Abstract
Children with myelomeningocele have a high prevalence of obesity and excess fat accumulation in their lower extremities. However, it is not known if this is subcutaneous or intramuscular fat, the latter of which has been associated with insulin resistance and metabolic disorders. This study quantified lower leg bone, muscle and adipose tissue volume in children with myelomeningocele, classifying adipose as subcutaneous or muscle-associated. Eighty-eight children with myelomeningocele and 113 children without myelomeningocele underwent lower leg computed tomography scans. Subcutaneous and muscle-associated adipose were classified based on location relative to the crural fascia. No differences were seen in subcutaneous adipose. Higher level disease severity was associated with increased muscle-associated adipose volume and decreased muscle volume. Bone volume tended to decrease with higher levels of involvement. Increases in lower leg adiposity in children with myelomeningocele are primarily attributable to accumulation of muscle-associated adipose, which may signify increased risk for metabolic disorders.
Keywords: myelomeningocele, adipose tissue, tissue distribution, tissue volume, spina bifida
INTRODUCTION
Myelomeningocele is the most common and severe type of spina bifida and the impact on patients’ health extends beyond the characteristic neurologic deficits. While folic acid fortification has reduced the incidence of myelomeningocele, this condition is estimated to affect about 3.4 per 10,000 live births in the United States1. The incidence of myelomeningocele is even higher among certain subpopulations, including Hispanic Americans2, 3. In addition to direct neurologic deficits, patients with myelomeningocele also have an increased risk for other adverse health outcomes including osteopenia, pathologic fractures and obesity4.
Recent work has shown that even in the absence of obesity, children with myelomeningocele accumulate excess fat in their lower extremities5, 6. It is not well understood, however, whether this fat is subcutaneous, within and between muscles or both. This distinction is important as the specific health risks of adiposity depend largely on its location. While total leg fat content has been correlated with favorable insulin sensitivity7, adipose tissue within the deep fascia of leg muscles has been associated with insulin resistance8. Indeed, total lower extremity fat may be neutral or even protective with regard to metabolic markers, whereas adipose tissue within and between leg muscles has been associated with unfavorable lipid profiles, insulin resistance and increased fracture risk 7-12. With increasing life expectancy for those with myelomeningocele, it is important to understand the patterns of excess adipose accumulation in this group and the associated long term health risks.
Conventional methods of assessing adipose distribution, such as dual-energy X-ray absorptiometry and skin fold measurement, fail to distinguish between subcutaneous and deep adipose tissue. In contrast, computed tomography (CT) allows for effective three dimensional tissue segmentation based on signal attenuation13. CT is widely used to quantify abdominal and extremity adipose tissue volumes, as well as to distinguish between subcutaneous and internal adipose tissue8, 13. Estimates of tissue volume by CT segmentation are highly correlated with tissue volumes measured physically upon cadaver limb dissection14, and CT and magnetic resonance imaging have comparable utility in differentiating subcutaneous and visceral adipose tissue15, 16.
The purpose of this study was to quantitatively assess bone, muscle and adipose tissue distribution in the lower legs of children with myelomeningocele. Adipose was classified as either subcutaneous or muscle-associated based on its location relative to the crural fascia. It was hypothesized that children with myelomeningocele would have more adipose tissue, both subcutaneous and muscle-associated, and less muscle and bone compared with typically developing controls and that these patterns would be accentuated at higher neurosegmental levels.
SUBJECTS and METHODS
This cross sectional study included convenience samples of both ambulatory and non-ambulatory children with myelomeningocele between the ages of 6 and 14 years who were seen at a tertiary children's hospital from 2010-2012, as well as a convenience sample of children without disability (control group) in the same age range. Children with myelomeningocele were recruited from spina bifida clinics and medical therapy units. Children in the control group were recruited by word of mouth. The control group was screened for any medical conditions or medication use that could affect growth or development. Children with myelomeningocele were excluded if they had bilateral metal implants in the lower legs, currently used glucocorticoid or seizure medications, or had additional chronic conditions other than myelomeningocele and hydrocephalus. Participants with myelomeningocele were classified according to the International Myelodysplasia Study Group (IMSG) criteria to determine functional neurosegmental level based on manual muscle testing17. All study procedures were approved by our institutional review board, and written informed assent and consent were obtained from all participants and their guardians.
