Abstract
Objective
Although reasoning and attention are two cognitive processes necessary for ensuring the efficiency of many everyday activities in older adults, the role of white matter integrity in these processes has been little studied. This is an important question due to the role of white matter integrity as a neural substrate of cognitive aging. Here, we sought to examine the white matter tracts subserving reasoning and visuospatial attention in healthy older adults.
Method
Sixty-one adults aged 60 and older completed a battery of cognitive tests to assess reasoning and visuospatial attention. In addition, diffusion tensor images were collected to assess Fractional Anisotropy (FA) – a measure of white matter integrity. A principle component analysis of the test scores yielded two components: reasoning and visuospatial attention. Whole-brain correlations between FA and the cognitive components were submitted to probabilistic tractography analyses for visualization of cortical targets of tracts.
Results
For reasoning, bilateral thalamo-anterior prefrontal, anterior corpus callosum, and corpus callosum body tracts interconnecting the superior frontal cortices and right cingulum bundle were found. For visuospatial attention, a right inferior fronto-parietal tract, and bilateral parietal and temporal connections were found.
Conclusions
We conclude that in older adults, prefrontal cortex white matter tracts and interhemispheric communication are important in higher order cognitive functioning. On the other hand, right-sided fronto-parietal tracts appear to be critical for supporting control of cognitive processes, such as redirecting attention. Researchers may use our results to develop neuroscience-based interventions for older adults targeting brain mechanisms involved in cognitive plasticity.
Keywords: aging, diffusion tensor imaging, reasoning, visuospatial attention, white matter integrity
1.0 INTRODUCTION
It is well known that cognitive functioning declines, on average, in healthy older adults. Despite this knowledge, the neural substrate of age-related cognitive decline is not fully understood. There are age-related changes in gray matter volume (Raz et al., 1997) and cortical thickness (Fjell et al., 2006), but white matter integrity is the only brain measure to be reliably associated with age-related cognitive change (Bennett & Madden, 2014; Greenwood, 2007; Madden, Bennett, & Song, 2009; Ziegler et al., 2010). In order to identify the substrate of cognitive aging, we sought to understand the relation between white matter integrity and two cognitive abilities that are particularly important for normal functioning in daily life–reasoning and visuospatial attention.
1.1 Reasoning/Visuospatial Attention and Cognitive Aging
Reasoning is important in a number of everyday activities of older people, including medical and financial decision-making needed to maintain independence (Agarwal, Driscoll, Gabaix, & Laibson, 2009; Boyle et al., 2012; Moye & Marson, 2007). A large literature demonstrates that reasoning abilities decline with age (e.g., Salthouse, 2005), leaving older adults impaired at these important everyday activities. Attention, and in particular visuospatial attention, is also important in many everyday activities, notably in driving (Ball, Owsley, Sloane, Roenker, & Bruni, 1993; Uc et al., 2006), finding objects (Greenwood, Lambert, Sunderland, & Parasuraman, 2005), spatial navigation (Moffat, Elkins, & Resnick, 2006), and even playing bingo (Laudate et al., 2012). Similar to reasoning, visuospatial attention declines with age (e.g., Greenwood & Parasuraman, 2004; Greenwood, Parasuraman, & Haxby, 1993), leaving older adults impaired at these and many other important everyday activities. With the possibility that a decline in reasoning and visuospatial attention has detrimental effects on the everyday functioning of older adults, and with the increasing proportion of older adults in the United States and in many other countries (United Nations, 2013), it is important to better understand reasoning and visuospatial attention in older adults with a view to developing techniques to improve these cognitive processes.
Although both reasoning and visuospatial attention decline with age (Greenwood et al., 1993; Mitchell et al., 2013), both cognitive processes have also been shown to be amenable to change following cognitive training – one of the few promising interventions shown to enhance older adults' cognitive function (Klingberg, 2010; Mackey, Whitaker, & Bunge, 2012; Rebok et al., 2014; Strenziok et al., 2014; Tang & Posner, 2009; Willis et al., 2006). Training-induced cognitive change in older adults was accompanied by altered white matter integrity (Engvig et al., 2012; Lövdén et al., 2010; Strenziok et al., 2014), further pointing to the importance of white matter integrity in old age. Although training has been aimed at other cognitive processes (e.g., working memory [Takeuchi et al., 2010] and perception [Berry et al., 2010; Strenziok et al., 2014]), there has yet to be a neuroscience-based cognitive training intervention specifically targeting reasoning and visuospatial attention. To advance the goal of designing training interventions that help improve reasoning and visuospatial attention in older adults, presumably by means of increased white matter integrity as has been shown for working memory (Takeuchi et al., 2010), it is important to know the specific white matter tracts underlying communication in brain circuits that subserve these cognitive processes. Although there is substantial evidence concerning the brain regions important for reasoning and visuospatial attention in young adults, few studies have examined the white matter tracts associated with reasoning and visuospatial attention in older adults. This weakness in the literature limits the ability of clinicians and researchers to develop neuroscience-based reasoning and visuospatial attention training interventions in older adults.
1.2 Brain Organization Underlying Reasoning
What is the evidence that there are age-related changes in the brain organization underlying reasoning? For gray matter, the only relevant study found age-related functional magnetic resonance imaging (fMRI) activation changes during reasoning in bilateral frontopolar, temporal, and cerebellar cortices (Esposito, Kirkby, Van Horn, Ellmore, & Berman, 1999). There have been many other reasoning-fMRI studies, but not within the context of aging. A recent meta-analysis of 28 fMRI studies in young adults pointed to a primarily left-lateralized network of gray matter regions important for deductive reasoning, including the left lateral and medial frontal cortices, parietal cortex, and basal ganglia (Prado, Chadha, & Booth, 2011). However, white matter tracts important for reasoning have only been studied within older adults to date. For white matter, a previous diffusion tensor imaging (DTI) study in older adults suggested that performance on the Primary Mental Abilities analogical reasoning test (Thurstone & Thurstone, 1949) depends on (a) left white matter tracts connecting the prefrontal cortex (PFC) with the thalamus via the anterior corona radiata, (b) fibers connecting left inferior PFC with medial temporal regions via the uncinate fasciculus, and (c) left anterior corpus callosum fibers interconnecting the PFC bilaterally (Borghesani et al., 2013). Strenziok, Greenwood, Santa Cruz, Thompson, and Parasuraman (2013) previously showed that matrix reasoning performance depends primarily on the white matter integrity of corpus callosum body fibers that subserve bilateral rostral and caudal dorsolateral prefrontal cortices in middle-aged and older adults. They also found a widespread prefrontal white matter network extending into the premotor cortex, superior and inferior parietal cortices, left posterior parahippocampalgyrus, bilateral anterior corona radiata, and bilateral splenium of the corpus callosum. Another group found that matrix reasoning depends on widespread white matter tracts, including bilateral prefrontal, temporal, parietal, and occipital regions in middle-aged and older adults (Haász et al., 2013).
