Abstract
Objective
To determine treatment frequency and duration of H2RA/PPI use among infants hospitalized within US children’s hospital NICUs and evaluate diagnoses/demographic factors associated with use.
Study design
We retrospectively analyzed a cohort of NICU infants admitted to 43 US children’s hospitals within the Pediatric Health Information System (PHIS) database between January 2006-March 2013 to determine H2RA/PPI treatment frequency, timing/duration of treatment, factors associated with use, percent of infants remaining on treatment at discharge, and inter-hospital prescribing variation. We used a modified Poisson regression to calculate the adjusted probability of infants ever receiving H2RAs/PPIs in relation to diagnosis, gestation, and sex.
Results
Of the 122,002 infants evaluated, 23.8% (n=28,989) ever received an H2RA or PPI; 19.0% received H2RAs (n=23187) and 10.5% (n=12823) received PPIs. Extremely preterm infants and term infants were the most likely to receive H2RA and PPI treatment. Infants with GERD [relative risk (RR): 3.13] and congenital heart disease (RR=2.41) had the highest H2RA/PPI treatment probabilities followed by those with an ENT diagnoses (RR=2.34; p<0.05). The majority of treated infants remained treated at discharge.
Conclusion
Despite limited evidence and increasing safety concerns, H2RAs/PPIs are frequently prescribed to extremely preterm neonates and those with congenital anomalies and continued through discharge. Our findings support the need for innovative studies to examine the comparative effectiveness and safety of H2RA/PPIs versus no treatment in these high-risk neonatal populations.
Keywords: neonatal, H2-receptor antagonist, proton pump inhibitor, pharmacoepidemiology, drug utilization, practice variation, gastroesophageal reflux disease, stress ulcer prophylaxis, comparative effectiveness, patient-centered outcomes
Acid suppressive medications including histamine-2 receptor antagonists (H2RAs) and proton pump inhibitors (PPIs), have been increasingly prescribed to neonates in recent years,[1–4] presumably for treatment of gastroesophageal reflux disease (GERD) and stress ulcer prophylaxis. However, neonatal patterns of reflux-type symptoms are different than those of older children or adults,[5–9] for which these drugs were US Food and Drug Administration (FDA) approved. Neonatal trials have not demonstrated improvement in GERD-attributed clinical symptoms following H2RA or PPI treatment.[10–12] Additionally, the effectiveness and safety of stress ulcer prophylaxis with H2RAs/PPIs is questioned, in adults and older children.[13–15] To our knowledge, no outcome-based data on prophylaxis or definitive therapy exists for neonates. H2RAs/PPIs are associated with adverse effects, [16–19] and the use of these acid suppressive medications in preterm infants has been associated with infections, necrotizing enterocolitis (NEC), and increased mortality risk.[20–24] Guidelines have cautioned against the liberal use of acid suppression medications, including H2RAs/PPIs in neonates.[25, 26] Others have reported that PPIs/H2RAs are frequently prescribed to infants.[1, 3, 4, 27, 28] However, to our knowledge, the duration of H2RA/PPI use among infants hospitalized in NICUs and the risk factors/comorbidities associated with H2RA/PPI treatment have never been fully examined. Identifying these variables will inform the design of trials to examine the comparative effectiveness of acid suppression versus non-treatment in the neonatal population. Therefore, our investigational objectives were to determine treatment frequency and duration for H2RA/PPI use among infants hospitalized in neonatal ICUs (NICUs) at US children’s hospitals, and to evaluate the association of clinical diagnoses, gestational age, and other demographic factors with H2RA/PPI prescribing patterns.
METHODS
We conducted a retrospective cohort study of infants admitted to NICUs within the 43 US children’s hospitals with NICUs participating in the Pediatric Health Information System (PHIS) (Children’s Hospital Association, Overland Park, KS) between January 2006 and March 2013. The Nationwide Children’s Institutional Review Board determined this was not human subjects’ research, because it was an analysis of pre-existing, de-identified data and involved no patient contact. PHIS, an administrative database, contains daily medication records as well as diagnostic data, duration of hospitalization, and demographic characteristics. The reliability and validity of PHIS has been documented in several previous studies.[29, 30]
We included all NICU patients with recorded gestational age that were admitted on postnatal day 0 or 1 and hospitalized in the NICU for a minimum of 7 days. We excluded infants admitted after postnatal day one to minimize exposure to unmeasured acid-suppressive treatments at outside hospitals and included only those who remained hospitalized ≥7 days to eliminate infants with short hospitalizations due to milder illnesses who might be treated in newborn nurseries instead of NICUs at some institutions and who were also unlikely to receive acid-suppressive medications.
Thompson-Reuters Healthcare (Ann Arbor, MI), the PHIS data processing partner, reorganizes each hospital’s daily charge codes into Clinical Transaction Classification (CTC) codes to ensure comparability of charge data among institutions. We evaluated the following PPIs: esomeprazole (CTC: 147201), dexlansoprazole (147203), lansoprazole (147205), pantoprazole (147211), omeprazole (147215), and rabeprazole (147221) and H2RAs: cimetidine (147101), famotidine (147105), ranitidine (147111) and nizatidine (147115).
International Statistical Classification of Diseases, 9th Revision (ICD-9) diagnostic codes: <24 weeks (765.21), 24 weeks (765.22), 25–26 weeks (765.23), 27–28 weeks (765.24), 29–30 weeks (765.25), 31–32 weeks (765.26), 33–34 weeks (765.27), 35–36 weeks (765.28), and ≥37 weeks (765.29) were used to classify gestational age at delivery. We gave precedence to ICD-9 codes because allowable billing charges are based on gestational age as reported via ICD-9. In instances when gestational age was indeterminable due to absent ICD-9 codes, gestational age reports from non-billing records were incorporated.
We created binary, composite variables for nine diagnostic categories [gastroesophageal reflux disease (GERD), otorhinolaryngology surgery (ENT), GI abnormality, congenital diaphragmatic hernia (CDH), congenital heart disease, congenital lung disease, infection, and neurologic abnormality] using ICD-9 codes (Table I; available at www.jpeds.com).
Table 1.
