Abstract
Background
In patients with bilateral colorectal liver metastases (CLM) not resectable in one operation, 2-stage hepatectomy is the standard surgical approach. The objective of this study was to determine factors associated with safety and efficacy of 2-stage hepatectomy.
Study Design
The study included all 109 patients for whom two-stage hepatectomy for CLM was planned during 2003-2014. RAS mutation status and other clinicopathologic factors were evaluated for association with major complications and survival using multivariate analysis.
Results
Two-stage hepatectomy was completed in 89 of 109 patients (82%). Reasons for dropout after first stage were disease progression (n=12), insufficient liver growth (n=5), and complications after first stage or portal vein embolization (n=3). More than six cycles of preoperative chemotherapy were associated with failure to proceed to second stage (p=0.009). Rates of major complications (26% vs. 6%; p<0.001) and 90-day mortality (7% vs. 0%; p=0.006) were higher after second stage. The cumulative rate of major complications was 15% (n=29). Factors independently associated with major complications were rectal primary tumor, metachronous CLM, and more than one lesion resected at first stage. At median follow-up of 29.5 months, 3-year (68% vs. 6%; p<0.001) and 5-year overall survival rates (49% vs. 0%; p<0.001) were better after two-stage hepatectomy completion than noncompletion. Factors independently associated with poor overall survival were rectal primary tumor (p=0.044), more than five CLM (p=0.043), need for chemotherapy after first stage (p=0.046), and RAS mutation (p<0.001).
Conclusions
RAS mutation independently predicts the oncologic efficacy of two-stage hepatectomy and may help guide patient selection for this aggressive surgical strategy.
Introduction
Advances in chemotherapy and surgical techniques during the past decade have improved the prognosis of patients with colorectal liver metastases (CLM) considerably. Two-stage hepatectomy, first described in 2000, (1) in combination with systemic chemotherapy has become the standard of care for patients with bilateral CLM that cannot be resected in one operation because of insufficient volume of the future liver remnant.(2) The main problems with two-stage hepatectomy are morbidity rates of 20% to 59%, mortality rates of up to 7%, (2-8) and progression after first-stage resection resulting in noncompletion of second-stage resection in 20% of patients.(9)
During the past decade, mutations in the rat sarcoma viral oncogene homolog (RAS) have been found in 15% to 35% of patients with resectable CLM and have been associated with worse survival.(10-12) Clinical parameters initially reported as prognostic factors after CLM resection, including CLM size, number of CLM, and carcinoembryonic antigen level, have limited clinical value in the era of modern chemotherapy.(13) Clinicopathological score (3) and pathological response (14, 15) have been reported to be major prognostic factors but can only be evaluated postoperatively following examination of the resected surgical specimen. Unlike clinical parameters, RAS mutation appears to remain a reliable prognostic factor over time, even after interval chemotherapy, (16) and does not depend on pathological analysis of the resected surgical specimen. Recently, RAS mutation status was reported to be a major biological prognostic factor after liver resection for CLM.(17) To date, no study has evaluated the effect of RAS mutation status on outcome after two-stage hepatectomy.
Because of the low numbers of patients with bilateral CLM amenable to resection, it has been difficult to determine factors influencing postoperative outcomes and survival after two-stage hepatectomy. The purpose of our study was to determine factors associated with safety and oncologic efficacy after two-stage hepatectomy using a large institutional prospective database of patients undergoing resection of bilateral CLM.
Methods
Patient population
The Institutional Review Board of The University of Texas MD Anderson Cancer Center approved this study protocol (IRB 15-0203). A total of 1502 patients underwent surgical resection of CLM between November 2003 and September 2014 and had their clinicopathologic factors prospectively recorded in a liver-resection database. Of these patients, 109 were considered for two-stage hepatectomy. Their computerized medical records were queried for data on clinicopathologic factors, including RAS mutation status, treatment variables, perioperative details, pathologic response, recurrence, and survival.
RAS Mutation Profiling
DNA from CLM was used to determine RAS mutation status, as previously described.(12) Routine polymerase chain reaction-based primer extension assay was performed to screen for mutations in KRAS codons 12 and 13 in all patients and for mutations in KRAS codons 61 and 146 and NRAS codons 12, 13, and 61 in the majority of patients treated since 2012. The lower limit of detection of this assay was approximately one mutant allele in a background of nine wild-type alleles. Single mutations in the various codons of KRAS and NRAS were analyzed together and reported as RAS mutations.
Two-stage hepatectomy
Two-stage hepatectomy was considered for patients with advanced bilateral CLM who responded to chemotherapy when CLM could be completely resected with a future liver remnant volume of 20% to 30% of the total liver volume with adequate inflow and outflow.(18) Portal vein embolization (PVE) was performed before second-stage resection when the future liver remnant volume was deemed insufficient, as previously described.(19) Interval chemotherapy was not used routinely but was used in patients with progressive disease or insufficient growth of the future liver remnant after first-stage resection. For patients with progressive disease after first-stage resection, response was reevaluated after 2 months of chemotherapy, and if disease had remained stable or responded, second-stage resection was performed. For patients with insufficient growth of the future liver remnant, repeat PVE including segment IV or hepatic vein embolization was considered. Liver growth was reevaluated 4 weeks after repeat embolization, and if the future liver remnant was deemed sufficient at that time, second-stage resection was considered. For patients in whom two-stage hepatectomy was completed, adjuvant chemotherapy was recommended to complete a total of 12 cycles, including preoperative and postoperative chemotherapy.(20) Regarding the management of the primary tumor, a reverse staged approach was preferred, but a combined primary resection could be considered during the first stage. Extra-hepatic disease at the time of the first-stage hepatectomy was not considered a contraindication to liver resection if the extra-hepatic lesion was deemed resectable and at least stable after systemic chemotherapy.
