Abstract
The most promising alternatives to heart transplantation are left ventricular assist devices and artificial hearts; however their use has been limited by thrombotic complications. To reduce these, sintered titanium (Ti) surfaces were developed, but thrombosis still occurs in ~7.5% of patients. We have invented a rapid-seeding technology to minimize the risk of thrombosis by rapid endothelialization of sintered Ti with human cord blood-derived endothelial cells (hCB-ECs). hCB-ECs were seeded within minutes onto sintered Ti and exposed to thrombosis-prone low fluid flow shear stresses. The hCB-ECs adhered and formed a confluent endothelial monolayer on sintered Ti. The exposure of sintered Ti to 4.4 dynes/cm2 for 20 hr immediately following rapid-seeding resulted in ~70% cell adherence. The cell adherence was not significantly increased by additional ex vivo static culture of rapid-seeded sintered Ti prior to flow exposure. In addition, adherent hCB-ECs remained functional on sintered Ti, as indicated by flow-induced increase in nitric oxide secretion and reduction in platelet adhesion. After 15-day ex vivo static culture, the adherent hCB-ECs remained metabolically active, expressed EC functional marker thrombomodulin, and reduced platelet adhesion. In conclusion, our results demonstrate the feasibility of rapid-seeding sintered Ti with blood-derived hCB-ECs to generate a living antithrombotic surface.
Keywords: ventricular assist device, biomaterials, endothelium, stem cells, thrombosis
INTRODUCTION
Over 23 million people worldwide suffer from heart failure.1 Late-stage heart failure is best treated with heart transplantation; however, the demand for suitable donor organs outnumbers the supply by approximately 50-fold.2 The only promising alternatives to heart transplantation are mechanical circulatory assist devices, such as ventricular assist devices (VADs). These devices have areas of low and high fluid flow shear stresses.3 Very high shear stresses lead to destruction of von Willebrand Factor (vWF), leading to bleeding complication rates of up to 65% in the first year after VAD placement.4,5 Despite the risk of life-threatening bleeding in 5–10% of VAD recipients per year,6,7 anticoagulation with warfarin is still needed to prevent thrombotic complications of VADs, specifically in the area of stagnant and low shear stresses (<10 dynes/cm2).8–10
The risk of thrombosis of VADs was addressed by modifying the blood-contacting surface with sintered titanium (Ti) microspheres of approximately 50–75 μm in diameter.11 Sintered surfaces create a ‘pseudointima’ of adherent collagen and fibrin deposits, and thus reduce the build-up of large thrombi and the corresponding risk of distal embolization.11,12 Despite these advances, thrombosis still occurs in ~7.5% of adult patients per year and the risk is even higher in pediatric patients.13 Pediatric patients are in dire need of devices that can operate at low flow and are at an even higher risk of pump thrombosis.14,15
The ideal approach to reduce the thrombosis risk of circulatory assist device application is to coat the blood-contacting Ti surfaces with endothelial cells (ECs) to reproduce the native antithrombotic lining of blood vessels and the heart. The benefit of artificial surface endothelialization has been previously demonstrated, but its clinical application has been hindered by the need for an invasive procedure to surgically harvest ECs from native vessels.16,17 Further, the need for prolonged ex vivo culture of ECs on circulatory assist devices for surface endothelialization has made this method clinically impractical. Hence, the preferred approach to endothelialize artificial surfaces would be to rapidly seed devices with ECs obtained by a minimally invasive method, such as a peripheral blood draw. Peripheral blood is a source of late-outgrowth endothelial progenitor cells, also known as endothelial colony forming cells, which display all typical EC characteristics.18 Our prior work has shown that these cells protect against thrombosis in vivo in a porcine model utilizing smooth Ti implantable devices.19
The method that we term “rapid-seeding” allows the cells to be added to a device at the point-of-care, just minutes prior to implantation without prior culture on the device. We have already demonstrated the efficacy of this procedure in our prior studies in a porcine model.19 Any prolonged culture of the cells on the device ex vivo would be less practical for translation into clinical practice because it would require additional and expensive incubation facilities.20 In contrast, our rapid-seeding technology can be performed inside the operating room and reduces the time a patient would have to wait after cell harvest and before implantation of the endothelialized devices.
The overall goal of the present study is to investigate the feasibility of rapidly seeding sintered Ti with patients’ own blood-derived ECs minutes prior to VAD implantation to create a living antithrombotic surface, specifically in the thrombosis-prone regions (low shear stress areas). Our previous studies demonstrated the efficacy of rapid-seeded ECs on smooth Ti surfaces, which we now extend to sintered Ti with the same surface structure and composition as the blood-contacting surface of the most commonly implanted ventricular assist device, the Thoratec HeartMateII.21
Pediatric circulatory assist devices are at an especially high risk of thrombosis and we previously investigated the outgrowth of endothelial cells from pediatric patients. Our results established that colonies of highly functional endothelial cells can be grown out as quickly as 8.3 ± 1.2 days after blood collection from human umbilical cord blood. We are utilizing these hCB-ECs for the following proof-of-concept studies.22,23 This technology may generate a personalized and living anti-thrombotic coating on VADs to minimize the risk of thrombosis and potentially eliminate the need for anticoagulation.
