Abstract
Objective
To determine the contemporary etiology, burden, and short-term outcomes of seizures in neonates monitored with continuous video-electroencephalogram (cEEG).
Study design
We prospectively collected data from 426 consecutive neonates (56% male, 88% term) ≤44 weeks postmenstrual age with clinically suspected seizures and/or electrographic seizures. Subjects were assessed between January 2013 and April 2015 at seven U.S. tertiary care pediatric centers following American Clinical Neurophysiology Society (ACNS) guidelines for cEEG for at risk neonates. Seizure etiology, burden, management and outcome were determined by chart review using a case report form designed at study onset.
Results
The most common seizure etiologies were hypoxic-ischemic encephalopathy (38%), ischemic stroke (18%), and intracranial hemorrhage (11%). Seizure burden was high, with 59% having ≥7 electrographic seizures and 16% having status epilepticus; 52% received ≥2 anti-seizure medications. During the neonatal admission, 17% died; 49% of survivors had abnormal neurological examination at hospital discharge. In an adjusted analysis, high seizure burden was a significant risk factor for mortality, length of hospital stay, and abnormal neurological examination at discharge.
Conclusions
In this large contemporary profile of consecutively enrolled newborns with seizures treated at centers using cEEG per ACNS guidelines, about half had high seizure burden, received ≥2 anti-seizure medications, and/or died or had abnormal examination at discharge. Higher seizure burden was associated with increased morbidity and mortality. These findings underscore the importance of accurate determination of neonatal seizure frequency and etiology, and a potential for improved outcome if seizure burden is reduced.
Keywords: Neonatal seizures, EEG, Electroencephalogram, Epilepsy, Neurocritical Care, Infant, Critical care, Neonatal Encephalopathy, Hypoxic-Ischemic Encephalopathy
Seizures are a common manifestation of neurological disorders in neonates and are associated with unfavorable short- and long-term developmental outcomes.1 More than 50% of survivors experience considerable disability across a range of developmental domains, most frequently cerebral palsy, post-neonatal epilepsy, and/or intellectual disability,1,2 requiring costly, lifelong therapies and social and academic support.
Advances in the accurate diagnosis and management of seizures in neonates have been limited by several important factors: (1) seizures are difficult to diagnose as almost any abnormal movement can be due to seizures, yet electrographic seizures frequently do not have a clinical correlate;3,4 (2) commonly used medications have limited efficacy;5 and (3) the relatively rare occurrence of seizures (1–4/1000 live term births) requires multicenter collaborative efforts.6–8 Most studies of neonatal seizures have used either single-center data or population-based information that relied primarily on observation of clinical seizures rather than seizures identified by electroencphalography (EEG)..
To address these limitations, we developed the Neonatal Seizure Registry, a multicenter collaboration of tertiary centers across the United States that follow the American Clinical Neurophysiology Society (ACNS) guidelines for continuous video-EEG (cEEG) monitoring for at-risk neonates.9 The aim of this study was to use registry data to identify the contemporary profile of seizure etiologies and characteristics of seizures in a large, prospective, consecutive cohort.
METHODS
Subjects were consecutive neonates (<44 weeks postmenstrual age) with clinical events suspicious for seizures and/or confirmed EEG seizures, who were admitted from January 2013 to April 2015 to one of the 7 participating tertiary care centers. All centers followed the 2011 ACNS guidelines for brain monitoring in neonates9 which recommend cEEG for the following indications: (1) to assess differential diagnosis of paroxysmal events (i.e., patients with one or more clinical events suggestive of seizure); (2) to detect seizures in high-risk populations (ie, neonates with acute encephalopathy, need for extracorporeal membrane oxygenation (ECMO), central nervous system infection, or, intracranial bleeding); and/or (3) to assess for background abnormalities during acute encephalopathy. The ACNS guidelines recommended duration of cEEG monitoring is: until index clinical events are captured, for a minimum of 24 hours, or until at least 24 hours after resolution of electrographic seizures. All centers used cEEG for neonates treated with therapeutic hypothermia during hypothermia and rewarming. Study data were collected and managed using REDCap (Research Electronic Data Capture) tools, hosted at University of California, San Francisco.13 The local Institutional Review Board or Committee on Human Research approved a waiver of consent for data collection at each site.
