Abstract
Objective
Little is known about the extent of cortical and subcortical volumetric alterations that may occur within the first year of HIV infection (primary HIV infection; PHI).
Design
We used structural MRI in this prospective cross-sectional neuroimaging study to determine the extent of volumetric changes in early HIV infection.
Methods
CSF, blood, NP testing, and structural T1 magnetic resonance imaging scans were acquired from 18 HIV- and 47 PHI age-matched, antiretroviral-naïve male participants. Using FreeSurfer 5.1, volumetric measurements were obtained from the caudate, amygdala, corpus callosum, ventricles, putamen, thalamus, cortical white matter, and total grey matter. Regional volumes were compared groupwise and related to biomarkers in cerebrospinal fluid (CSF) [viral load (VL), neopterin, and neurofilament light-chain (NFL)], blood (VL, CD4+, and CD8+ T cell count), and neuropsychometric tests [digit-symbol (DSST), grooved pegboard (GPD), finger-tapping (FT), and timed gait (TG)].
Results
A trend-level moderate reduction of putamen volume (p=0.076, adjusted Cohen’s d=0.5 after controlling for age) was observed for PHI compared to HIV-uninfected individuals. Within the PHI group, putamen volume associated with CD4+ count (p=0.03), CD4+/CD8+ Ratio (p=0.045), infection duration (p=0.009), and worsening psychomotor performance on the DSST (p=0.028), FT (p=0.039), and TG (p=0.009) tests.
Conclusions
Our volumetric results suggest that the putamen is preferentially susceptible to early HIV-associated processes. Examining the natural course of early HIV infection longitudinally will allow for mapping of the trajectory of HIV-associated CNS changes, enabling creation of improved interventional strategies to potentially stabilize or reverse these observied structural changes.
Keywords: Putamen, Primary HIV Infection, Structural MRI, Brain Volume Loss, NFL
INTRODUCTION
HIV enters the central nervous system (CNS) soon after initial infection in the form of free virions or by way of immune cells travelling across the blood–brain barrier (BBB) and stimulates immune activation [1]. Markers of systemic and microglial immune activation, such as CD8+ T cell count and neopterin, are elevated in cerebrospinal fluid (CSF) during untreated primary HIV infection (PHI; defined as <1 year after exposure) and remain elevated with chronic HIV infection (CHI) (>1 year) in the absence of combination antiretroviral therapy (cART) [2, 3]. Viral and immunopathogenic changes that occur during PHI may facilitate the subsequent neurodegeneration and cortical and subcortical volume loss reported in CHI [4, 5].
However, the existence and extent of brain tissue volume changes early in the course of HIV infection are incompletely characterized. One prior study revealed reduced total cortical volume and enlarged third ventricle in PHI, though the study included HIV+ individuals receiving cART [6, 7]. Thus, the natural course of untreated HIV-dependent volume changes is not established, as early initiation of cART may preclude further neural injury. Early regional volume loss may be responsible for downstream cognitive performance deficits commonly associated with chronic disease [8]. For example, a reduction in putamen size is associated with impaired motor performance in CHI [9]. In this study, we obtained cortical and subcortical volumetrics in cART-naïve PHI individuals and investigated relationships among regional brain volumes, duration of infection, laboratory measures, and neuropsychological performance.
MATERIALS AND METHODS
Participants
This study included PHI (n=47) and HIV-uninfected (n=18) participants that have been previously described [2, 10, 11]. cART-naïve PHI individuals were assessed within 1 year of acquiring HIV as confirmed by recent negative result on HIV antibody testing or results of a less-sensitive EIA test [2]. The date of HIV exposure was estimated as 14 days before onset of seroconversion symptoms (n=38/47) or as the date halfway between the last negative and first positive HIV test (n=9/47). The institutional review board at the University of California San Francisco approved the protocol, and informed consent was obtained from all participants.
Specimen sampling, processing, and laboratory studies
Clinical examination (including medical and neurological) and laboratory studies (CSF and blood) were obtained. Detailed laboratory analysis for CD4+, CD8+ T cells, CSF neopterin, neurofilament light chain (NFL), CSF/plasma albumin ratio, and blood and CSF HIV RNA levels have been previously described [10, 11].