Demographic data including age, sex and ethnicity and clinical data including height, weight, body mass index (BMI) and Tanner stage of sexual development were obtained by a physician or physical therapist. Height was measured either standing or supine depending on the participant's ability to stand. Participants underwent CT imaging of the lower legs while lying supine. All CTs were acquired on the same scanner (Philips Gemini GXL Philips Medical Systems Inc., Cleveland, OH) using the same mineral reference phantom for simultaneous calibration (Model 3 CT Calibration Phantom, Mindways Software, Inc., Austin, TX). The following scanning parameters were used in order to minimize radiation exposure while acquiring contiguous 1 mm slices along the entire length of the tibias: 90 kVp, 32 mA (100 mA for scout scan), and 1 second rotation time. The effective radiation dose was estimated to be <0.05 mSv; the time required to complete each CT examination, including positioning, was less than 5 minutes.
Tissue volumes for adipose, muscle and bone were computed along the entire length of both tibias using custom scripting implemented in MATLAB (The MathWorks, Inc., Natick, MA). The length of the tibia was demarcated by the proximal surface of the intercondylar eminence and the distal surface of the medial malleolus. Tissue volumes were quantified by a semi-automated, threshold-based method using previously validated attenuation ranges of [−190, −30], [−29, 150] and [151, 1000] Hounsfield units (HU) for adipose tissue, muscle and bone, respectively14.
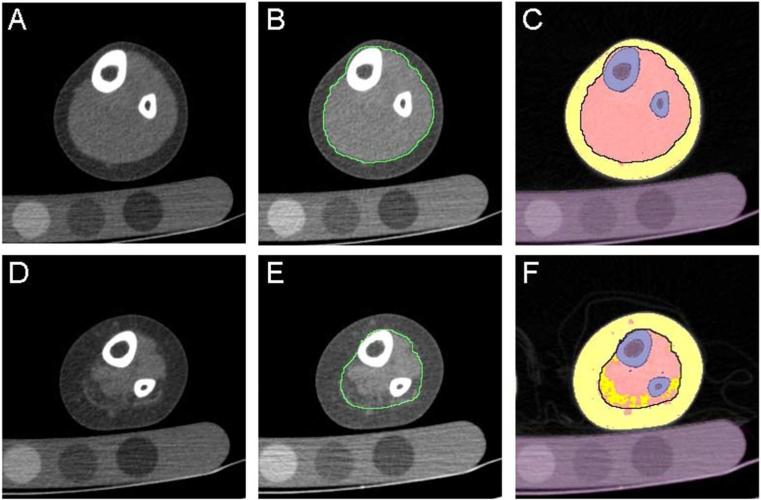
For the purpose of this study, classification of adipose tissue as subcutaneous or muscle-associated was based on work by Shen et al, which defined subcutaneous adipose tissue as that found between the dermis and the aponeurosis and deep fascia16. We then defined muscle-associated adipose tissue as total adipose minus subcutaneous adipose and bone marrow. Thus, muscle-associated adipose included adipose within the deep (crural) fascia as well as that between muscles and between muscle and bone (Figure 1)16. The boundary of the crural fascia in healthy controls was determined using Sobel operator based edge detection18. In patients with myelomeningocele, the crural fascia was manually delineated in slices at intervals of 20 mm, and the fascial boundaries in the intervening slices were estimated using shape-base interpolation of the manually delineated boundaries. Both semi-automated methods were found to have mean single user error of <3% compared to manual delineation on a variety of sample images (1.2% for edge detection in control participants and 2.9% for shape-based interpolation in myelomeningocele subjects). Tissue volumes in each leg were quantified separately with subsequent analyses using the sum of both legs for each subject.
Figure 1.
Sample segmented images. CT slice from (A) control participant and (D) myelomeningocele patient (low lumbar level) with (B,E) crural fascia highlighted and (C,F) with bone in blue, muscle in pink, muscle-associated adipose in dark yellow and SC adipose in light yellow. Purple denotes masked pixels not included in the analysis (bone marrow and calibration phantom).