Clinical data were compiled prospectively in a systematic manner using predetermined variable definitions. Patient demographic characteristics, duration of monitoring, and in-hospital neurologic outcomes were extracted from medical records by a trained research assistant at each site. A study investigator at each site reviewed medical records, including clinical, laboratory, EEG, and neuroimaging results, to determine the indication for EEG monitoring, seizure etiology, and burden, as well as EEG and examination findings. Seizures were defined as repetitive, evolving patterns, with a definite beginning and end, with a minimum duration of 10 seconds and a minimum amplitude of 2 microvolts.10,11 EEG seizure burden was defined a priori as follows: (1) none; (2) rare EEG seizures (<7); (3) many isolated EEG seizures (≥7); (4) frequent recurrent EEG seizures; (5) status epilepticus; or (6) documentation inadequate to quantify. Status epilepticus (SE) was defined as any electrographic recording with seizures lasting >50% over at least one hour of recording.11,12 Seizure burden was also dichotomized to “low” (<7 seizures) or “high” (≥7). Abnormal neurological examination was defined as abnormalities in consciousness, tone, and/or reflexes, as documented by the treating clinician(s). Anti-seizure medication administration was based on local guidelines at the discretion of the treating physicians. Each site obtained a neurologist consult on neonates with seizures as standard care.
To help ensure data integrity, the study principal investigator and coordinating center research assistant reviewed data from each site for completeness and outliers. In addition, five randomly chosen files from each center were re-abstracted in person by the study principal investigator and research assistant. During these study audits, data were checked for completeness and accuracy, and local investigators were asked to correct any systematic errors.
Statistical Analyses
Study results are presented as actual numbers with percentages, mean with standard deviation, or medians with interquartile ranges (IQR). The chi-squared test was used to examine the difference between proportions. Student t-test was used to compare means. Statistical analyses were performed using Stata 12 (StataCorp, College Station, Texas) and p-values <0.05 were considered significant. For the adjusted analysis, variables that were significant to p=0.1 were included in the multivariable model, which was then refined using backward stepwise regression as needed.
RESULTS
Seven sites enrolled 426 subjects who had suspected or confirmed seizures during the study period and were monitored with cEEG according to ACNS guidelines. The indication for cEEG was a suspicion of clinical seizures in 63%, and the remaining neonates were monitored for encephalopathy with or without suspicious clinical events (15% and 19%, respectively), or other indication in 4% (during ECMO or post cardiac surgery in 7 subjects, abnormal neuroimaging in 5, and unspecified/other in 3). Basic demographic data are presented in Table I.
Table 1.
Clinical characteristics and seizure etiology among 426 neonates with clinically suspected and/or EEG confirmed seizures who were monitored by cEEG.
| Overall N=426 | |
|---|---|
| Clinical Characteristics | |
| Male | 237 (56%) |
| Term (>36 weeks gestation) | 373 (88%) |
| Admission to the study center at <24 hours of age | 222 (52%) |
| Medical Comorbidities | |
| Congenital cardiac disease | 60 (14%) |
| ECMO | 27 (6%) |
| Dialysis | 6 (1%) |
| Congenital diaphragmatic hernia | 4 (1%) |
| Indication for cEEG Monitoring | |
| Clinical event suspicious for seizure | 267 (63%) |
| Encephalopathy | 82 (19%) |
| Clinical event and encephalopathy | 62 (15%) |
| Other | 15 (4%) |
| Seizure Etiology | |
| Hypoxic ischemic encephalopathy | 163 (38%) |
| Ischemic stroke | 75 (18%) |
| Intracranial hemorrhage | 49 (12%) |
| Epileptic encephalopathy/Genetic epilepsy | 24 (6%) |
| Intracranial infection | 19 (4%) |
| Brain Malformation | 18 (4%) |
| Transient metabolic (hypoglycemia or electrolyte disturbance) | 16 (4%) |
| Inborn error of metabolism | 13 (3%) |
| Benign familial neonatal epilepsy | 11 (3%) |
| Other/Unknown | 38 (9%) |
| Short Term Outcomes | |
| Death or transfer to hospice | 72 17%) |
| Abnormal mental status, tone or reflexes among survivors at discharge/transfer | 173 (49%) |
| Length of hospital stay among survivors discharged home, days | 14 (10, 28) |
Data are presented as N(%), median(interquartile range)
ECMO extracorporeal membrane oxygenation, cEEG continuous video-EEG monitoring
The most common seizure etiologies were hypoxic-ischemic encephalopathy (HIE, 38%), arterial or venous ischemic stroke (18%), and intracranial hemorrhage (ICH, 11%) (Table I). Neonatal onset epilepsy was present in 13%, due to epileptic encephalopathy/genetic epilepsy syndrome in 6% and congenital brain malformation in 4%; benign familial neonatal epilepsy was identified in 3%. Most subjects (79%) had a single identified etiology; those with more than one etiology usually had a combination of acute symptomatic and/or transient metabolic etiologies.