Despite this previous work, there remain important, unresolved questions. The existing literature has not clarified the role of bilateral connections underlying reasoning in older adults. Regardless of age, the literature agrees on a role for PFC gray and white matter structures in reasoning, but some studies suggest the importance of left-lateralized intrahemispheric communication (Borghesani et al., 2013; Prado et al., 2011) and left-lateralized cortico-subcortical communication involving the PFC (Borghesani et al., 2013; Prado et al., 2011). In middle-aged and older adults, some studies found a role for both left and right-sided white matter tracts, as well as interconnecting corpus callosum fibers (Borghesani et al., 2013; Haász et al., 2013; Strenziok et al., 2013). There is also functional imaging evidence of the importance of bilateral engagement in older people seen in increased bilateral brain activation during reasoning (Esposito et al., 1999) and other higher order cognitive functions (for reviews see Cabeza, 2002; Park & Reuter-Lorenz, 2009). Such findings have been interpreted as signaling a general brain aging mechanism of either dedifferentiation of brain networks (Li & Lindenberger, 1999) or compensation through engagement of bilateral structures with aging (Cabeza, 2002). In order to advance knowledge about white matter tracts important for reasoning in older adults, it is important to address this question of lateralization of function in white matter tracts. Another weakness in the existing literature is that previous DTI studies selected reasoning measures idiosyncratically, which limits the applicability of their findings to one aspect of reasoning. Finally, in light of the importance of reasoning to the daily lives of older people, it is important to assess those abilities broadly, including in everyday reasoning performance.
1.3 Brain Organization Underlying Visuospatial Attention
Regarding visuospatial attention, abundant evidence in young adults suggests that it relies on a dorsal fronto-parietal network (Bressler, Tang, Sylvester, Shulman, & Corbetta, 2008; Corbetta & Shulman, 2002). For gray matter, the fMRI visual search literature shows lower occipital and temporal, but greater fronto-parietal cortex activation in older adults (Madden et al., 2007; Madden et al., 2002). For white matter, results are less clear. Findings from a DTI study in young and middle-aged adults suggest that dorsal fibers connecting the temporo-parietal and prefrontal cortices, and ventral fibers connecting the temporo-parietal, prefrontal, and insular cortices are important in visuospatial attention (Umarova et al., 2010). However, only right-sided white matter structures were assessed, limiting conclusions. Madden et al. (2007) measured white matter integrity in older people during visual search, but used a biased approach by limiting their analysis to regions of interest. They found that white matter integrity in the superior parietal lobe did not mediate the relationship between parietal activation and visual search performance. Overall, given the limitations of the existing literature, the white matter tracts important for visuospatial attention in older adults remain unclear.
1.4 Current Study
The goal of the present study was to investigate the white matter tracts subserving reasoning and visuospatial attention in older adults. Clinically, this may further the development of neuroscience-based reasoning and visuospatial attention training interventions for older adults. Here, we examined the relation between white matter integrity and performance on a series of tasks designed to assess reasoning and visuospatial attention. Correlations were computed between (a) reasoning and visuospatial attention factors derived from a principal component analysis (PCA) and (b) an index of white matter integrity. To determine likely cortical targets of white matter tracts that subserve reasoning and visuospatial attention, results from the correlations were submitted to probabilistic tractography analyses. Based on the existing literature, our hypothesis predicted the following for our sample: (a) reasoning varies with the integrity of anterior corpus callosum fibers interconnecting the bilateral PFC (Borghesani et al., 2013; Cabeza, 2002; Esposito et al., 1999; Strenziok et al., 2013); (b) visuospatial attention varies with the integrity of white matter tracts connecting the temporo-parietal cortex dorsally to the prefrontal cortex and ventrally to the prefrontal and insular cortices (Umarova et al., 2010). Given the increased dependence on bilateral brain activation in cognitive processing in healthy older adults and indications that visuospatial attention relies on brain activation in both hemispheres, at least in young but perhaps also in older people (Corbetta, Patel, & Shulman, 2008; Corbetta & Shulman, 2002), we predicted that bilateral fronto-parieto-temporal tracts would be important for visuospatial attention in healthy older adults.
2.0 METHODS
2.1 Sample
Sixty-one healthy adults aged 60 to 83 (mean = 69.2, SD = 5.3) years were recruited for this study. Participants were screened with a self-report questionnaire for diseases or injury with neurological implications such as stroke, head injury, psychiatric conditions, and diabetes. The Mini Mental State Examination (MMSE) was used to screen for possible cognitive dysfunction (Folstein, Folstein, & McHugh, 1975); individuals with scores lower than 24 were excluded based on the Alzheimer’s Disease Neuroimaging Initiative’s standards. The Beck Depression Inventory was administered to assess for signs of clinical depression (Beck, Ward, Mendelson, Mock, & Erbaugh, 1961); individuals with scores higher than 18 were excluded. In addition, significant injuries and brain pathology were assessed by a neuroradiologist using T2-weighted FLAIR MRI images sensitive to lesions. No exclusions were necessary based on these criteria. The study group was predominantly female (60.7%) and right-handed (85.2%). Racially, the group included 95.1% White (n = 58), 1.6% Asian (n = 1), 1.6% African-American (n = 1), and 1.6% other (n = 1) participants. All study procedures were approved by the George Mason University Institutional Review Board. Prior to testing, written informed consent was obtained from each participant.
2.2 Neuropsychological and Laboratory Assessment
A battery of neuropsychological and laboratory tests was administered to examine reasoning and visuospatial attention. Reasoning was assessed with the Matrix Reasoning test of the Wechsler Adult Intelligence Scale-III (WAIS-III; Wechsler, 1997), Letter/Word Series tests of the Schaie-Thurstone Adult Mental Abilities Test (STAMAT; Schaie, 1985), and the Everyday Problems Test (EPT; Willis & Marsiske, 1993). In the Matrix Reasoning test, participants were shown a partially completed design and were asked to select from five choices the one part that would complete the whole design. The number of correct trials was recorded. In the Letter and Word Series tasks, participants were presented with either a series of letters or words and were asked to infer and choose the letter or word that should come next in the series based on the pattern. The total number of correct responses was recorded to compute percentage of correct trials. In the EPT, participants were presented with a series of everyday tasks and were asked to answer questions and make inferences based upon them. The total number of correct responses (accuracy) and time to respond were retained for further analyses.
Visuospatial attention was assessed with a Posner-type cued attention task (Greenwood, Sunderland, Friz, & Parasuraman, 2000) and a visuospatial working memory task (Greenwood et al., 2005). In the cued attention task (Greenwood et al., 2000), participants were presented with a fixation cross on a computer screen (displayed for 500 ms) followed by a centered location cue (an arrow pointing to the left, right, or in both directions). In the valid cues condition (62.5% of trials), the cue correctly predicted the target location. In the invalid cues condition (18.75% of trials), the cue incorrectly predicted the target location. In the neutral cues condition (18.75% of trials), both the left- and right-pointed arrows appeared providing no indication of target location. The centered location cue appeared for a variable cue-target stimulus onset asynchrony (SOA) of 200, 500, or 2000 ms, followed by a letter target appearing 6.7° (visual angle) to the right or left of fixation. A speeded response was required, identifying the target as either a consonant or a vowel by pressing one of two response buttons within 2000 ms. Total cue validity reaction time (invalidly cued response time – validly cued response time) of the cued attention task with a 2s cue-target SOA was the measure of interest.