MATERIALS AND METHODS
See Supplemental Methods for further details.
Isolation and Characterization of hCB-ECs
hCB-ECs were isolated from human umbilical cord blood and characterized for EC phenotypic markers by flow cytometry and immunocytochemistry, as previously described.20 The isolated hCB-ECs were utilized at passages 6–9 for all experiments.
Rapid-Seeding of hCB-ECs onto Sintered Ti
Sintered Ti materials were provided by Thoratec Corporation (Pleasanton, CA). For rapid-seeding of sintered Ti, Ti tubes (outer radius: 0.91 cm, inner radius of 0.63 cm) were seeded with hCB-ECs suspended in 1.5 mL serum-free Leibovitz’s L-15 medium (Life Technologies, NY) at various concentrations and then placed in a rotating seeding device (10 revolutions/hr for 45 min at 37°C, 5% CO2).19 Cell adherence was determined by quantifying the number of cells in the remaining hCB-EC suspension and subtracting this number from the total number initially seeded. The cells were stained with 1 mM Cell-Tracker Green (Life Technologies, NY) and then imaged with a confocal microscope (Zeiss 780 upright, Oberkochen, Germany) and scanning electron microscope (Philips XL30 Environmental SEM).
Flow Experiments
Rapid-seeded sintered Ti tubes were exposed to steady flow to mimic the continuous flow conditions of current generation VADs, specifically at low shear stresses in the range of 4.4 dynes/cm2 for 20 hr with a modified cardiopulmonary bypass circuit, as described previously.19
Cell Adherence and Function Tests
To quantify the number of hCB-ECs retained on the Ti surface after flow exposure, Cell Counting Kit – 8 Assay (CCK-8 Sigma–Aldrich) was utilized. To assess the functions of hCB-ECs on Ti surface following fluid flow exposure, we quantified nitric oxide (NO) secretion and performed platelet adhesion assay, as previously described.21
RESULTS
Characterization of hCB-ECs
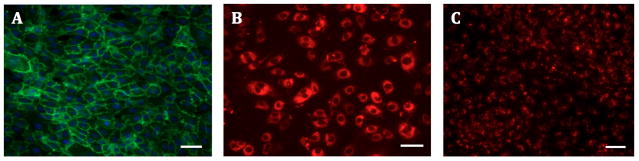
The isolated hCB-ECs exhibited characteristic EC cobblestone morphology (Figure 1A). Flow cytometry indicated positive expression of EC surface markers CD31, CD105 and CD146 (Figure 1B), and absence of leukocyte markers CD14 and CD45 (Figure 1C). Immunocytochemistry further confirmed EC-like properties of the isolated hCB-ECs. The cells stained positive for EC markers CD31 and vWF (Figure 2A,B) and took up Dil-Ac-LDL (Figure 2C).