Patients with myelomeningocele were divided into neurosegmental groups based on motor level of disease (IMSG classification). These groups were: sacral (S1-S3), low lumbar (L4 to L5-S1), mid lumbar (L3 to L3-4), and high lumbar level and above (T10-L2). First, differences in demographic and clinical characteristics among controls and neurosegmental groups were assessed using Chi-Square tests for categorical variables and analysis of variance (ANOVA) with Bonferroni post-hoc tests for continuous variables. Due to some variables, such as ethnicity, not being equally distributed among groups, we then conducted univariate regression analysis of muscle-associated adipose volume on demographic and clinical variables for control and myelomeningocele participants separately. Demographic and clinical variables showing a significant association with muscle-associated adipose tissue in univariate regression were considered for inclusion in a multivariate model using a step-down approach. Muscle-associated adipose, subcutaneous adipose, muscle, and bone volumes were then compared among the neurosegmental groups using ANOVA with Bonferroni post-hoc tests. Lastly, BMI was evaluated as a predictor of muscle-associated adipose volume in stratified analyses of each neurosegmental group using multivariate regression. All statistical analyses were performed in Stata 12 (StataCorp, College Station, TX).
RESULTS
The study sample included 88 children with myelomeningocele (49 males; 10y0m SD 2y8m) and 113 children without disability (62 males; 10y3m SD 2y3m). Among the 88 children with myelomeningocele, 21 were classified as sacral level, 13 as low lumbar level, 44 as mid lumbar level and 10 as high lumbar level or above (Table 1). All children in the control, sacral and low lumbar groups were ambulatory; 1 child in the mid-lumbar group was non-ambulatory, and all children in the high lumbar and above group were non-ambulatory. The study groups were well matched for sex distribution (p=0.54), weight (p=0.14) and Tanner stage (p=0.54). The study groups were also of similar age, except that the low lumbar group was significantly younger than the control group (p=0.04). The low, mid and high lumbar groups were significantly shorter than controls (p<0.001). The high lumbar group had a greater BMI than the control group (p=0.01) and the low lumbar group (p=0.02). There was also a higher proportion of Hispanic patients among the mid lumbar compared to the control group (p=0.03).
Table 1.
Comparison of demographic and clinical characteristics among neurosegmental levels. (mean, sd)
| Characteristic | Control (n=113) | Sacral (n=21) | Low Lumbar (n=13) | Mid Lumbar (n=44) | High Lumbar and above (n=10) | p |
|---|---|---|---|---|---|---|
| Age (yr) | 10y3m (2y3m) | 10y0m (2y11m) | 8y3m (2y0m)* | 10y3m (2y6m) | 10yr4m (2y8m) | 0.09 |
| Male (n, %) | 62 (55%) | 9 (43%) | 6 (46%) | 28 (64%) | 6 (60%) | 0.54 |
| Height (cm) | 142.5 (14.6) | 136.9 (19.8) | 123.8 (15.4)* | 130.0 (15.4)* | 122.4(11.8) * | <0.001 |
| Weight (kg) | 42.0 (15.8) | 39.2 (20.9) | 29.3 (10.8) | 41.9 (20.2) | 39.2 (14.6) | 0.14 |
| BMI (kg/m2) | 20.0 (4.6) | 19.6 (5.3) | 18.4 (3.0) | 22.7 (7.0) | 25.2 (5.8) *, † | 0.001 |
| Tanner Stage | 0.87 | |||||
| 1 | 51 (45%) | 10 (48%) | 9 (69%) | 22 (50%) | 6 (60%) | |
| 2 | 11 (10%) | 1 (5%) | 1 (8%) | 5 (11%) | 1 (10%) | |
| 3 | 19 (17%) | 3 (14%) | 1 (8%) | 5 (11%) | 1 (10%) | |
| 4 | 16 (14%) | 1 (5%) | 2 (15%) | 5 (11%) | 0 (0%) | |
| 5 | 16 (14%) | 6 (29%) | 0 (0%) | 7 (16%) | 2 (20%) | |
| Hispanic (n, %) | 85 (75%) | 17 (81%) | 12 (92%) | 42 (95%)* | 10 (100%) | 0.02 |
indicates significant difference from control group at p≤0.05
×Indicates significant difference from sacral group at p≤0.05
indicates significant difference from low lumbar group at p≤0.05
When considering the control and myelomeningocele participants separately, with the exception of sex (p≤0.32) and ethnicity (p≤0.11), all demographic and clinical variables, including age, height, weight, BMI and Tanner stage, were found to have significant predictive capacity in univariate regression with muscle-associated adipose tissue (p≤0.001). These variables were considered for inclusion in the multivariate model, and the final model included height, age and BMI as covariates. It is worth noting that in multivariate analysis stratified by neurosegmental group, BMI was found to be a significant predictor of muscle-associated adipose volume in control subjects (p<0.001) and all neurosegmental groups (p<0.01) except for the high lumbar group (p=0.14).