Morbidity and mortality
Postoperative 90-day morbidity and mortality were prospectively recorded. Morbidity was graded according to the Dindo classification.(21) Postoperative hepatic insufficiency was defined as a peak total bilirubin level greater than 7 mg/dL.(22)
Statistical analysis
Quantitative and qualitative variables were expressed as median and range and percentage. Categorical variables were compared using the chi-square test, and continuous variables were compared using the Mann-Whitney U test. For the detection of factors associated with completion of second-stage resection or major complications, variables with p value of less than 0.1 from univariate analyses were entered into multivariate analysis using binary logistic regression with enter method.
Overall (OS) and progression-free survival (PFS) rates were calculated using the Kaplan-Meier method and compared using the log-rank test. Survival was calculated from the date of first-stage resection. PFS was calculated from the date of first-stage resection for patients who did not undergo second-stage resection and from the date of first progression for patients in whom two-stage hepatectomy was completed. Survival was analyzed on an intent-to-treat basis, including patients who died from surgery and patients who did not undergo second-stage resection. Variables with p value of less than 0.1 from univariate analyses were entered into Cox regression survival analyses with backward conditional method to assess predictors of survival. Completion of second-stage resection was highly correlated with OS and PFS and was not included in the multivariate model because of a linear dependence with other covariates. P<0.05 was considered statistically significant. Statistical analysis was performed using SPSS, version 22 (SPSS Inc., IBM, Chicago, IL).
Results
Patient characteristics
Clinicopathologic factors of the 109 patients for whom two-stage hepatectomy was planned are listed in Table 1. A major hepatectomy (>3 segments) was performed for 3% (3/109) of patients during the first-stage, and 82% (73/89) during the second-stage, among them 49 (55%) underwent an extended liver resection. Twenty patients (18%) who underwent first-stage resection did not undergo second-stage resection because of disease progression (n=12), insufficient future liver remnant volume (n=5), or complications after first-stage resection or PVE (n=3). Complications precluding second-stage resection were hepatic insufficiency after first-stage resection in 1 patient, portal hypertension after PVE in 1 patient, and portal vein thrombosis extending to the future liver remnant after PVE in 1 patient. Regarding extra-hepatic disease at time of first-stage, 13 presented lung metastases, 1 ovarian metastases, 1 peritoneal implant and 1 retrocaval nodes, and half of them could underwent a resection of the metastatic disease.
Table 1. Clinicopathologic Factors of 109 Patients for Whom 2-Stage Hepatectomy Was Planned.
| Characteristic | Only first-stage resection completed (n=20) | Second-stage resection completed (n=89) | p Value |
|---|---|---|---|
| Median age, y (range) | 51 (26-68) | 52 (23-72) | 0.416 |
| Sex, n (%) | 0.680 | ||
| Male | 14 (70) | 58 (65) | |
| Female | 6 (30) | 6 (30) | |
| Primary tumor location, n (%) | 0.642 | ||
| Colon | 16 (80) | 75 (84) | |
| Rectum | 4 (20) | 14 (16) | |
| Primary tumor nodal status, n (%) | 0.650 | ||
| Positive | 14 (70) | 58 (65) | |
| Negative | 2 (10) | 12 (14) | |
| Unknown | 4 (20) | 19 (21) | |
| Synchronous presentation of CLM, n (%) | 19 (95) | 74 (83) | 0.176 |
| Combined primary resection at first-stage*, n (%) | 4 (20) | 21 (24) | 0.730 |
| Extrahepatic metastases at time of first-stage resection, n (%) | 0 | 16 (18) | 0.040 |
| No. of CLM on preoperative imaging, median (range) | 9 (4-60) | 6 (2-25) | 0.004 |
| Median diameter of largest metastasis on preoperative imaging, mm (range) | 45 (18-120) | 29 (10-144) | 0.011 |
| Median no. of cycles of chemotherapy before first-stage resection (range) | 10 (3-26) | 6 (1-24) | 0.006 |
| Interval chemotherapy administered after first-stage resection, n (%) | 19 (95) | 39 (44) | <0.001 |
| Positive margin on first- or second-stage resection, n (%) | 2 (10) | 22 (25) | 0.150 |
| Portal vein embolization, n (%) | 18 (90) | 62 (70) | 0.063 |
| RAS mutation†, n (%) | 8 (40) | 32 (36) | 0.378 |
| Pathological response on final resection (first- or second-stage), n (%) | |||
| Complete | NA | 5 (5.5) | NA |
| Major | 22 (24.5) | ||
| Minor | 37 (42) | ||
| Unknown | 25 (28) |
In addition, only one patients underwent a combined primary resection at time of second stage.
Sixteen patients (15%) did not undergo evaluation of RAS mutation status, and no patient had NRAS mutations.
CLM, colorectal liver metastases.