In the visuospatial working memory task (Greenwood et al., 2005), a fixation cross appeared for 1s followed by either one, two, or three black dots (0.67 degrees in diameter) that were displayed for 500 ms in randomly chosen locations. Next, a fixation cross appeared alone during a 3s delay. After the delay period, a single red test dot appeared on the screen either in the same location as one of the target dots (match) or at a different location (non-match, target-test distance 2, 4, or 8 degrees of visual angle). Participants were asked to indicate whether or not the location of the test dot matched the location of one of the target dots by pressing one of two response buttons (same or different location). Percentage correct of the visuospatial working memory task under the three-dot load and 1 cm (0.67 degrees in diameter) distance between the test dot and target dot was the measure of interest.
2.3 MRI Data Acquisition
A Siemens Allegra 3 Tesla head-only MRI scanner equipped with a standard quadrature transmit-receive head coil was used to collect diffusion-weighted MRI images. A single-shot EPI sequence with 12 gradient directions (b-value = 1000 s/mm2; TE = 75 ms; TR = 10000 ms; 50 slices; slice thickness = 3 mm; acquisition matrix = 128 mmx 128 mm) and 4 repetitions was used to acquire axial images. The four repetitions of our DTI sequence were used to increase the signal-to-noise ratio in the acquired diffusion-weighted images.
2.4 Principal Component Analysis
SPSS Statistics 20.0 was used to perform the PCA with varimax rotation to obtain cognitive components to be used as input data for the brain-cognition correlation analyses. The PCA was computed on all the measures collected in the current study. These were the Matrix Reasoning raw score, average of the percentage correct of the Letter and Word Series tasks, percentage correct of the EPT, total cue validity reaction time (invalidly cued response time – validly cued response time) of the cued attention task with a 2s variable cue-target SOA, and percentage correct of the visuospatial working memory task under the three dot load and 1 cm (0.67 degrees in diameter) distance between test dot and target dot. An extraction criterion of eigenvalues larger than 1 was used.
2.5 Diffusion Imaging Data Processing
Diffusion image processing was performed using FSL 4.1.8. (Smith et al., 2004). Preprocessing included head movement and eddy currents correction and voxel-wise fitting of the diffusion tensor model yielding FA maps for each participant (Smith et al., 2006). FA values assess the directionality of diffusion, with a FA value of zero indicating non-directional (isotropic) diffusion and a value of one indicating entirely directional (anisotropic) diffusion. Tract-based spatial statistics (TBSS; Smith et al., 2006) included non-linear registration of individual FA maps to the FMRIB58-FA standard space and linear transformation of registration parameters into 1 mm isotropic MNI152-TI-1 mm space. Normalized FA maps were averaged across subjects and thresholded at 0.2 resulting in a white matter skeleton, which was fed into voxel-wise covariance analyses.
2.6 Statistical Analyses of Fractional Anisotropy
The skeletonized mean FA was subjected to a one-sample voxel-wise whole brain t-test (general linear model, GLM). Two cognitive components – reasoning and visuospatial attention – derived from the PCA were included as predictors in the GLM. Age, sex, and z-transformed number of years of education served as nuisance predictors. MMSE scores were not included in this analysis because this screening instrument yields ceiling effects. Contrasts were computed to assess positive and negative FA-cognition components correlations. Correlation maps corrected for multiple comparisons were obtained using FSL’s randomise tool and the family-wise error (FWE) correction method on threshold-free cluster-enhanced images. For anatomic reference, correlation maps were overlaid onto a rendered brain volume (MNI152_TI_1 mm). In order to label white matter tracts that yielded significant correlations between FA and cognition, the Juelich histological atlas (Eickhoff et al., 2005), JHU ICBM-DTI-81 white matter labels (Mori, Wakana, Van Zijl, & Nagae-Poetscher, 2005), and JHU white-matter tractography atlas (Hua et al., 2008) were used.
2.7 Probabilistic Fiber Tractography Analyses
In order to visualize both (a) the white matter tracts that were found to be associated with the reasoning and visuospatial attention components and (b) the cortical targets of those tracts, probabilistic fiber tractography analyses were conducted. This information is not captured in the FA-cognitive component correlations. The tractography analysis was intended to be purely descriptive. Locations of voxels with the highest signal intensity values in the FA-cognition component correlation maps were chosen as seeds to track pathways that are important for reasoning and visuospatial attention. Small spherical seeds were used for the probabilistic fiber tractography analyses because we were interested in examining the cortical targets of white matter tracts innervating regions most strongly associated with the cognitive components as found in the TBSS analyses. Three 2 mm diameter spherical seeds were chosen based on voxels with the highest signal intensity values from the TBSS analyses in the right anterior corona radiata (seed 1: MNI, 14, 25, 20; reasoning), right corpus callosum body (seed 2: MNI, 8, 7, 25; reasoning), and right corpus callosum splenium (seed 3: MNI, 28, −42, 24; visuospatial attention), which were created in 1 mm isotropic MNI152_TI standard space. Probabilistic tractography (5000 samples; step length = 0.5 mm, curvature threshold = 0.2) was performed from these seeds for each cognitive component using a fully automated Bayesian method (BedpostX; Behrens, Berg, Jbabdi, Rushworth, & Woolrich, 2007). Connectivity maps were computed and thresholded at 50% for each seed, which revealed the tracts that were drawn through each voxel of the seed regions in at least half of the sample. Maps were then binarized and added to obtain a group overlay of pathways. The Juelich histological atlas (Eickhoff et al., 2005) and the Harvard-Oxford cortical and subcortical structural atlases (Desikan et al., 2006) were used to label gray matter targets of the white matter tracts.
3.0 RESULTS
3.1 Principal Components
Demographics and cognitive performance results are displayed in Table 1. The PCA using an extraction criterion of eigenvalues larger than 1 yielded a factor solution with satisfactory communalities across variables (Matrix Reasoning, 0.69; Letter/Word Series, 0.70; EPT, 0.75; cued attention task, 0.55; visuospatial working memory task, 0.62), indicating that the percent of variance of each of the five variables that is accounted for by the components is sufficiently high to accept the PCA solution. Two components were retained that accounted for a cumulative variance of 66.1%. Component 1– called reasoning – loaded high on Matrix Reasoning, Letter/Word Series, and EPT and explained 42.7% of the total variance. Component 2– called visuospatial attention – loaded high on the cued attention task and the visuospatial working memory task and explained 23.5% of the total variance. The rotated component matrix revealed independence of the two components (Table 2).
Table 1.
Age, Years of Education, and Cognitive Test Results for 61 Healthy Older Adults.
| Variable | M (SD) | n |
|---|---|---|
| Age (years) | 69.2 (5.3) | |
| Education (years) | 16.7 (2.5) | |
| MMSE (raw score) | 28.7 (1.4) | |
| Matrix Reasoning (raw score) | 16.9 (4.4) | |
| Letter/Word series (average % correct) | 57.8 (19.8) | |
| EPT (% correct) | 87.5 (8.4) | |
| Cued attention (total cue validity RT) | 79.082 (52.377) | |
| Visuospatial working memory (% correct)a | 47.8 (18.8) | |
| Gender (male/female) | 24/37 |
Note. MMSE = Mini-Mental State Examination; EPT = Everyday Problems Test; RT = reaction time.
Percentage correct of trials with three dot load and 1 cm (0.67 degrees in diameter) distance between test dot and target dot.
Table 2.