Neuropsychometric performance evaluation
A brief neuropsychometric performance battery was administered to participants and included four assessments: timed gait, grooved pegboard, finger tapping, and digit symbol as previously described [10]. To control for social and demographic variability in the groups, z-scores for neuropsychological testing were calculated based on comparing raw scores to age, sex, ethnicity, and level of education matched norms. An NPZ-4 score was calculated by averaging the four z-scores from the battery [12].
Neuroimaging acquisition and analyses
T1-weighted three-dimensional magnetization-prepared rapid acquisition gradient echo (MPRAGE, TR/T1/TE=2300/950/3 ms, voxel size=1.0×1.0×1.0mm3, flip angle 7°, bandwidth=200 Hz/pixel) scans were acquired using a 4T Siemens/Bruker MedSpec scanner (Siemens AG, Erlangen, Germany).
Volumetric segmentation was performed using the Freesurfer 5.1 image analysis suite. (Harvard University, Boston, MA) [13]. Regional volumes were normalized to total intracranial volume (ICV) using a least squares residual regression model. ICV was similar across the two groups (p=0.383). Normalized volumes were calculated for the amygdala, caudate, putamen, thalamus, third ventricle, corpus callosum, and total white and gray matter, regions previously implicated in HIV infection [4, 7].
Statistical analysis
Demographic characteristics are summarized using means and standard deviations for the continuous variables with approximately normal distributions (age, education, NPZ-4), and median and first and third quartiles (Q1, Q3) for skewed continuous variables (CD4+ T cells, CD8+ T cells, plasma and CSF neopterin, CSF:plasma albumin ratio, CSF WBC), and compared between groups using Student’s t-t and Wilcoxon rank-sum test, respectively. For groupwise regional volume comparisons, one-way analysis of covariance (ANCOVA) was used with age as a covariate. Regions that showed adjusted volumetric differences between the PHI and HIV- group at the 0.10 level were further studied in the PHI group using two-tailed partial correlation analyses (partialling out age) to examine the associations of regional volumes to measures of HIV infection, neuropsychological performance, or duration of infection. Appropriate normality transformations (square root and logarithm) were used for the skewed variables. No corrections for multiple comparisons were included in analysis because of the exploratory nature of the analysis. All statistical analyses used SPSS 22 (IBM Corp., Armonk, NY).
RESULTS
Participants
PHI participants were assessed at a median 106 days post estimated infection. Table 1 compares the demographic and laboratory data between the two groups. The PHI and control groups did not differ significantly in gender, age, education, or ethnicity. As expected, PHI had lower CD4+ T cell count and higher CD8+ T cell and CSF WBC counts. CSF: plasma albumin ratio, a measure of BBB integrity, was similar in the two groups, while CSF neopterin, a marker of neuroinflammation, was elevated in the PHI group.
Table 1.
Demographic and clinical information for study participants.
| HIV- (n=18) |
PHI (n=47) |
P-value1 | |
|---|---|---|---|
| Sex (% male) | 18 (100) | 47 (100) | 1.0 |
| Age (years) | 35.2 (9.7) | 37.1 (9.3) | 0.449 |
| Ethnicity, n (%) | 0.843 | ||
| Caucasian | 12 (67) | 34 (72) | |
| African-American | 4 (22) | 6 (13) | |
| Asian | 2 (11) | 4 (9) | |
| Other | 0 (0) | 3 (6) | |
| Education in years (SD) | 16.1 (2.7) | 15.4 (2.4) | 0.330 |
| CD4+ T cell count (IQR) | 793 (739, 1003) | 539 (387, 723) | <0.001 |
| CD8+ T cell count | 476 (316, 744) | 924 (714, 1195) | <0.001 |
| Plasma Neopterin | 4.5 (3.3,7.3) | 12.6 (9.0,18.0) | <0.001 |
| CSF Neopterin | 4.5 (4.3,4.6) | 8.6 (6.8,15.6) | <0.001 |
| Plasma HIV-1 viral load (log10) | 4.6 (4.0, 5.1) | ||
| CSF HIV-1 viral load (log10) | 2.6 (1.7,3.1) | ||
| CSF:Plasma albumin ratio | 4.5 (3.3,7.3) | 4.6 (3.8,6.7) | 0.861 |
| CSF WBC | 1.0 (1.0,3.0) | 6.0 (3.5, 9.5) | <0.001 |
| CSF NFL | 519 (398, 740) | ||
| Duration of Infection (months) (SD)2 |
4.0 (2.1) | ||
| NPZ-4 | −0.38 (0.9) | −0.03 (0.72) | 0.151 |
Mean (SD), Median (Q1, Q3), or Number (%) per group are shown
Determined using Student’s t-test (age, education, NPZ-4), Wilcoxon rank-sum test (CD4+ T cells, CD8+ T cells, plasma and CSF neopterin, CSF/plasma albumin ratio, CSF White Blood Cell count), and Fisher’s exact test (ethnicity).