Seizure Characteristics
Eighty-two percent of subjects had electrographic seizures detected by cEEG. The remainder had only clinical events suspicious for seizures that resolved prior to cEEG recording, or electrographic seizures recorded at the referral hospital but no confirmed seizures on the study center cEEG. Sixty-two percent of subjects had at least one electrographic seizure without clinical correlate (i.e. subclinical seizure) and 16% had only electrographic seizures without clinical correlate. Subclinical seizures occurred equally among neonates with at least one seizure captured on EEG, regardless of seizure burden.
Monitoring with cEEG was maintained for a median duration of 66 hours (IQR 40, 96 hours), with 90% of subjects monitored for ≥24 hours, and 98% monitored for >12 hours. cEEG monitoring was initiated at a median age of 50 hours (IQR 15 hours, 5 days) for term neonates, whose first clinically suspected seizure was reported at a median age of 27 hours (IQR 11, 80 hours). This was significantly different from preterm newborns for whom cEEG monitoring was initiated at a median 11 days (IQR 27 hours, 33 days, p<0.0005 compared with term neonates), whose first suspected seizure was noted at a median of 14 days (IQR 3, 33 days). The median time to electrographic seizure detection from the onset of recording was 7 hours (IQR 3, 17 hours) and was not significantly different between term and preterm neonates (p=0.09) or by indication for monitoring (p=0.5).
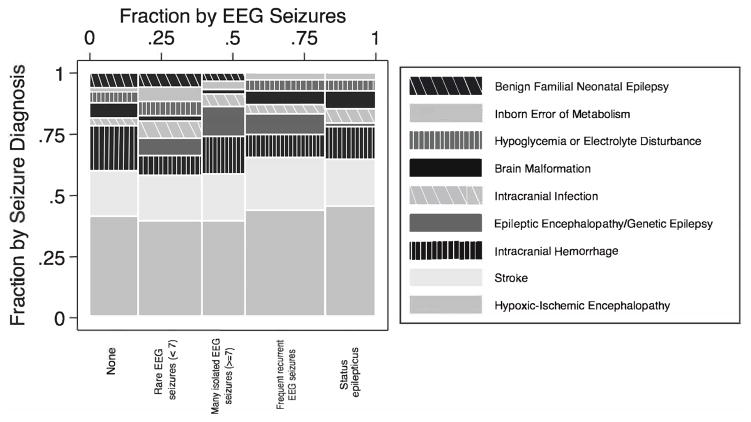
The seizure burden was high, with 59% of subjects having ≥7 electrographic seizures and 16% having status epilepticus (Figure). There was no significant difference in seizure burden between preterm and term neonates or among the three most common causes of seizure (HIE, ischemic stroke, and ICH, p=0.9). Term newborns with HIE treated with hypothermia showed no difference in EEG seizure frequency when compared with those not treated with hypothermia (65/76, 85% vs 28/38, 74% with any EEG seizures, p=0.12), excluding term newborns with other medical diagnoses and preterm newborns.
Figure.
Mosaic plot of seizure burden by etiology. The proportions on the x-axis represent the number of observations for each level of seizure burden, whereas the proportions on the y-axis represent the proportion by seizure etiology.
Medical Management of Seizures
Phenobarbital was the most common medication used during the hospital admission (94%) and for initial bolus dosing (93%). Forty-three percent of subjects were treated with phenobarbital prior to monitoring; subjects without seizures on EEG were more likely to have been treated with phenobarbital prior to monitoring (54/76, 71% vs 22/76, 29% p<0.001). The next most commonly used medications were levetiracetam and fosphenytoin, followed by benzodiazepines for either intermittent or infusion dosing (Tables II and III). Topiramate, carbamazepine/oxcarbazepine, lidocaine, and lacosamide were administered to <5% of subjects.
Table 2.
