Abstract
Background
Numerous systems exist to model the blood-brain barrier (BBB) with the goal of understanding the regulation of passage into the central nervous system (CNS) and the potential impact of selected insults on BBB function. These models typically focus on the intrinsic cellular properties of the BBB, yet studies of peripheral cell migration are often excluded due to technical restraints.
New Method
This method allows for the study of in vitro cellular transmigration following exposure to any treatment of interest through optimization of co-culture conditions for the human brain microvascular endothelial cells (BMEC) cell line, hCMEC/D3, and primary human peripheral blood mononuclear cells (PBMCs).
Results
hCMEC/D3 cells form functionally confluent monolayers on collagen coated polytetrafluoroethylene (PTFE) transwell inserts, as assessed by microscopy and tracer molecule (FITC-dextran (FITC-D)) exclusion. Two components of complete hCMEC/D3 media, EBM-2 base-media and hydrocortisone (HC), were determined to be cytotoxic to PBMCs. By combining the remaining components of complete hCMEC/D3 media with complete PBMC media a resulting co-culture media was established for use in hCMEC/D3 – PBMC co-culture functional assays.
Comparison with existing methods
Through this method, issues of extensive differences in culture media conditions are resolved allowing for treatments and functional assays to be conducted on the two cell populations co-cultured simultaneously.
Conclusion
Described here is an in vitro co-culture model of the BBB, consisting of the hCMEC/D3 cell line and primary human PBMCs. The co-culture media will now allow for the study of exposure to potential insults to BBB function over prolonged time courses.
Keywords: Blood-brain barrier, peripheral blood mononuclear cells, brain microvascular endothelial cells, hCMEC/D3 cell line
1. Introduction
The blood-brain barrier (BBB) regulates passage between peripheral circulation and the central nervous system (CNS). Aside from limiting permeability to circulating proteins, ions, and therapeutic compounds, the BBB also controls cellular transmigration and immune surveillance (Loeffler et al., 2011; Strazza et al., 2011). In comparison to other tissues and organs throughout the body, immune surveillance of the CNS is more limited and tightly regulated in order to control the neuroinflammatory response and to minimize the risk of deleterious neuroinflammation within the brain (Miner and Diamond, 2016; Ousman and Kubes, 2012; Ransohoff and Engelhardt, 2012). In this regard, accelerated immune cell extravasation has been linked to a number of disease pathologies (Katsetos et al., 1999; Ryan et al., 2005; Song et al., 2015). Understanding the impact of compounds or proteins that alter proper BBB function is a critical first step in developing appropriate interventions to specific neuropathologic conditions.
In order to study BBB integrity following exposure to experimental conditions, model systems must be utilized. These model systems, either in vivo or in vitro, incorporate, at minimum, brain microvascular endothelial cells (BMEC), which comprise the BBB endothelium into selected co-culture combinations with astrocytes, pericytes, and under certain circumstances, neurons. In vitro models using human cells are generally preferred to animal models when addressing many types of experimental questions, and the culture of primary human BMECs is generally accepted to require or be greatly enhanced by co-culture with primary human astrocytes (Eugenin and Berman, 2003; Hayashi et al., 1997). The in vitro primary co-culture model maintains the BBB characteristics for a window of one to two days beyond establishing confluence, which can limit the duration of experimental exposures. The recent development of the immortalized human BMEC cell line, hCMEC/D3 cells, has led to the development of another human in vitro BBB model; the hCMEC/D3 cell line was established through the in vitro immortalization of BMECs isolated from the temporal lobe (Weksler et al., 2005). Critical to defining the hCMEC/D3 cell line as a BBB model, these cells were shown to express BBB-specific markers, including ZO-1, JAM-A, occludin, and claudin-5 (Eigenmann et al., 2013; Helms et al., 2016; Huang et al., 2009; Pu et al., 2005; Weksler et al., 2005). In addition, hCMEC/D3 cells maintain the functional characteristics of the BBB in monoculture for several days beyond confluence, allowing for extended duration exposures that are critical to drug studies as well as models of chronic inflammatory diseases (Daniels et al., 2013; Jacob et al., 2015; Urich et al., 2012; Weksler et al., 2013a; Weksler et al., 2005). Recent studies have been aimed at defining the specific attributes of hCMEC/D3 cells, as well as other endothelial cell lines that are emerging in popularity as BBB models (Helms et al., 2016). These studies confirmed tight junction protein expression and low transendothelial permeability that was not enhanced through the co-culture with astrocytes or pericytes, and conclude that the mono-culture of the immortalized human cell line will provide another valuable tool in the in vitro studies of the BBB (Cucullo et al., 2008; Eigenmann et al., 2013; Weksler et al., 2005).
Beyond studying characteristics of the BBB such as transendothelial electrical resistance (TEER), tight junction protein expression and localization, cell adhesion molecule expression, and small molecule permeability, the interaction between immune cells and the BBB during the process of transmigration must also be assessed following an experimental exposure in order to fully understand the nature of the dysfunction induced. Transmigration functional assays require the co-culture of BMECs with immune cell populations, in the absence or presence of astrocytes, pericytes, and cells relevant to a functional BBB. The merging of these cell types into a common culture setup creates the obstacle of also bringing together the culture conditions that were previously defined and optimized for monoculture of each unique cell type. In the case of the hCMEC/D3 cell line, the defined media for cell growth contains a complex combination of media components, each serving distinct roles while collectively promoting the in vitro growth and maintenance of the cells. In contrast, primary human PBMCs are often maintained in a simple media formulation consisting of a few essential supplements to the base media. Presented here is a unique media formulation that maintains viability and function of primary human PBMCs and hCMEC/D3 endothelial cells.