In general, total tissue volume decreased with greater disease severity as did muscle and bone volume (Table 2). After including age, height and BMI in the model, muscle volume was significantly lower in all neurosegmental groups compared to the control group (p<0.001) and was significantly decreased in all lumbar groups compared to the sacral group (p<0.001). The mid lumbar and high lumbar groups had lower bone volume than control and sacral groups (p<0.01).
Table 2.
Tissue volumes by neurosegmental level. (mean, sd)
| Tissue Volume (cm3) | Control (n=113) | Sacral (n=21) | Low Lumbar (n=13) | Mid Lumbar (n=44) | High Lumbar and above (n=10) |
|---|---|---|---|---|---|
| Total | 6210 (1770) | 5247 (2126)** | 3436 (1338)**,Δ | 4486 (1759)**Δ | 4133 (1064) ** |
| Bone | 807 (233) | 698 (274) | 483 (195) | 544 (207) **,Δ | 293 (99) **,Δ,• |
| Muscle | 3210 (889) | 2212 (988) ** | 910 (565) **,Δ | 908 (577) **,Δ | 558 (273) **,Δ |
| Total Adipose | 2194 (875) | 2336 (1134) | 2042 (958) | 3034 (1340) ** | 3282 (989) |
| Muscle-associated | 84 (70) | 307 (288) ** | 534 (311) **,Δ | 897 (513) **,Δ | 861 (382) **,Δ |
| Subcutaneous | 2110 (823) | 2030 (931) | 1509 (673) | 2138 (931) | 2421 (645) |
indicates significant difference from control group at p≤0.001
indicates significant difference from sacral group at p≤0.05
indicates significant difference from mid lumbar group at p≤0.05
Total adipose volume generally increased with disease severity but only the mid and high lumbar groups had significantly more total adipose tissue compared to controls (p<0.001) and only the high lumbar group had more adipose than the sacral group (p<0.05). The increase in total adipose tissue volume was primarily attributable to more muscle-associated adipose, which generally was associated with increasing disease severity (p<0.05) though the lumbar groups did not differ significantly from each other (p>0.99). There was no significant difference in subcutaneous adipose among any of the groups (p≥0.16).
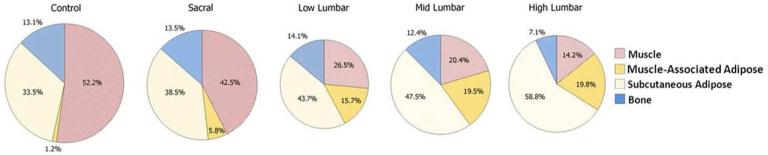
The proportion of total tissue volume comprised of muscle was lower, and muscle-associated and subcutaneous adipose proportions was higher with each increasing neurosegmental level (Figure 2). These differences were significant between the control, sacral, and lumbar groups (p<0.05) except for between the low lumbar and sacral groups for subcutaneous proportion (p=0.38). Proportions did not differ significantly among the lumbar groups (p≥0.11).
Figure 2.
Proportional tissue volumes by neurosegmental level. The size of each pie chart is proportional to mean total lower leg volume.
DISCUSSION
Past studies have shown that children with myelomeningocele are more likely to be overweight or obese compared to peers without myelomeningocele, and more recent work has shown that children with myelomeningocele accumulate a disproportionate amount of body fat in their lower extremities regardless of BMI 5, 6, 19, 20. The results of the current study indicate that this excess adipose is muscle-associated rather than subcutaneous.. This previously unknown finding may impact our understanding of long term health risks in this population.
Muscle-associated adipose has been associated with type 2 diabetes mellitus, insulin resistance and increased serum cholesterol levels, while there have been no similar relationships seen for subcutaneous adipose21-23. Several studies have found leg muscle-associated adipose specifically to be strongly associated with abnormal glucose metabolism8, 24-26. In both diabetic and non-diabetic adults, Goodpasture et al. found that leg muscle-associated adipose was associated with increased insulin resistance while subcutaneous adipose had no association, based on data from a single mid-thigh CT slice8. A later study by Goodpasture et al. also found a similar association in a study of over 2000 elderly adults25. Boettcher et al. found that adipose within the deep fascia of the calf was associated with insulin resistance in non-diabetic adults using quantification from magnetic resonance images26. Also using magnetic resonance imaging, Elder et al. found that thigh muscle-associated adipose accounted for about 70% of plasma glucose variance in adults with complete lower extremity paralysis from spinal cord injury24. The same study also found that there was a strong correlation between lower muscle volume and elevated blood glucose. The present study found that muscle-associated adipose tended to be higher and muscle volume lower in more severe myelomeningocele, which raises the concern for dysregulation of glucose and lipid metabolism in this population.