ICD-9 codes used to generate composite diagnostic variables
| Diagnostic Variable | ICD-9 Codes |
|---|---|
| Gastroesophageal reflux disease (GERD) | 530.81 |
| Congenital diaphragmatic hernia | 756.6 |
| GI abnormalities | bowel wall defect (756.72, 756.73); other GI anomalies (751.0, 751.1, 752.2, 751.3, 751.4, 751.5, 751.61, 751.62, 751.69, 751.7, 751.8), necrotizing enterocolitis (NEC) (777.50 777.51 777.52 777.53), short bowel syndrome (579.3), bowel perforation (779.3) |
| Operative ENT diagnoses | 748.0, 748.2, 748.3, 749.0, 749.2, 750.3, 747.00, 749.01, 749.02, 749.03, 749.04, 749.20, 749.21, 749.22, 749.23, 749.24, 749.25 |
| Congenital lung anomalies | 748.5, 748.61, 748.60, 748.69 |
| Congenital heart disease | 745.0, 745.69, 745.7, 745.1, 745.10, 745.11, 745.12, 745.19, 745.2, 745.3, 745.6, 745.0, 746.01, 746.02, 746.1, 746.2, 746.3, 746.4, 746.5, 746.6, 746.7, 746.81, 746.82, 746.83, 746.85, 746.87, 747.1, 747.11, 747.21, 747.22, 747.31, 747.40, 747.41, 747.42, 747.49 |
| Neurologic | periventricular leukomalacia (779.7), kernicterus (773.4, 774.7), seizures in newborn (779.0); congenital brain anomalies (740, 740.1, 740.2, 742.0, 742.1, 742.2, 742.3, 742.4) |
| Infections | 381, 382, 383, 383.4, 385, 386, 387, 388, 389, 771.81, 771.83, urinary tract infection of newborn (771.82), congenital pneumonia (774.0), congenital cytomegalovirus infection (771.1), listeria/malaria/toxoplasma (771.2, 771.8, 771.89) |
Daily, billed respiratory support as recorded in PHIS was used to assign a diagnosis of bronchopulmonary dysplasia (BPD). These infants must have been born before 29 weeks of gestation with a birth weight <1500 grams. We chose these gestational (<29 weeks) and birth weight (<1500 g) cutoffs to include >97% of infants with BPD.[31] We designated those infants with moderate or severe BPD according to the National Institutes of Health (NIH) Consensus Definition,[32] as modified by Ehrenkranz et al,[33] as having BPD by using respiratory charge data to identify those infants who received mechanical ventilation (CTC=521166), continuous positive airway pressure (CPAP) (CTC=521162), supplemental oxygen (CTC=521171), or a combination of these modalities for the first 28 consecutive postnatal days and who remained on supplemental oxygen, CPAP, or mechanical ventilation at 36 weeks postmenstrual age.
Data Analyses
All analyses were conducted by using Stata 14.0 (StataCorp, College Station, TX). We chose to calculate risk ratios rather than odds ratios because H2RA and PPI administration were common occurrences in our dataset. We used a modified Poisson regression with a robust between-cluster variance estimator to estimate adjusted risk ratios (relative risk) for H2RA/PPI receipt while controlling for clustering at the hospital level.[34, 35] Linear regression with a random intercept for hospitals was used to evaluate the association of clinical risk factors and diagnoses with the timing of the first H2RA or PPI doses in treated infants.
RESULTS
A total of 122,002 infants met inclusion criteria, of which 23.8% (n=28,989) ever received either an H2RA or PPI. The proportion of infants who ever received H2RAs was 19.0% (n=23,187), while 10.5% (n=12,823) ever received PPIs and 5.8% (n=7,021) ever received both at some point during their hospital stay. Patient characteristics including birth gestation and diagnoses are shown in Table II (available at www.jpeds.com). Table III (available at www.jpeds.com) demonstrates the frequency of H2RA/PPI receipt by gestation, sex, and major diagnoses. Within the cohort, 11.2% (n=13,625) of patients received an ICD-9 diagnosis of GERD. Of these infants diagnosed with GERD, 74.3% (n=10,127) were ever treated with an H2RA or PPI; 53.8% (n=7,330) ever received an H2RA and 46.5% (n=6,331) a PPI.
Table 2.
Cohort Demographics
| Full Sample N=122,002 |
Gestational age < 35 weeks N=53,727 (44.0%) |
Gestational age ≥ 35 weeks N=66,809 (54.8%) |
|
|---|---|---|---|
| n (%) | n (%) | n (%) | |
| Birth Gestation | |||
| ≤ 24 weeks | 2547 (2.1%) | 2547 (4.7%) | - |
| 25–26 weeks | 4764 (3.9%) | 4764 (8.9%) | - |
| 27–28 weeks | 6289 (5.2%) | 6289 (11.7%) | - |
| 29–30 weeks | 7681 (6.3%) | 7681 (14.3%) | - |
| 31–32 weeks | 11879 (9.7%) | 11879 (22.1%) | - |
| 33–34 weeks | 20567 (16.9%) | 20567 (38.3%) | - |
| 35–36 weeks | 22327 (18.3%) | - | 22327 (33.4%) |
| ≥ 37 weeks | 44482 (36.5%) | - | 44482 (66.6%) |
| GA missing | 1466 (1.2%) | - | - |
| Sex | |||
| Female | 53355 (43.7%) | 24606 (45.8%) | 28144 (42.1%) |
| Early Diagnoses | |||
| CDH | 2500 (2.1%) | 258 (0.5%) | 2195 (3.3%) |
| Congenital Lung Anomaly | 2265 (1.9%) | 878 (1.6%) | 1352 (2.0%) |
| Congenital Heart Anomaly | 13624 (11.2%) | 2522 (4.7%) | 10918 (16.3%) |
| Neurologic | 14833 (12.2%) | 6572 (12.2%) | 7994 (12.0%) |
| Operative ENT diagnosis | 5742 (4.7%) | 1346 (2.5%) | 4290 (6.4%) |
| Later Outcomes | |||
| BPD at 28 days | 6296 (5.2%) | 6257 (11.7%) | 39 (0.06%) |
| Moderate or Severe BPD at | 3263 (2.7%) | 3239 (6.0%) | 24 (0.04%) |
| 36 weeks | |||
| Discharged home | 100291 (82.2%) | 42258 (78.7%) | 56950 (85.2%) |
| Transferred | 16153 (13.2%) | 8805 (16.4%) | 7060 (10.6%) |
| Discharge missing | 623 (0.5%) | 254 (0.5%) | 358 (0.5%) |
| Died | 4935 (4.1%) | 2410 (4.5%) | 2441 (3.7%) |
| Early or late diagnosis | |||
| GERD | 13625 (11.2%) | 7310 (13.6%) | 6193 (9.3%) |
| GI abnormality | 11092 (9.1%) | 4148 (7.7%) | 6782 (10.2%) |
| Infection | 33751 (27.7%) | 15957 (29.7%) | 17282 (25.9%) |
Percentiles were derived by using column N as the denominator.
Table 3.