2. Methods
2.1 Materials
Mannitol and FITC-dextran (70 kDa) (FITC-D) were purchased from Sigma Aldrich. SDF-1α was purchased from eBioscience. IL-2 was obtained through the NIH AIDS Reagent Program, Division of AIDS, NIAID, NIH, from Dr. Maurice Gately, Hoffmann - La Roche Inc (Lahm and Stein, 1985).
2.2 Cell culture and treatment
The human BMEC line, hCMEC/D3, was obtained from Dr. Babette Weksler (Weill Cornell Medical College) and cultured in EBM-2 (Lonza) supplemented with heat inactivated fetal bovine serum (FBS; 5%, GemBio), penicillin-streptomycin (1%, CellGro), hydrocortisone (1.4 μM, CellGro), ascorbic acid (5 μg/mL, Sigma Aldrich), chemically defined lipid concentrate (1%, Invitrogen), HEPES (10 mM, CellGro), and basic fibroblast growth factor (1 ng/mL, Sigma Aldrich) (designated herein as “CMEC media”). All experiments were conducted between cell passages 27–32. Cells were grown on petri dishes or 12-well plates coated with Cultrex rat collagen I (150 μg/mL, Trevigen) in H2O. hCMEC/D3 cells were seeded on petri dishes and 12-well plates at a density of 37,000 cells/cm2 or on transwell inserts (details below). At confluence on plates or in transwells, hCMEC/D3 cells were treated where indicated with mannitol (1.4 M, Sigma Aldrich) for 30 minutes.
Primary human PBMCs were obtained from the Comprehensive NeuroAIDS Center (Basic Science Core 1, Temple University School of Medicine). Cells were cultured overnight under unstimulated, non-adherent conditions in polypropylene 50 mL conical tubes at a density of 1 x 106 cells/mL in RPMI media supplemented with heat-inactivated FBS (10%), penicillin-streptomycin (1%), and IL-2 (20 U/ml) (designated herein as “PBMC media”).
Co-culture studies were performed in “co-culture media.” Co-culture media consists of RPMI supplemented with heat-inactivated FBS (5%), penicillin-streptomycin (1%), ascorbic acid (5 μg/mL), chemically defined lipid concentrate (1%), HEPES (10 mM), basic fibroblast growth factor (1 ng/mL), and IL-2 (20 U/ml).
2.3 In vitro blood-brain barrier model
hCMEC/D3 cells were seeded on collagen-coated porous polytetrafluoroethylene (PTFE) transwell inserts (Corning, NY) at a density of 4.5 x 104 cells/cm2 and grown for 10 days to achieve confluence for use in FITC-D and PBMC transmigration assays. Of note, transwell inserts were purchased pre-coated with collagen, limiting the variability to which coating with collagen prior to each experiment may influence cell growth and membrane permeability. Addition of treatments to the upper chamber of the transwell inserts was used to mimic a peripheral exposure.
2.4 FITC-Dextran permeability assay
Any indicated treatments of the hCMEC/D3 monolayers were performed at confluence on porous PTFE transwell inserts (0.4 μm). The monolayers were subsequently washed with 10 mM HEPES in 1X HBSS and the FITC-dextran (FITC-D) permeability assay was performed as previously described (Weksler et al., 2005) with minor modification. The use of the FITC-D assay to monitor confluence and small-molecule permeability is the preferred method for hCMEC/D3 cells due to the well-published fact that many of the immortalized human BMEC cell lines have consistently low TEER values, therefore, necessitating the use of other tools to assess barrier tightness or integrity throughout the duration of culture while maintaining protein expression markers of the BBB (Deli et al., 2005; Eigenmann et al., 2013; Helms et al., 2016; Reichel et al., 2003; Weksler et al., 2005). Briefly, 70 kDa FITC-dextran (FITC-D; 2 mg/mL) in CMEC media was added to the upper chamber. Passage through the monolayer was monitored through sampling from the lower chamber at 5 minute intervals throughout a 30- minute time course (6 time points); these samples were read in optical bottom plates for fluorescence intensity. The increase in frequency of sampling from 10 to 5 minutes allows for increased accuracy in plotting the curves of cleared volume over time that are critical to the calculation of the permeability coefficient (Pe), or rate of passage. Some transwell inserts were maintained in the absence of cells, and these wells were used to determine the rate of passage across the filter alone, another necessary value in the calculation of Pe. Fluorescence intensity was then used to calculate the Pe of the monolayer utilizing the following equation: Pe = PS/s, where PS (clearance) is the permeability surface area of the endothelial monolayer and s is the surface area of the filter (1.12 cm2).
PS is given by 1/PS = 1/me – 1/mf, where me and mf are the slopes of the curves corresponding to endothelial cells on filters and to filters only, respectively, with me and mf calculated by plotting the cleared volume against time.
The cleared volume was calculated by (AUa – AUb)/Fi, where AUa is the total fluorescence (arbitrary units) in the basal compartment, AUb is the background fluorescence and Fi is the fluorescence of the initial solution (AU/ml).
2.5 PBMC viability assay
Following overnight non-adherent culture, as described above, 1 x 106 PBMCs were incubated for 24 hours in the indicated media conditions in 12-well plates. An MTS assay (CellTiter 96® AQueous One Solution Cell Proliferation Assay System, Promega, Madison, WI) was then performed, as described by the manufacturer (Promega), to determine the viability of the PBMCs under selected culture conditions. Cell viability in normal PBMC media was set to 100% viability.
