease; CVD, cerebrovascular disease; CHF, congestive heart failure.
P < .05
P < .001
missing values for 52 patients
Reasons for vascular access failure
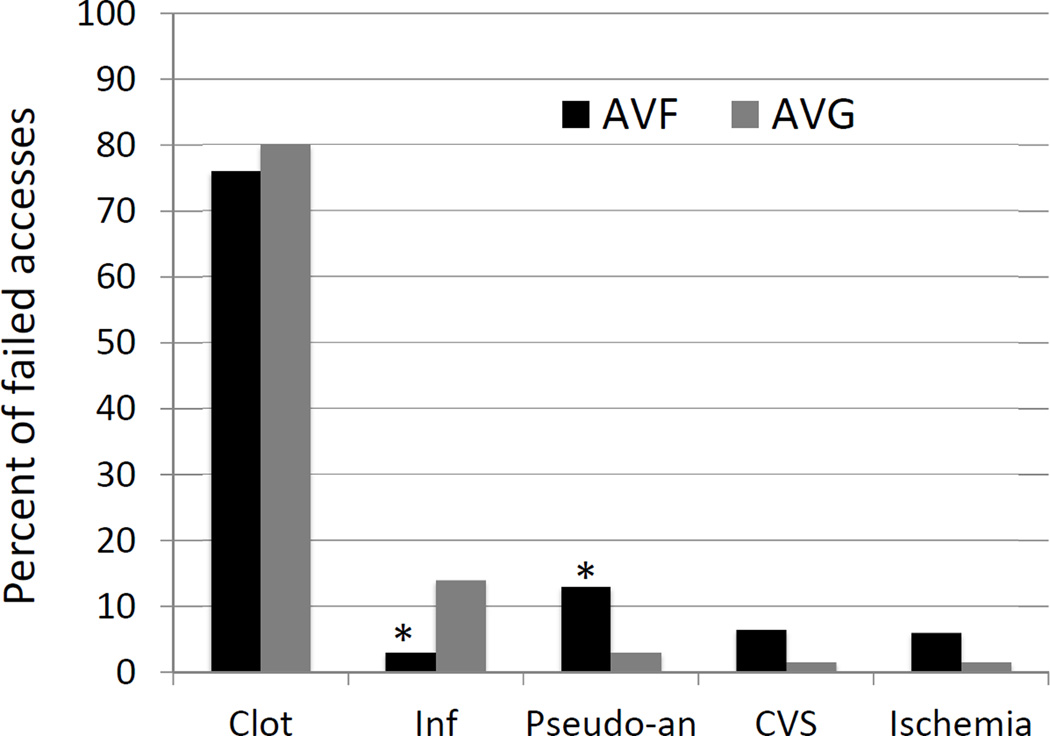
Access failure was observed in 128 AVF and 130 AVG. Thrombosis was the major cause of failure in both groups (76% of AVF and 80% of AVG)(Figure 2). Infection was a more common cause of failure in AVG (14 vs 3%, P = .002), whereas a pseudo-aneurysm with impending rupture was a more common cause in AVF (13 vs 3%, P = .003). Vascular steal and central vein stenosis with debilitating ipsilateral edema were less common causes of access failure.
Fig 2. Causes of AVF and AVG failure.
Inf, infection; Pseudo-an, pseudoaneurysm; CVC, central vein stenosis. * P < .01.