Medication use by seizure etiology among 426 neonates with clinically suspected and/or EEG confirmed seizures who were monitored by cEEG.
| Phenobarbital | Levetiracetam | Fosphenytoin | |
|---|---|---|---|
| Term | 346 (93%) | 116 (31%) | 109 (29%) |
| Preterm | 47 (89%) | 18 (34%) | 10 (19%) |
| Hypoxic ischemic encephalopathy | 153 (94%) | 45 (28%) | 44 (27%) |
| Ischemic stroke | 71 (95%) | 23 (31%) | 28 (37%) |
| Intracranial hemorrhage | 45 (92%) | 19 (39%) | 17 (35%) |
| Epileptic encephalopathy/Genetic epilepsy | 23 (96%) | 15 (63%) | 6 (25%) |
| Intracranial infection | 18 (95%) | 9 (47%) | 4 (21%) |
| Brain Malformation | 18 (100%) | 10 (56%) | 8 (44%) |
| Benign familial neonatal epilepsy | 9 (82%) | 1 (9%) | 0 |
| Inborn error of metabolism | 10 (77%) | 3 (23%) | 2 (15%) |
| Transient metabolic (hypoglycemia or electrolyte disturbance) | 15 (93%) | 2 (13%) | 3 (19%) |
| Other/Unknown | 31 (82%) | 7 (18%) | 7 (18%) |
Data are presented as N and row %
Table 3.
Seizure management among 426 neonates with clinically suspected and/or EEG confirmed seizures who were monitored by cEEG.
| Overall N=426 | |
|---|---|
| Initial loading medication and dose | |
| Phenobarbital (20mg/kg, IQR 20, 20mg/kg) | 379 (89%) |
| Levetiracetam (20mg/kg, IQR 20, 32mg/kg) | 22 (5%) |
| Fosphenytoin (20m/kg, IQR 15, 20mg/kg) | 4 (1%) |
| No loading dose | 18 (4%) |
| Seizure medications administered during the admission | |
| Phenobarbital | 393 (92%) |
| Levetiracetam | 134 (31%) |
| Fosphenytoin | 119 (28%) |
| Benzodiazepine – intermittent doses | 84 (20%) |
| Benzodiazepine infusion | 31 (7%) |
| Topiramate | 17 (4%) |
| Carbamazepine/oxcarbazepine | 9 (2%) |
| Vitamin(s): (pyridoxine, folic acid, pyridoxal 5 phosphate) | 32 (8%) |
| Number of anti-seizure medications administered | |
| 0 | 10 (2%) |
| 1 | 194 (46%) |
| 2 | 101 (24%) |
| 3 | 68 (16%) |
| ≥4 | 53 (12%) |
Overall, 64% of subjects had electrographic seizures that were refractory to the initial loading dose of anti-seizure medication (Table III). There was no significant difference in response to initial medication among term and preterm neonates. There was a significant difference in rates of refractory seizures following initial medication given when all etiologies were compared (overall p=0.01), but there was no significant difference among the three most common etiologies, where the rate of seizures refractory to initial medication was high (HIE 62%, stroke 66%, ICH 70%, p=0.3). Neonates with inborn errors of metabolism were least likely to have refractory seizures (33% refractory), followed by neonates with benign familial neonatal seizures (40% refractory). Median loading dose with phenobarbital was 20mg/kg (IQR 20, 20mg/kg), and 245/379 subjects (65%) had subsequent electrographic seizures. Median loading dose with levetiracetam was 20mg/kg (IQR 20, 32mg/kg) and 14/22 subjects (64%) who received levetiracetam as their initial loading medication had subsequent electrographic seizures. Median loading dose of fosphenytoin was 20mg/kg (IQR 15, 20mg/kg) and all 4 subjects (100%) who received fosphenytoin as their initial loading medication had subsequent electrographic seizures.
Seizures were treated with at least one medication in >97% of subjects; and 52% were treated with ≥2 anti-seizure medications during the inpatient stay (Table III). There was no difference in the number of medications used among term vs preterm neonates and among the three most common diagnoses (HIE, ICH, and ischemic stroke, p>0.3).