2.6 PBMC transmigration assay
Primary human PBMCs were obtained from the Comprehensive NeuroAIDS Center (Basic Science Core 1, Temple University School of Medicine) and cultured at 1 x 106 cells/mL in the presence of IL-2 (20 U/ml) in polypropylene 50 mL conical tubes to minimize monocyte differentiation and adherence. Meanwhile, hCMEC/D3 cells were grown to confluence on porous PTFE transwell inserts (3 μm). At confluence, a suspension of 1 x 106 PBMCs in co-culture media were then added to the upper chamber. As a positive control of enhanced PBMC transmigration, SDF-1α (100 ng/mL) was added to the lower chamber. In both cases, untreated or SDF-1α-treated, the media in the lower chamber was co-culture media. Following a 24 hour incubation, cellular transmigration to the lower chamber was determined using flow cytometry. Briefly, media from the lower chamber was collected, and transmigrated cells were counted using the Beckton Dickson FACSCalibur and the results were analyzed using FlowJo software.
2.7 Statistical analysis
Statistics were calculated using the student’s t-test. P values less than 0.05 were considered significant in all assays.
3. Results
3.1 hCMEC/D3 cells as an in vitro model of the BBB
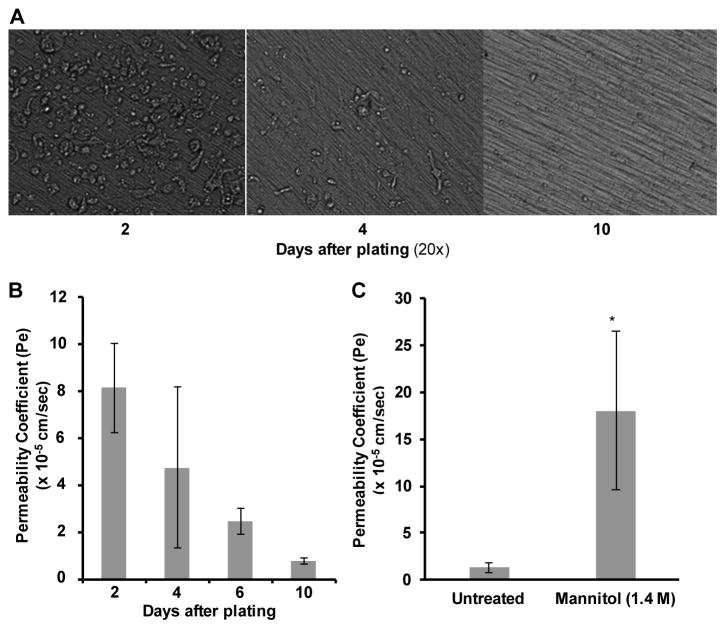
To establish the growth pattern of hCMEC/D3 cells on collagen-coated PTFE transwell inserts, cells were seeded at 4.5 x 104 cells/cm2 and monitored microscopically for 10 days (Figure 1A). After 2 days in culture, hCMEC/D3 cells remained on the membrane with a rounded morphology. On day 4, the cells were partially confluent with areas of cells still rounded and proliferating. By 10 days in culture, hCMEC/D3 cells were flat against the membrane, and appeared to be fully confluent. To complement the visual analysis, confluence was monitored through a FITC-D permeability coefficient assay. FITC-D is available in multiple sizes ranging from 4 – 70 kDa, and for these studies the 70 kDa size was used since it most closely mimics tracer molecules, such as Evan’s blue labeled albumin, often used in in vivo models. Following 2, 4, 6, or 10 days in culture, 70 kDa FITC-D (2 mg/mL) in CMEC media was added to the upper chamber of the transwell inserts, and passage through the monolayer to the lower chamber was monitored at 5 minute intervals over a 30 minute period and permeability coefficients (Pe) were calculated (Figure 1B). After 10 days in culture, a Pe comparable to published values for this cell line was observed, and, notably, this Pe was lower than those of other commonly used in vitro BBB models (Weksler et al., 2005). Additionally, treatment of the confluent monolayer with mannitol (1.4 M), a compound commonly used clinically to enhance transport of therapeutics across the BBB, induced a significant increase in Pe (Figure 1C), indicating that the low Pe observed at 10 days was not due to cell piling and a physical blockade of the insert pores. From these studies it can be concluded that hCMEC/D3 cells form a functionally confluent monolayer on collagen-coated PTFE transwell inserts after 10 days of growth. For all subsequent transwell studies, hCMEC/D3 cells were grown for 10 days prior to any treatments or experiments.
Figure 1. hCMEC/D3 cells form a confluent monolayer following 10 days in culture.
(A) hCMEC/D3 cells were seeded on porous PTFE transwell inserts (0.4 μM) at time zero and cultured for 10 days. Throughout this time course, monolayer growth was visualized under 20X Relief Contrast Microscopy using an Olympus IX81 de-convolution microscope. (B) hCMEC/D3 cells remained in culture 2, 4, 6, or 10 days (as in A) prior to addition of 2 mg/mL 70 kDa FITC-D to the upper chamber. Samples were taken from the lower chamber at 5 minute intervals over 30 minutes in order to calculate Pe. (C) hCMEC/D3 cells were cultured (as in A) for 10 days prior to treatment of the monolayer with mannitol (1.4 M) for 30 minutes at 37°C in CO2 (5%). Following treatment, a FITC-D assay was performed (as in B) to calculate Pe. Bars represent the mean of three independent experiments performed in triplicate with standard deviation. Statistical analysis was performed with a two-tailed Student’s t-test. *p < 0.0001.