Figure 1. Morphologic and Flow Cytometric Characterization.
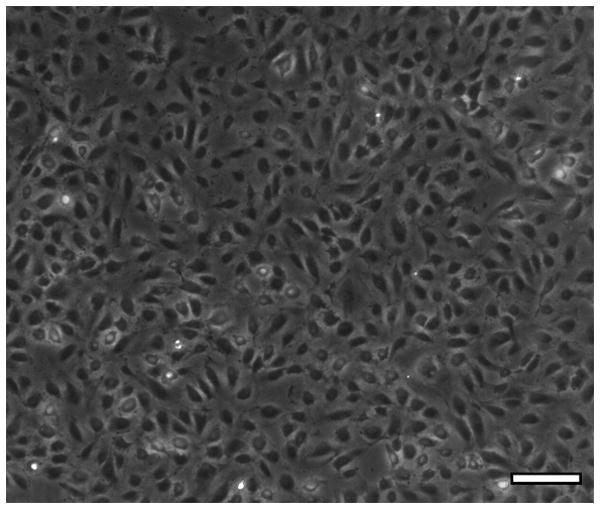
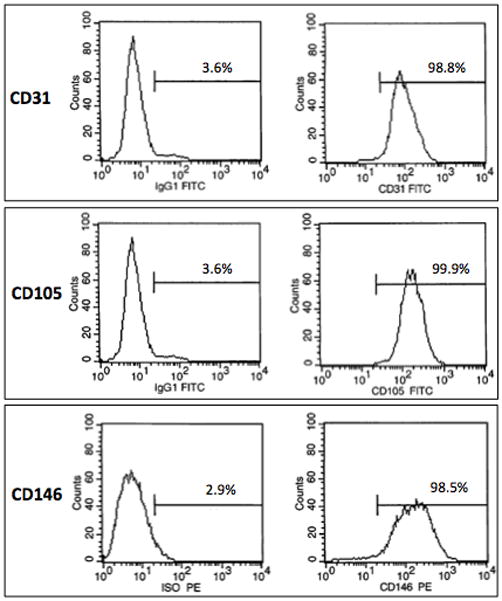
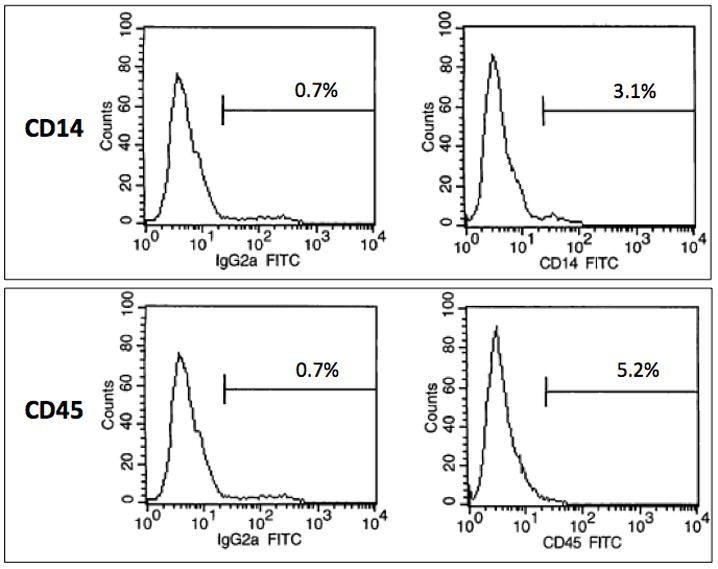
The isolated hCB-ECs showed characteristic EC properties: (A) cobblestone morphology; (B) positive expression for EC surface markers CD31, CD105 and CD146; (C) negative expression for leukocyte markers CD14 and CD45. (Scale bar: 100 μm)
Figure 2. Immunocytochemistry Staining.
The isolated hCB-ECs were stained positive for characteristic EC markers: (A) CD31 (green), (B) vWF (red) and (C) DiI-Ac-LDL (red). (Scale bars: 100 μm)
Rapid-Seeding of hCB-ECs on Sintered Ti
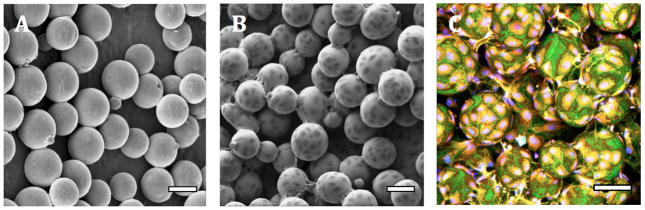
The density of rapid-seeded hCB-ECs on sintered Ti (cells/cm2) linearly correlated with the initial concentration of hCB-ECs (/mL) used for rapid-seeding (Figure 3). Immediately after rapid-seeding, hCB-ECs were initially concentrated in the valleys between Ti microspheres. The distribution of hCB-ECs over sintered Ti microspheres was not affected by increasing seeding concentrations from 0.9 × 105 cells/cm2 up to 4.5 × 105 cells/cm2 (Figure 3). Despite this initial distribution in valleys, hCB-ECs grew over the Ti microspheres and formed a confluent monolayer after ex vivo static culture (37°C, 5% CO2, full EC medium) for 12 hr without any surface precoating (Figure 4).
Figure 3. Rapid-Seeding Density.
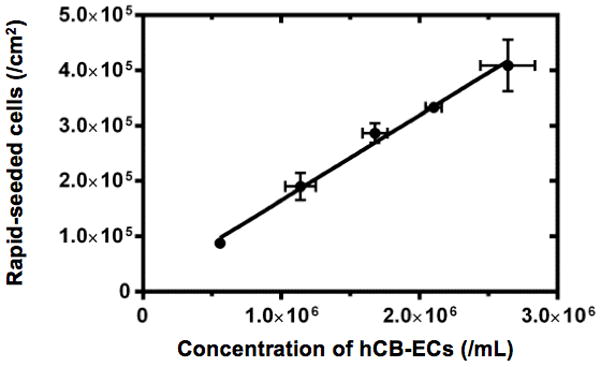
The graph shows linear correlation between initial cell seeding concentration (/mL) and the resulting density of rapid-seeded cells on sintered Ti (/cm2). (Error bars: SEMs)
Figure 4. Confluent Cell Coating on Sintered Ti.
After ex vivo static culture (12 hr), rapid-seeded hCB-ECs formed a confluent cell monolayer on sintered Ti. (A) Control sintered Ti surfaces without hCB-ECs (SEM); (B) Rapid-seeded sintered Ti (SEM); (C) Rapid-seeded sintered Ti (Confocal Microscopy). (Rapid-seeding density: 0.9 × 105 cells/cm2; blue, red and green color: nuclei, cytoplasm and cell junctions, respectively; scale bars: 100 μm.)
Rapid-Seeding of Sintered Ti Inhibits Platelet Adhesion
As shown in Figure 5, significantly fewer platelets adhered on rapid-seeded sintered Ti as compared to control sintered Ti without hCB-ECs (n=3, p=0.02, paired t-test).
Figure 5. Platelet Adhesion Assay on Rapid-Seeded Sintered Ti that Had a Confluent Cell Coating.