The findings of this study may have important implications for long term health management in persons with myelomeningocele. Firstly, these findings suggest that persons with myelomeningocele may be at increased risk for type 2 diabetes mellitus which, to our knowledge, has not yet been studied in the spina bifida population. This study also found that higher BMI was a significant predictor of more muscle-associated adipose in all groups except for high lumbar, which demonstrated the same trend despite a small sample size. This suggests that lowering BMI through dietary and exercise interventions might decrease muscle-associated adipose volumes and associated disease risks.
The findings of more muscle-associated adipose tissue and less muscle volume are not unexpected given that myelomeningocele results in some degree of denervation of the lower extremity, which has been previously associated with decreased muscle volume as well as increased adipose tissue within and around muscles27, 28. Interestingly, prior studies of obesity in spina bifida and paralyzed limbs have found higher skinfold thickness, indicating higher volumes of subcutaneous adipose, in the legs of children with myelomeningocele29, 30. In contrast, this study found comparable subcutaneous adipose volumes between children with and without myelomeningocele. The cause of this discrepancy is not immediately clear. One contributing factor may be a higher percentage of body fat among our control group compared to contemporary comparisons for Hayes-Allen and Tring's 1973 study, inferred from population trends. It was also noted that subcutaneous adipose comprised a greater proportion of total tissue volume in more severe disease, which might plausibly lead to a thicker layer of subcutaneous adipose for the same absolute volume. We also noted that total bone volume tended to be lower in more severe myelomeningocele, although only the mid and high lumbar groups had significantly lower bone volume after accounting for differences in height, BMI and age. While decreased total bone volume does not directly address bone strength, this finding is consistent with the increased risk of lower extremity fracture in this population31.
We note some limitations on the present study. Relatively small samples of children with low lumbar and high lumbar level myelomeningocele may have limited our ability to detect differences involving these groups. These two groups included lower proportions of patients Tanner stage 4-5, which may suggest that some differences in tissue volumes may be attributable an earlier developmental stage. Our technique of manual demarcation coupled with automated interpolation, while a widely used method of volume estimation, may result in some amount of error. We attempted to minimize measurement variability by having a single user perform manual demarcation. Limitations of CT imaging included limited visualization of muscle fascia and dermis, as well as limitations of resolution including edge effects and inability to detect scattered adipocytes. Though this study included a high proportion of Hispanic patients which may limit the generalizability of the results to other populations, it should be noted that myelomeningocele has a high prevalence in Hispanics32, 33. Lastly, height was measured standing or supine, which may underestimate height for some myelomeningocele subjects and thus overestimate BMI.
CONCLUSIONS
The results of this study suggest that greater lower leg adipose tissue volume in children with myelomeningocele is primarily attributable to higher volumes of muscle-associated adipose, particularly in patients affected at higher neurosegmental levels. In contrast, subcutaneous adipose volumes were comparable in all groups examined. Since muscle-associated adipose is more strongly associated with negative health outcomes than subcutaneous adipose, children with myelomeningocele may have an increased risk of adverse health effects. It may be possible to reduce muscle-associated adipose through diet and exercise interventions, and it may be beneficial to monitor and tailor treatment protocols to consider the anatomical distribution of adipose tissue when treating children with myelomeningocele.