3.2 CMEC media decreases PBMC Viability
Assays of BBB function requires co-culture of both PBMCs and hCMEC/D3 cells; therefore, necessitating the merging of the cell culture requirements of the cell types. Due to the differences between PBMC and CMEC media components (Table 1), PBMC viability was assessed to establish optimal co-culture conditions.
Table 1.
Components of PBMC and hCMEC/D3 complete media
| PBMC media | hCMEC/D3 media (CMEC) |
|---|---|
| RPMI-1640 | EBM-2 |
| Fetal Bovine Serum (10%) | Fetal Bovine Serum (5%) |
| Penicillin/Streptomycin | Penicillin/Streptomycin |
| IL-2 | Hydrocortisone |
| Ascorbic Acid | |
| Chemically Defined Lipid Concentrate | |
| HEPES | |
| bFGF |
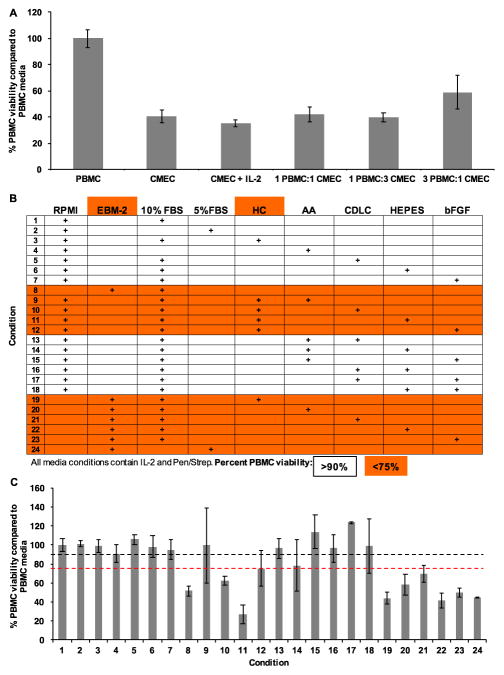
PBMCs were grown under selected combinations of PBMC and CMEC media conditions for 24 hours prior to an MTS assay. Compared to PBMC media, PBMC viability in CMEC media was 40% (Figure 2A). Importantly, the addition of IL-2 did not rescue viability (35%), nor did any ratio combinations of PBMC and CMEC media. The low viability (59%) observed when PBMCs were grown in 3 parts PBMC media and 1 part CMEC media suggested that one or more components of CMEC media may be toxic to PBMCs (Figure 2A). To identify toxic components, PBMCs were grown under 24 media conditions, each with either an RPMI or EBM-2 media base and the indicated combinations of CMEC media supplements, for 24 hours prior to an MTS assay. All media conditions included IL-2 and Pen/Strep (Figure 2B). Any condition with less than 75% viability was considered toxic.
Figure 2. CMEC media decreases PBMC viability.
(A) PBMCs were grown under selected media conditions at 1 x 106 cells/mL at 37°C in 5% CO2 for 24 hours prior to an MTS assay to assess cellular viability. Media conditions: complete PBMC media; complete CMEC media; CMEC media supplemented with IL-2; combination of one part PBMC and one part CMEC; combination of one part PBMC and three parts CMEC; three parts PBMC and one part CMEC. PBMC viability within complete PBMC media was normalized to 100%, and all further media conditions were compared to complete PBMC media. (B) PBMCs were grown as in (A). Media conditions included either an RPMI or EBM-2 media base with the addition of selected components of complete CMEC media. HC – hydrocortisone, AA – ascorbic acid, CDLC – chemically defined lipid concentrate, and bFGF – basic fibroblast growth factor. All media conditions included IL-2 and Pen/Strep. An MTS assay was performed to assess PBMC viability. PBMC viability within complete PBMC media (condition 1) was normalized to 100%, and all further media conditions were compared to complete PBMC media (condition 1). Media formulations and components highlighted in orange indicate conditions which yielded less than 75% viability. All conditions with less than 75% viability contain either EBM-2 or HC. (C) Percent PBMC viability as determined by MTS assay. Conditions refer to those defined in (B). Black line: 90%. Red line: 75%.
Interestingly, all toxic conditions included either EBM-2 or hydrocortisone (HC) (Figure 2C). These results suggested that EBM-2 and HC are specifically cytotoxic for PBMCs. The toxicity of HC may be due to an induced decrease in IL-2 receptors on T cells within the PBMCs. Based on these observations, a co-culture media formulation was developed and included an RPMI base with IL-2, Pen/Strep, 10% FBS, ascorbic acid, chemically defined lipid concentrate (CDLC), HEPES, and bFGF.