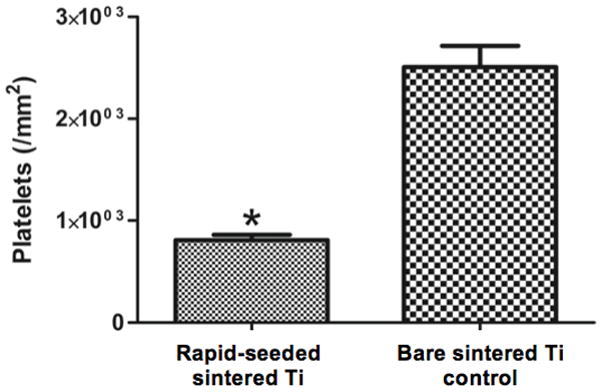
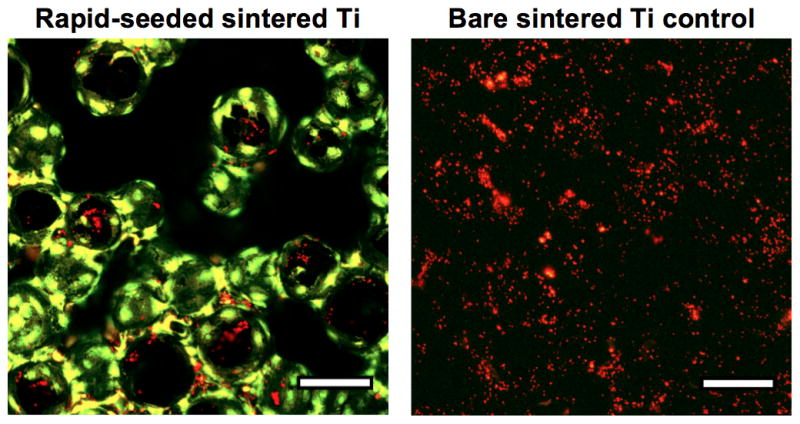
(A) The quantification and (B) representative confocal images of platelets on rapid-seeded sintered Ti after the hCB-ECs had formed a confluent monolayer. (Rapid-seeding density: 0.9 × 105 cells/cm2; n=3; green: ECs; red: platelets; error bars: SEMs; black holes in confocal images were due to different focal plane layers, scale bars: 100 μm.)
Adherence of Rapid-Seeded Cells after Exposure to Flow Shear Stresses
Exposure of the rapid-seeded sintered Ti to fluid flow shear stresses of 4.4 dynes/cm2 for 20 hr resulted in 67.5 ± 0.9% cell adherence (n= 3, rapid-seeding density: 3.3 ± 0.7 × 105 cell/cm2). When the rapid-seeded sintered Ti was preincubated under ex vivo static culture (37°C, 5% CO2, full EC medium) for 6 hr prior to flow exposure, the cell adherence on sintered Ti was similarly high (69.1 ± 3.0 %; n=3, rapid-seeding density: 4.0 ± 0.7 × 105 cells/cm2).
Monolayer Cell Coating on Rapid-Seeded Sintered Ti after Flow Exposure
The hCB-ECs that withstood 4.4 dynes/cm2 for 20 hr on sintered Ti were able to spread and grow to a monolayer under flow (Figure 6A). This finding was observed when the rapid-seeded sintered Ti was either pre-incubated or not pre-incubated under ex vivo static culture prior to flow exposure. As shown in Figure 6, prior incubation of rapid-seeded sintered Ti (6 hr static ex vivo culture) did not increase the extent of hCB-EC coverage on sintered Ti surface (Figure 6B). Therefore, these findings demonstrate the ability of rapid-seeded sintered Ti to withstand low fluid flow shear stresses without the need for additional ex vivo culture on the Ti surface in order to create an endothelial coating.
Figure 6. Rapid-Seeded Sintered Ti After Fluid Flow Exposure.