Principal Component Analysis Results: Estimates of Correlations between Cognitive Tests and Estimated Components (Rotated Component Matrix).
| Component | ||
|---|---|---|
| Cognitive Test | 1 | 2 |
| Matrix Reasoning | 0.830 | 0.010 |
| Letter/Word series | 0.809 | 0.212 |
| Everyday Problems Test | 0.866 | 0.050 |
| Cued attention | 0.004 | 0.743 |
| Visuospatial working memory | 0.073 | 0.781 |
Note. PCA, Principal Component Analysis. Component: 1 = reasoning, 2 = visuospatial attention. Data in italics indicate non-trivial correlations (greater than 0.4).
3.2 Reasoning Component
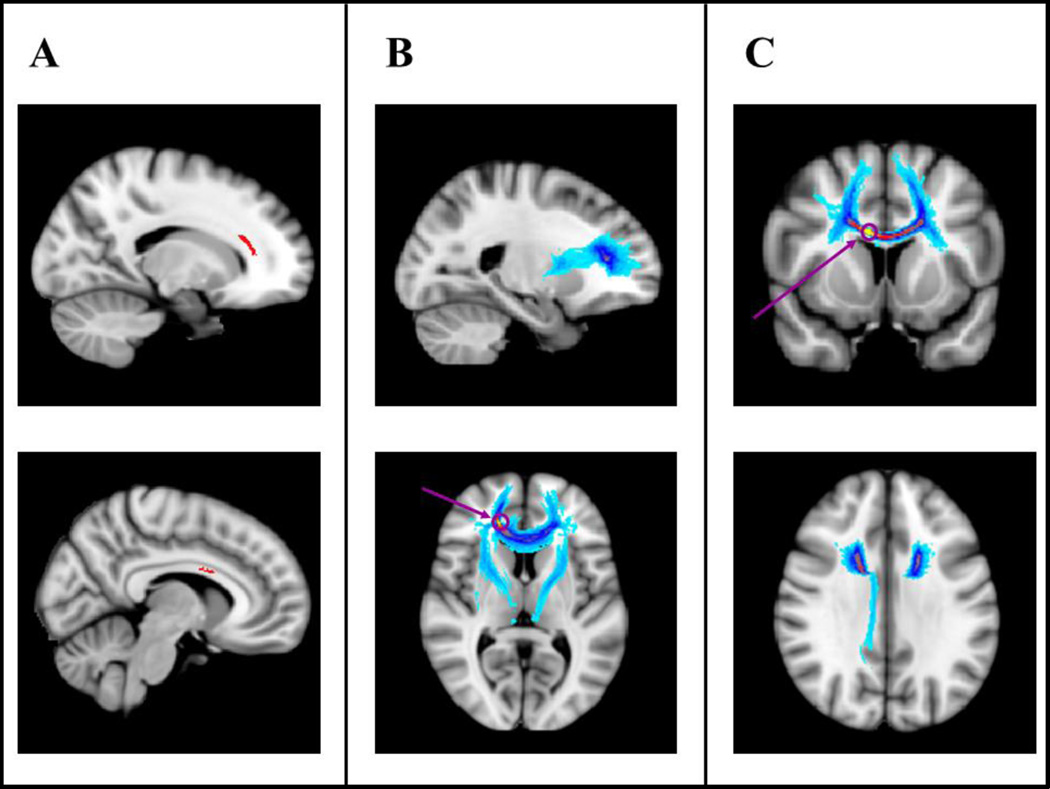
Results from the regression analysis on FA revealed that significantly increased FA in right anterior corona radiata and right corpus callosum body were both associated with better performance on the reasoning component (Fig. 1A; p<0.05, FWE corrected based on the threshold-free cluster-enhanced statistical image); the negative reasoning component-FA correlation contrast did not yield any statistically significant results. The voxels with the highest signal intensity values were found in the right anterior corona radiata and right corpus callosum body. These locations were used as seeds for subsequent fiber tracking analyses. Tracking from the right anterior corona radiata seed revealed (a) bilateral thalamo-anterior prefrontal tracts and (b) corpus callosum tracts connecting bilateral anterior PFC (Fig. 1B). Tracking from the right corpus callosum body seed revealed corpus callosum body tracts interconnecting the superior frontal cortices and right cingulum bundle, the latter connecting medial parietal regions with the PFC, thalamus, and the medial temporal lobe (Fig. 1C).
Fig. 1. Reasoning component tract-based and probabilistic tractography results.
For the tract-based spatial statistics results (TBSS; Panel A), white matter tracts shown in black (in red in the online version of this figure) represent statistically significant positive correlations between fractional anisotropy (FA) and the reasoning component (p < .05, corrected). The TBSS results revealed that significantly increased FA in right anterior corona radiata and corpus callosum body was associated with better performance on the reasoning component. Images are shown at x= 17 (top) and x = 8 (bottom).
For the probabilistic tractography results (Panels B and C), the color code represents the number of participants for which connectivity distributions were found in a given voxel, with lighter shades of gray (warm colors in the online version of this figure) indicating larger participant overlap and darker shades of gray (cold colors in the online version of this figure) indicating smaller participant overlap (light gray = largest overlap, black = smallest overlap; in the online version of this figure, yellow = largest overlap, light blue = smallest overlap). Seed 1 (Panel B) was placed in the right corona radiata (MNI, 14, 25, 20), and Seed 2 (Panel C) in the right corpus callosum body (MNI, 8, 7, 25). The gray (purple in the online version of this figure) circles and arrows indicate the locations of the seeds. Images in Panel B are shown at x = 22 (top) and z = 7 (bottom). Images in Panel C are shown at y = 10 (top) and z = 31 (bottom). All results are shown overlaid onto MNI152_T1_1 mm standard space (radiological convention).
3.3 Visuospatial Attention Component
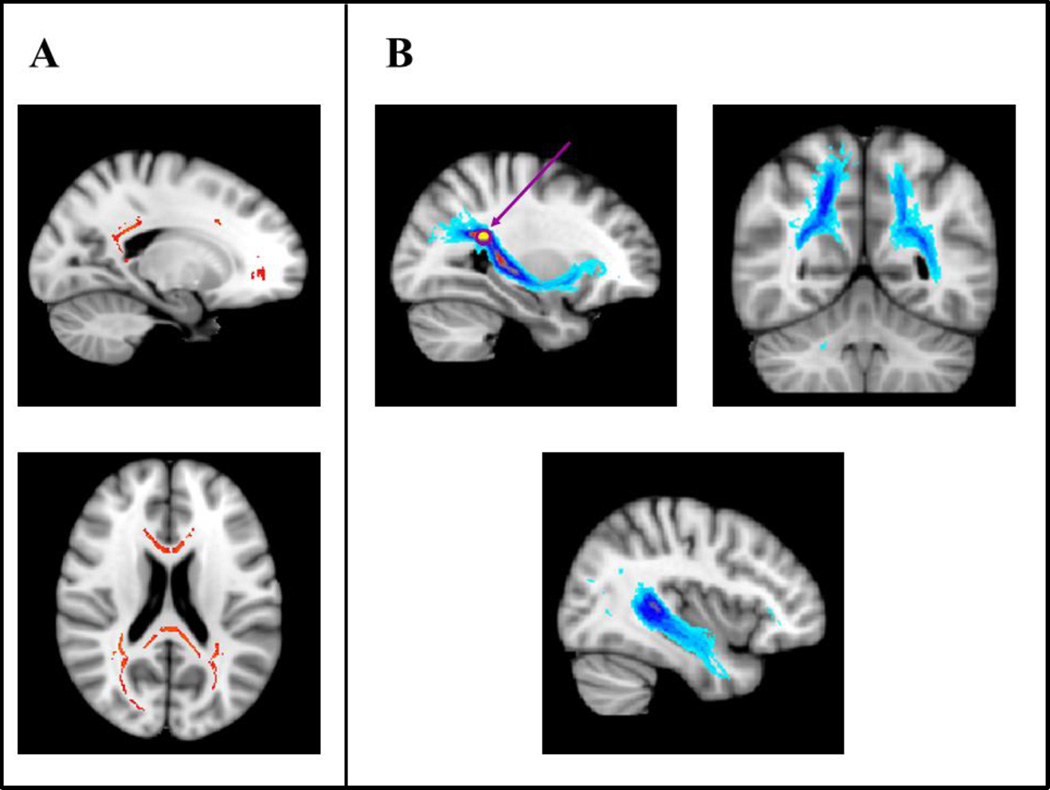
Results from the regression analysis on FA revealed that significantly increased FA in widespread bilateral frontal and parietal areas was associated with better performance on the visuospatial attention component (Fig. 2A; p<0.05, FWE corrected based on the threshold-free cluster-enhanced statistical image); the negative visuospatial attention component-FA correlation contrast did not yield any statistically significant results. The voxels with the highest signal intensity values were found in the right corpus callosum splenium. Fiber tracking from this location revealed a right inferior fronto-parietal tract. We also found bilateral parietal and temporal tracts (Fig. 2B).