Completion of two-stage hepatectomy
Patients who did not complete the two-stage hepatectomy presented significantly more extrahepatic metastases, larger size and number of metastases, more cycles of chemotherapy before FS, and need for chemotherapy after FS (table 1). When evaluating factors available after FS, on multivariate analysis, the only factor independently associated with noncompletion of two-stage hepatectomy was receipt of more than six cycles of chemotherapy before first-stage resection (Table 2). RAS mutation status was not associated with noncompletion of two-stage hepatectomy.
Table 2. Factors Predicting Completion of 2-Stage Hepatectomy.
| Factor | Univariate p value | Multivariate* | |||
|---|---|---|---|---|---|
| Odds ratio | 95% Confidence interval | p Value | |||
| Lower | Upper | ||||
| Rectal primary tumor | 0.643 | ||||
| Primary tumor nodal status positive | 0.651 | ||||
| Synchronous presentation of CLM | 0.205 | ||||
| Extrahepatic metastases | 0.998 | ||||
| > 5 CLM b | 0.055 | 2.026 | 0.467 | 8.785 | 0.346 |
| Diameter of largest metastasis > 5 cm† | 0.026 | 2.862 | 0.919 | 8.919 | 0.070 |
| > 6 cycles of chemotherapy before first-stage resection | 0.001 | 4.51 | 1.463 | 13.897 | 0.009 |
| Two lines of chemotherapy before first-stage resection | 0.238 | ||||
| Combined primary surgery at first-stage resection | 0.73 | ||||
| > 1 lesion resected at first-stage resection | 0.502 | ||||
| Portal vein embolization | 0.08 | 2.460 | 0.446 | 13.556 | 0.301 |
| RAS mutation | 0.381 | ||||
| Complete pathologic response at first-stage resection | 0.193 | ||||
| Major complication of first-stage resection | 0.059 | 2.859 | 0.379 | 21.576 | 0.308 |
CLM, colorectal liver metastases.
Binary logistic regression, using enter methods with 0.1 selection.
Based on computed tomography before chemotherapy.
Morbidity and mortality
Postoperative morbidity and mortality are summarized in Table 3. Major complications occurred after first-stage resection in 6 of 109 patients (6%) and after second-stage resection in 23 of 89 patients (26%). One patient suffered major complications after both first-stage and second-stage resection; therefore, 28 patients presented with major complications after first- or second-stage resection. Hepatic insufficiency, bile leak, major complications, need for interventional radiology procedure and death within 90 days after resection were significantly more frequent after second-stage resection than after first-stage resection. Factors independently associated with major complications when complications after first-stage and second-stage resection were analyzed together were rectal primary tumor, metachronous CLM, and more than one lesion resected during first-stage resection (Table 4). The same factors were independently associated with complications when the analysis was limited to complications after second-stage resection (data not shown).
Table 3. Postoperative Morbidity and Mortality after First- and Second-Stage Resection.
| Outcomes | First-stage resection (n=109) | Second-stage resection (n=89) | p Value |
|---|---|---|---|
| Postoperative hepatic insufficiency, n (%) | 1 (1) | 6 (7) | 0.030 |
| Bile leak, n (%) | 2 (2) | 11 (12) | 0.003 |
| Major complication, n (%) | 6 (6) | 23 (26) | <0.001 |
| Admission to intensive care unit, n (%) | 1 (1) | 4 (4.5) | 0.110 |
| Need for interventional radiology procedure, n (%) | 2 (2) | 19 (21) | <0.001 |
| Reoperation, n (%) | 2 (2) | 0 | 0.199 |
| Death < 30 d (%) | 0 | 2 (2) | 0.110 |
| Death < 90 d (%) | 0 | 6 (7) | 0.006 |
Table 4. Risk Factors for Major Complications with 2-Stage Hepatectomy.
| Risk factor | Univariate p value | Multivariate* | |||
|---|---|---|---|---|---|
| Odds ratio | 95% Confidence interval | p Value | |||
| Lower | Upper | ||||
| Rectal primary tumor | 0.052 | 3.904 | 1.248 | 12.211 | 0.019 |
| Primary tumor nodal status positive | 0.347 | ||||
| Synchronous presentation of CLM | 0.081 | 0.295 | 0.091 | 0.953 | 0.041 |
| > 5 CLM† | 0.295 | ||||
| Diameter of largest metastasis > 5 cm† | 0.701 | ||||
| > 6 cycles of chemotherapy before first-stage resection | 0.9 | ||||
| Bevacizumab before first-stage resection | 0.468 | ||||
| Interval chemotherapy after first-stage resection | 0.875 | ||||
| Combined primary surgery at first-stage resection | 0.763 | ||||
| Portal vein embolization | 0.474 | ||||
| > 1 lesion resected during first-stage resection | 0.028 | 3.594 | 1.313 | 9.842 | 0.013 |
| > 1 lesion resected during second-stage resection | 0.225 | ||||
| RAS mutation | 0.308 | ||||
Binary logistic regression, using enter method with 0.1 selection.
Based on computed tomography before chemotherapy.
CLM, colorectal liver metastases.
Survival
At a median follow-up time of 29.5 months (range, 2.5-135.5 months), median OS was 40 months (95% CI, 25-56 months). Median OS times for patients who did and did not undergo second-stage resection were 56.8 months and 20.4 months, respectively (p<0.001, Figure 1). Three- and 5-year survival rates were 68% and 49%, respectively, for patients who underwent second-stage resection and 6% and 0%, respectively, for patients who did not. Three- and 5-year survival were significantly better for patients who underwent second-stage resection (p<0.001). Median PFS for patients who underwent second-stage resection was 12.5 months.