3.3 hCMEC/D3 monolayers maintain low permeability in co-culture media
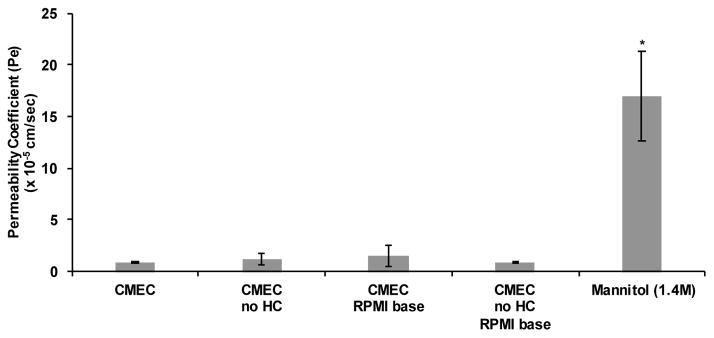
A critical next step was to confirm that a media lacking HC and made with RPMI would not alter proper hCMEC/D3 monolayer function. To establish the impact of the new co-culture media formulation on the hCMEC/D3 monolayer a FITC-D permeability coefficient assay was performed. The hCMEC/D3 monolayer was grown to confluence in full CMEC media on 0.4 μm pore size transwell inserts. Following 10 days in culture in CMEC media, when confluence was reached, media in the upper and lower chambers was changed to the new co-culture media and cells were incubated for 24 hours. The absence of HC and substitution of an RPMI base did not significantly increase permeability of the hCMEC/D3 monlayer after 24 hours of culture (Figure 3). Therefore, these results suggest that co-culture media can be used for assays of BBB function.
Figure 3. Co-culture media does not induce leakiness of the hCMEC/D3 barrier.
The effect of co-culture media on hCMEC/D3 structure and function was assessed through a FITC-D permeability coefficient assay as previously described (Figure 1). Media conditions: complete CMEC media; CMEC media without HC; CMEC media made with an RPMI base (no EBM-2); CMEC media without HC made with an RPMI base. A 30 minute treatment with mannitol (1.4 M) is included as a positive control of barrier compromise for the assay. Statistical analysis was performed with a two-tailed Student’s t-test. *p = 0.04
3.4 Co-culture media supports PBMC and hCMEC/D3 transmigration assays
To confirm that the co-culture media supports functional assays utilizing both cell populations, transmigration of PBMCs across the hCMEC/D3 monolayer was examined. hCMEC/D3 cells were grown to confluence in CMEC media on collagen-coated PTFE transwell inserts (3 μm pores), as described above. At confluence, media in lower chambers was replaced with either co-culture media or co-culture media supplemented with SDF-1α (100 ng/mL), added as a chemoattractant to promote PBMC transmigration. Additionally, primary human PBMCs suspended in co-culture media were added to the upper chamber and incubated for 24 hours at 37°C in 5% CO2 to allow transmigration to occur. After 24 hours, the media in the lower chambers was collected, and the number of transmigrated cells was quantified by flow cytometry. Events were gated based on forward and side scatter to distinguish PBMCs, and the number of events within this gate was quantified for each sample. Of note, this method can be expanded to include staining the transmigrated PBMCs for surface proteins of interest in order to assess subpopulations of cells that migrate preferentially in response to stimuli. As expected, SDF-1α significantly increased PBMC transmigration (Figure 4). This result demonstrated that the untreated hCMEC/D3 monolayer maintained functional integrity and that both the hCMEC/D3 cells and the PBMCs maintained the ability to become activated and participate in the transmigration process in co-culture media for 24 hours. Overall, these studies demonstrate that a co-culture media formulation for the hCMEC/D3 cell line and primary human PBMCs allows for functional studies of the two populations and this co-culture model will provide an additional tool to examine the structure and function of the BBB and its relationship with other cellular compartments and pathogenic agents.
Figure 4. Co-culture media supports the PBMC transmigration assay.
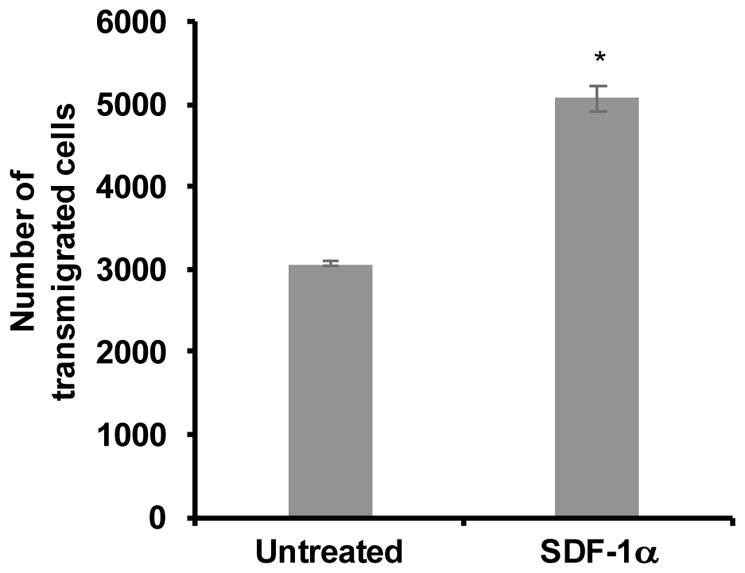
hCMEC/D3 cells were cultured for 10 days on porous PTFE transwell inserts (3 μM). Separately, PBMCs were maintained in non-adherent culture in the presence of IL-2 for 24 hours. At confluence of the monolayer, the CMEC media was removed from both chambers, new co-culture media was added to the lower chambers, in the absence or presence SDF-1α (100 ng/mL), and a suspension of 1 x 106 PBMCs in co-culture media was added to the upper chambers. Transmigration was allowed to occur over a period of 24 hours during incubation at 37°C in 5% CO2. Following transmigration, cells were collected from the lower chambers and analyzed by flow cytometry. Cells were characterized based on forward and side scatter, to identify the PBMC population, and quantified. All bars represent the average two independent experiments performed in triplicate treatments +/− standard deviation. All treatments were significantly increased when compared to untreated as determined by student’s t-test. *p < 0.0013
4. Discussion
The development of the hCMEC/D3 cell line has allowed for great advances in the field of BBB research, due in part to the ease of acquisition, amplification of cell number, and extended culture time to facilitate experimentation (Chen et al., 2015; Daniels et al., 2013; Helms et al., 2016; Urich et al., 2012; Weksler et al., 2013b; Weksler et al., 2005). However, despite these benefits, this cell line has been shown to require numerous media supplements for optimal growth and function. This requirement has led to a unique challenge for co-culturing hCMEC/D3 cells with primary human PBMCs, and perhaps other cell types, due to the contrasting simplicity of typical PBMC culture media. We have been able to identify a unique media formulation for co-culturing both hCMEC/D3 and PBMC cells to permit in vitro investigations focused on mechanisms that may facilitate cellular transmigration. In this regard, we have first identified specific components of the CMEC media that are cytotoxic to PBMCs and a co-culture media was then formulated that was shown to maintain both cell populations for use in co-culture studies. Importantly, both the hCMEC/D3 cells and PBMCs were grown initially in complete CMEC and PBMC medias, respectively, and were then transferred into co-culture media at the onset of the functional assay. This process ensures normal growth and proliferation of the two cell types individually before their behavior is examined under selected experimental conditions. Additionally, the studies performed in this investigation demonstrated that co-culture media alone did not induce altered BBB permeability or PBMC viability.