Frequency of access interventions after successful use
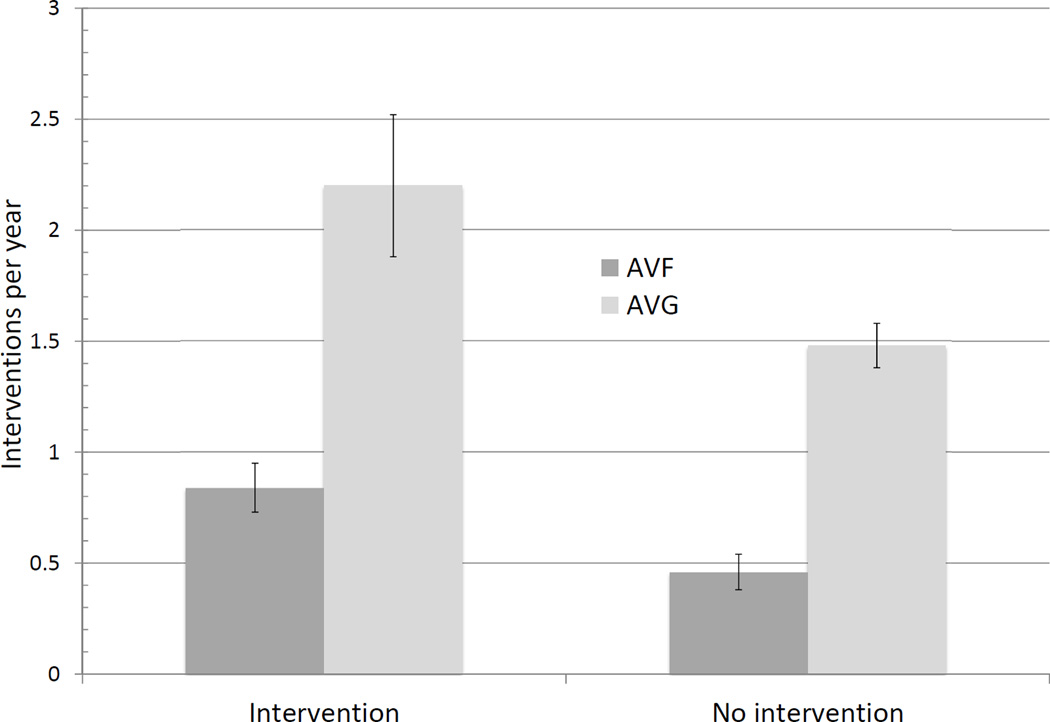
The mean follow-up time was 2.30 years for AVFs and 2.01 years for AVGs. Among accesses used successfully for dialysis without prior interventions, AVG required approximately 2.5-fold more interventions than did AVF (Table III). However, the frequency of interventions to maintain access patency was dependent on whether the access required an intervention prior to successful use. Thus, AVF with an intervention prior to maturation required almost twice as many interventions than AVF that matured without an intervention (0.84 [95% CI 0.74–0.95] vs 0.46 [95% CI 0.40–0.54], P < .0001)(Figure 3, Table II). Likewise, AVG with an intervention prior to successful use required approximately 50% more interventions to maintain patency for dialysis, as compared to AVG that were used for dialysis without a prior intervention (2.20 [95% CI 1.90–2.52] vs 1.48 [95% CI 1.37–1.58], P < .0001]. Finally, the frequency of interventions to maintain patency for dialysis was about 40% lower for AVF that required an intervention prior to maturation, as compared to AVG that were successfully used without prior intervention (0.84 [95% CI 0.74–0.95] vs 1.48 [95% CI 1.37–1.58], P = .01).
Table III.
Comparison of access outcomes and interventions in different groups.
| Secondary access patency | Access interventions per year | |||
|---|---|---|---|---|
| Comparison | HR (95% CI) | P-value | Rate ratio | P-value |
| All AVG vs all AVF | 1.06 (0.83–1.35) | .64 | 2.50 (2.22–2.78) | <.0001 |
| AVF-1 vs AVF-0 | 1.84 (1.30–2.60) | < .0001 | 1.81 (1.49–2.20) | < .0001 |
| AVG-1 vs AVG-0 | 1.98 (1.52–4.02) | < .0001 | 1.49 (1.27–1.74) | < .0001 |
| AVG-0 vs AVF-0 | 1.28 (0.91–1.78) | .16 | 3.18 (2.70–3.76) | < .0001 |
| AVF-1 vs AVG-0 | 1.45 (1.08–2.01) | .01 | 0.57 (0.49–0.66) | < .0001 |
AVF-1, AVF with interventions prior to maturation
AVF-0, AVF without intervention prior to maturation
AVG-1, AVG with interventions prior to successful cannulation
AVG-0, AVG without interventions prior to successful cannulation
A HR or rate ratio >1 indicates that the first subgroup has a worse outcome than the second subgroup. A HR or rate ratio <1 indicates that that first subgroup has a better outcome than the second subgroup.