For PHI, duration of infection was calculated from date of estimated HIV transmission in the context of laboratory-confirmed recent infection.
PHI= primary HIV infection; NPZ-4= neuropsychological performance Z score; CSF= cerebrospinal fluid; NFL= neurofilament light chain; SD= standard deviation; IQR= interquartile range; WBC=white blood count
Putamen volume is reduced in PHI
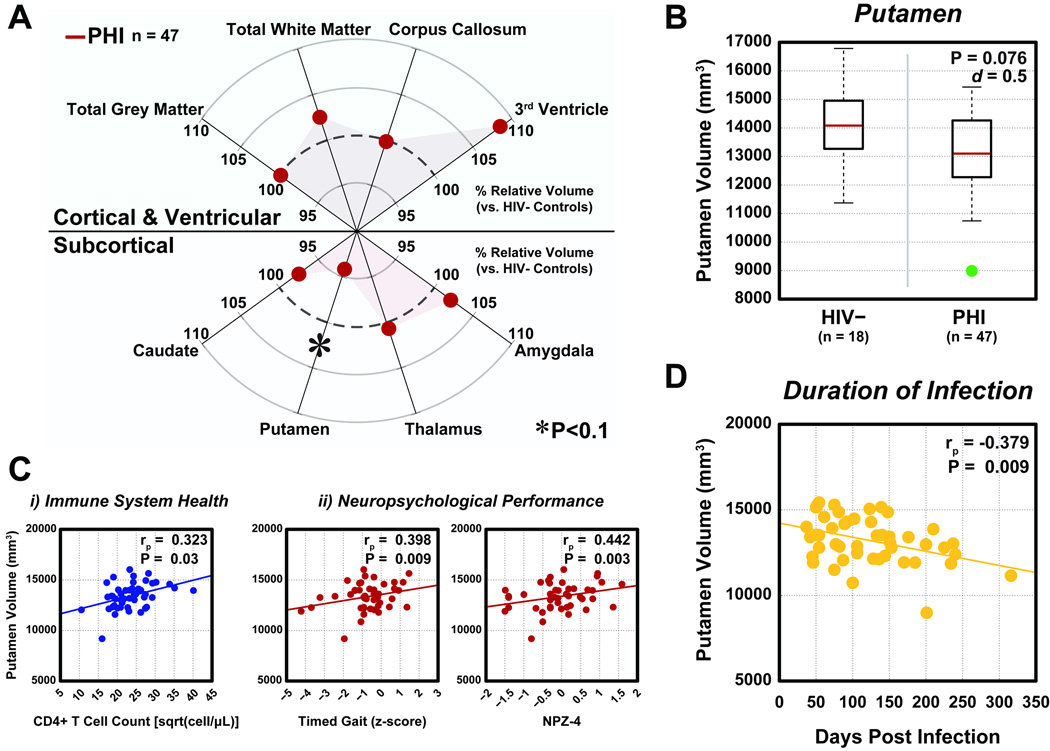
The relative group-wise comparisons of each of the eight target regions can be seen in Figure 1A. Total gray and white matter volumes were similar for the two groups, whereas a trend-level moderate reduction of putamen volume (p=0.076, adjusted Cohen’s d=0.5 after controlling for age) was seen for PHI compared to HIV-uninfected individuals (Figure 1B). Cohen’s d is a measure of effect size and is in units of standard deviation. This finding of d=0.5 describes a medium-level effect, that, taken together with the trend-level p-value, points to a probable, appreciable reduction in putamen volume. It should be noted that although the 3rd ventricle appears to have a larger relative groupwise volume difference, this difference is not significant (p=0.36) and potentially reflects the high coefficient of variation in this region compared to the putamen (average CV3rdVentricle=28% versus average CVPutamen=9%). Because of this marginal evidence of a putamen volume reduction in early HIV infection, we next explored associations between volume and laboratory measures.