Figure 1.
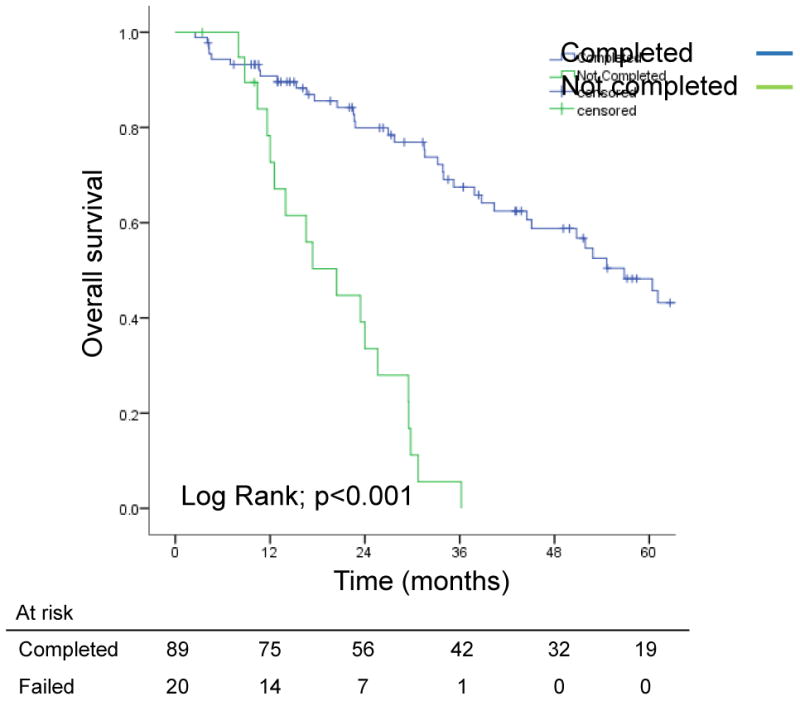
Overall survival from the date of first-stage resection in patients with bilateral colorectal liver metastases in whom 2-stage hepatectomy was and was not completed.
On intent-to-treat analysis, median OS times for patients with RAS mutated and RAS wild-type tumors were 33.9 months and 101.6 months, respectively (p<0.001; Figure 2). Similarly, median PFS was shorter for patients with RAS mutated tumors (9.2 vs. 12.2 months, p<0.006). Among patients who underwent second-stage resection, median OS times for patients with RAS mutated and RAS wild-type tumors were 44.5 months and 101.6 months, respectively (p<0.001), and 5-year survival rates were 12% and 67%, respectively (Figure 3a). Similarly, among patients who underwent second-stage resection, median PFS was shorter for patients with RAS mutated tumors (11 vs. 15.3 months, p=0.009; Figure 3b).
Figure 2.
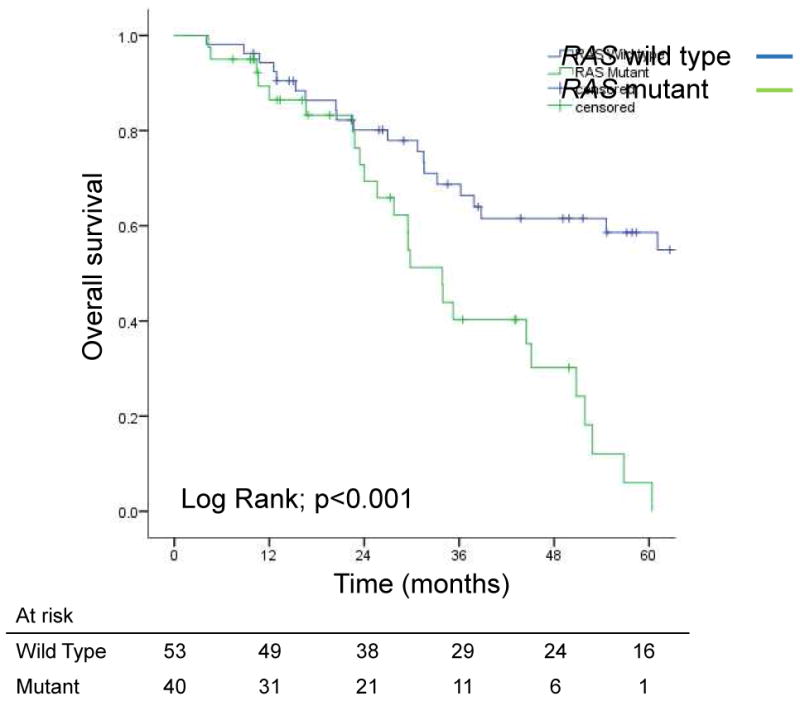
Overall survival on intent-to-treat from the date of first-stage resection in patients with bilateral colorectal liver metastases for whom 2-stage resection was planned, by RAS status.
Figure 3.