Numerous in vitro BBB models have been established and studied. Primary BMECs of animal origin, including bovine (Salmeri et al., 2013), murine (Ludewig et al., 2013), and porcine (Pieper et al., 2013), have often been used to mimic the human BBB. While in vitro cell culture models share common characteristics with the human BBB, they are certainly not optimal or ideal. Culture of primary human BMECs in combination with primary human astrocytes has widely been thought to be a relevant model of barrier function, and has been utilized in many different types of studies (Bethel-Brown et al., 2012; Gandhi et al., 2010). A difficulty of primary cell models is the limited window beyond confluence during which the cells maintain viability and function. Consequently, primary cell models of the BBB are incompatible with extended duration exposure studies. The hCMEC/D3 cell line, which has been characterized for several days beyond establishing confluence, has been previously used for co-culture studies with PBMCs, and has been shown to yield results comparable to primary human BMECs both in the absence or presence of astrocytes (Daniels et al., 2013). Previous studies combining hCMEC/D3 cells with PBMCs introduced PBMCs to the upper chamber of the transwell system in PBMC media while CMEC media, minus HC, was maintained in the lower chamber (Daniels et al., 2013). While these culture conditions were sufficient for showing the capacity of the model to recapitulate a primary cell culture model, the mixed conditions are not ideal for studies concerning the impact of selected treatments on barrier function due to the added variable of media differences. The comparable results obtained from hCMEC/D3 cells and primary human BMECs in the previous investigations open the possibility that the co-culture media described here may additionally optimize PBMC co-culture with primary human BMECs and astrocytes.
5. Conclusions
These studies have established the optimal conditions for the co-culture of the hCMEC/D3 cell line with primary human PBMCs for use in BBB functional assays, in vitro. It was demonstrated that EBM-2 media and HC are cytotoxic to PBMCs, and the removal of these two components from a new media formulation yields an improved co-culture media. The results demonstrate that PBMCs cultured in this co-culture media remain viable for at least 24 hours. Additionally, incubation of hCMEC/D3 cells in co-culture media for 24 hours did not induce leakiness of the monolayer.
The new media formulation can thus be used for the simultaneous exposure of the two cell populations to additional treatment conditions or potential BBB insult of interest. Another key benefit of this co-culture media formulation has been the ability to study the interaction of the two cell populations following such treatments, allowing for the assessment of BBB function at both the endothelial and migrating cell level. Proper BBB function involves not only small molecule and ion exclusion, but, importantly, the regulation of cellular passage and immune surveillance between the periphery and the CNS. In the future, this co-culture media formulation may be used for the study of therapeutics, infectious diseases, autoimmunity, exogenous proteins, neuroinflammation, and other disease processes involving deregulation of cellular passage between the periphery and the CNS.
Highlights.
The immortalized human brain microvascular endothelial cells (BMEC) cell line, hCMEC/D3, in vitro blood brain barrier model is useful in long-term functional studies
hCMEC/D3 media components EBM-2 and hydrocortisone are cytotoxic to peripheral blood mononuclear cells (PBMCs)
Co-culture media excluding the toxic components, EBM-2 and hydrocortisone, support hCMEC/D3 barrier function as well as continued PBMC viability
Co-culture media can be used in assays of PBMC transmigration
Acknowledgments
These studies were funded in part by the Public Health Service, National Institutes of Health, through grants from the National Institute of Mental Health Comprehensive NeuroAIDS Core Center (CNAC) Developmental Grant, 1P30 MH-092177-01A (Nonnemacher, principal investigator), National Institute of Neurological Disorders and Stroke, NS32092 and NS46263, the National Institute of Drug Abuse, DA19807 (Wigdahl, principal investigator), and under the Ruth L. Kirschstein National Research Service Award 5T32MH079785 (Rappaport, principal investigator and Wigdahl, principal investigator of subcontract to Drexel University College of Medicine).