Fig 3. Annual frequency of AVF and AVG interventions after successful access use.
Values are 95% CI.
P < .0001 for AVF with prior intervention vs AVF without intervention.
P < .0001 for AVG with prior intervention vs AVG without intervention.
P < .0001 for AVF without intervention vs AVG without intervention.
P < .0001 for AVF with intervention vs AVG without intervention.
Types of AVF interventions and their impact on secondary AVF patency and frequency of interventions
Among the 146 patients who required an AVF intervention prior to successful use, the most common interventions were transposition (n=72), angioplasty of an anastomotic stenosis (n=31) and surgical revision of the stenosis (n=31). As compared to patients whose AVF matured without a prior intervention, those who required an angioplasty or transposition before successful use had a significantly shorter secondary access patency (Table IV). There was also a trend of shorter secondary AVF patency in patients who required a surgical stenosis revision, although this difference did not quite achieve statistical significance (p= .06). The frequency of AVF interventions after AVF maturation was higher in each of the groups requiring one of these interventions, as compared to the group with AVF that matured without an intervention (Table IV). The annual frequency of interventions (mean [95%CI]) was .46 [.40– .54] for AVF without prior interventions, 1.00 [.75–1.30] for AVF with prior angioplasty, .69 [.50 – .93] for AVF with prior surgical stenosis revision, and .79 [.65– .94] for AVF requiring transposition.
Table IV.
Comparison of AVF outcomes with specific interventions prior to successful use with AVF with no interventions.
| Secondary access patency | Access interventions per year | |||
|---|---|---|---|---|
| Comparison | HR (95% CI) | P-value | Rate ratio | P-value |
| PTA vs no intervention | 2.70 (2.02–7.95) | < .0001 | 2.15 (1.59–2.91) | < .0001 |
| Stenosis revision vs no intervention | 1.71 (.96– 3.79) | 0.06 | 1.49 (1.07–2.07) | .02 |
| Transposition vs no intervention | 1.56 (1.01–2.59) | .047 | 1.70 (1.34–2.14) | < .0001 |
There were 143 AVFs without an intervention prior to successful use, 31 with PTA (percutaneous transluminal angioplasty), 31 with surgical stenosis revision, and 72 with a transposition procedure.
A HR or rate ratio >1 indicates that the first subgroup has a worse outcome than the second subgroup. A HR or rate ratio <1 indicates that that first subgroup has a better outcome than the second subgroup.
Duration of catheter dependence
Intervention prior to successful access use was associated with significant prolongation of catheter-dependence prior to the initial access use, and this was observed for both AVF and AVG (Table V). In patients with AVFs the requirement for intervention prior to successful use was also associated with a longer duration of catheter days after the initial access use. A similar trend was observed in patients with AVGs, although it did not achieve statistical significance (p= .07). Finally, the requirement for an intervention prior to successful access use was associated with a greater duration of catheter dependence from initial access use to the end of patient follow-up, and this was true for both patients with AVFs and those with AVGs.
Table V.
Duration of catheter dependence after placement of new vascular access
| Access group | Catheter days prior to initial access use Mean [95% CI] |
Catheter days after initial access use Mean [95% CI] |
Total catheter days after access placement Mean [95% CI] |
|---|---|---|---|
| AVF w/o prior intervention | 99 [92–106] | 78 [40–116] | 177 [137–216] |
| VF w/ prior intervention | 159 [148–170] a | 145 [99–191] c | 304 [257–352] a |
| AVG w/o prior intervention | 38 [36–41] a | 127 [86–167] | 165 [125–206] |
| AVG w/ prior intervention | 79 [65–94] b | 221 [102–339] | 300 [180–420] d |
p< .0001 vs AVF w/o prior intervention
p< .0001 vs AVG w/p prior intervention
p= .03 vs AVF w/o prior intervention
p= .002 vs AVG w/o prior interventions
Discussion
Our study highlights the adverse impact of interventions in AVF and AVG prior to their successful cannulation for dialysis on the long-term clinical access outcomes. Specifically, such interventions are associated with shorter cumulative access patency and more frequent interventions to maintain long-term access patency, and this observation was true for both AVF and AVG. The injury from such interventions causes accelerated intimal hyperplasia, leading to recurrent stenosis, additional interventions, and shortened access patency16. Alternatively, the need for more frequent access intervention prior to successful use may be a surrogate marker for poor vascular quality in certain patient subsets. Importantly, patients with AVF were much more like than those with AVG to require interventions prior to successful access cannulation. For the half of patients receiving an AVF that required an intervention to promote access maturation, cumulative access patency was significantly worse than for those who had an AVG without prior interventions. This advantage of AVG was counterbalanced, however, by the higher rate of interventions required to maintain long-term AVG patency after successful use. In other words, AVG required fewer interventions prior to successful cannulation, but more interventions after successful use. The clinical decision about the optimal access choice in a patient dialyzing with a CVC should consider the anticipated access patency, the frequency of interventions to maintain access patency for dialysis, and the patient’s life expectancy.