Figure 1.
Groupwise regional brain volumetric comparisons. A) Among eight regions included in this analysis, the putamen was reduced (p<0.1) in primary HIV infection (PHI) compared to HIV- individuals. B) Magnitude of putamen volume reduction in PHI. Red line: median volume. Green: outlier (>2.7σ). C) Significant (p<0.05) correlations were observed between putamen volume and CD4+ T cell count (i) or neuropsychological measures (ii) in PHI individuals. D) A decline in cross-sectional putamen volume was associated with increasing duration of infection for PHI participants (p<0.01). All correlation coefficients (rp) are determined using partial correlation analysis, adjusted for the effects of age.
Putamen volume correlates with systemic immune health measures
In PHI participants, both CD4+ T cell count and CD4+/CD8+ T cell ratio, measures of HIV-related immune status, showed significant positive correlations with putamen volume (CD4+ T cell: rp=0.323, p=0.030; CD4+/CD8+ Ratio: rp=0.304, p=0.045) (Figure 1Ci). PHI putamen volume did not significantly correlate with plasma HIV RNA or other CSF measures.
Putamen volume correlates with neuropsychometric performance
The putamen is a basal ganglia structure and is an important component of motor control pathways. Therefore, we anticipated a relationship between PHI putamen volume and psychomotor performance. We observed significant correlations between putamen volume and normed z-scores from timed gait (rp=0.398; p=0.009), finger tapping (rp=0.312; p=0.039), digit symbol substitution (rp=0.331; p=0.028), and the global NPZ-4 score (rp=0.442; p=0.003) (Figure 1Cii). Associations between putamen volume and performance from timed gait and NPZ4 were only significant in the PHI cohort.
Putamen volume decreases with longer duration of infection
Putamen volume had a negative relationship with increasing estimated duration of infection (p=0.009) (Figure 1D). Though this study is cross-sectional, this finding may imply a continuous relationship between duration of infection and putamenal volume loss even during the first year of infection.
DISCUSSION
In this study, we examined regional volumetric differences between individuals within the first year of HIV infection and HIV-uninfected individuals. We observed a reduction in putamen volume in PHI that associated with duration of infection, neuropsychometric performance, and immune system measures.
The putamen (and the basal ganglia collectively) has been shown to be selectively injured in a number of diseases, with volume regional loss reported in Type 1 Diabetes [14], multiple sclerosis [15], and Alzheimer’s disease [16]. The mechanistic relationships between these conditions and HIV may be potentially related to inflammation or vascular injury, and warrant further investigation. Though previous studies have reported basal ganglia volume loss in HIV, including in chronically infected participants with cognitive impairment [17], the mechanism underlying the selective injury to this area remains unclear. In HIV-associated dementia, HIV replication preferentially occurs in perivascular macrophages in the basal ganglia [18], which in its early stages associates with basal ganglia hypermetabolism on brain positron emission tomography [19]. Interestingly, the basal ganglia is the earliest region to manifest inflammation in magnetic resonance studies of acute HIV infection [20], possibly a result of selective impairment of endothelial tight junctions observed in this brain region in HIV [21]. Thus, it is possible that even early during infection, due to a locally compromised blood brain barrier in the basal ganglia, the putamen is an early target of viral infection and inflammatory injury, leading to early tissue compromise and volume loss.
These findings, along with previous reports from the same cohort using multiple modalites [2, 10, 11], imply a comprehensive narrative of the mechanisms that underlie observed pathophsyiological changes seen with early HIV infection. The elevated CSF:plasma albumin ratio observed in a larger sample from this cohort and elevated CSF WBC and neopterin reported here implies compromised BBB integrity and CNS immune activation in PHI [2]. The observed elevated NFL in some HIV+ individuals in this cohort [10] suggest neuronal degradation, however we observed no association between NFL and putamen volume (rp=−0.103, p=0.497) or other brain volumes. Previously observed changes in white matter integrity seen during the first year of infection [11] and our current report of putamen volume changes support the concept that early inflammation and neuronal injury measurably impact brain structure. While the putamenal volumetric difference was moderate (p=0.076, d=0.5), its biological meaning may be supported by the correlations we observed with markers of systemic immune status and duration of HIV infection, possibly reflecting cumulative exposure to immune and viral pathogenesis. Furthermore, the biologic significance of putaminal volume changes is supported by the relationships noted between psychomotor performance and putamen volume. These suggest that subtle changes in brain volume could underlie neuropsychological deficits described during early infection [7]. Finally, with increasing duration of infection, both functional and structrual connections (e.g. prominent white matter tracts) are affected, possibly leading to the more widespread neurocognitive changes seen with CHI [11, 22].