Short-Term Outcomes
Overall, 72 subjects (17%) died prior to discharge or were transferred to hospice care, and mortality was higher for preterm compared with term neonates (32% versus 15%, p=0.002). Seizure etiology was associated with death. Neonates with brain malformation had the highest rate of death (33%). Among the three most common causes of seizure, the highest mortality was among neonates with HIE (26%) as compared with those with ICH (13%) and ischemic stroke (4%, p<0.0005). Mortality was strongly associated with seizure burden, with higher mortality among those neonates who experienced a higher seizure burden. Neonates with <7 seizures captured on EEG at the study center had a mortality of 6%, and those with ≥7 seizures had a 24% mortality (p<0.0005). Similarly, neonates without status epilepticus had a mortality of 15%, and those with status epilepticus had a mortality of 26% (p=0.03). Neonates whose seizures were refractory to a loading dose of medication were twice as likely to die (54/264, 20%) as compared with neonates whose seizures were controlled with the initial loading dose of medication (13/143, 9%, p=0.009). Neonates who had only subclinical seizures had higher mortality (20/67, 30%) than neonates who had clinical manifestations with or without electrographic correlate (52/359, 14%, p=0.002). In an adjusted analysis, seizure etiology, higher seizure burden, and preterm birth all remained significant risk factors for death. Accounting for only those neonates with acute symptomatic seizures, etiology, higher seizure burden and preterm birth remained significant risk factors for death.
Median length of hospital stay among survivors was 13 days (IQR 9, 24) for term neonates and 46 days (IQR 19, 91) for preterm neonates (p<0.0005). Seizure etiology was associated with length of hospital stay, which was longer for subjects who had ≥7 seizures (median 16, IQR 11, 35 days) compared with those with <7 seizures (median 12, IQR 8, 27 days, p=0.02). In an adjusted analysis, seizure etiology, higher seizure burden and preterm birth were all significantly associated with length of hospital stay. Accounting for only those neonates with acute symptomatic seizures, etiology, higher seizure burden, and preterm birth remained significant risk factors for length of stay.
Among survivors, 49% had an abnormal examination (abnormality of consciousness, tone or reflexes) at the time of discharge or transfer. There was no difference in rates of abnormal examination between term and preterm neonates (p=0.2). Rates of abnormal examination were highest among survivors with brain malformation (10/12, 83%), neonatal onset epileptic encephalopathies/genetic epilepsies (13/22, 59%), and HIE (69/121, 57%). Seizure burden was significantly associated with abnormal examination at the time of hospital discharge among survivors. Neonates with ≥7 electrographic seizures had a higher rate of abnormal examination at the time of hospital discharge (112/191, 59%) compared with those with <7 seizures (61/163, 37%, p<0.0005). Among survivors of status epilepticus, 38/52 (73%) had a higher rate of abnormal neurological examination at the time of hospital discharge compared with those without status epilepticus 135/302 (45%). In an adjusted analysis, seizure etiology and burden remained significant for abnormal neurological examination at the time of discharge. Accounting for only those neonates with acute symptomatic seizures, etiology was no longer a significant risk factor; however, high seizure burden remained a significant risk factor for abnormal neurological examination.
DISCUSSION
Our multicenter, collaborative effort from 7 tertiary centers that use continuous video-EEG according to ACNS guidelines9 provides important data to examine and improve management of neonates with seizures. In particular, our data show that higher seizure burden is associated with mortality, longer length of hospital stay, and abnormal neurological examination at the time of hospital discharge, independent of seizure etiology and preterm birth. This finding underscores the importance of detecting and characterizing neonatal seizures, and the potential for improving outcome with better seizure control. We furthermore confirm that neonatal seizures are associated with a high need for specialized neurological care, as more than half of subjects had ≥7 seizures that were refractory to initial loading doses of anti-seizure medication, received ≥2 antiseizure medications, and/or were deceased or had an abnormal neurologic examination at the time of discharge.
Data from these 7 centers following ACNS guidelines add to the literature that supports the ACNS recommendations to monitor at-risk neonates with conventional video-EEG for at least 24 hours of continuous monitoring to identify subclinical seizures and confirm electrographic correlates of paroxysmal events9,14. High frequency of subclinical seizures has been reported in several previous studies of neonates,3,15,16 and our much larger study population supports and extends this finding. Furthermore, we found that seizures were detected within a median of 7 hours of cEEG monitoring onset (and more than 75% were detected within 24 hours) and that subclinical seizures were associated with high mortality.