The high likelihood of an intervention to promote AVF maturation in the present study is notable, and consistent with prior reports. Three previous series reported such interventions in 35, 42, and 44% of patients undergoing AVF surgery7,9,17. This worrisome trend is not surprising, given the national consensus guidelines urging surgeons, nephrologists, and dialysis units to consider AVF placement in almost all hemodialysis patients. In previous studies including ones from our medical center), the cumulative patency of AVF was superior to that of AVG, once primary failures were excluded10,18–20. The present study demonstrates that AVF creation in patients with high co-morbidity leads to aggressive efforts to salvage a large proportion of immature AVFs, and in turn results in a loss of the patency advantage of AVFs over AVGs. It is also noteworthy that even a transposition procedure, which is often a planned surgical intervention to promote ease of AVF cannulation, was associated with shorter secondary AVF patency and more frequent AVF interventions after successful AVF use.
In a previous study we reported that female dialysis patients were almost twice as likely to require an AVF intervention prior to maturation, as compared to male patients (42 vs 23%)21. These findings are consistent with the current study that observed a much higher frequency of interventions prior to AVF maturation in women than in men (68 vs 42%). The reason for the greater requirement for interventions prior to AVF non-maturation is not apparent, since the patients underwent routine preoperative mapping to ensure selection of vessels meeting the minimal thresholds. In marked contrast, there was no gender difference in the frequency of interventions prior to successful cannulation of AVG (18 vs 17%).
AVG intervention prior to successful cannulation was associated negatively with hypertension and positively with coronary artery disease. The early AVG interventions were primarily due to early thrombosis. Patients with lower baseline blood pressures are more susceptible to intra-dialytic hypotension, an occurrence that has been associated with vascular access thrombosis22. The association between coronary artery disease and early AVG thrombosis is harder to explain, but perhaps coronary artery disease is a surrogate marker for accelerated intimal hyperplasia that leads to rapid AVG stenosis and thrombosis23. Finally, the association of an ipsilateral catheter and shortened AVG patency is consistent with a prior publication from our center24.
An important strength of the current study is the use of a prospective vascular access database to ensure comprehensive data on access patency, as well interventions before and after successful cannulation for dialysis. Such detailed information is not readily available from national databases. Our study also has some limitations. First, it represents the experience of a single large dialysis center, and the results may not generalize to some centers. For example, the vast majority of our patients were black, and the findings may not be true for other races. Second, we restricted our analysis to patients who were already dialysis dependent when their AVF or AVG was created. It may well be that the clinical outcomes of AVF and AVG that are placed in pre-dialysis patients and are suitable for cannulation upon initiation of dialysis differ from the outcomes obtained in vascular accesses placed after dialysis initiation. However, given that about 80% of incident U.S. dialysis patients use a CVC, the findings of our study are relevant to a substantial proportion of dialysis patients25. Third, we included only those vascular accesses that were successfully used for dialysis. This was by design, as the goal of the study was to evaluate the outcomes of AVF and AVG after they were successfully used for dialysis. It is likely, however, that inclusion of accesses that were placed but not used for dialysis would have made AVF look even worse, given that AVFs have a higher primary failure rate than do AVGs. For example, a previous large series from our center observed a primary access failure rate of 38% in AVF vs 15% in AVG19.