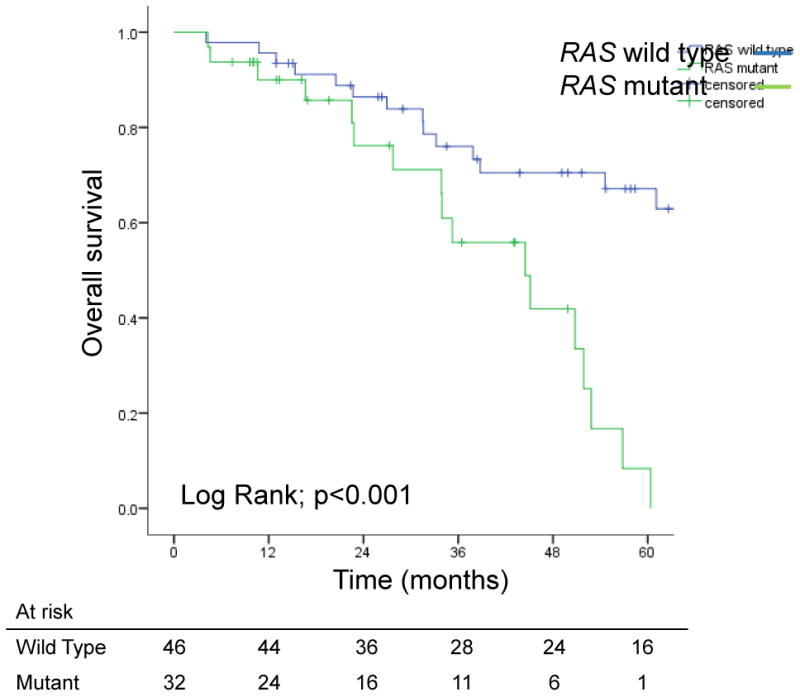

(A) Overall survival and (B) progression-free survival in patients with bilateral colorectal liver metastases who underwent second-stage resection, by RAS status.
For all patients, factors independently associated with poor OS were rectal primary tumor, more than five CLM, need for chemotherapy after first-stage resection, and RAS mutation (Table 5). For patients who underwent second-stage resection, RAS mutation was the only factor independently associated with OS (data not shown). Factors independently associated with poor PFS were synchronous CLM, more than six cycles of chemotherapy before first-stage resection, and RAS mutation (Table 5). For patients who underwent second-stage resection, RAS mutation was the only factor independently associated with PFS (data not shown).
Table 5. Univariate and multivariate analyses of overall and progression-free survival after two-stage hepatectomy (intent-to-treat analysis).
| Risk factor | Overall survival | Progression-free survival | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate p value | Multivariate* | Univariate p value | Multivariate* | ||||||||||||||||
| Odds ratio | 95% CI | p Value | Odds ratio | 95% CI | p Value | ||||||||||||||
| Lower | Upper | Lower | Upper | ||||||||||||||||
| Rectal primary tumor | 0.079 | 2.9 | 1.029 | 8.176 | 0.044 | 0.212 | |||||||||||||
| Primary tumor nodal status positive | 0.285 | 0.724 | |||||||||||||||||
| Synchronous presentation of CLM | 0.405 | 0.072 | 2.326 | 1.053 | 5.138 | 0.037 | |||||||||||||
| Extrahepatic metastases | 0.325 | 0.959 | |||||||||||||||||
| > 5 CLM† | 0.01 | 2.357 | 1.026 | 5.416 | 0.043 | 0.009 | 1.627 | 0.972 | 2.722 | 0.064 | |||||||||
| Diameter of largest metastasis > 5 cm† | 0.679 | 0.918 | |||||||||||||||||
| > 1 lesion resected at first-stage resection | 0.744 | 0.359 | |||||||||||||||||
| Positive margin on first- or second-stage resection | 0.857 | 0.3 | |||||||||||||||||
| > 6 cycles of chemotherapy before first-stage resection | 0.014 | 1.912 | 0.919 | 3.980 | 0.083 | 0.004 | 1.654 | 1.024 | 2.673 | 0.040 | |||||||||
| Two lines of chemotherapy before first-stage resection | 0.333 | 0.442 | |||||||||||||||||
| Interval chemotherapy after first-stage resection | 0.01 | 2.064 | 1.013 | 4.206 | 0.046 | 0.081 | 1.607 | 0.988 | 2.615 | 0.056 | |||||||||
| Portal vein embolization | 0.22 | 0.636 | |||||||||||||||||
| RAS mutation | <0.001 | 4.821 | 2.109 | 11.021 | <0.001 | 0.009 | 2.037 | 1.232 | 3.366 | 0.006 | |||||||||
| Complete pathological response after second-stage resection | 0.614 | 0.709 | |||||||||||||||||
| Major complication after first-stage resection | 0.113 | 0.084 | |||||||||||||||||
| Hepatic insufficiency after second-stage resection | 0.008 | 0.295 | |||||||||||||||||
| Major complication after second-stage resection | 0.014 | 0.176 | |||||||||||||||||
Cox regression, using backward conditional with 0.1 selection.
Based on computed tomography before chemotherapy.
CLM, colorectal liver metastases.