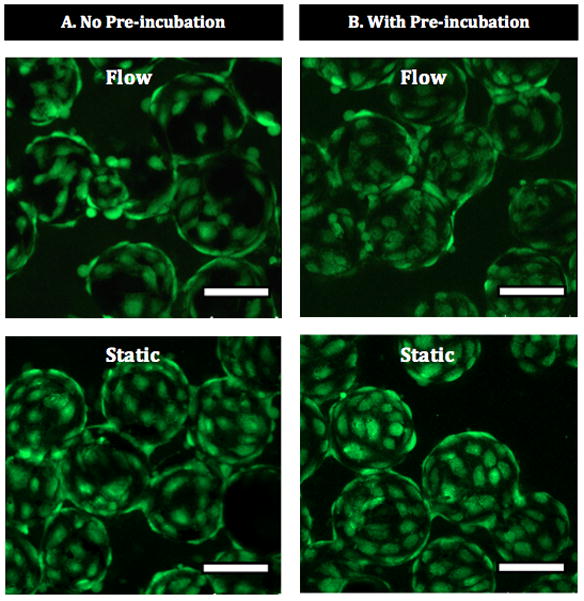
After flow exposure of rapid-seeded sintered Ti, ECs formed a monolayer cell coating on sintered Ti either (A) without prior incubation or (B) with prior incubation (6 hr ex vivo static culture) following rapid-seeding. (Scale bars: 100 μm)
Function of Rapid-Seeded hCB-ECs on Sintered Ti after Flow Exposure
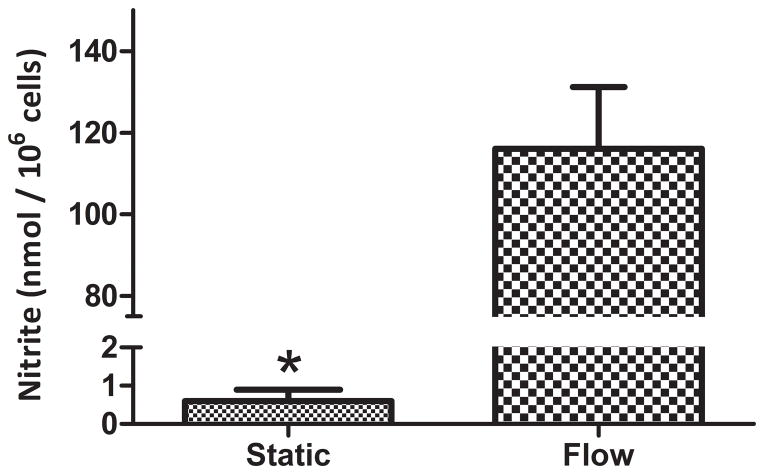
The function of rapid-seeded sintered Ti after exposure to 4.4 dynes/cm2 for 20 hr was investigated with a platelet adhesion and NO secretion assay. Significantly fewer platelets adhered on hCB-EC rapid-seeded sintered Ti as compared to unseeded bare metal Ti controls without hCB-ECs (Figure 7, n=3, p=0.01, paired t-test). Further, hCB-ECs on rapid-seeded sintered Ti significantly increased the secretion of NO by >150-fold after flow exposure as compared to hCB-ECs on static controls (Figure 8, n=3, p<0.01, paired t-test).
Figure 7. Platelet Adhesion Assays on Flow-Exposed Rapid-Seeded Sintered Ti.
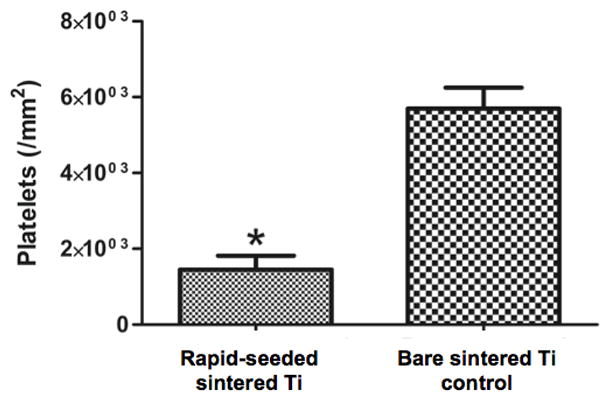
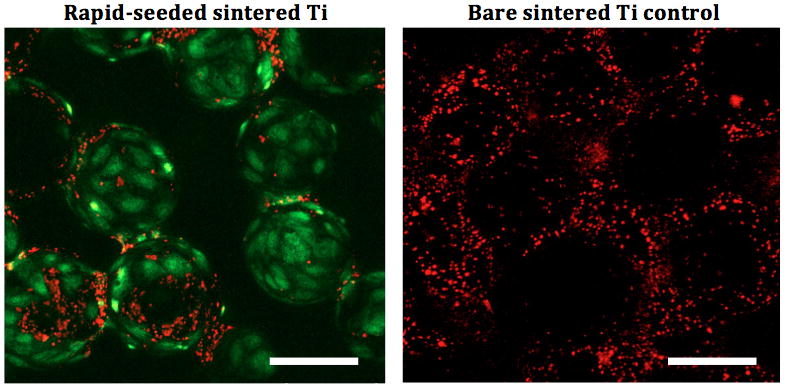
(A) The quantification and (B) confocal images of platelets (red) on rapid-seeded (green) sintered Ti was compared with the ones on control sintered Ti surfaces without hCB-ECs. (Rapid-seeding density: 4×105 cells/cm2; n=3; error bars: SEMs; black holes in confocal images were due to the different focal plane layers; scale bars: 100 μm)
Figure 8. NO Secretion after Exposure of Rapid-Seeded Sintered Ti to Flow.