Conclusions
This study highlights important tradeoffs arising from aggressive efforts to maximize AVF use in the hemodialysis population, particularly in patients who initiate dialysis with a CVC. Placement of AVFs in these patients is often associated with prolonged catheter dependence and a high frequency of catheter-related bacteremia. These complications are often viewed as an acceptable short-term price for achieving a long-term gain, namely an AVF with prolonged cumulative patency. Contrary to these assumptions, AVF’s that required an intervention to promote maturation had inferior secondary patency to that obtained with most AVGs. Thus, placement of an AVG may be a better choice in some patients initiating dialysis with a CVC who are referred for a permanent access. Of course, it is difficult to predict a priori which AVF will require an intervention prior to successful use. However, our results suggest that the intervention rate is particularly high in women receiving an AVF. Conversely, the higher rate of AVG interventions after successful use may favor AVF in some patients, particularly those with longer life expectancy.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Portions of this manuscript were presented at the National Kidney Foundation spring clinical meeting in Dallas, TX on March 25-29, 2015.
References
- 1.KDOQI clinical practice guidelines and clinical practice recommendations for vascular access 2006. Am J Kidney Dis. 2006;48(suppl 1):S176–S322. doi: 10.1053/j.ajkd.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 2.Allon M. Current management of vascular access. Clin J Am Soc Nephrol. 2007;2:786–800. doi: 10.2215/CJN.00860207. [DOI] [PubMed] [Google Scholar]
- 3.Lynch JR, Wasse H, Armistead NC, McClellan WM. Achieving the goal of the Fistula First Breakthrough Initiative for prevalent maintenance hemodialysis patients. Am J Kidney Dis. 2011;57:78–89. doi: 10.1053/j.ajkd.2010.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lok CE, Allon M, Moist LM, Oliver MJ, Shah H, Zimmerman D. REDUCE FTM I (Risk equation determining unsuccessful cannulation events and failure to maturation in arteriovenous fistulas) J Am Soc Nephrol. 2006;17:3204–3212. doi: 10.1681/ASN.2006030190. [DOI] [PubMed] [Google Scholar]
- 5.Richardson AI, Leake A, Schmieder GC, Biuckians A, Stokes GK, Panneton JM, et al. Should fistulas really be first in the elderly patient? J Vasc Access. 2009;10(3):199–202. doi: 10.1177/112972980901000311. [DOI] [PubMed] [Google Scholar]
- 6.Peterson WJ, Barker J, Allon M. Disparities in fistula maturation persist despite preoperative vascular mapping. Clin J Am Soc Nephrol. 2008;3:437–441. doi: 10.2215/CJN.03480807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Falk A. Maintenance and salvage of arteriovenous fistulas. J Vasc Interv Radiol. 2006;17:807–813. doi: 10.1097/01.RVI.0000217928.43396.35. [DOI] [PubMed] [Google Scholar]
- 8.Asif A, Roy-Chaudhury P, Beathard GA. Early arteriovenous fistula failure: A logical proposal for when and how to intervene. Clin J Am Soc Nephrol. 2006;1:332–339. doi: 10.2215/CJN.00850805. [DOI] [PubMed] [Google Scholar]
- 9.Lee T, Ullah A, Allon M, Succop P, El-Khatib M, Munda R, et al. Decreased cumulative access patency in arteriovenous fistulas requiring interventions to promote maturation. Clin J Am Soc Nephrol. 2011;6:575–581. doi: 10.2215/CJN.06630810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allon M, Lockhart ME, Lilly RZ, Gallichio MH, Young CJ, Barker J, et al. Effect of preoperative sonographic mapping on vascular access outcomes in hemodialysis patients. Kidney Int. 2001;60:2013–2020. doi: 10.1046/j.1523-1755.2001.00031.x. [DOI] [PubMed] [Google Scholar]