Fig. 2. Visuospatial attention component tract-based and probabilistic tractography results.
For the tract-based spatial statistics results (TBSS; Panel A), white matter tracts shown in black (in red in the online version of this figure) represent statistically significant positive correlations between fractional anisotropy (FA) and the visuospatial attention component (p < .05, corrected). The TBSS results revealed that significantly increased FA in widespread frontal and parietal areas was associated with better performance on the visuospatial attention component. Images are shown at x = −18 (top) and z = 20 (bottom).
For the probabilistic tractography results (Panel B), the color code represents the number of participants for which connectivity distributions were found in a given voxel, with lighter shades of gray (warm colors in the online version of this figure) indicating larger participant overlap and darker shades of gray (cold colors in the online version of this figure) indicating smaller participant overlap (light gray = largest overlap, black = smallest overlap; for color version, yellow = largest overlap, light blue = smallest overlap). Seed 3 was placed in the right corpus callosum splenium (MNI, 28, −42, 24). The gray (purple in the online version of this figure) circle and arrow indicates the location of the seed. Images are shown at x = 29 (top left), y = −54 (top right), and x = 38 (bottom). All results are shown overlaid onto MNI152_T1_1 mm standard space (radiological convention).
4.0 DISCUSSION
Our results largely supported our hypothesis that the integrity of specific PFC white matter tracts is associated with reasoning and visuospatial attention performance in healthy older adults.
4.1 Reasoning
We hypothesized that reasoning is associated with integrity of white matter tracts connecting the anterior PFC (Borghesani et al., 2013; Cabeza, 2002; Esposito et al., 1999; Strenziok et al., 2013). We tested that hypothesis with a PCA-derived reasoning factor that included a range of reasoning tasks, including those relevant for older adults' everyday functioning. The results were consistent with the hypothesis. In our sample of healthy older adults, we found associations with the integrity of the corpus callosum likely interconnecting anterior PFC. Anterior PFC is thought to be involved in the processing of relationally complex, abstract information (e.g., Koechlin, Ody, & Kouneiher, 2003), such as those presented in reasoning tasks. Our present finding implicating anterior callosal interconnections in reasoning supports the hypothesis of greater bilateral engagement in healthy older adults compared to young adults during processing of higher order cognitive functions involving the PFC (Cabeza, 2002; Park & Reuter-Lorenz, 2009). Nevertheless, it is important to note that the lack of a young comparison group prevents us from concluding that this result is specific to older adults.
In addition to anterior PFC, dorsolateral PFC and parietal brain regions have been associated with reasoning –fluid ability, deductive reasoning, and abstract thinking (Kroger et al., 2002; Perfetti et al., 2009; Prabhakara, Smith, Desmond, Glover, & Habrieli, 1997; Preusse, van der Meer, Deshpande, Krueger, & Wartenburger, 2011). In the present DTI study, we found that healthy older people relied for reasoning on corpus callosum body tracts interconnecting the superior frontal cortices. Similar evidence was previously reported by our group (Strenziok et al., 2013) and further supports the idea of a prominent role of the PFC and bilateral integration in reasoning processing in older adults. We also found the cingulum bundle to be important for reasoning in older adults, previously found to be important for the integration of frontal, parietal, temporal, and thalamic regions (Croxson et al., 2005; Morris, Pandya, & Petrides, 1999). Although we did not predict that the cingulum bundle would be associated with reasoning performance, that result is consistent with previous findings that measures of fluid ability depend on white matter integrity in a widespread fronto-parieto-temporo-thalamic network, including the cingulum bundle (Penke et al., 2012).
We also found evidence that cortico-thalamic connections are important for reasoning in healthy older people, as revealed by the association we observed between integrity of the anterior corona radiata and reasoning performance. Our results are in accordance with previous DTI studies conducted in older adults (e.g., Borghesani et al., 2013). Borghesani et al. (2013) also found that the anterior corona radiata is involved in reasoning. Furthermore, Penke et al.(2012) found that general intelligence assessed with a composite measure of fluid intelligence, including reasoning, varied with the white matter integrity of subcortico-cortical fibers. The observed importance of subcortico-cortical integration for reasoning suggests that subcortical areas may modulate cortical neurons in response magnitude, firing mode, and synchrony in the service of cognition (Saalmann & Kastner, 2011).
Lastly, we did not find any evidence that better reasoning performance is associated with decreased white matter integrity. This finding agrees with an abundant literature demonstrating that better cognitive performance is associated with greater white matter integrity in older adults (Bennett & Madden, 2014; Madden et al., 2009; Ziegler et al., 2010).
4.2 Visuospatial Attention
For visuospatial attention, we hypothesized that visuospatial attention function varies with the integrity of white matter tracts connecting the temporo-parietal cortex dorsally to the prefrontal cortex and ventrally to the prefrontal and insular cortices, and to frontal and parietal white matter tracts (Corbetta et al., 2008; Corbetta & Shulman, 2002; Umarova et al., 2010). Our results are consistent with our hypothesis insofar as we found that right inferior fronto-parietal white matter and additional bilateral parietal and temporal white matter were important for visuospatial attention performance in our sample of older adults.
What is the role of ventral fronto-parietal connections in visuospatial attention? The right ventral attention network has been claimed to be involved in reorienting attention toward visual targets appearing at unattended locations (Corbetta et al., 2008; Corbetta & Shulman, 2002). The visuospatial attention tasks we included in the PCA required attentional reorienting to detect validly- and invalidly-cued targets, consistent with our observed associations with ventral fronto-parietal pathways. In older adults, the reorienting response may become particularly important based on evidence suggesting that older adults are more susceptible to disruptions from task-irrelevant stimuli (Healey, Campbell, & Hasher, 2008), and, therefore, may rely more on neurocognitive processes that facilitate reorienting following disruptions. One functional imaging study showed that older adults have a diminished ability to disengage from the processing of an interruption and to reengage task-related functional networks (Clapp, Rubens, Sabharwal, & Gazzaley, 2011). This evidence combined with our current DTI findings indicate that healthy older adults rely on ventral fronto-parietal brain circuits known to be important for the reorientation of visuospatial attention.