The level of surrogate marker nitrite (nmol/106 hCB-ECs) was measured after rapid-seeded sintered Ti was exposed to fluid flow shear stresses, and compared with static control. (n=3, error bars: SEMs)
The Function of Rapid-Seeded hCB-ECs on Sintered Ti after Longer-Term Maintenance
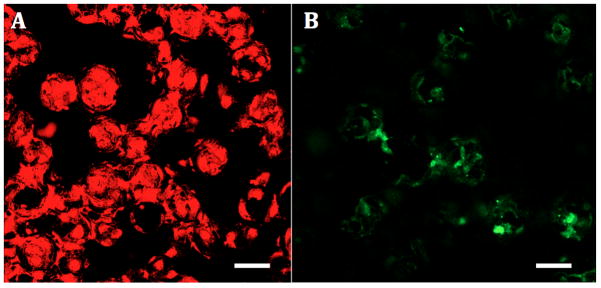
To examine EC function on rapid-seeded sintered Ti after longer-term maintenance, we tested the metabolic activity of the rapid-seeded hCB-ECs after 15 days of ex vivo static culture. hCB-ECs remained metabolically active as shown by increased absorbance readings on a CCK-8 metabolic assay from 0.35 ± 0.02 (at 0 hr) to 1.25 ± 0.04 (at 3 hr) by hCB-ECs (n=3; comparable to increased absorbance readings by hCB-ECs after 1 day of ex vivo static culture). In addition, the hCB-ECs also retained the EC characteristic properties, i.e. Di-Ac-LDL uptake and thrombomodulin expression (Figure 9). Furthermore, rapid-seeded sintered Ti remained antithrombotic as shown by a persistent significant reduction in the number of adherent platelets, as compared to control sintered Ti surfaces without hCB-ECs (1.4 ± 0.3 vs. 4.7 ± 0.9 × 103 platelets/mm2; n=3, p=0.039, paired t-test).
Figure 9. Retained Function of Rapid-Seeded ECs on Sintered Ti after Longer-Term Maintenance.
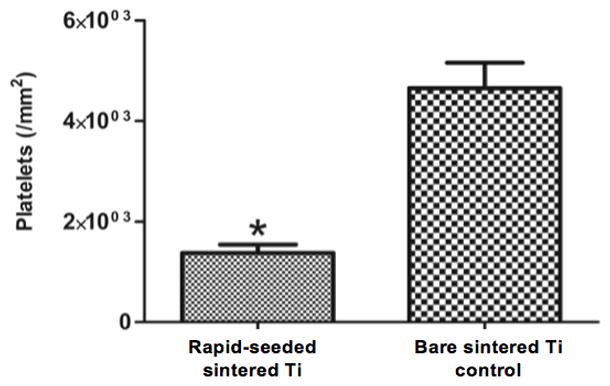
After ex vivo static culture (15 days), rapid-seeded hCB-ECs on sintered Ti retained the EC characteristic properties: (A) Dil-Ac-LDL uptake (red), (B) Thrombomodulin expression (green†), (C) inhibition of platelet adhesion. (n=3, p=0.039, paired t-test; error bars: SEMs; scale bars: 100 μm)
†As thrombomodulin was expressed on the surface of ECs, the detection of thrombomodulin expression was limited by different focal plane layers.
DISCUSSION
We have previously demonstrated that blood-derived ECs adhere well to smooth Ti surfaces – a finding that can be attributed to the naturally formed titanium dioxide film.21,25 The present study shows that hCB-ECs are also able to adhere to sintered Ti without any surface precoatings, which may otherwise render the blood-contacting surface prothrombotic.21,25
In addition to showing excellent cell adherence on sintered Ti under static conditions, our study demonstrates that hCB-EC seeding on sintered Ti could be performed within minutes to create a monolayer that withstands low fluid flow shear stresses (4.4 dynes/cm2) resulting in ~ 70% cell adherence. Cell coverage within the low shear stress areas of mechanical circulatory assist devices is clinically relevant because these regions are especially prone to platelet adhesion and thrombus formation.8–10
Longer ex vivo static culture following rapid-seeding did not increase cell adherence or the cells’ ability to form a monolayer on sintered Ti under shear stresses. Without the need for additional ex vivo culture, this rapid-seeding technology may be practical to apply in the operating room, e.g. on the back table while the patient is placed on cardiopulmonary bypass in preparation for VAD insertion.
Rapid-seeded hCB-ECs appeared to be functional under flow on sintered Ti as evidenced by the cells’ ability to secrete significantly more of the antithrombotic mediator NO (>150-fold) under flow than under static conditions, indicating a normal physiological response characteristic of healthy ECs. 23,24,26 Further, consistent with our prior work showing a dramatic reduction in platelet adhesion on EC-coated smooth Ti,21 there was a remarkable reduction in the number of platelets adhering on hCB-EC covered sintered Ti, as compared to control sintered Ti surfaces without hCB-ECs.
The function of hCB-ECs on sintered Ti was retained after 15 day ex vivo static culture. hCB-ECs retained platelet inhibition capacity on sintered Ti, as well as metabolic activity and expression of characteristic markers of functional ECs, such Di-Ac-LDL uptake and thrombomodulin expression.
The present study has several limitations. Firstly, as this study was primarily aimed at assessing the feasibility of rapid-seeding ECs onto sintered Ti to prevent thrombosis in the thrombosis-prone low shear stress regions (<10 dynes/cm2), the properties of rapid-seeded hCB-ECs above 4.4 dynes/cm2 were not investigated. Secondly, the function of hCB-ECs on sintered Ti was tested within relatively short time periods because long-term flow experiments do not adequately reproduce in vivo environment in which blood-derived circulating cells can potentially replace sheared-off ECs. Thirdly, ECs were derived from human umbilical cord because hCB-ECs have been shown to behave similarly with peripheral blood-derived ECs.25,27 Future works should assess adult blood-derived ECs, and also perform in vivo studies for a proof of concept in the long term.