- 11.Robbin ML, Gallichio ML, Deierhoi MH, Young CJ, Weber TM, Allon M. US vascular mapping before hemodialysis access placement. Radiology. 2000;217:83–88. doi: 10.1148/radiology.217.1.r00oc2883. [DOI] [PubMed] [Google Scholar]
- 12.Allon M, Lok CE. Dialysis fistula or graft: The role for randomized clinical trials. Clin J Am Soc Nephrol. 2010;5:2348–2354. doi: 10.2215/CJN.06050710. [DOI] [PubMed] [Google Scholar]
- 13.Maya ID, Oser R, Saddekni S, Barker J, Allon M. Vascular access stenosis: Comparison of arteriovenous grafts and fistulas. Am J Kidney Dis. 2004;44:859–865. [PubMed] [Google Scholar]
- 14.Allon M, Bailey R, Ballard R, Deierhoi MH, Hamrick K, Oser R, et al. A multidisciplinary approach to hemodialysis access: prospective evaluation. Kidney Int. 1998;53:473–479. doi: 10.1046/j.1523-1755.1998.00761.x. [DOI] [PubMed] [Google Scholar]
- 15.Liu J, Huang Z, Gilbertson DT, Foley RN, Collins AJ. An improved co-morbidity index for outcome analyses among dialysis patients. Kidney Int. 2010;77:141–151. doi: 10.1038/ki.2009.413. [DOI] [PubMed] [Google Scholar]
- 16.Chang CJ, Ko PJ, Hsu LA, Ko YS, Ko YL, Chen CF, et al. Highly increased cell proliferation activity in restenotic hemodialysis vascular access after percutaneous transluminal angioplasty: Implication in prevention of stenosis. Am J Kidney Dis. 2004;43:74–84. doi: 10.1053/j.ajkd.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 17.Asif A, Cherla G, Merrill D, Cipleu CD, Briones P, Pennell P. Conversion of tunneled hemodialysis catheter-consigned patients to arteriovenous fistula. Kidney Int. 2005;67:2399–2406. doi: 10.1111/j.1523-1755.2005.00347.x. [DOI] [PubMed] [Google Scholar]
- 18.Lok CE, Sontrop JM, Tomlinson G, Rajan DK, Cattral M, Oreopoulos G, et al. Cumulative patency of contemporary fistulas versus graft (2000–2010) Clin J Am Soc Nephrol. 2013;8:810–818. doi: 10.2215/CJN.00730112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maya ID, O'Neal JC, Young CJ, Barker-Finkel J, Allon M. Outcomes of brachiocephalic fistulas, transposed brachiobasilic fistulas, and upper arm grafts. Clin J Am Soc Nephrol. 2009;4:86–92. doi: 10.2215/CJN.02910608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oliver MJ, McCann RL, Indridason OS, Butterly DW, Schwab SJ. Comparison of transposed brachiobasilic fistulas to upper arm grafts and brachiocephalic fistulas. Kidney Int. 2001;60:1532–1539. doi: 10.1046/j.1523-1755.2001.00956.x. [DOI] [PubMed] [Google Scholar]
- 21.Miller CD, Robbin ML, Allon M. Gender differences in outcomes of arteriovenous fistulas in hemodialysis patients. Kidney Int. 2003;63:346–352. doi: 10.1046/j.1523-1755.2003.00740.x. [DOI] [PubMed] [Google Scholar]
- 22.Chang TI, Palk J, Greene T, Desai M, Bech F, Cheung AK, et al. Intradialytic hypotension and vascular access thrombosis. J Am Soc Nephrol. 2011;22:1526–1533. doi: 10.1681/ASN.2010101119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roy-Chaudhury P, Kelly BS, Zhang J, Narayana A, Desai P, Melham M, et al. Hemodialysis vascular access dysfunction: from pathophysiology to novel therapies. Blood Purif. 2003;21(1):99–110. doi: 10.1159/000067863. [DOI] [PubMed] [Google Scholar]
- 24.Shingarev R, Barker-Finkel J, Allon M. Association of hemodialysis central venous catheter use with ipsilateral arteriovenous access patency. Am J Kidney Dis. 2012;60:510–513. doi: 10.1053/j.ajkd.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Collins AJ, Foley RN, Gilbertson DT, Chen SC. The state of chronic kidney disease, ESRD, and morbidity and mortality in the first year of dialysis. Clin J Am Soc Nephrol. 2009;4(Suppl 1):S5–S11. doi: 10.2215/CJN.05980809. [DOI] [PubMed] [Google Scholar]