Also, we found that better visuospatial attention performance was not associated with decreased white matter integrity. This is consistent with a large literature showing that better cognitive performance is associated with greater white matter integrity (Bennett & Madden, 2014; Madden et al., 2009; Ziegler et al., 2010).
4.3 Strengths and Limitations of the Current Study
The methods used in the present study have some advantages over prior work. First, the use of a PCA allowed us to avoid a bias toward composite or idiosyncratically-chosen test scores that can limit findings to individual aspects of reasoning and visuospatial attention. Second, because reasoning ability is an important aspect of older people's daily lives, we assessed reasoning broadly, including in everyday problem solving. Third, the use of fiber tractography analyses to describe the cortical targets of white matter tracts important for reasoning and visuospatial attention in older adults allowed interpretations supplementing the FA-behavior correlations. In addition, our fiber tractography analyses add to information available through published fiber tractography atlases by showing the specific tracts that are likely important for reasoning and visuospatial attention in older adults based on the white matter voxels derived from the FA-behavior correlations.
Although the present study does contain several methodological strengths, there are some limitations. First, higher FA values are known to be biased toward larger white matter tracts, such as the cingulum bundle (Vos, Jones, Viergever, & Leemans, 2011), and away from white matter tracts with fibers that cross at oblique angles (Chepuri et al., 2002; Oouchi et al., 2007). It is possible that white matter integrity variation in smaller white matter tracts and white matter tracks with low fiber direction coherence were not detected due to this methodological limitation. Second, we did not utilize high angular resolution diffusion MRI, which limits our ability to resolve fibers that cross at oblique angles within our tractography analyses (for a review see Savadjiev et al., 2008). Third, this study cannot detect causality between white matter integrity and cognitive function; the results only indicate that reasoning and visuospatial attention performance are associated with white matter integrity in the tracts discussed above. It may be that reduced white matter integrity causes cognitive decline, but it is also possible that decline in cognitive function, caused by changes in another neural substrate (e.g., gray matter), results in reduced white matter integrity. Lastly, because a population of young adults was not included in the study, it cannot be confirmed that the correlations discussed above are specific to older adults. Future studies could include a similar experimental design with both young and older adults to directly examine the effect of age on white matter associations with reasoning and visuospatial attention.
4.4 Conclusions
The present results indicate that (a) white matter integrity is an important brain substrate for reasoning and visuospatial attention, and (b) that integrity of white matter connections of the PFC are important for good cognitive performance in older adults. Our results are also important in their support to the idea that at least some cognitive processes, such as reasoning, depend on bilateral processing in older adults, supporting fMRI-based evidence of greater bilateral processing in older compared to young adults (Cabeza, 2002). In addition, we avoided some limitations of past reasoning- and visuospatial attention-DTI studies in older adults by using a PCA approach and including tasks relevant to everyday functioning. Also, unlike past visuospatial attention-DTI studies in older adults, we did not limit our analysis to regions of interest. That decision allowed us to observe effects of white matter tracts in older adults that previously may not have been assessed for a role in visuospatial attention.
Reasoning and visuospatial attention are cognitive processes that are essential for everyday activities in older adults. To the extent that training on reasoning and visuospatial attention could increase integrity in white matter tracts (as has been shown for working memory training [Takeuchi et al., 2010] and perception training [Berry et al., 2010; Strenziok et al., 2014]), such training could have particular benefits for cognition in healthy older people. Therefore, understanding the white matter connections important for these cognitive processes can inform researchers about white matter networks that may be usefully targeted by cognitive training. Such information could provide health professionals with information about training interventions that are effective in modulating age-related cognitive decline.
Acknowledgments
This work was supported by National Institutes of Health (NIH) grant AG019653 (to R.P.) and Air Force Office of Scientific Research grant FA9550-10-1-0385 (to R.P.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
REFERENCES
- Agarwal S, Driscoll JC, Gabaix X, Laibson D. The age of reason: Financial decisions over the life cycle and implications for regulation. Brookings Papers on Economic Activity, Fall 2009. 2009;2009:51–117. [Google Scholar]
- Ball K, Owsley C, Sloane ME, Roenker DL, Bruni JR. Visual attention problems as a predictor of vehicle crashes in older drivers. Investigative Ophthalmology & Visual Science. 1993;34(11):3110–3123. [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory of measuring depression. Archives of General Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Behrens TEJ, Berg HJ, Jbabdi S, Rushworth MFS, Woolrich MW. Probabilistic diffusion tractography with multiple fibre orientations: What can we gain? Neuroimage. 2007;34(1):144–155. doi: 10.1016/j.neuroimage.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett I, Madden D. Disconnected aging: Cerebral white matter integrity and age-related differences in cognition. Neuroscience. 2014 doi: 10.1016/j.neuroscience.2013.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry AS, Zanto TP, Clapp WC, Hardy JL, Delahunt PB, Mahncke HW, Gazzaley A. The influence of perceptual training on working memory in older adults. PloS one. 2010;5(7):e11537. doi: 10.1371/journal.pone.0011537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghesani PR, Madhyastha TM, Aylward EH, Reiter MA, Swarny BR, Schaie KW, Willis SL. The association between higher order abilities, processing speed, and age are variably mediated by white matter integrity during typical aging. Neuropsychologia. 2013;51:1435–1444. doi: 10.1016/j.neuropsychologia.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle PA, Yu L, Wilson RS, Gamble K, Buchman AS, Bennett DA. Poor decision making is a consequence of cognitive decline among older persons without Alzheimer's disease or mild cognitive impairment. PloS one. 2012;7(8):e43647. doi: 10.1371/journal.pone.0043647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bressler SL, Tang W, Sylvester CM, Shulman GL, Corbetta M. Top-down control of human visual cortex by frontal and parietal cortex in anticipatory visual spatial attention. The Journal of neuroscience. 2008;28(40):10056–10061. doi: 10.1523/JNEUROSCI.1776-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R. Hemispheric Asymmetry Reduction in Older Adults: The HAROLD Model. Psychology and Aging. 2002;17(1):85–100. doi: 10.1037//0882-7974.17.1.85. [DOI] [PubMed] [Google Scholar]