Acknowledgments
Funding
Support provided by NIH-NICHD Grant # 5R01HD059826 from the National Institutes of Health – Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Footnotes
Conflict of interest
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Boulet SL, Yang Q, Mai C, et al. Trends in the postfortification prevalence of spina bifida and anencephaly in the United States. Birth Defects Res Part A-Clin Mol Teratol. 2008;82:527–532. doi: 10.1002/bdra.20468. [DOI] [PubMed] [Google Scholar]
- 2.Canfield MA, Annegers JF, Brender JD, Cooper SP, Greenberg F. Hispanic Origin and Neural Tube Defects in Houston/Harris County, Texas: II. Risk Factors. American Journal of Epidemiology. 1996;143:12–24. doi: 10.1093/oxfordjournals.aje.a008653. [DOI] [PubMed] [Google Scholar]
- 3.Shin M, Besser LM, Siffel C, et al. Prevalence of Spina Bifida Among Children and Adolescents in 10 Regions in the United States. Pediatrics. 2010;126:274–279. doi: 10.1542/peds.2009-2084. [DOI] [PubMed] [Google Scholar]
- 4.Mitchell LE, Adzick NS, Melchionne J, Pasquariello PS, Sutton LN, Whitehead AS. Spina bifida. Lancet. 2004;364:1885–1895. doi: 10.1016/S0140-6736(04)17445-X. [DOI] [PubMed] [Google Scholar]
- 5.Dosa NP, Foley JT, Eckrich M, Woodall-Ruff D, Liptak GS. Obesity across the lifespan among persons with spina bifida. Disability and Rehabilitation. 2009;31:914–920. doi: 10.1080/09638280802356476. [DOI] [PubMed] [Google Scholar]
- 6.Mueske NM, Ryan DD, Van Speybroeck AL, Chan LS, Wren TAL. Fat distribution in children and adolescents with myelomeningocele. Developmental Medicine & Child Neurology. 2014 doi: 10.1111/dmcn.12591. n/a-n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Pelt R, Jankowski C, Gozansky W, Schwartz R, Kohrt W. Lower-body adiposity and metabolic protection in postmenopausal women. The Journal of Clinical Endocrinology & Metabolism. 2005;90:4573–4578. doi: 10.1210/jc.2004-1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goodpaster BH, Thaete FL, Kelley DE. Thigh adipose tissue distribution is associated with insulin resistance in obesity and in type 2 diabetes mellitus. Am J Clin Nutr. 2000;71:885–892. doi: 10.1093/ajcn/71.4.885. [DOI] [PubMed] [Google Scholar]
- 9.Lang T, Cauley JA, Tylavsky F, Bauer D, Cummings S, Harris TB. Computed tomographic measurements of thigh muscle cross-sectional area and attenuation coefficient predict hip fracture: The health, aging, and body composition study. Journal of Bone and Mineral Research. 2010;25:513–519. doi: 10.1359/jbmr.090807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Pelt RE, Evans EM, Schechtman KB, Ehsani AA, Kohrt WM. Contributions of total and regional fat mass to risk for cardiovascular disease in older women2002. doi: 10.1152/ajpendo.00467.2001. [DOI] [PubMed] [Google Scholar]
- 11.Schafer AL, Vittinghoff E, Lang TF, et al. Fat infiltration of muscle, diabetes, and clinical fracture risk in older adults. The Journal of Clinical Endocrinology & Metabolism. 2010;95:E368–E372. doi: 10.1210/jc.2010-0780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mojtahedi MC, Valentine RJ, Arngrimsson SA, Wilund KR, Evans EM. The association between regional body composition and metabolic outcomes in athletes with spinal cord injury. Spinal Cord. 2007;46:192–197. doi: 10.1038/sj.sc.3102076. [DOI] [PubMed] [Google Scholar]
- 13.Mourtzakis M, Prado CM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab. 2008;33:997–1006. doi: 10.1139/H08-075. [DOI] [PubMed] [Google Scholar]
- 14.Mitsiopoulos N, Baumgartner RN, Heymsfield SB, Lyons W, Gallagher D, Ross R. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol (1985) 1998;85:115–122. doi: 10.1152/jappl.1998.85.1.115. [DOI] [PubMed] [Google Scholar]
- 15.Seidell JC, Bakker CJ, van der Kooy K. Imaging techniques for measuring adipose-tissue distribution--a comparison between computed tomography and 1.5-T magnetic resonance. The American Journal of Clinical Nutrition. 1990;51:953–957. doi: 10.1093/ajcn/51.6.953. [DOI] [PubMed] [Google Scholar]