Frequency of H2RA and PPI Use by Gestational Age and Diagnosis
| Cohort N=122,002 | Ever received either a PPI or an H2RA N=28989 (23.8%) |
Ever received an H2RA N=23187 (19.0%) |
Ever received a PPI N=12823 (10.5%) |
Ever received both a PPI and an H2RA N=7021 (5.8%) |
|
|---|---|---|---|---|---|
| n(%) | n(%) | n(%) | n(%) | ||
| Birth Gestation | |||||
| ≤ 24 weeks (n=2547) | 962 (3.3%) (37.8%; ≤24 wks) | 731 (3.2%) (28.7%; ≤24 wks) | 461 (3.6%) (18.1%; ≤24 wks) | 230 (3.3%) (9.0%; ≤24 wks) | |
| 25–26 weeks (n=4764) | 1795 (6.2%) (37.7%; 25–26 wks) | 1315 (5.7%) (27.6%; 25–26 wks) | 932 (7.3%) (19.6%; 25–26 wks) | 452 (6.4%) (9.5%; 25–26 wks) | |
| 27–28 weeks (n=6289) | 1839 (6.3%) (29.2%; 27–28 wks) | 1315 (5.7%) (20.9%; 27–28 wks) | 935 (7.3%) (14.9%; 27–28 wks) | 411 (5.9%) (6.5%; 27–28 wks) | |
| 29–30 weeks (n=7681) | 1591 (5.5%) (20.7%; 29–30 wks) | 1129 (4.9%) (14.7%; 29–30 wks) | 791 (6.2%) (10.3%; 29–30 wks) | 329 (4.7%) (4.3%; 29–30 wks) | |
| 31–32 weeks (n=11879) | 1737 (6.0%) (14.6%; 31–32 wks) | 1299 (5.6%) (10.9%; 31–32 wks) | 854 (6.7%) (7.2%; 31–32 wks) | 416 (5.9%) (3.5%; 31–32 wks) | |
| 33–34 weeks (n=20567) | 2421 (8.4%) (11.8%; 33–34 wks) | 1891 (8.2%) (9.2%; 33–34 wks) | 1109 (8.7%) (5.4%; 33–34 wks) | 579 (8.3%) (2.8%; 33–34 wks) | |
| 35–36 weeks (n=22327) | 4942 (17.1%) (22.1%; 35–36 wks) | 4008 (17.3%) (18.0%; 35–36 wks) | 2182 (17.0%) (9.8%; 35–36 wks) | 1248 (17.8%) (5.6%; 35–36 wks) | |
| ≥ 37 weeks (n=44482) | 13214 (45.6%) (29.7%; ≥37 weeks) | 11111 (47.9%) (25.0%; ≥37 weeks) | 5329 (41.6%) (12.0%; ≥37 weeks) | 3226 (46.0%) (7.3%; ≥37 weeks) | |
| GA missing (n=1466) | 488 (1.7%) | 388 (1.7%) | 230 (1.8%) | 130 (1.9%) | |
| Sex | |||||
| Female (n=53355) | 12605 (43.5%) | 10091 (43.5%) | 5626 (43.9%) | 3112 (44.3%) | |
| Early Diagnoses | |||||
| CDH (n=2500) | 1787 (6.2%) | 1472 (6.4%) | 1017 (7.9%) | 702 (10.0%) | |
| Congenital Lung Anomaly (n=2265) | 1269 (4.4%) | 1064 (4.6%) | 682 (5.3%) | 477 (6.8%) | |
| Congenital Heart Anomaly (n=13624) | 8016 (27.7%) | 7079 (30.5%) | 3192 (25.0%) | 2255 (32.1%) | |
| Neurologic (n=14833) | 4855 (16.8%) | 3868 (16.7%) | 2242 (17.5%) | 1255 (17.9%) | |
| Operative ENT diagnosis (n=5742) | 3843 (13.3%) | 2913 (12.6%) | 2400 (18.7%) | 1470 (20.9%) | |
| Later Outcomes | |||||
| BPD at 28 days (n=6296) | 2551 (8.8%) | 1867 (8.1%) | 1339 (10.4%) | 655 (9.3%) | |
| Moderate or Severe BPD at 36 weeks (n=3263) | 1638 (5.6%) | 1201 (5.2%) | 914 (7.1%) | 477 (6.8%) | |
| Discharged home (n=100291) | 21776 (75.1%) | 17306 (74.6%) | 9453 (73.7%) | 4983 (71.0%) | |
| Transferred (n=16153) | 4863 (16.8%) | 3829 (16.5%) | 2416 (18.8%) | 1382 (19.6%) | |
| Discharge missing (n=623) | 212 (0.7%) | 163 (0.7%) | 131 (1.0%) | 82 (1.2%) | |
| Died (n=4935) | 2138 (7.4%) | 1889 (8.2%) | 823 (6.4%) | 574 (8.2%) | |
| Early or late diagnosis | |||||
| GERD (n=13625) | 10127 (34.9%) | 7330 (31.6%) | 6331 (49.3%) | 3534 (50.3%) | |
| GI abnormality (n=11092) | 5935 (20.5%) | 4803 (20.7%) | 2926 (22.8%) | 1794 (25.6%) | |
| Infection (n=33751) | 10490 (36.2%) | 8362 (36.1%) | 5040 (39.3%) | 2912 (41.5%) | |
Percentiles were derived using column N as the denominator. A row N (in italics) denominator is also reported for gestational age categories with column percentiles adding to 100%; row percentiles do not.
Timing of Treatment
Within the whole cohort, the median postnatal age for first H2RA or PPI receipt was 10 days (25th–75th%: 3–28) (range 0–361). Median treatment duration was 15 days (25th–75th%: 6–35) (range 1–1244). Initial H2RA treatment was at a median of 7 days (25th–75th%: 2–23), earlier than the 15-day median (25th–75th%: 4–37) for first PPI treatment. Median H2RA (10 days with 25th–75th%: 4–22) treatment duration was shorter than for PPIs (19 days with 25th–75th%: 8–39).
Treatment Timing and Frequency by Gestational Age
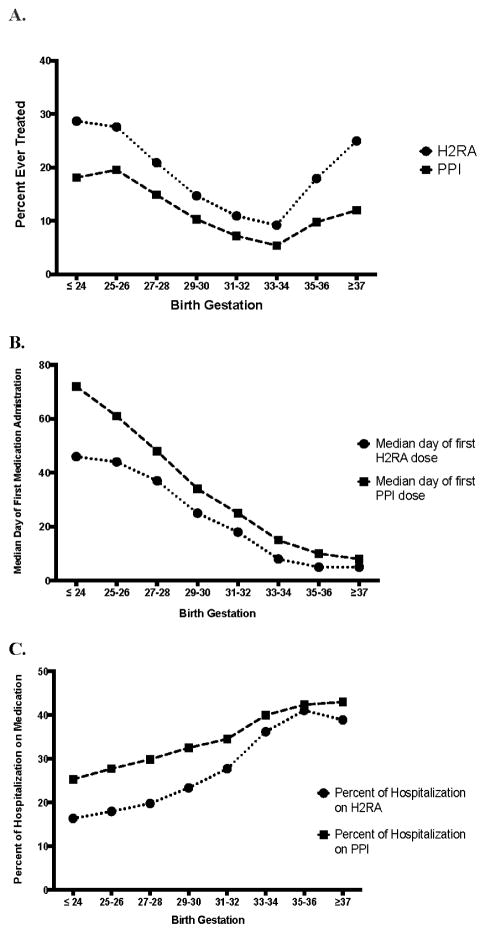
Most treated infants were born at late-preterm (35–36 weeks) or term (≥37 weeks) gestations (Table III). However, when viewed proportionally by birth gestation, H2RA and PPI treatment percentiles take on a bimodal, U-shaped distribution with extremely-preterm infants as or even more likely to be treated relative to late-preterm or term infants (Figure 1, A). Among infants born at ≤24-weeks gestation, 28.7% were ultimately treated with H2RAs and 18.1% with PPIs; with 27.6% H2RA treatment and 19.6% PPI treatment at 26–26 weeks gestations. In comparison, among term infants, 25.0% were treated with H2RAs and only 12.0% with PPIs.
Figure 1.
A. Percent of infants ever treated with an H2RA or PPI by gestational age. B. Median day that treated infants received their first dose of H2RAs by gestational age. C. Mean percent of days that treated infants received H2RAs and PPIs during their hospitalization (total days treated/total days hospitalized) by gestational age.
An infant’s postnatal age at first H2RA and PPI receipt decreased with increasing gestational age at birth in a near-linear fashion (Figure 1, B). The percent of the total hospitalization that an infant received H2RAs/PPIs increased with gestational maturity at birth (Figure 1, C). Although the majority of the most preterm infants (birth gestation ≤ 26 weeks) were treated later in their hospitalization, 36.1% ever treated with H2RAs and 17.1% ever treated with PPIs received their first medication dose in the neonatal period (≤ postnatal day 28).