Our rapid-seeding technology could potentially be applied to both pediatric and adult patients. In pediatric patients, for whom congenital heart disease can be diagnosed before birth, a small amount of umbilical cord blood can be harvested at birth and will be sufficient to generate enough cells for surface coatings due to hCB-ECs’ high proliferative capacity.28 In adult patients, the source of ECs can be from peripheral blood. Despite not being enriched with ECs, with even 30% less yield in heart failure patients,29 peripheral blood can yield a sufficient number of ECs, e.g. with a mobilizing agent (FDA-approved Plerixafor), in combination with apheresis.30,31,32
The presented rapid-seeding technology has shown promise that warrants future investigation to develop a novel technology utilizing patients’ own blood-derived ECs to create living personalized antithrombotic coatings in thrombosis-prone low flow regions of VADs and artificial hearts. With less thrombotic complications, future devices could be specifically designed to operate at lower shear stresses, which will prevent vWF destruction. Combined with the possibility of reducing or eliminating the use of anticoagulation, life-threatening bleeding complications may be prevented.13 Such advances utilizing a rapid seeding technology could broaden device indications, e.g. for elective placement or as destination therapy in less sick patients.
Supplementary Material
Acknowledgments
Source of Funding: We are thankful to Thoratec Corporation for providing financial and material support.
Footnotes
Disclaimers: None
Conflict of Interest: None
References
- 1.Liu L, Eisen H. Epidemiology of heart failure and scope of the problem. Cardiol Clin. 2014;32:1–8. doi: 10.1016/j.ccl.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 2.Health Resources and Services Administration HSB, Division of Transplantation; US Department of Health and Human Services, editor. Annual Report of the US Organ Procurement and Transplantation Network and the Scientific Registry of Transplant Recipients: transplant data 1999–2008. Rockville: 2009. [Google Scholar]
- 3.Fraser K, Zhang T, Taskin M, Griffith B, Wu Z. A quantitative comparison of mechanical blood damage parameters in rotary ventricular assist devices: shear stress, exposure time and hemolysis index. J of Biomech Eng. 2012;134:1–11. doi: 10.1115/1.4007092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crow S, Chen D, Milano C, et al. Acquired von Willebrand syndrome in continuous-flow ventricular assist device recipients. Ann Thorac Surg. 2010;90:1263–1269. doi: 10.1016/j.athoracsur.2010.04.099. [DOI] [PubMed] [Google Scholar]
- 5.Boyle AJ, Jorde UP, Sun B, et al. Pre-operative risk factors of bleeding and stroke during left ventricular assist device support: an analysis of more than 900 HeartMate II outpatients. J Am Coll Cardiol. 2014;63:880–888. doi: 10.1016/j.jacc.2013.08.1656. [DOI] [PubMed] [Google Scholar]
- 6.Slaughter MS, Naka Y, John R, et al. Post-operative heparin may not be required for transitioning patients with a HeartMate II left ventricular assist system to long-term warfarin therapy. J Heart Lung Transplant. 2010;29:616–624. doi: 10.1016/j.healun.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 7.Slaughter MS, Pagani FD, Rogers JG, et al. Clinical management of continuous-flow left ventricular assist devices in advanced heart failure. J Heart Lung Transplant. 2010;29:S1–39. doi: 10.1016/j.healun.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 8.Papaioannou T, Stefanadis C. Vascular wall shear stress: basic principles and methods. Hellenic J Cardiol. 2005;46:9–15. [PubMed] [Google Scholar]
- 9.Hochareon P, Manning K, Fontaine A, Tarbell J, Deutsch S. Correlation of in vivo clot deposition with the flow characteristics in the 50 cc penn state artificial heart: a preliminary study. ASAIO J. 2004;50:537–542. doi: 10.1097/01.mat.0000145694.40637.a0. [DOI] [PubMed] [Google Scholar]
- 10.Turitto V, Hall C. Mechanical factors affecting hemostasis and thrombosis. Thromb Res. 1998;92:S25–31. doi: 10.1016/s0049-3848(98)00157-1. [DOI] [PubMed] [Google Scholar]
- 11.Menconi M, Pockwinse S, Owen T, Dasse K, Stein G, Lian J. Properties of blood-contacting surfaces of clinically implanted cardiac assist devices: gene expression, matrix composition, and ultrastructural characterization of cellular linings. J Cell Biochem. 1995;57:557–573. doi: 10.1002/jcb.240570320. [DOI] [PubMed] [Google Scholar]
- 12.Dasse K, Chipman S, Sherman C, Levine A, Frazier O. Clinical experience with textured blood contacting surfaces in ventricular assist devices. ASAIO Trans. 1987;33:418–425. [PubMed] [Google Scholar]