- 16.Shen W, Wang Z, Punyanita M, et al. Adipose tissue quantification by imaging methods: a proposed classification. Obesity research. 2003;11:5–16. doi: 10.1038/oby.2003.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wright J. Neurosegmental level and functional status.. In: Sarwark JF, Lubicky JP, editors. Caring for the child with spina bifida : Shriners Hospitals for Children; Symposium; Oak Brook, Illinois. April 14-16, 2000; Rosemont, Ill.: American Academy of Orthopaedic Surgeon; 2001. p. xiv.p. 657. [Google Scholar]
- 18.Maini R, Aggarwai H. Study and Comparison of Various Image Edge Detection Techniques. Int J Image Processing. 2009;3:1–12. [Google Scholar]
- 19.Roberts D, Shepherd RW, Shepherd K. Anthropometry and obesity in myelomeningocele. Journal of Paediatrics and Child Health. 1991;27:83–90. doi: 10.1111/j.1440-1754.1991.tb00358.x. [DOI] [PubMed] [Google Scholar]
- 20.Shepherd K, Roberts D, Golding S, Thomas BJ, Shepherd RW. Body composition in myelomeningocele. Am J Clin Nutr. 1991;53:1–6. doi: 10.1093/ajcn/53.1.1. [DOI] [PubMed] [Google Scholar]
- 21.Gallagher D, Kelley DE, Yim J-E, et al. Adipose tissue distribution is different in type 2 diabetes. The American Journal of Clinical Nutrition. 2009;89:807–814. doi: 10.3945/ajcn.2008.26955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yim JE, Heshka S, Albu J, et al. Intermuscular adipose tissue rivals visceral adipose tissue in independent associations with cardiovascular risk. International journal of obesity. 2007;31:1400–1405. doi: 10.1038/sj.ijo.0803621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yim JE, Heshka S, Albu JB, Heymsfield S, Gallagher D. Femoral-gluteal subcutaneous and intermuscular adipose tissues have independent and opposing relationships with CVD risk. J Appl Physiol (1985) 2008;104:700–707. doi: 10.1152/japplphysiol.01035.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elder CP, Apple DF, Bickel CS, Meyer RA, Dudley GA. Intramuscular fat and glucose tolerance after spinal cord injury - a cross-sectional study. Spinal Cord. 2004;42:711–716. doi: 10.1038/sj.sc.3101652. [DOI] [PubMed] [Google Scholar]
- 25.Goodpaster BH, Krishnaswami S, Resnick H, et al. Association between regional adipose tissue distribution and both type 2 diabetes and impaired glucose tolerance in elderly men and women. Diabetes care. 2003;26:372–379. doi: 10.2337/diacare.26.2.372. [DOI] [PubMed] [Google Scholar]
- 26.Boettcher M, Machann J, Stefan N, et al. Intermuscular adipose tissue (IMAT): Association with other adipose tissue compartments and insulin sensitivity. Journal of Magnetic Resonance Imaging. 2009;29:1340–1345. doi: 10.1002/jmri.21754. [DOI] [PubMed] [Google Scholar]
- 27.Wren TAL, Ponrartana S, Van Speybroeck A, Ryan DD, Chia JM, Hu HH. Heterogeneity of muscle fat infiltration in children with spina bifida. Research in Developmental Disabilities. 2014;35:215–222. doi: 10.1016/j.ridd.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sproule DM, Punyanitya M, Shen W, et al. Muscle Volume Estimation by Magnetic Resonance Imaging in Spinal Muscular Atrophy. Journal of Child Neurology. 2011;26:309–317. doi: 10.1177/0883073810380457. [DOI] [PubMed] [Google Scholar]
- 29.Hayes-Allen MC, Tring FC. Obesity: another hazard for spina bifida children. British journal of preventive & social medicine. 1973;27:192–196. doi: 10.1136/jech.27.3.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee MM. Thickening of the subcutaneous tissues in paralyzed limbs in chronic hemiplegia. Human biology. 1959;31:187–193. [PubMed] [Google Scholar]
- 31.Akbar M, Bresch B, Raiss P, et al. Fractures in myelomeningocele. J Orthop Traumatol. 2010;11:175–182. doi: 10.1007/s10195-010-0102-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shin M, Besser LM, Siffel C, et al. Prevalence of spina bifida among children and adolescents in 10 regions in the United States. Pediatrics. 2010;126:274–279. doi: 10.1542/peds.2009-2084. [DOI] [PubMed] [Google Scholar]
- 33.Williams LJ, Rasmussen SA, Flores A, Kirby RS, Edmonds LD. Decline in the prevalence of spina bifida and anencephaly by race/ethnicity: 1995–2002. Pediatrics. 2005;116:580–586. doi: 10.1542/peds.2005-0592. [DOI] [PubMed] [Google Scholar]