List of abbreviations
- BBB
Blood-brain barrier
- BMEC
Brain microvascular endothelial cells
- CNS
Central nervous system
- FITC-D
Fluorescein isothiocyanate dextran
- PBMC
Peripheral blood mononuclear cells
- Pe
Permeability coefficient
- PTFE
Polytetrafluoroethylene
- TEER
Transendothelial electrical resistance
Footnotes
7. Conflict of interest
The authors declare that they have no conflicts of interest.
The contents of the paper are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Marianne Strazza, Email: ms996@drexel.edu.
Monique E. Maubert, Email: mem446@drexel.edu.
Vanessa Pirrone, Email: vpirrone@drexelmed.edu.
Brian Wigdahl, Email: bwigdahl@drexelmed.edu.
Michael R. Nonnemacher, Email: mnonnema@drexelmed.edu.
References
- Bethel-Brown C, Yao H, Hu G, Buch S. Platelet-derived growth factor (PDGF)-BB-mediated induction of monocyte chemoattractant protein 1 in human astrocytes: implications for HIV-associated neuroinflammation. Journal of neuroinflammation. 2012;9:262. doi: 10.1186/1742-2094-9-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Liu W, Wang P, Xue Y, Su Q, Zeng C, Shang X. Endophilin-1 regulates blood-brain barrier permeability via EGFR-JNK signaling pathway. Brain Res. 2015;1606:44–53. doi: 10.1016/j.brainres.2015.02.032. [DOI] [PubMed] [Google Scholar]
- Cucullo L, Couraud PO, Weksler B, Romero IA, Hossain M, Rapp E, Janigro D. Immortalized human brain endothelial cells and flow-based vascular modeling: a marriage of convenience for rational neurovascular studies. J Cereb Blood Flow Metab. 2008;28:312–28. doi: 10.1038/sj.jcbfm.9600525. [DOI] [PubMed] [Google Scholar]
- Daniels BP, Cruz-Orengo L, Pasieka TJ, Couraud PO, Romero IA, Weksler B, Cooper JA, Doering TL, Klein RS. Immortalized human cerebral microvascular endothelial cells maintain the properties of primary cells in an in vitro model of immune migration across the blood brain barrier. J Neurosci Methods. 2013;212:173–9. doi: 10.1016/j.jneumeth.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deli MA, Abraham CS, Kataoka Y, Niwa M. Permeability studies on in vitro blood-brain barrier models: physiology, pathology, and pharmacology. Cellular and molecular neurobiology. 2005;25:59–127. doi: 10.1007/s10571-004-1377-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eigenmann DE, Xue G, Kim KS, Moses AV, Hamburger M, Oufir M. Comparative study of four immortalized human brain capillary endothelial cell lines, hCMEC/D3, hBMEC, TY10, and BB19, and optimization of culture conditions, for an in vitro blood-brain barrier model for drug permeability studies. Fluids and barriers of the CNS. 2013;10:33. doi: 10.1186/2045-8118-10-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eugenin EA, Berman JW. Chemokine-dependent mechanisms of leukocyte trafficking across a model of the blood-brain barrier. Methods. 2003;29:351–61. doi: 10.1016/s1046-2023(02)00359-6. [DOI] [PubMed] [Google Scholar]
- Gandhi N, Saiyed ZM, Napuri J, Samikkannu T, Reddy PV, Agudelo M, Khatavkar P, Saxena SK, Nair MP. Interactive role of human immunodeficiency virus type 1 (HIV-1) clade-specific Tat protein and cocaine in blood-brain barrier dysfunction: implications for HIV-1-associated neurocognitive disorder. J Neurovirol. 2010;16:294–305. doi: 10.3109/13550284.2010.499891. [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Nomura M, Yamagishi S, Harada S, Yamashita J, Yamamoto H. Induction of various blood-brain barrier properties in non-neural endothelial cells by close apposition to co-cultured astrocytes. Glia. 1997;19:13–26. [PubMed] [Google Scholar]
- Helms HC, Abbott NJ, Burek M, Cecchelli R, Couraud PO, Deli MA, Forster C, Galla HJ, Romero IA, Shusta EV, Stebbins MJ, Vandenhaute E, Weksler B, Brodin B. In vitro models of the blood-brain barrier: An overview of commonly used brain endothelial cell culture models and guidelines for their use. J Cereb Blood Flow Metab. 2016 doi: 10.1177/0271678X16630991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Eum SY, Andras IE, Hennig B, Toborek M. PPARalpha and PPARgamma attenuate HIV-induced dysregulation of tight junction proteins by modulations of matrix metalloproteinase and proteasome activities. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2009;23:1596–606. doi: 10.1096/fj.08-121624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob A, Potin S, Chapy H, Crete D, Glacial F, Ganeshamoorthy K, Couraud PO, Scherrmann JM, Decleves X. Aryl hydrocarbon receptor regulates CYP1B1 but not ABCB1 and ABCG2 in hCMEC/D3 human cerebral microvascular endothelial cells after TCDD exposure. Brain Res. 2015;1613:27–36. doi: 10.1016/j.brainres.2015.03.049. [DOI] [PubMed] [Google Scholar]