Treatment Initiation by Diagnosis
In addition to gestational age, specific medical diagnoses had a large impact on the timing of H2RA/PPI administration (Table IV; available at www.jpeds.com). After accounting for gestational age, sex, and other diagnoses, infants with a GERD diagnosis received their first H2RA dose an average of 5.2 days (95% CI: 4.5, 5.8) later than other cohort infants that were treated with H2RAs. However, initial PPI dose timing was not significantly different [0.8 days (95% CI: −0.1, 1.7)] in infants with a GERD diagnosis. A diagnosis of severe BPD at 36 weeks postmenstrual age was associated with a 19.2 day increase (95% CI: 17.7, 20.7) in the H2RA treatment start date and 20.4 day increase in PPI start date (95%CI: 18.5, 22.4), after controlling for gestational age and other diagnoses. Infants with ENT anomalies, congenital lung anomalies, congenital heart disease, and congenital diaphragmatic hernia were more likely to be treated early in their hospitalization.
Table 4.
Median Postnatal Age at First H2RA and PPI Administration
| Diagnoses | First Dose H2RA (Postnatal Days) | First Dose PPI (Postnatal Days) | ||
|---|---|---|---|---|
| Median First Dose | Linear regressiona | Median First Dose | Linear regressiona | |
| Postnatal days (25th–75th percentile) | Coefficient (95% CI) | Postnatal days (25th–75th percentile) | Coefficient (95% CI) | |
| All treated patients | 7 (2–23) | 15 (4–37) | ||
| Diagnoses | ||||
| GERD | 14 (4–36) | 5.2 (4.5, 5.8) | 19 (6–42) | 0.8 (−0.1, 1.7) |
| Cong. Heart Disease | 6 (3–13) | −2.5 (−3.2, −1.8) | 8 (4–20) | −5.1 (−6.2, −4.0) |
| GI | 8 (2–27) | 3.9 (3.2, 4.6) | 14 (3–36) | 3.6 (2.5, 4.7) |
| ENT | 7 (2–20) | 1.3 (0.5, 2.2) | 9 (2–25) | −0.6 (−1.7, 0.6) |
| CDH | 4 (1–13) | −3.0 (−4.3, −1.7) | 6 (1–18) | −5.4 (−6.2, −4.0) |
| Moderate/Sever e BPD | 53 (25–85) | 19.2 (17.7, 20.7) | 65 (38–91) | 20.4 (18.5, 22.4) |
| Infection | 10 (3–33) | 4.0 (3.3, 4.6) | 21 (6–51) | 5.6 (4.7, 6.6) |
| Neurologic | 12 (4–36) | 3.2 (2.4, 4.0) | 21 (7–54) | 4.2 (3.0, 5.3) |
| Cong. lung anomaly | 8 (2–27) | 3.8 (2.3, 5.2) | 12 (2–35) | 5.6 (3.5, 7.7) |
| Birth Gestation | ||||
| ≥37 weeks (reference) | 5 (2–11) | - | 8 (2–17) | - |
| 35–36 weeks | 5 (1–14) | 0.9 (0.1, 1.7) | 10 (2–24) | 1.1 (−0.1, 2.4) |
| 33–34 weeks | 8 (2–22) | 4.2 (3.1, 5.3) | 15 (4–30) | 4.6 (3.0, 6.3) |
| 31–32 weeks | 18 (5–35) | 9.9 (8.6, 11.2) | 25 (10–41) | 10.6 (8.7, 12.4) |
| 29–30 weeks | 25 (7–48) | 15.1 (13.7, 16.5) | 34 (18–52) | 16.9 (15.0, 18.8) |
| 27–28 weeks | 37 (15–64) | 19.8 (18.5, 21.2) | 48 (28–69) | 20.3 (18.4, 22.2) |
| 25–26 weeks | 44 (18–80) | 24.0 (22.5, 25.4) | 61 (35–85) | 28.7 (26.7, 30.7) |
| ≥24 weeks | 46 (21–92) | 34.0 (31.0, 37.0) | 72 (42–105) | 45.3 (40.8, 49.8) |
| Sex | ||||
| Female | 7 (2–23) | 0.2 (−0.3, 0.8) | 15 (4–37) | 0.6 (−0.2, 1.5) |
| Male | 7 (2–22) | - | 15 (4–37) | - |
Linear regression with a random intercept for hospital
Multivariable Adjusted Relative Risk of Ever Receiving H2RA or PPI
In our multivariable model, the largest influence on the probability of an infant ever receiving an H2RA/PPI, after controlling for gestation and sex, was receipt of a GERD diagnosis (Table V). Relative to infants without a GERD diagnosis, those diagnosed with GERD had 2.68 (95% CI: 2.36, 3.03) times the risk of ever receiving an H2RA and 5.37 (95% CI: 4.46, 6.46) the risk of ever receiving a PPI. Infants with congenital heart disease had the next highest probability of having ever received an H2RA or PPI (relative risk 2.41 (95% CI: 2.11, 2.75). ENT diagnoses, typically co-managed by pediatric otorhinolaryngologists, had the second highest relative risk of ever receiving a PPI [2.34 (95% CI 2.13, 2.58)] after infants with a GERD diagnosis. Diagnoses in the categories of GI abnormality, congenital diaphragmatic hernia, moderate/severe BPD at 36 weeks, infection, congenital lung abnormality, and neurologic abnormality were also all associated with an increased probability of receiving H2RA/PPI treatment.
Table 5.
Multivariable Adjusted Risk of Receiving H2RA or PPI
| Ever Received H2RA or PPI | Ever Received H2RA | Ever Received PPI | |
|---|---|---|---|
| Relative Risk (95% CI) | Relative Risk (95% CI) | Relative Risk (95% CI) | |
| Diagnoses | |||
| GERD | 3.13 (2.72, 3.59) | 2.68 (2.36, 3.03) | 5.37 (4.46, 6.46) |
| Cong. Heart Disease | 2.41 (2.11, 2.75) | 2.77 (2.30, 3.35) | 1.96 (1.67, 2.29) |
| GI | 1.98 (1.85, 2.12) | 2.03 (1.84, 2.24) | 2.08 (1.89, 2.29) |
| CDH | 1.79 (1.61, 1.98) | 1.83 (1.63, 2.06) | 2.08 (1.78, 2.42) |
| ENT | 1.74 (1.62, 1.87) | 1.63 (1.49, 1.80) | 2.34 (2.13, 2.58) |
| Moderate/Severe BPD | 1.34 (1.19, 1.50) | 1.34 (1.17, 1.53) | 1.42 (1.16, 1.74) |
| Infection | 1.23 (1.12, 1.35) | 1.26 (1.12, 1.41) | 1.29 (1.16, 1.43) |
| Cong. lung anomaly | 1.15 (1.06, 1.26) | 1.21 (1.10, 1.34) | 1.15 (0.98, 1.34) |
| Neurologic | 1.12 (1.06, 1.18) | 1.13 (1.05, 1.22) | 1.09 (1.02, 1.17) |
| Birth Gestation | |||
| ≥37 weeks (reference) | - | - | - |
| 35–36 weeks | 0.88 (0.82, 0.94) | 0.86 (0.80, 0.93) | 0.97 (0.90, 1.04) |
| 33–34 weeks | 0.56 (0.50, 0.62) | 0.54 (0.47, 0.60) | 0.66 (0.59, 0.75) |
| 31–32 weeks | 0.64 (0.59, 0.70) | 0.60 (0.53, 0.67) | 0.78 (0.69, 0.88) |
| 29–30 weeks | 0.80 (0.72, 0.89) | 0.71 (0.63, 0.81) | 0.92 (0.78, 1.09) |
| 27–28 weeks | 0.94 (0.84, 1.06) | 0.85 (0.74, 0.99) | 1.04 (0.88, 1.23) |
| 25–26 weeks | 1.08 (0.95, 1.22) | 0.998 (0.85, 1.2) | 1.18 (1.01, 1.38) |
| ≤24 weeks | 1.08 (0.90, 1.29) | 1.04 (0.82, 1.31) | 1.10 (0.84, 1.43) |
| Sex | |||
| Female | 0.98 (0.96, 0.997) | 0.98 (0.96, 1.0) | 0.99 (0.95, 1.02) |
Data are shown as adjusted risk ratios (95% confidence interval). Regression risk ratios for gestational age are reported relative to a reference group of ≥37-week gestation infants. Risk ratios were determined by using a modified Poisson regression model with a robust between-cluster variance estimator with a random intercept for hospital. Race/ethnicity categories were excluded from the model after they were found to not have a statistically significant influence on the probability of ever receiving an H2RA or PPI.