Discussion
Patients with bilateral CLM have a high risk of recurrence and poor survival; therefore, appropriate tools are needed for deciding which such patients are appropriate candidates for hepatic resection. The main finding of our study was the impact of RAS mutation status on survival for patients with bilateral CLM considered for two-stage hepatectomy. Patients with RAS mutation had significantly worse long-term survival than patients with RAS wild-type tumors. For patients who underwent second-stage resection, RAS mutation was the only factor independently associated with OS and PFS. A recent meta-analysis evaluating the impact of KRAS mutations in 1181 patients undergoing resection of CLM showed a negative impact of KRAS mutation on OS and PFS, irrespective of preoperative chemotherapy regimen. (17) In the studies analyzed, clinical and morphological parameters were considered, and KRAS mutations remained an independent predictor of OS and PFS. RAS mutation status is highly concordant between colorectal primary tumors and metastases (23, 24) and remains stable over time, even after interval chemotherapy.(16) Therefore, RAS mutation status can be determined in a biopsy specimen of the primary tumor or metastases, a primary tumor resection specimen, or the surgical specimen from first-stage hepatectomy. In this study, among patients in whom two-stage hepatectomy was completed, the only factor independently associated with OS was RAS mutation status, a fact that identifies RAS mutation status as a biologic selection factor that trumps other factors.
For patients with bilateral CLM that cannot be resected in one operation, two-stage hepatectomy offers the best chance for prolonged survival.(2) However, two-stage hepatectomy is a demanding treatment strategy that includes systemic chemotherapy, two consecutive hepatic resections, and in most patients interval PVE. Given this extensive and stressful treatment strategy, selection criteria are pivotal. In the past, selection factors for hepatectomy have included number and size of CLM, serum carcinoembryonic antigen level, and primary tumor nodal status.(2, 7, 8, 25) However, these traditional factors are less relevant with the use of effective perioperative chemotherapy. Our group previously demonstrated the importance of pathologic response to preoperative chemotherapy as a surrogate marker of tumor biology and predictor of survival after resection of CLM.(15) More recently, we identified specific radiographic response criteria that predict pathologic response, providing a powerful preoperative selection tool.(26) The current study highlights the evolution of selection tools to a biologic factor, RAS, which was the only factor independently associated with survival in patients with completed two-stage hepatectomy. Patients with RAS mutation had no hope of cure, as they all had recurrence within 18 months after first-stage resection. Furthermore, among all patients, only 1 patient with RAS mutation was alive 5 years after the first-stage, compared to 16 patients without RAS mutation. As such it defines 2 different populations of colorectal liver metastases patients: those who undergo palliative resection and those who benefit from curative resection.
This study affirms prior reports showing that two-stage hepatectomy offers oncologic benefits by allowing assessment of response to preoperative chemotherapy and avoiding the second-stage, major hepatectomy in patients with aggressive tumor biology. A prospective study evaluating 54 patients considered for two-stage hepatectomy reported a 20% rate of noncompletion of second-stage resection because of disease progression.(9) In our study, 18% of patients did not undergo second-stage resection, and these patients presented initially with significantly more and larger CLM, received more cycles of chemotherapy before first-stage resection, and were more likely to need interval chemotherapy. In multivariate analysis, more than six cycles of chemotherapy before first-stage resection was the only independent predictor of noncompletion of two-stage hepatectomy. These findings support a recent multi-institutional study of 130 patients that showed that tumor progression during preoperative chemotherapy predicted failure to complete two-stage hepatectomy.(25) At our institution, we use response to chemotherapy as a selection factor for patients considered for two-stage hepatectomy.(2) Chemosensitivity appears to be a major predictor of completion of two-stage hepatectomy, but better predictors of response to chemotherapy need to be determined.
One-stage hepatectomy (27, 28) and Associating Liver Partition and Portal Vein Ligation for Staged Hepatectomy (ALPPS) (29) have both been proposed to decrease the risk of dropout after first-stage resection. In a study by Torzilli et al, one-stage hepatectomy for bilateral CLM was associated with no local recurrences. However, this study included only 29 patients and had a short median follow-up of 14 months. To increase resectability of bilateral CLM in one-stage hepatectomy, concomitant radiofrequency ablation has been performed.(28) A recent case-matched study of one-stage hepatectomy with radiofrequency ablation compared to two-stage hepatectomy reported comparable OS and PFS.(30) However, patients treated with one-stage hepatectomy received significantly more postoperative intra-arterial chemotherapy than patients treated with two-stage hepatectomy, which could bias the results. For solitary CLM, the local recurrence rate after radiofrequency ablation is significantly higher than after liver resection, (31) and a recent study suggested limiting radiofrequency ablation to solitary, small CLM (<2 cm).(32) Regarding ALPPS, the mortality rate remains elevated, and no long-term outcomes have yet been reported.(33)
We were somewhat surprised that our findings did not show significant improvement in postoperative morbidity and mortality rates compared to the findings from our prior report on two-stage hepatectomy, which covered the years 2002-2010(2). However, the kinetic growth rate, defined as the degree of hypertrophy divided by number of weeks elapsed after PVE, has only recently been routinely used to avoid postoperative hepatic insufficiency, and we expect future improvements in complication rates resulting from routine use of this measure.(34)
A limitation of this study is the fact that methods for RAS determination have changed over time; mutations in KRAS codons 61 and 146 and NRAS codons 12, 13, and 61 were evaluated only in recent years. It is likely that a number of patients with RAS mutation were included among patient without RAS mutation but these are rare mutations and their inclusions would only have increased the observed differences in survival between groups.
In conclusion, this study demonstrates the importance of RAS as a biological marker to select patients with bilateral CLM for surgical treatment; the long-term survival benefit of two-stage hepatectomy is limited in patients with RAS mutation. On the other hand, in patients with RAS wild-type tumors, the 5-year OS rate of 67% underscores the benefit of two-stage hepatectomy in patients with bilateral CLM previously considered borderline resectable. Further studies evaluating predictors of chemosensitivity and more effective systemic agents for patients with RAS mutated tumors are needed to improve patient outcomes after two-stage hepatectomy.