- Chepuri NB, Yen Y, Burdette JH, Li H, Moody DM, Maldjian JA. Diffusion anisotropy in the corpus callosum. American Journal of Neuroradiology. 2002;23(5):803–808. [PMC free article] [PubMed] [Google Scholar]
- Clapp WC, Rubens MT, Sabharwal J, Gazzaley A. Deficit in switching between functional brain networks underlies the impact of multitasking on working memory in older adults. Proceedings of the National Academy of Sciences. 2011;108(17):7212–7217. doi: 10.1073/pnas.1015297108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: From environment to theory of mind. Neuron. 2008;58(3):306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Controls of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience. 2002;3:201–214. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Croxson PL, Johansen-Berg H, Behrens TE, Robson MD, Pinsk MA, Gross CG, Rushworth MF. Quantitative investigation of connections of the prefrontal cortex in the human and macaque using probabilistic diffusion tractography. The Journal of neuroscience. 2005;25(39):8854–8866. doi: 10.1523/JNEUROSCI.1311-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Hyman BT. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 2005;25(4):1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Engvig A, Fjell AM, Westlye LT, Moberget T, Øyvind S, Larsen VA, Walhovd KB. Memory training impacts short-term changes in aging white matter: A longitudinal diffusion tensor imaging study. Human brain mapping. 2012;33:2390–2406. doi: 10.1002/hbm.21370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito G, Kirkby BS, Van Horn JD, Ellmore TM, Berman KF. Context-dependent, neural system-specific neurophysiological concomitants of ageing: Mapping PET correlates during cognitive activation. Brain. 1999;122:963–979. doi: 10.1093/brain/122.5.963. [DOI] [PubMed] [Google Scholar]
- Fjell AM, Walhovd KB, Reinvang I, Lundervold A, Salat D, Quinn BT, Dale AM. Selective increase of cortical thickness in high-performing elderly—structural indices of optimal cognitive aging. Neuroimage. 2006;29(3):984–994. doi: 10.1016/j.neuroimage.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Greenwood PM. Functional plasticity in cognitive aging: Review and hypothesis. Neuropsychology. 2007;21(6):657. doi: 10.1037/0894-4105.21.6.657. [DOI] [PubMed] [Google Scholar]
- Greenwood PM, Lambert C, Sunderland T, Parasuraman R. Effects of Apolipoprotein in E genotype on spatial attention, working memory, and their interaction in healthy, middle-aged adults: Results from the National Institute of Mental Health’s BIOCARD study. Neuropsychology. 2005;19:199–211. doi: 10.1037/0894-4105.19.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood PM, Parasuraman R. The scaling of spatial attention in visual search and its modification in healthy aging. Perception & Psychophysics. 2004;66(1):3–22. doi: 10.3758/bf03194857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood PM, Parasuraman R, Haxby JV. Changes in visuospatial attention over the adult lifespan. Neuropsychologia. 1993;31:471–485. doi: 10.1016/0028-3932(93)90061-4. [DOI] [PubMed] [Google Scholar]
- Greenwood PM, Sunderland T, Friz JL, Parasuraman R. Genetics and visual attention: Selective deficits in healthy adult carriers of the ε4 allele of the apolipoprotein E gene. Proceedings of the National Academy of Sciences. 2000;97:11661–11666. doi: 10.1073/pnas.97.21.11661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haász J, Westlye ET, Fjær S, Espeseth T, Lundervold A, Lundervold AJ. General fluid-type intelligence is related to indices of white matter structure in middle-aged and old adults. Neuroimage. 2013;83:372–383. doi: 10.1016/j.neuroimage.2013.06.040. [DOI] [PubMed] [Google Scholar]
- Healey MK, Campbell KL, Hasher L. Cognitive aging and increased distractibility: Costs and potential benefits. In: Sossin WS, Lacaille JC, Castellucci VF, Belleville S, editors. Progress in brain research. Vol. 169. London, UK: Progress in Brain Research; 2008. pp. 353–363. [DOI] [PubMed] [Google Scholar]
- Hua K, Zhang J, Wakana S, Jiang H, Li X, Reich DS, Mori S. Tract probability maps in stereotaxic spaces: Analyses of white matter anatomy and tract-specific quantification. Neuroimage. 2008;39(1):336–347. doi: 10.1016/j.neuroimage.2007.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingberg T. Training and plasticity of working memory. Trends in cognitive sciences. 2010;14:317–324. doi: 10.1016/j.tics.2010.05.002. [DOI] [PubMed] [Google Scholar]
- Koechlin E, Ody C, Kouneiher F. The architecture of cognitive control in the human prefrontal cortex. Science. 2003;302(5648):1181–1185. doi: 10.1126/science.1088545. [DOI] [PubMed] [Google Scholar]
- Kroger JK, Sabb FW, Fales CL, Bookheimer SY, Cohen MS, Holyoak KJ. Recruitment of anterior dorsolateral prefrontal cortex in human reasoning: A parametric study of relational complexity. Cerebral Cortex. 2002;12(5):477–485. doi: 10.1093/cercor/12.5.477. [DOI] [PubMed] [Google Scholar]
- Laudate TM, Neargarder S, Dunne TE, Sullivan KD, Joshi P, Gilmore GC, Cronin-Golomb A. Bingo! Externally supported performance intervention for deficient visual search in normal aging, Parkinson’s disease, and Alzheimer’s disease. Aging, Neuropsychology, and Cognition. 2012;19:102–121. doi: 10.1080/13825585.2011.621930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S-C, Lindenberger U. Cross-level unification: A computational exploration of the link between deterioration of neurotransmitter systems and dedifferentiation of cognitive abilities in old age. In: Lars-Göran N, Markowitsch HJ, editors. Cognitive Neuroscience of Memory. Ashland, Ohio: Hogrefe & Huber Publishers; 1999. pp. 103–146. [Google Scholar]
- Lövdén M, Bodammer NC, Kühn S, Kaufmann J, Schütze H, Tempelmann C, Lindenberger U. Experience-dependent plasticity of white-matter microstructure extends into old age. Neuropsychologia. 2010;48:3878–3883. doi: 10.1016/j.neuropsychologia.2010.08.026. [DOI] [PubMed] [Google Scholar]
- Mackey AP, Whitaker KJ, Bunge SA. Experience-dependent plasticity in white matter microstructure: Reasoning training alters structural connectivity. Frontier in Neuroanatomy. 2012;6 doi: 10.3389/fnana.2012.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, Bennett IJ, Song AW. Cerebral white matter integrity and cognitive aging: Contributions from diffusion tensor imaging. Neuropsychological Review. 2009;19(4):415–435. doi: 10.1007/s11065-009-9113-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, Spaniol J, Whiting WL, Bucur B, Provenzale JM, Cabeza R, Huettel SA. Adult age differences in the functional neuroanatomy of visual attention: A combined fMRI and DTI study. Neurobiology of Aging. 2007;28(3):459–476. doi: 10.1016/j.neurobiolaging.2006.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, Turkington TG, Provenzale JM, Denny LL, Langley LK, Hawk TC, Coleman RE. Aging and attention guidance during visual search: Functional neuroanatomy by positron emission tomography. Psychology and Aging. 2002;17(1):24–43. doi: 10.1037//0882-7974.17.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell MB, Miller LS, Woodard JL, Davey A, Martin P, Poon LW. Norms from the Georgia Centenarian Study: Measures of verbal abstract reasoning, fluency, memory, and motor function. Aging, Neuropsychology, and Cognition: A Journal on Normal and Dysfunctional Development. 2013;20(5):620–637. doi: 10.1080/13825585.2012.761671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffat SD, Elkins W, Resnick SM. Age differences in the neural systems supporting human allocentric spatial navigation. Neurobiology of Aging. 2006;27(7):965–972. doi: 10.1016/j.neurobiolaging.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Mori S, Wakana S, Van Zijl PC, Nagae-Poetscher L. MRI atlas of human white matter. Vol. 16. Amsterdam, The Netherlands: American Society of Neuroradiology; 2005. [Google Scholar]