H2RA and PPI Use at Discharge
Among treated patients, 56.0% (n=16230) ever treated with either an H2RA or PPI were still receiving an H2RA/PPI at discharge. An even higher proportion of infants that ever received a PPI remained on a PPI at discharge (75.7%; n=9701). Among those that ever received an H2RA, 51.7% (n=11993) were receiving an H2RA at discharge.
National and Hospital-Level Trends in H2RA and PPI Utilization
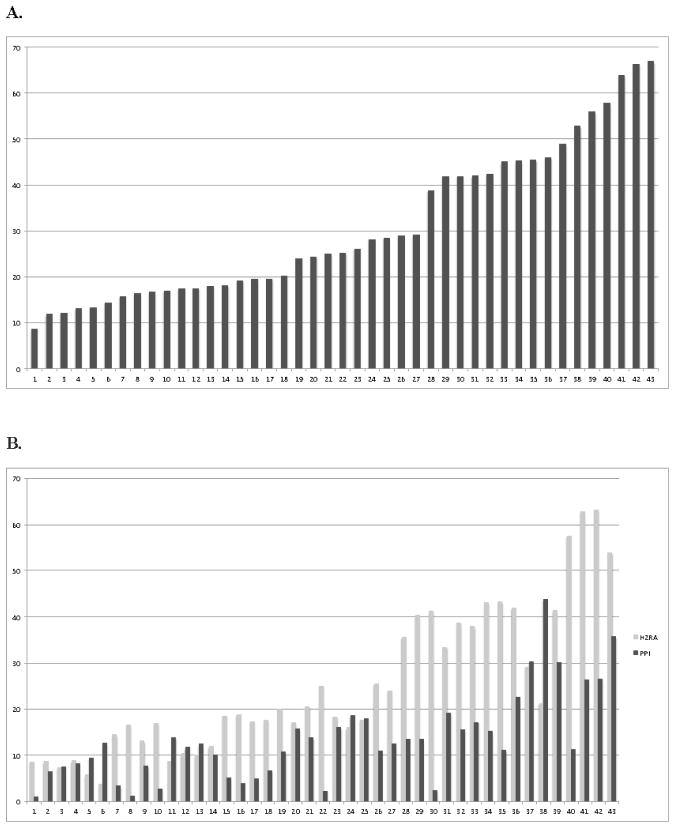
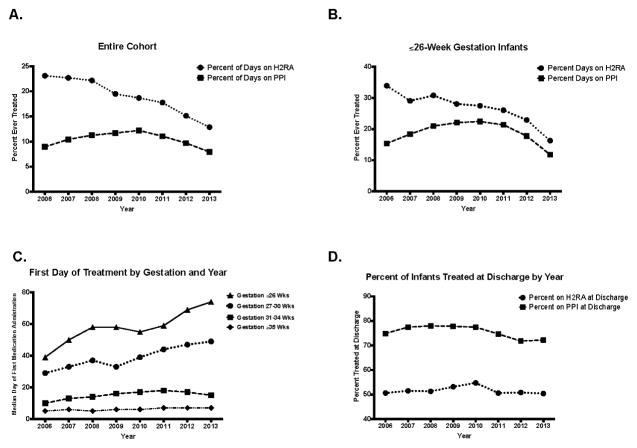
Hospitals varied both in frequency of H2RA/PPI utilization and ratio of H2RA to PPI use (Figure 2, A and B). H2RAs predominated at most hospitals, but a minority (23.3%; n=10 of 43 hospitals) prescribed PPIs more frequently. H2RA utilization declined annually (23.1% in 2006 vs. 12.9% in 2013). In contrast, PPI use increased from 2006 until 2010, reaching a peak of 12.2%, before declining to 7.9% in 2013 (Figure 3, A). H2RA and PPI use in the most preterm infants (≤26-weeks gestation) followed similar patterns (Figure 3, B). The median postnatal age at first H2RA and PPI treatment steadily increased in ≤30-week gestation infants between 2006–2013 (Figure 3, C). However, for all gestational ages, there was little change in the percentage of infants discharged on an H2RA or PPI over the study period (Figure 3, D).
Figure 2.
A, Percent H2RA and PPI utilization by hospital for infants that ever received either an H2RA or PPI during their hospitalization. B, Percent ever receiving a H2RA and percent ever receiving a PPI by hospital.
Figure 3.
A. Percent of all cohort infants ever treated with an H2RA or PPI by year. B. Percent of infants born at ≤26-weeks gestation that were ever treated with an H2RA or PPI by year. C. Gestational age-specific median day of first H2RA and PPI administration by year. D. Percent of cohort infants that continue to be treated with an H2RA or PPI at the time of NICU discharge.
DISCUSSION
Our investigation demonstrates H2RAs/PPIs are frequently prescribed to neonates in US children’s hospitals, often to discharge, despite a lack of published evidence for improved outcomes following their administration[10–12, 36–38] and increasing concerns for adverse effects.[25, 26, 39] By proportion, the highest frequencies of overall acid suppressive treatment occurred in extremely-preterm, late-preterm, and term infants. The highest frequency of PPI treatment occurred in extremely-preterm infants. The majority of infants ever started on an H2RA or PPI, and ~75% of PPI users, remained on treatment at discharge. Although, as expected, the highest adjusted probability of H2RA and/or PPI treatment occurred in infants with a GERD diagnosis, we unexpectedly found those with congenital heart disease had the next highest treatment probability with either drug (H2RA or PPI) and those with ENT diagnoses had the second highest probability of receiving PPIs.