This study has several limitations. Because this cohort was designed specifically to examine early HIV-associated changes, the HIV-uninfected group was small compared to the PHI group. Additionally no adjustments for multiple comparisons were made, exposing the results to potential false positive results. Further confirmation of our findings is needed from independent studies. Finally, we did not classify the participants according to HIV-associated cognitive disorder (HAND) criteria for this analysis as we included a limited testing battery and did not formally assess functional status in this cohort [23].
CONCLUSION
The results reported here add to the contuinally growing body of evidence describing central nervous system changes seen early after HIV infection. Collectively, they suggest that early intervention may mitigate brain damage, but additional clinical trials are needed.
Acknowledgments
Study concept and design: Ances, Meyerhoff, Price, Spudich, Wright
Acquisition of data: Lee, Meyerhoff, Peterson, Price, Spudich, Walter, Zetterberg
Analysis and interpretation of data: Ances, Pyakurel, Fuchs, Price, Robertson, Spudich, Vaida, Wright, Zetterberg
Drafting of the manuscript: Ances, Spudich, Vaida, Wright
Critical revision of the manuscript for important intellectual content: All authors
Statistical analysis: Vaida, Wright
Administrative, technical, or material support: Lee, Peterson, Walter
Study supervision: Ances, Spudich
Design and conduct of the study, collection, management, analysis and interpretation of the data were supported by the National Institute of Mental Health [R01 MH081772, R21 MH099979, K23 MH074466, K23 MH081786], National Institute of Allergy and Infectious Diseases [P01 A1071713, M01 RR00083]; National Institute of Nursing Research [R01 NR012907, R01 NR014449, R01 NR014449, R01 NR015738] UCSF AIDS Research Institute, UCSF Academic Senate, UCSF REAC, and the Radiology Research Service at the SF VA Medical Center. Funding organizations had no role in preparation, review, or approval of the manuscript or the decision to submit the manuscript for publication.
We also sincerely thank the study participants who enrolled in this project and the staff at the UCSF Options Project and Magnet, as well as Liz Westerhaus, M.A. at Washington University in Saint Louis for administrative assistance.
Footnotes
No authors report any conflicts of interest.
REFERENCES
- 1.Spudich S, Gonzalez-Scarano F. HIV-1-Related Central Nervous System Disease: Current Issues in Pathogenesis, Diagnosis, and Treatment. Cold Spring Harb Perspect Med. 2012;2:a007120. doi: 10.1101/cshperspect.a007120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spudich S, Gisslen M, Hagberg L, Lee E, Liegler T, Brew B, et al. Central nervous system immune activation characterizes primary human immunodeficiency virus 1 infection even in participants with minimal cerebrospinal fluid viral burden. J Infect Dis. 2011;204:753–760. doi: 10.1093/infdis/jir387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sinclair E, Ronquillo R, Lollo N, Deeks SG, Hunt P, Yiannoutsos CT, et al. Antiretroviral treatment effect on immune activation reduces cerebrospinal fluid HIV-1 infection. J Acquir Immune Defic Syndr. 2008;47:544–552. doi: 10.1097/QAI.0b013e318162754f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ances BM, Ortega M, Vaida F, Heaps J, Paul R. Independent effects of HIV, aging, and HAART on brain volumetric measures. J Acquir Immune Defic Syndr. 2012;59:469–477. doi: 10.1097/QAI.0b013e318249db17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gongvatana A, Correia S, Dunsiger S, Gauthier L, Devlin KN, Ross S, et al. Plasma cytokine levels are related to brain volumes in HIV-infected individuals. J Neuroimmune Pharmacol. 2014;9:740–750. doi: 10.1007/s11481-014-9567-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Du H, Wu Y, Ochs R, Edelman RR, Epstein LG, McArthur J, et al. A comparative evaluation of quantitative neuroimaging measurements of brain status in HIV infection. Psychiatry Res. 2012;203:95–99. doi: 10.1016/j.pscychresns.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ragin AB, Wu Y, Gao Y, Keating S, Du H, Sammet C, et al. Brain alterations within the first 100 days of HIV infection. Ann Clin Transl Neurol. 2015;2:12–21. doi: 10.1002/acn3.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ances BM, Ellis RJ. Dementia and neurocognitive disorders due to HIV-1 infection. Semin Neurol. 2007;27:86–92. doi: 10.1055/s-2006-956759. [DOI] [PubMed] [Google Scholar]