- Morris R, Pandya DN, Petrides M. Fiber system linking the mid-dorsolateral frontal cortex with the retrosplenial/presubicular region in the rhesus monkey. Journal of Comparative Neurology. 1999;407(2):183–192. doi: 10.1002/(sici)1096-9861(19990503)407:2<183::aid-cne3>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Moye J, Marson DC. Assessment of decision-making capacity in older adults: An emerging area of practice and research. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2007;62:P3–P11. doi: 10.1093/geronb/62.1.p3. [DOI] [PubMed] [Google Scholar]
- Oouchi H, Yamada K, Sakai K, Kizu O, Kubota T, Ito H, Nishimura T. Diffusion anisotropy measurement of brain white matter is affected by voxel size: Underestimation occurs in areas with crossing fibers. American Journal of Neuroradiology. 2007;28(6):1102–1106. doi: 10.3174/ajnr.A0488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DC, Reuter-Lorenz P. The adaptive brain: aging and neurocognitive scaffolding. Annual review of psychology. 2009;60:173–196. doi: 10.1146/annurev.psych.59.103006.093656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penke L, Muñoz Maniega S, Bastin ME, Valdés Hernández MC, Murray C, Royle NA, Deary IJ. Brain white matter tract integrity as a neural foundation for general intelligence. Molecular Psychiatry. 2012;17(10):1026–1030. doi: 10.1038/mp.2012.66. [DOI] [PubMed] [Google Scholar]
- Perfetti B, Saggino A, Ferretti A, Caulo M, Romani GL, Onofrj M. Differential patterns of cortical activation as a function of fluid reasoning complexity. Human brain mapping. 2009;30:497–510. doi: 10.1002/hbm.20519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakara V, Smith JAL, Desmond JE, Glover GH, Habrieli JDE. Neural substrates of fluid reasoning: An fMRI study of neocortical activation during performance of the Raven’s Progressive Matrices test. Cognitive Psychology. 1997;33:43–63. doi: 10.1006/cogp.1997.0659. [DOI] [PubMed] [Google Scholar]
- Prado J, Chadha A, Booth JR. The brain network for dedutive reasoning: A quantitative meta-analysis of 28 neuroimaging studies. Journal of cognitive neuroscience. 2011;23(11):3483–3497. doi: 10.1162/jocn_a_00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preusse F, van der Meer E, Deshpande G, Krueger F, Wartenburger I. Fluid intelligence allows flexible recruitment of the parieto-frontal network in analogical reasoning. Frontiers in Human Neuroscience. 2011;5 doi: 10.3389/fnhum.2011.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Gunning FM, Head D, Dupuis JH, McQuain J, Briggs SD, Acker JD. Selective aging of the human cerebral cortex observed in vivo: Differential vulnerabiliy of the prefrontal gray matter. Cerebral Cortex. 1997;7(3):268–282. doi: 10.1093/cercor/7.3.268. [DOI] [PubMed] [Google Scholar]
- Rebok GW, Ball K, Guey LT, Jones RN, Kim H-Y, King JW, Willis SL. Ten-year effects of the Advanced Cognitive Training for Independent and Vital Elderly cognitive training trial on cognition and everyday functioning in older adults. Journal of the American Geriatrics Society. 2014;62(1):16–24. doi: 10.1111/jgs.12607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saalmann YB, Kastner S. Cognitive and perceptual functions of the visual thalamus. Neuron. 2011;71(2):209–223. doi: 10.1016/j.neuron.2011.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA. Effects of aging on reasoning. In: Holyoak KJ, Morrison RG, editors. The Cambridge handbook of thinking and reasoning. New York, NY: Cambridge University Press; 2005. pp. 589–605. [Google Scholar]
- Savadjiev P, Campbell JS, Descoteaux M, Deriche R, Pike GB, Siddiqi K. Labeling of ambiguous subvoxel fibre bundle configurations in high angular resolution diffusion MRI. Neuroimage. 2008;41(1):58–68. doi: 10.1016/j.neuroimage.2008.01.028. [DOI] [PubMed] [Google Scholar]
- Schaie KW. Manual for the Schaie-Thurstone Test of Adult Mental Abilities (STAMAT) Palo Alto, CA: Consulting Psychologists Press; 1985. [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Behrens TEJ. Tract-based spatial statistics: Voxel wise analysis of multi-subject diffusion data. Neuroimage. 2006;31(4):1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Supplement 1(0)):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Strenziok M, Greenwood PM, Santa Cruz SA, Thompson JC, Parasuraman R. Differential contributions of dorso-ventral and rostro-caudal prefrontal white matter tracts to cognitive control in healthy older adults. PloS one. 2013;8(12):e81410. doi: 10.1371/journal.pone.0081410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strenziok M, Parasuraman R, Clarke E, Cisler DS, Thompson JC, Greenwood PM. Neurocognitive enhancement in older adults: Comparison of three cognitive training tasks to test a hypothesis of training transfer in brain connectivity. Neuroimage. 2014;85:1027–1039. doi: 10.1016/j.neuroimage.2013.07.069. [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Sekiguchi A, Taki Y, Yokoyama S, Yomogida Y, Komuro N, Kawashima R. Training of working memory impacts structural connectivity. The Journal of neuroscience. 2010;30(9):3297–3303. doi: 10.1523/JNEUROSCI.4611-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Posner MI. Attention training and attention state training. Trends in cognitive sciences. 2009;13:222–227. doi: 10.1016/j.tics.2009.01.009. [DOI] [PubMed] [Google Scholar]
- Thurstone LL, Thurstone TG. Examiner manual for the SRA Primary Mental Abilities test. Chicago, IL: Science Research Associates; 1949. [Google Scholar]
- Uc EY, Rizzo M, Anderson SW, Sparks J, Rodnitzky RL, Dawson JD. Impaired visual search in drivers with Parkinson’s disease. Annals of neurology. 2006;60:407–413. doi: 10.1002/ana.20958. [DOI] [PubMed] [Google Scholar]
- Umarova RM, Saur D, Schnell S, Kaller CP, Vry M, Glauche V, Weiller C. Structural connectivity of visuospatial attention: Significance of ventral pathways. Cerebral Cortex. 2010;20:121–129. doi: 10.1093/cercor/bhp086. [DOI] [PubMed] [Google Scholar]
- United Nations. World Population Prospects: The 2012 Revision. [November 1, 2014];2013 Retrieved from http://esa.un.org/wpp on. [Google Scholar]
- Vos SB, Jones DK, Viergever MA, Leemans A. Partial volume effect as a hidden covariate in DTI analyses. Neuroimage. 2011;55(4):1566–1576. doi: 10.1016/j.neuroimage.2011.01.048. [DOI] [PubMed] [Google Scholar]
- Wechsler D. WAIS-III: Administration and scoring manual: Wechsler adult intelligence scale. San Antonio, TX: Psychological Corporation; 1997. [Google Scholar]
- Willis SL, Marsiske M. Everyday Problems Test. University Park, PA: Pennsylvania State University; 1993. [Google Scholar]
- Willis SL, Tennstedt SL, Marsiske M, Ball K, Elias J, Mann Koepke K, Wright E. Long-term effects of cognitive training on everyday functional outcomes in older adults. JAMA. 2006;296:2805–2814. doi: 10.1001/jama.296.23.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler DA, Piguet O, Salat DH, Prince K, Connally E, Corkin S. Cognition in healthy aging is related to regional white matter integrity, but not cortical thickness. Neurobiology of Aging. 2010;31(11):1912–1926. doi: 10.1016/j.neurobiolaging.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]