The median age at first H2RA/PPI treatment decreased nearly linearly with increasing birth gestation. As a result, treated term infants spent the greatest proportion of their hospitalization on acid suppressive medications. On average, most preterm infants started H2RAs/PPIs at a median postmenstrual age near normal oral feeding introduction (33–34 weeks). Therefore, we speculate that aerodigestive symptoms including feeding concerns and respiratory events purported to be due to GERD likely played a large role in clinicians’ decisions to start H2RAs/PPIs for many preterm infants. Nevertheless, ~25% of infants born at or below 26 weeks were started on H2RAs/PPIs during the first postnatal month, a time early in their development when they are at high risk for infection and morbidities such as NEC that have been associated with acid suppression. Total use decreased and the median first day of treatment increased in ≤30-week gestation infants over 2006–2013, perhaps indicating that physicians have become more cautious with H2RA/PPI use given increasing safety concerns. Nonetheless, there was little change in the proportion of infants remaining on treatment at discharge.
Clinical diagnoses affected the timing of treatment initiation. Although 74% of infants with a GERD diagnosis were treated with H2RA/PPIs, the number of treated patients without a GERD diagnosis, just slightly under two-thirds, was unexpected. Median timing of H2RA/PPI treatment initiation began at an older age for infants with a GERD diagnosis than the overall population. On average, preterm infants diagnosed with moderate/severe BPD began acid suppressive medications late in their hospitalization, similar to other preterm infants. Conversely, infants with congenital anomalies received H2RAs/PPIs at an earlier than median age. Because treatment initiation for all the diagnoses we examined, except for moderate/severe BPD, occurred prior to the median treatment date of infants with a GERD diagnosis (14 postnatal days), this likely suggests that clinicians are starting acid suppressive medication for other reasons such as stress ulcer prophylaxis. We speculate that the sustained decrease in H2RA utilization since 2006 and decrease in PPI utilization since 2010 may be due to changes in clinical practice related to published evidence showing lack of efficacy and even harm for H2RA and PPI use in infants.[21–24]
The utility of pharmacologic acid suppression in neonates has been questioned because the gastric pH of infants typically remains > 4.0 for 75–90% of the time due to milk buffering. [40, 41] Uncomplicated GER symptoms usually resolve by 12 months of age without treatment.[42] The few available studies in preterm infants failed to show a reduction in symptoms clinically attributed to GERD following acid suppression.[10, 11, 25, 37]
More worrisome is the concern gastric acid suppression may be harmful because stomach acidity plays an important role in the gastrointestinal tract host defense as a barrier to harmful pathogens.[16, 23, 39, 43] H2RA treatment of infants has been associated with a higher bloodstream infection risk.[44–46] Acid suppression in 4–36 month-olds with increased pneumonia and gastroenteritis.[47]. A prospective cohort showed ranitidine to be associated with infection, NEC, and increased mortality.[24] A nested case-control demonstrated a significant relationship between prior H2RA use and NEC.[22] A double-blinded, multicenter RCT by Orenstein et al found no improvement in GERD-associated events following omeprazole administration to infants; instead serious adverse events including respiratory events were more common.[11] In adults, PPIs have been associated with hospital-acquired pneumonia,[17] acute interstitial nephritis[48], hypergastrinemia-induced hyperplasia of histamine-secreting gastric enterochromaffin-like cells,[48] osteoporosis-related fractures[18], vitamin B12 deficiency,[49] and myocardial infarction.[19] The effects of long-term acid suppression on the developing gastric mucosa and bones of preterm infants are unknown.[36] Given these findings and two multicenter RCTs showing no improvement in GERD-associated events in infants,[10, 11] the FDA concluded PPIs should only be prescribed to infants with documented acid-induced conditions following a trial of conservative measures.[25] Almost all trials and large studies examining the effect of acid suppression on GERD relied on non-specific clinical symptoms commonly attributed to GERD.[12] To determine if H2RAs/PPIs are beneficial for neonatal GERD management, future comparative effectiveness trials should differentiate true GERD-related clinical events from similar non-GERD related clinical events via a clear physiological GERD diagnosis prior to randomization.
H2RAs/PPIs are used in ill adults and older children for stress ulcer prophylaxis and there is controversy regarding the safety and effectiveness of population-wide, empiric prophylactic therapy.[13–15, 50] Given the early timing of H2RA/PPI use for cohort patients without a GERD diagnosis and our clinical experiences, we speculate most early acid suppression in ill neonates is for prophylactic reasons or bloody nasogastric tube aspirates. In light of a lack of studies demonstrating the safety and effectiveness of these practices in improving important outcomes, our findings support the need for further study.
Our study design has multiple strengths including a generous sample size and rich pharmacological information within PHIS that allowed us to determine daily H2RA/PPI utilization for each patient. Although our investigation was limited to 43 freestanding US Children’s Hospitals, our findings are likely generalizable to other US NICUs because most included hospitals serve as major neonatal training centers.
Our study is limited by its retrospective design. The data in PHIS was initially collected for hospital administrative purposes instead of research. Although we used hospital charge data for specific date of service to determine days on mechanical ventilation, CPAP, and oxygen, and dates of H2RA/PPI administration, we had to rely on less specific ICD-9 codes for discharge diagnoses of GERD, IVH, PDA, and NEC. Potential recording errors might reduce the accuracy of our diagnoses. PHIS data is rigorously screened for errors and rejected when quality thresholds are not met. A reliance on discharge diagnoses prevented us from examining temporal associations between medication administration and diagnoses, including the impact of H2RAs and PPIs on NEC and sepsis incidence. We therefore chose not to study the causal effects of H2RAs/ PPIs on important clinical outcomes such as mortality, feeding milestones, and length of stay or evaluate the H2RA/PPI safety.
Many infants identified in this study as having GERD diagnoses may not have had true pathologic GERD, as diagnosed by pH impedance and manometry, similar to recent RCTs that randomized infants with presumed GERD to acid suppressive therapy.[10, 11, 51] The ICD-9 diagnosis of GERD itself is highly relevant because it indicates a clinical presumption that the infant had GERD, and reflects providers’ practice thought patterns.[52] Clinical presumptions, not only concrete diagnoses, often drive treatment decisions.
Our findings support the need for further studies to examine the comparative effectiveness of pharmacologic acid suppression versus no treatment, as well as the safety of these medications for neonatal patients.