- 9.Paul RH, Ernst T, Brickman AM, Yiannoutsos CT, Tate DF, Cohen RA, et al. Relative sensitivity of magnetic resonance spectroscopy and quantitative magnetic resonance imaging to cognitive function among nondemented individuals infected with HIV. J Int Neuropsychol Soc. 2008;14:725–733. doi: 10.1017/S1355617708080910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peluso MJ, Meyerhoff DJ, Price RW, Peterson J, Lee E, Young AC, et al. Cerebrospinal fluid and neuroimaging biomarker abnormalities suggest early neurological injury in a subset of individuals during primary HIV infection. J Infect Dis. 2013;207:1703–1712. doi: 10.1093/infdis/jit088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wright PW, Vaida FF, Fernandez RJ, Rutlin J, Price RW, Lee E, et al. Cerebral white matter integrity during primary HIV infection. AIDS. 2015;29:433–442. doi: 10.1097/QAD.0000000000000560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S, et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol. 2011;17:3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- 14.van Duinkerken E, Schoonheim MM, Steenwijk MD, Klein M, RG IJ, Moll AC, et al. Ventral striatum, but not cortical volume loss, is related to cognitive dysfunction in type 1 diabetic patients with and without microangiopathy. Diabetes Care. 2014;37:2483–2490. doi: 10.2337/dc14-0016. [DOI] [PubMed] [Google Scholar]
- 15.Kramer J, Meuth SG, Tenberge JG, Schiffler P, Wiendl H, Deppe M. Early and Degressive Putamen Atrophy in Multiple Sclerosis. Int J Mol Sci. 2015;16:23195–23209. doi: 10.3390/ijms161023195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Jong LW, van der Hiele K, Veer IM, Houwing JJ, Westendorp RG, Bollen EL, et al. Strongly reduced volumes of putamen and thalamus in Alzheimer's disease: an MRI study. Brain. 2008;131:3277–3285. doi: 10.1093/brain/awn278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aylward EH, Henderer JD, McArthur JC, Brettschneider PD, Harris GJ, Barta PE, et al. Reduced basal ganglia volume in HIV-1-associated dementia: results from quantitative neuroimaging. Neurology. 1993;43:2099–2104. doi: 10.1212/wnl.43.10.2099. [DOI] [PubMed] [Google Scholar]
- 18.Pumarola-Sune T, Navia BA, Cordon-Cardo C, Cho ES, Price RW. HIV antigen in the brains of patients with the AIDS dementia complex. Ann Neurol. 1987;21:490–496. doi: 10.1002/ana.410210513. [DOI] [PubMed] [Google Scholar]
- 19.Rottenberg DA, Moeller JR, Strother SC, Sidtis JJ, Navia BA, Dhawan V, et al. The metabolic pathology of the AIDS dementia complex. Ann Neurol. 1987;22:700–706. doi: 10.1002/ana.410220605. [DOI] [PubMed] [Google Scholar]
- 20.Sailasuta N, Ross W, Ananworanich J, Chalermchai T, DeGruttola V, Lerdlum S, et al. Change in brain magnetic resonance spectroscopy after treatment during acute HIV infection. PLoS One. 2012;7:e49272. doi: 10.1371/journal.pone.0049272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dallasta LM, Pisarov LA, Esplen JE, Werley JV, Moses AV, Nelson JA, et al. Blood-brain barrier tight junction disruption in human immunodeficiency virus-1 encephalitis. Am J Pathol. 1999;155:1915–1927. doi: 10.1016/S0002-9440(10)65511-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ortega M, Brier MR, Ances BM. Effects of HIV and combination antiretroviral therapy on cortico-striatal functional connectivity. AIDS. 2015;29:703–712. doi: 10.1097/QAD.0000000000000611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]