Our data also confirm the high burden of seizures among neonates with the most common etiologies (HIE, stroke, and ICH), with >40% of these neonates having frequent recurrent seizures or status epilepticus. Notably, higher seizure burden was a significant risk factor for mortality, longer length of stay, and abnormal examination at discharge among newborns with these three acute symptomatic etiologies. This association between higher seizure burden and worse short-term outcome further supports the possibility that improved seizure control might improve neurologic outcome in these newborns. Additionally, we found that HIE remains the most common cause of seizures despite reports suggesting a lower seizure burden among neonates treated with hypothermia.17–19
Similar to previous reports, we found that >50% of neonates have electrographic seizures refractory to the initial medication.5,20 There was no significant difference in response to the three most common initial medications, phenobarbital, levetiracetam, and fosphenytoin. These data suggest that phenobarbital, fosphenytoin, and levetiracetam are incompletely effective for neonates with the most refractory seizures. Clinical trials are needed to determine which medication, or combination of medications, and which doses are most effective.
Although we report a large cohort from 7 pediatric centers that follow the latest ACNS guidelines for monitoring in neonates, our study has limitations. First, we relied on chart review, including EEG reports and determination of seizure etiology. However, each study center included a child neurologist and neurophysiologist with special interest in neonatal neurology, factors that strengthen both clinical reporting and data collection for the study. Second, although study investigators regularly monitored inpatient services for eligible subjects, it is possible some at-risk patients with shorter stay or milder clinical manifestations were missed, and thus, our data might be skewed toward more severely affected subjects. Third, our large study cohort precluded collection of detailed data that would be helpful to further elucidate the etiology, characteristics, and management of neonatal seizures, such as detailed maternal and fetal data, the precise number, localization, and duration of seizures, rationale for individual medication choices, and effect of medications on EEG seizure burden. Lastly, the distribution of seizure etiology in our study may not reflect the distribution in the general population, as tertiary centers typically care for more neonates with rare neonatal onset epilepsies and more severe and complex medical diseases. Nonetheless, our data set includes a large number of neonates with the most common seizure etiologies. This greater representation of rare etiologies is helpful to delineate differences between the rare and common seizure etiologies.
In conclusion, we have shown that seizures are a frequent manifestation of neurological disorders in neonates and are associated with high morbidity and mortality. Optimizing seizure identification and management may improve outcome. Randomized controlled trials and large longitudinal cohort studies to examine the relationship between management and outcomes are urgently needed. The degree to which seizure burden results in or reflects a risk for increased morbidity and mortality should be addressed in future clinical trials and prospective studies that control for underlying seizure etiology.
Acknowledgments
Supported by the Pediatric Epilepsy Research Foundation. H.G. is supported by the National Institutes of Health (NIH)/National Institute of Neurological Disorders and Stroke (K23NS066137) and the Neonatal Brain Research Institute at University of California, San Francisco. J.S. is supported by Boston Children’s Hospital Intellectual and Developmental Disabilities Research Center,(P30 HD18655). REDCap (Research Electronic Data Capture) tools is supported by University of California, San Francisco.
Abbreviations and Acronyms
- ACNS
American Clinical Neurophysiology Society
- cEEG
continuous video-EEG
- ECMO
extracorporeal membrane oxygenation
- EEG
electroencephalogram
- HIE
hypoxic-ischemic encephalopathy
- ICH
intracranial hemorrhage
- IQR
interquartile range
Appendix
Additional members of the Neonatal Seizure Registry Study Group include:
Ann Marie Bergin, MB, ScM, MRCP (UK), Boston Children’s Hospital and Harvard Medical School (neurophysiology data analysis and interpretation), Boston, MA; Taeun Chang, MD, Children’s National Health System, George Washington University School of Medicine, Washington, DC; Dennis Dlugos, MD, MSCE, Children’s Hospital of Philadelphia and University of Pennsylvania School of Medicine, Philadelphia, PA; Donna M. Ferriero, MD, MS, UCSF Benioff Children’s Hospital and University of California San Francisco School of Medicine, San Francisco, CA; and Kevin Staley, MD, Massachusetts General Hospital and Harvard Medical School, Boston, MA.