- Katsetos CD, Fincke JE, Legido A, Lischner HW, de Chadarevian JP, Kaye EM, Platsoucas CD, Oleszak EL. Angiocentric CD3(+) T-cell infiltrates in human immunodeficiency virus type 1-associated central nervous system disease in children. Clinical and diagnostic laboratory immunology. 1999;6:105–14. doi: 10.1128/cdli.6.1.105-114.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahm HW, Stein S. Characterization of recombinant human interleukin-2 with micromethods. J Chromatogr. 1985;326:357–61. doi: 10.1016/s0021-9673(01)87461-6. [DOI] [PubMed] [Google Scholar]
- Loeffler C, Dietz K, Schleich A, Schlaszus H, Stoll M, Meyermann R, Mittelbronn M. Immune surveillance of the normal human CNS takes place in dependence of the locoregional blood-brain barrier configuration and is mainly performed by CD3(+)/CD8(+) lymphocytes. Neuropathology : official journal of the Japanese Society of Neuropathology. 2011;31:230–8. doi: 10.1111/j.1440-1789.2010.01167.x. [DOI] [PubMed] [Google Scholar]
- Ludewig P, Sedlacik J, Gelderblom M, Bernreuther C, Korkusuz Y, Wagener C, Gerloff C, Fiehler J, Magnus T, Horst AK. CEACAM1 Inhibits MMP-9-Mediated Blood-Brain-Barrier Breakdown in a Mouse Model for Ischemic Stroke. Circulation research. 2013 doi: 10.1161/CIRCRESAHA.113.301207. [DOI] [PubMed] [Google Scholar]
- Miner JJ, Diamond MS. Mechanisms of restriction of viral neuroinvasion at the blood-brain barrier. Curr Opin Immunol. 2016;38:18–23. doi: 10.1016/j.coi.2015.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ousman SS, Kubes P. Immune surveillance in the central nervous system. Nature neuroscience. 2012;15:1096–101. doi: 10.1038/nn.3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieper C, Pieloch P, Galla HJ. Pericytes support neutrophil transmigration via interleukin-8 across a porcine co-culture model of the blood-brain barrier. Brain research. 2013;1524:1–11. doi: 10.1016/j.brainres.2013.05.047. [DOI] [PubMed] [Google Scholar]
- Pu H, Tian J, Andras IE, Hayashi K, Flora G, Hennig B, Toborek M. HIV-1 Tat protein-induced alterations of ZO-1 expression are mediated by redox-regulated ERK 1/2 activation. J Cereb Blood Flow Metab. 2005;25:1325–35. doi: 10.1038/sj.jcbfm.9600125. [DOI] [PubMed] [Google Scholar]
- Ransohoff RM, Engelhardt B. The anatomical and cellular basis of immune surveillance in the central nervous system. Nature reviews Immunology. 2012;12:623–35. doi: 10.1038/nri3265. [DOI] [PubMed] [Google Scholar]
- Reichel A, Begley DJ, Abbott NJ. An overview of in vitro techniques for blood-brain barrier studies. Methods in molecular medicine. 2003;89:307–24. doi: 10.1385/1-59259-419-0:307. [DOI] [PubMed] [Google Scholar]
- Ryan G, Grimes T, Brankin B, Mabruk MJ, Hosie MJ, Jarrett O, Callanan JJ. Neuropathology associated with feline immunodeficiency virus infection highlights prominent lymphocyte trafficking through both the blood-brain and blood-choroid plexus barriers. Journal of neurovirology. 2005;11:337–45. doi: 10.1080/13550280500186445. [DOI] [PubMed] [Google Scholar]
- Salmeri M, Motta C, Anfuso CD, Amodeo A, Scalia M, Toscano MA, Alberghina M, Lupo G. VEGF receptor-1 involvement in pericyte loss induced by Escherichia coli in an in vitro model of blood brain barrier. Cellular microbiology. 2013;15:1367–84. doi: 10.1111/cmi.12121. [DOI] [PubMed] [Google Scholar]
- Song J, Wu C, Korpos E, Zhang X, Agrawal SM, Wang Y, Faber C, Schafers M, Korner H, Opdenakker G, Hallmann R, Sorokin L. Focal MMP-2 and MMP-9 activity at the blood-brain barrier promotes chemokine-induced leukocyte migration. Cell Rep. 2015;10:1040–54. doi: 10.1016/j.celrep.2015.01.037. [DOI] [PubMed] [Google Scholar]
- Strazza M, Pirrone V, Wigdahl B, Nonnemacher MR. Breaking down the barrier: the effects of HIV-1 on the blood-brain barrier. Brain Res. 2011;1399:96–115. doi: 10.1016/j.brainres.2011.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urich E, Lazic SE, Molnos J, Wells I, Freskgard PO. Transcriptional profiling of human brain endothelial cells reveals key properties crucial for predictive in vitro blood-brain barrier models. PLoS One. 2012;7:e38149. doi: 10.1371/journal.pone.0038149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weksler B, Romero AI, Couraud PO. The hCMEC/D3 cell line as a model of the human blood brain barrier. Fluids and barriers of the CNS. 2013a;10 doi: 10.1186/2045-8118-10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weksler B, Romero IA, Couraud PO. The hCMEC/D3 cell line as a model of the human blood brain barrier. Fluids and barriers of the CNS. 2013b;10:16. doi: 10.1186/2045-8118-10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weksler BB, Subileau EA, Perriere N, Charneau P, Holloway K, Leveque M, Tricoire-Leignel H, Nicotra A, Bourdoulous S, Turowski P, Male DK, Roux F, Greenwood J, Romero IA, Couraud PO. Blood-brain barrier-specific properties of a human adult brain endothelial cell line. FASEB J. 2005;19:1872–4. doi: 10.1096/fj.04-3458fje. [DOI] [PubMed] [Google Scholar]