Acknowledgments
Supported by the National Institutes of Health (NIH; 1K08HL121182 [to J.S.] and 2R01DK068158 [to S.J.]). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Abbreviations
- CHA
Children’s Hospital Association
- CI
confidence interval
- CPAP
continuous positive airway pressure
- H2RA
H2-receptor antagonist
- ICD-9
International Classification of Diseases, Ninth Revision
- IVH
intraventricular hemorrhage
- NEC
necrotizing enterocolitis
- NICU
neonatal intensive care unit
- PDA
patent ductus arteriosus
- PHIS
Pediatric Health Information System
- PPI
proton pump inhibitor
- RR
relative risk
- SD
standard deviation
Footnotes
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Clark RH, Bloom BT, Spitzer AR, Gerstmann DR. Reported medication use in the neonatal intensive care unit: data from a large national data set. Pediatrics. 2006;117:1979–87. doi: 10.1542/peds.2005-1707. [DOI] [PubMed] [Google Scholar]
- 2.Illueca M, Alemayehu B, Shoetan N, Yang H. Proton pump inhibitor prescribing patterns in newborns and infants. J Pediatr Pharmacol Ther. 2014;19:283–7. doi: 10.5863/1551-6776-19.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Malcolm WF, Gantz M, Martin RJ, Goldstein RF, Goldberg RN, Cotten CM. Use of medications for gastroesophageal reflux at discharge among extremely low birth weight infants. Pediatrics. 2008;121:22–7. doi: 10.1542/peds.2007-0381. [DOI] [PubMed] [Google Scholar]
- 4.Hsieh EM, Hornik CP, Clark RH, Laughon MM, Benjamin DK, Jr, Smith PB. Medication use in the neonatal intensive care unit. Am J Perinatol. 2014;31:811–22. doi: 10.1055/s-0033-1361933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jadcherla SR. Gastroesophageal reflux in the neonate. Clin Perinatol. 2002;29:135–58. doi: 10.1016/s0095-5108(03)00068-x. [DOI] [PubMed] [Google Scholar]
- 6.Jadcherla SR, Parks VN, Peng J, Dzodzomenyo S, Fernandez S, Shaker R, et al. Esophageal sensation in premature human neonates: temporal relationships and implications of aerodigestive reflexes and electrocortical arousals. Am J Physiol Gastrointest Liver Physiol. 2012;302:G134–44. doi: 10.1152/ajpgi.00067.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jadcherla SR, Duong HQ, Hoffmann RG, Shaker R. Esophageal body and upper esophageal sphincter motor responses to esophageal provocation during maturation in preterm newborns. J Pediatr. 2003;143:31–8. doi: 10.1016/S0022-3476(03)00242-7. [DOI] [PubMed] [Google Scholar]
- 8.Jadcherla SR, Gupta A, Fernandez S, Nelin LD, Castile R, Gest AL, et al. Spatiotemporal characteristics of acid refluxate and relationship to symptoms in premature and term infants with chronic lung disease. Am J Gastroenterol. 2008;103:720–8. doi: 10.1111/j.1572-0241.2007.01748.x. [DOI] [PubMed] [Google Scholar]
- 9.Jadcherla SR. Pathophysiology of aerodigestive pulmonary disorders in the neonate. Clin Perinatol. 2012;39:639–54. doi: 10.1016/j.clp.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davidson G, Wenzl TG, Thomson M, Omari T, Barker P, Lundborg P, et al. Efficacy and safety of once-daily esomeprazole for the treatment of gastroesophageal reflux disease in neonatal patients. J Pediatr. 2013;163:692–8. e1–2. doi: 10.1016/j.jpeds.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 11.Orenstein SR, Hassall E, Furmaga-Jablonska W, Atkinson S, Raanan M. Multicenter, double-blind, randomized, placebo-controlled trial assessing the efficacy and safety of proton pump inhibitor lansoprazole in infants with symptoms of gastroesophageal reflux disease. J Pediatr. 2009;154:514–20. e4. doi: 10.1016/j.jpeds.2008.09.054. [DOI] [PubMed] [Google Scholar]
- 12.Poets CF. Gastroesophageal reflux: a critical review of its role in preterm infants. Pediatrics. 2004;113:e128–32. doi: 10.1542/peds.113.2.e128. [DOI] [PubMed] [Google Scholar]
- 13.Reveiz L, Guerrero-Lozano R, Camacho A, Yara L, Mosquera PA. Stress ulcer, gastritis, and gastrointestinal bleeding prophylaxis in critically ill pediatric patients: a systematic review. Pediatr Crit Care Med. 2010;11:124–32. doi: 10.1097/PCC.0b013e3181b80e70. [DOI] [PubMed] [Google Scholar]
- 14.Allen ME, Kopp BJ, Erstad BL. Stress ulcer prophylaxis in the postoperative period. Am J Health Syst Pharm. 2004;61:588–96. doi: 10.1093/ajhp/61.6.588. [DOI] [PubMed] [Google Scholar]
- 15.Chanpura T, Yende S. Weighing risks and benefits of stress ulcer prophylaxis in critically ill patients. Crit Care. 2012;16:322. doi: 10.1186/cc11819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bavishi C, Dupont HL. Systematic review: the use of proton pump inhibitors and increased susceptibility to enteric infection. Aliment Pharmacol Ther. 2011;34:1269–81. doi: 10.1111/j.1365-2036.2011.04874.x. [DOI] [PubMed] [Google Scholar]
- 17.Herzig SJ, Howell MD, Ngo LH, Marcantonio ER. Acid-suppressive medication use and the risk for hospital-acquired pneumonia. JAMA. 2009;301:2120–8. doi: 10.1001/jama.2009.722. [DOI] [PubMed] [Google Scholar]
- 18.Reimer C, Sondergaard B, Hilsted L, Bytzer P. Proton-pump inhibitor therapy induces acid-related symptoms in healthy volunteers after withdrawal of therapy. Gastroenterology. 2009;137:80–7. 7e1. doi: 10.1053/j.gastro.2009.03.058. [DOI] [PubMed] [Google Scholar]
- 19.Shah NH, LePendu P, Bauer-Mehren A, Ghebremariam YT, Iyer SV, Marcus J, et al. Proton Pump Inhibitor Usage and the Risk of Myocardial Infarction in the General Population. PLoS One. 2015;10:e0124653. doi: 10.1371/journal.pone.0124653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chung EY, Yardley J. Are there risks associated with empiric acid suppression treatment of infants and children suspected of having gastroesophageal reflux disease? Hosp Pediatr. 2013;3:16–23. doi: 10.1542/hpeds.2012-0077. [DOI] [PubMed] [Google Scholar]
- 21.Graham PL, 3rd, Begg MD, Larson E, Della-Latta P, Allen A, Saiman L. Risk factors for late onset gram-negative sepsis in low birth weight infants hospitalized in the neonatal intensive care unit. Pediatr Infect Dis J. 2006;25:113–7. doi: 10.1097/01.inf.0000199310.52875.10. [DOI] [PubMed] [Google Scholar]
- 22.Guillet R, Stoll BJ, Cotten CM, Gantz M, McDonald S, Poole WK, et al. Association of H2-blocker therapy and higher incidence of necrotizing enterocolitis in very low birth weight infants. Pediatrics. 2006;117:e137–42. doi: 10.1542/peds.2005-1543. [DOI] [PubMed] [Google Scholar]
- 23.Smith A, Saiman L, Zhou J, Della-Latta P, Jia H, Graham PL., 3rd Concordance of Gastrointestinal Tract Colonization and Subsequent Bloodstream Infections With Gram-negative Bacilli in Very Low Birth Weight Infants in the Neonatal Intensive Care Unit. Pediatr Infect Dis J. 2010;29:831–5. doi: 10.1097/INF.0b013e3181e7884f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Terrin G, Passariello A, De Curtis M, Manguso F, Salvia G, Lega L, et al. Ranitidine is associated with infections, necrotizing enterocolitis, and fatal outcome in newborns. Pediatrics. 2012;129:e40–5. doi: 10.1542/peds.2011-0796. [DOI] [PubMed] [Google Scholar]