- 13.Slaughter M, Naka Y, John R, et al. Post-operative heparin may not be required for transitioning patients with a HeartMate II left ventricular assist system to long-term warfarin therapy. J Heart Lung Transplant. 2010;29:616–624. doi: 10.1016/j.healun.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 14.Gandolfo F, Rita FD, Hasan A, Griselli M. Mechanical circulatory support in pediatrics. Ann Cardiothorac Surg. 2014;3:507–512. doi: 10.3978/j.issn.2225-319X.2014.08.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kar B, Delgado R, Radovancevic B, et al. Vascular Thrombosis During Support with Continuous Flow Ventricular Assist Devices: Correlation with Computerized Flow Simulations. Congestive Heart Failure. 2007;11:182–187. doi: 10.1111/j.1527-5299.2005.04474.x. [DOI] [PubMed] [Google Scholar]
- 16.Deutsch M, Meinhart J, Fischlein T, Preiss P, Zilla P. Clinical autologous in vitro endothelialization of infrainguinal ePTFE grafts in 100 patients: a 9-year experience. Surgery. 1999;126:847–855. [PubMed] [Google Scholar]
- 17.Magometschnigg H, Kadletz M, Vodrazka M, et al. Prospective clinical study with in vitro endothelial cell lining of expanded polytetrafluoroethylene grafts in crural repeat reconstruction. J Vasc Surg. 1992;15:527–535. [PubMed] [Google Scholar]
- 18.Yoder M, Mead L, Prater D, et al. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007;109:1801–1809. doi: 10.1182/blood-2006-08-043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jantzen AE, Lane WO, Gage SM, et al. Use of autologous blood-derived endothelial progenitor cells at point-of-care to protect against implant thrombosis in a large animal model. Biomaterials. 2011;32:8356–8363. doi: 10.1016/j.biomaterials.2011.07.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Müller-Glauser W, Zilla P, Lachat M, et al. Immediate shear stress resistance of endothelial cell monolayers seeded in vitro on fibrin glue-coated ePTFE prostheses. Eur J Vasc Endovasc Surg. 1993;7:324–328. doi: 10.1016/s0950-821x(05)80017-9. [DOI] [PubMed] [Google Scholar]
- 21.Achneck HE, Jamiolkowski RM, Jantzen AE, et al. The biocompatibility of titanium cardiovascular devices seeded with autologous blood-derived endothelial progenitor cells EPC-seeded antithrombotic Ti Implants. Biomaterials. 2011;32:10–18. doi: 10.1016/j.biomaterials.2010.08.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fuchs A, Netz H. Ventricular assist devices in pediatrics. Images Paediatr Cardiol. 2001;3:24–54. [PMC free article] [PubMed] [Google Scholar]
- 23.Kang S, Carlon T, Jantzen A, et al. Isolation of functional human endothelial cells from small volumes of umbilical cord blood. Ann Biomed Eng. 2013;41:2181–2192. doi: 10.1007/s10439-013-0807-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brown MA, Wallace CS, Angelos M, Truskey GA. Characterization of Umbilical Cord Blood-Derived Late Outgrowth Endothelial Progenitor Cells Exposed to Laminar Shear Stress. Tissue Eng Part A. 2009;15:3575–3587. doi: 10.1089/ten.tea.2008.0444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yeh H, Lu S, Tian T, Hong R, Lee W, Tsai C. Comparison of endothelial cells grown on different stent materials. J Biomed Mater Res A. 2006;76:835–841. doi: 10.1002/jbm.a.30595. [DOI] [PubMed] [Google Scholar]
- 26.Achneck H, Sileshi B, Lawson J. Review of the biology of bleeding and clotting in the surgical patient. Vascular. 2008;16:S6–13. [PubMed] [Google Scholar]
- 27.Ingram D, Mead L, Tanaka H, et al. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Hemostasis, Thrombosis, and Vascular Biology. 2004;104:2752–2760. doi: 10.1182/blood-2004-04-1396. [DOI] [PubMed] [Google Scholar]
- 28.Ingram DA, Mead LE, Tanaka H, et al. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood. 2004;104:2752–2760. doi: 10.1182/blood-2004-04-1396. [DOI] [PubMed] [Google Scholar]
- 29.Berezin A, Kremzer A. Circulating endothelial progenitor cells as markers for severity of ischemic chronic heart failure. J Card Fail. 2014;20:438–447. doi: 10.1016/j.cardfail.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 30.Capoccia B, Shepherd R, Link D. G-CSF and AMD3100 mobilize monocytes into the blood that stimulate angiogenesis in vivo through a paracrine mechanism. Blood. 2006;108:2438–2445. doi: 10.1182/blood-2006-04-013755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stroncek J, Grant B, Brown M, Povsic T, Truskey G, Reichert W. Comparison of endothelial cell phenotypic markers of late-outgrowth endothelial progenitor cells isolated from patients with coronary artery disease and healthy volunteers. Tissue Eng Part A. 2009;15:3473–3486. doi: 10.1089/ten.tea.2008.0673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jamiolkowski R, Kang S, Rodriguez A, et al. Increased yield of endothelial cells from peripheral blood for cell therapies and tissue engineering. Regen Med. 2015;10:1–14. doi: 10.2217/rme.15.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.