Acknowledgments
The authors particularly thank Stephanie Deming (Department of Scientific Publications, MD Anderson Cancer Center) for copyediting the manuscript, and Ruth J Haynes (Department of Surgical Oncology, MD Anderson Cancer Center) for secretarial assistance in the preparation of the manuscript.
Support: This research was supported in part by the National Institutes of Health through MD Anderson's Cancer Center Support Grant, CA016672. Dr Passot was supported by the French Association of Surgery.
Abbreviations
- CLM
colorectal liver metastases
- OS
overall survival
- PFS
progression-free survival
- PVE
portal vein embolization
Footnotes
Disclosure Information: Nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Adam R, Laurent A, Azoulay D, et al. Two-stage hepatectomy: A planned strategy to treat irresectable liver tumors. Ann Surg. 2000;232:777–785. doi: 10.1097/00000658-200012000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brouquet A, Abdalla EK, Kopetz S, et al. High survival rate after two-stage resection of advanced colorectal liver metastases: response-based selection and complete resection define outcome. J Clin Oncol. 2011;29:1083–1090. doi: 10.1200/JCO.2010.32.6132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Faitot F, Soubrane O, Wendum D, et al. Feasibility and survival of 2-stage hepatectomy for colorectal metastases: definition of a simple and early clinicopathologic predicting score. Surgery. 2015;157:444–453. doi: 10.1016/j.surg.2014.09.033. [DOI] [PubMed] [Google Scholar]
- 4.Muratore A, Zimmitti G, Ribero D, et al. Chemotherapy between the first and second stages of a two-stage hepatectomy for colorectal liver metastases: should we routinely recommend it? Ann Surg Oncol. 2012;19:1310–1315. doi: 10.1245/s10434-011-2069-5. [DOI] [PubMed] [Google Scholar]
- 5.Narita M, Oussoultzoglou E, Jaeck D, et al. Two-stage hepatectomy for multiple bilobar colorectal liver metastases. Br J Surg. 2011;98:1463–1475. doi: 10.1002/bjs.7580. [DOI] [PubMed] [Google Scholar]
- 6.Tsai S, Marques HP, de Jong MC, et al. Two-stage strategy for patients with extensive bilateral colorectal liver metastases. HPB (Oxford) 2010;12:262–269. doi: 10.1111/j.1477-2574.2010.00161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turrini O, Ewald J, Viret F, et al. Two-stage hepatectomy: who will not jump over the second hurdle? Eur J Surg Oncol. 2012;38:266–273. doi: 10.1016/j.ejso.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 8.Wicherts DA, Miller R, de Haas RJ, et al. Long-term results of two-stage hepatectomy for irresectable colorectal cancer liver metastases. Ann Surg. 2008;248:994–1005. doi: 10.1097/SLA.0b013e3181907fd9. [DOI] [PubMed] [Google Scholar]
- 9.Dupre A, Lefranc A, Buc E, et al. Use of bioresorbable membranes to reduce abdominal and perihepatic adhesions in 2-stage hepatectomy of liver metastases from colorectal cancer: results of a prospective, randomized controlled phase II trial. Ann Surg. 2013;258:30–36. doi: 10.1097/SLA.0b013e3182854949. [DOI] [PubMed] [Google Scholar]
- 10.Margonis GA, Spolverato G, Kim Y, et al. Effect of KRAS Mutation on Long-Term Outcomes of Patients Undergoing Hepatic Resection for Colorectal Liver Metastases. Ann Surg Oncol. 2015 doi: 10.1245/s10434-015-4587-z. [DOI] [PubMed] [Google Scholar]
- 11.Karagkounis G, Torbenson MS, Daniel HD, et al. Incidence and prognostic impact of KRAS and BRAF mutation in patients undergoing liver surgery for colorectal metastases. Cancer. 2013;119:4137–4144. doi: 10.1002/cncr.28347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vauthey JN, Zimmitti G, Kopetz SE, et al. RAS mutation status predicts survival and patterns of recurrence in patients undergoing hepatectomy for colorectal liver metastases. Ann Surg. 2013;258:619–626. doi: 10.1097/SLA.0b013e3182a5025a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zakaria S, Donohue JH, Que FG, et al. Hepatic resection for colorectal metastases: value for risk scoring systems? Ann Surg. 2007;246:183–191. doi: 10.1097/SLA.0b013e3180603039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rubbia-Brandt L, Giostra E, Brezault C, et al. Importance of histological tumor response assessment in predicting the outcome in patients with colorectal liver metastases treated with neo-adjuvant chemotherapy followed by liver surgery. Ann Oncol. 2007;18:299–304. doi: 10.1093/annonc/mdl386. [DOI] [PubMed] [Google Scholar]
- 15.Blazer DG, 3rd, Kishi Y, Maru DM, et al. Pathologic response to preoperative chemotherapy: a new outcome end point after resection of hepatic colorectal metastases. J Clin Oncol. 2008;26:5344–5351. doi: 10.1200/JCO.2008.17.5299. [DOI] [PubMed] [Google Scholar]