- 25.Chen IL, Gao WY, Johnson AP, Niak A, Troiani J, Korvick J, et al. Proton pump inhibitor use in infants: FDA reviewer experience. J Pediatr Gastroenterol Nutr. 2012;54:8–14. doi: 10.1097/MPG.0b013e31823890b4. [DOI] [PubMed] [Google Scholar]
- 26.Vandenplas Y, Rudolph CD, Di Lorenzo C, Hassall E, Liptak G, Mazur L, et al. Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN) and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) J Pediatr Gastroenterol Nutr. 2009;49:498–547. doi: 10.1097/MPG.0b013e3181b7f563. [DOI] [PubMed] [Google Scholar]
- 27.Barron JJ, Tan H, Spalding J, Bakst AW, Singer J. Proton pump inhibitor utilization patterns in infants. J Pediatr Gastroenterol Nutr. 2007;45:421–7. doi: 10.1097/MPG.0b013e31812e0149. [DOI] [PubMed] [Google Scholar]
- 28.Golski CA, Rome ES, Martin RJ, Frank SH, Worley S, Sun Z, et al. Pediatric specialists’ beliefs about gastroesophageal reflux disease in premature infants. Pediatrics. 2010;125:96–104. doi: 10.1542/peds.2008-3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Puetz J, Witmer C, Huang YS, Raffini L. Widespread use of fresh frozen plasma in US children’s hospitals despite limited evidence demonstrating a beneficial effect. J Pediatr. 2012;160:210–5. e1. doi: 10.1016/j.jpeds.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 30.Slaughter JL, Stenger MR, Reagan PB. Variation in the use of diuretic therapy for infants with bronchopulmonary dysplasia. Pediatrics. 2013;131:716–23. doi: 10.1542/peds.2012-1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walsh MC, Szefler S, Davis J, Allen M, Van Marter L, Abman S, et al. Summary proceedings from the bronchopulmonary dysplasia group. Pediatrics. 2006;117:S52–6. doi: 10.1542/peds.2005-0620I. [DOI] [PubMed] [Google Scholar]
- 32.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163:1723–9. doi: 10.1164/ajrccm.163.7.2011060. [DOI] [PubMed] [Google Scholar]
- 33.Ehrenkranz RA, Walsh MC, Vohr BR, Jobe AH, Wright LL, Fanaroff AA, et al. Validation of the National Institutes of Health consensus definition of bronchopulmonary dysplasia. Pediatrics. 2005;116:1353–60. doi: 10.1542/peds.2005-0249. [DOI] [PubMed] [Google Scholar]
- 34.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–6. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 35.Williams RL. A note on robust variance estimation for cluster-correlated data. Biometrics. 2000;56:645–6. doi: 10.1111/j.0006-341x.2000.00645.x. [DOI] [PubMed] [Google Scholar]
- 36.Rudolph CD. Are proton pump inhibitors indicated for the treatment of gastroesophageal reflux in infants and children? J Pediatr Gastroenterol Nutr. 2003;37(Suppl 1):S60–4. doi: 10.1097/00005176-200311001-00012. [DOI] [PubMed] [Google Scholar]
- 37.van der Pol RJ, Smits MJ, van Wijk MP, Omari TI, Tabbers MM, Benninga MA. Efficacy of proton-pump inhibitors in children with gastroesophageal reflux disease: a systematic review. Pediatrics. 2011;127:925–35. doi: 10.1542/peds.2010-2719. [DOI] [PubMed] [Google Scholar]
- 38.van der Pol R, Langendam M, Benninga M, van Wijk M, Tabbers M. Efficacy and safety of histamine-2 receptor antagonists. JAMA Pediatr. 2014;168:947–54. doi: 10.1001/jamapediatrics.2014.1273. [DOI] [PubMed] [Google Scholar]
- 39.Cohen S, Bueno de Mesquita M, Mimouni FB. Adverse effects reported in the use of gastroesophageal reflux disease treatments in children: a 10 years literature review. Br J Clin Pharmacol. 2015;80:200–8. doi: 10.1111/bcp.12619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grant L, Cochran D. Can pH monitoring reliably detect gastro-oesophageal reflux in preterm infants? Arch Dis Child Fetal Neonatal Ed. 2001;85:F155–7. doi: 10.1136/fn.85.3.F155. discussion F7–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mitchell DJ, McClure BG, Tubman TR. Simultaneous monitoring of gastric and oesophageal pH reveals limitations of conventional oesophageal pH monitoring in milk fed infants. Arch Dis Child. 2001;84:273–6. doi: 10.1136/adc.84.3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nelson SP, Chen EH, Syniar GM, Christoffel KK. One-year follow-up of symptoms of gastroesophageal reflux during infancy. Pediatric Practice Research Group. Pediatrics. 1998;102:E67. doi: 10.1542/peds.102.6.e67. [DOI] [PubMed] [Google Scholar]
- 43.Tennant SM, Hartland EL, Phumoonna T, Lyras D, Rood JI, Robins-Browne RM, et al. Influence of gastric acid on susceptibility to infection with ingested bacterial pathogens. Infect Immun. 2008;76:639–45. doi: 10.1128/IAI.01138-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Beck-Sague CM, Azimi P, Fonseca SN, Baltimore RS, Powell DA, Bland LA, et al. Bloodstream infections in neonatal intensive care unit patients: results of a multicenter study. Pediatr Infect Dis J. 1994;13:1110–6. [PubMed] [Google Scholar]
- 45.Rojas MA, Efird MM, Lozano JM, Bose CL, Rojas MX, Rondon MA, et al. Risk factors for nosocomial infections in selected neonatal intensive care units in Colombia, South America. J Perinatol. 2005;25:537–41. doi: 10.1038/sj.jp.7211353. [DOI] [PubMed] [Google Scholar]
- 46.Bianconi S, Gudavalli M, Sutija VG, Lopez AL, Barillas-Arias L, Ron N. Ranitidine and late-onset sepsis in the neonatal intensive care unit. J Perinat Med. 2007;35:147–50. doi: 10.1515/JPM.2007.017. [DOI] [PubMed] [Google Scholar]
- 47.Canani RB, Cirillo P, Roggero P, Romano C, Malamisura B, Terrin G, et al. Therapy with gastric acidity inhibitors increases the risk of acute gastroenteritis and community-acquired pneumonia in children. Pediatrics. 2006;117:e817–20. doi: 10.1542/peds.2005-1655. [DOI] [PubMed] [Google Scholar]
- 48.Yang YX, Metz DC. Safety of proton pump inhibitor exposure. Gastroenterology. 2010;139:1115–27. doi: 10.1053/j.gastro.2010.08.023. [DOI] [PubMed] [Google Scholar]
- 49.Valuck RJ, Ruscin JM. A case-control study on adverse effects: H2 blocker or proton pump inhibitor use and risk of vitamin B12 deficiency in older adults. JClin Epidemiol. 2004;57:422–8. doi: 10.1016/j.jclinepi.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 50.Costarino AT, Dai D, Feng R, Feudtner C, Guevara JP. Gastric Acid Suppressant Prophylaxis in Pediatric Intensive Care: Current Practice as Reflected in a Large Administrative Database. Pediatr Crit Care Med. 2015 doi: 10.1097/PCC.0000000000000427. [DOI] [PubMed] [Google Scholar]
- 51.Wheatley E, Kennedy KA. Cross-over trial of treatment for bradycardia attributed to gastroesophageal reflux in preterm infants. J Pediatr. 2009;155:516–21. e1. doi: 10.1016/j.jpeds.2009.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jadcherla SR, Slaughter JL, Stenger MR, Klebanoff M, Kelleher K, Gardner W. Practice Variance, Prevalence, and Economic Burden of Premature Infants Diagnosed With GERD. Hosp Pediatr. 2013;3:335–41. doi: 10.1542/hpeds.2013-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]