- 16.Kawamoto Y, Tsuchihara K, Yoshino T, et al. KRAS mutations in primary tumours and post-FOLFOX metastatic lesions in cases of colorectal cancer. Br J Cancer. 2012;107:340–344. doi: 10.1038/bjc.2012.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brudvik KW, Kopetz SE, Li L, et al. Meta-analysis of KRAS mutations and survival after resection of colorectal liver metastases. Br J Surg. 2015 doi: 10.1002/bjs.9870. [DOI] [PubMed] [Google Scholar]
- 18.Shindoh J, Chun YS, Loyer EM, Vauthey JN. Non-size-based response criteria to preoperative chemotherapy in patients with colorectal liver metastases: the morphologic response criteria. Curr Colorectal Cancer Rep. 2013;9:198–202. doi: 10.1007/s11888-013-0164-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shindoh J, Tzeng CW, Aloia TA, et al. Safety and efficacy of portal vein embolization before planned major or extended hepatectomy: an institutional experience of 358 patients. J Gastrointest Surg. 2014;18:45–51. doi: 10.1007/s11605-013-2369-0. [DOI] [PubMed] [Google Scholar]
- 20.Kopetz S, Vauthey JN. Perioperative chemotherapy for resectable hepatic metastases. Lancet. 2008;371:963–965. doi: 10.1016/S0140-6736(08)60429-8. [DOI] [PubMed] [Google Scholar]
- 21.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mullen JT, Ribero D, Reddy SK, et al. Hepatic insufficiency and mortality in 1,059 noncirrhotic patients undergoing major hepatectomy. J Am Coll Surg. 2007;204:854–862. doi: 10.1016/j.jamcollsurg.2006.12.032. [DOI] [PubMed] [Google Scholar]
- 23.Italiano A, Hostein I, Soubeyran I, et al. KRAS and BRAF mutational status in primary colorectal tumors and related metastatic sites: biological and clinical implications. Ann Surg Oncol. 2010;17:1429–1434. doi: 10.1245/s10434-009-0864-z. [DOI] [PubMed] [Google Scholar]
- 24.Knijn N, Mekenkamp LJ, Klomp M, et al. KRAS mutation analysis: a comparison between primary tumours and matched liver metastases in 305 colorectal cancer patients. Br J Cancer. 2011;104:1020–1026. doi: 10.1038/bjc.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giuliante F, Ardito F, Ferrero A, et al. Tumor progression during preoperative chemotherapy predicts failure to complete 2-stage hepatectomy for colorectal liver metastases: results of an Italian multicenter analysis of 130 patients. J Am Coll Surg. 2014;219:285–294. doi: 10.1016/j.jamcollsurg.2014.01.063. [DOI] [PubMed] [Google Scholar]
- 26.Shindoh J, Loyer EM, Kopetz S, et al. Optimal morphologic response to preoperative chemotherapy: an alternate outcome end point before resection of hepatic colorectal metastases. J Clin Oncol. 2012;30:4566–4572. doi: 10.1200/JCO.2012.45.2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Torzilli G, Procopio F, Botea F, et al. One-stage ultrasonographically guided hepatectomy for multiple bilobar colorectal metastases: a feasible and effective alternative to the 2-stage approach. Surgery. 2009;146:60–71. doi: 10.1016/j.surg.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 28.Elias D, Baton O, Sideris L, et al. Hepatectomy plus intraoperative radiofrequency ablation and chemotherapy to treat technically unresectable multiple colorectal liver metastases. J Surg Oncol. 2005;90:36–42. doi: 10.1002/jso.20237. [DOI] [PubMed] [Google Scholar]
- 29.Schnitzbauer AA, Lang SA, Goessmann H, et al. Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling 2-staged extended right hepatic resection in small-for-size settings. Ann Surg. 2012;255:405–414. doi: 10.1097/SLA.0b013e31824856f5. [DOI] [PubMed] [Google Scholar]
- 30.Faitot F, Faron M, Adam R, et al. Two-stage hepatectomy versus 1-stage resection combined with radiofrequency for bilobar colorectal metastases: a case-matched analysis of surgical and oncological outcomes. Ann Surg. 2014;260:822–827. doi: 10.1097/SLA.0000000000000976. [DOI] [PubMed] [Google Scholar]
- 31.Aloia TA, Vauthey JN, Loyer EM, et al. Solitary colorectal liver metastasis: resection determines outcome. Arch Surg. 2006;141:460–466. doi: 10.1001/archsurg.141.5.460. [DOI] [PubMed] [Google Scholar]
- 32.Lee H, Heo JS, Cho YB, et al. Hepatectomy vs radiofrequency ablation for colorectal liver metastasis: A propensity score analysis. World J Gastroenterol. 2015;21:3300–3307. doi: 10.3748/wjg.v21.i11.3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schadde E, Schnitzbauer AA, Tschuor C, et al. Systematic Review and Meta-Analysis of Feasibility, Safety, and Efficacy of a Novel Procedure: Associating Liver Partition and Portal Vein Ligation for Staged Hepatectomy. Ann Surg Oncol. 2014 doi: 10.1245/s10434-014-4213-5. [DOI] [PubMed] [Google Scholar]
- 34.Shindoh J, Truty MJ, Aloia TA, et al. Kinetic growth rate after portal vein embolization predicts posthepatectomy outcomes: toward zero liver-related mortality in patients with colorectal liver metastases and small future liver remnant. J Am Coll Surg. 2013;216:201–209. doi: 10.1016/j.jamcollsurg.2012.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]