Footnotes
No reprints requested
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Uria-Avellanal C, Marlow N, Rennie JM. Outcome following neonatal seizures. Semin Fetal Neonatal Med. 2013;18:224–32. doi: 10.1016/j.siny.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 2.Ronen GM, Buckley D, Penney S, Streiner DL. Long-term prognosis in children with neonatal seizures: a population-based study. Neurology. 2007;69:1816–22. doi: 10.1212/01.wnl.0000279335.85797.2c. [DOI] [PubMed] [Google Scholar]
- 3.Clancy RR, Legido A, Lewis D. Occult neonatal seizures. Epilepsia. 1988;29:256–61. doi: 10.1111/j.1528-1157.1988.tb03715.x. [DOI] [PubMed] [Google Scholar]
- 4.Biagioni E, Ferrari F, Boldrini A, Roversi MF, Cioni G. Electroclinical correlation in neonatal seizures. Eur J Paediatr Neurol. 1998;2:117–25. doi: 10.1016/s1090-3798(98)80027-5. [DOI] [PubMed] [Google Scholar]
- 5.Painter MJ, Scher MS, Stein AD, Armatti S, Wang Z, Gardiner JC, et al. Phenobarbital compared with phenytoin for the treatment of neonatal seizures. N Engl J Med. 1999;341:485–9. doi: 10.1056/NEJM199908123410704. [DOI] [PubMed] [Google Scholar]
- 6.Glass HC, Pham TN, Danielsen B, Towner D, Glidden D, Wu YW. Antenatal and intrapartum risk factors for seizures in term newborns: a population-based study, California 1998–2002. J Pediatr. 2009;154:24–8. e1. doi: 10.1016/j.jpeds.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hall DA, Wadwa RP, Goldenberg NA, Norris JM. Maternal risk factors for term neonatal seizures: population-based study in Colorado, 1989–2003. J Child Neurol. 2006;21:795–8. doi: 10.1177/08830738060210092001. [DOI] [PubMed] [Google Scholar]
- 8.Saliba RM, Annegers JF, Waller DK, Tyson JE, Mizrahi EM. Incidence of neonatal seizures in Harris County, Texas, 1992–1994. Am J Epidemiol. 1999;150:763–9. doi: 10.1093/oxfordjournals.aje.a010079. [DOI] [PubMed] [Google Scholar]
- 9.Shellhaas RA, Chang T, Tsuchida T, Scher MS, Riviello JJ, Abend NS, et al. The American Clinical Neurophysiology Society’s Guideline on Continuous Electroencephalography Monitoring in Neonates. Journal of clinical neurophysiology: official publication of the American Electroencephalographic Society. 2011;28:611–7. doi: 10.1097/WNP.0b013e31823e96d7. [DOI] [PubMed] [Google Scholar]
- 10.Clancy RR, Legido A. The exact ictal and interictal duration of electroencephalographic neonatal seizures. Epilepsia. 1987;28:537–41. doi: 10.1111/j.1528-1157.1987.tb03685.x. [DOI] [PubMed] [Google Scholar]
- 11.Tsuchida TN, Wusthoff CJ, Shellhaas RA, Abend NS, Hahn CD, Sullivan JE, et al. American clinical neurophysiology society standardized EEG terminology and categorization for the description of continuous EEG monitoring in neonates: report of the American Clinical Neurophysiology Society critical care monitoring committee. Journal of clinical neurophysiology: official publication of the American Electroencephalographic Society. 2013;30:161–73. doi: 10.1097/WNP.0b013e3182872b24. [DOI] [PubMed] [Google Scholar]
- 12.Abend NS, Wusthoff CJ. Neonatal seizures and status epilepticus. J Clin Neurophysiol. 2012;29:441–8. doi: 10.1097/WNP.0b013e31826bd90d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of biomedical informatics. 2009;42:377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wietstock SO, Bonifacio SL, Sullivan JE, Nash KB, Glass HC. Continuous Video Electroencephalographic (EEG) Monitoring for Electrographic Seizure Diagnosis in Neonates: A Single-Center Study. J Child Neurol. 2015 doi: 10.1177/0883073815592224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nash KB, Bonifacio SL, Glass HC, Sullivan JE, Barkovich AJ, Ferriero DM, et al. Video-EEG monitoring in newborns with hypoxic-ischemic encephalopathy treated with hypothermia. Neurology. 2011;76:556–62. doi: 10.1212/WNL.0b013e31820af91a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scher MS. Neonatal seizures and brain damage. Pediatr Neurol. 2003;29:381–90. doi: 10.1016/s0887-8994(03)00399-0. [DOI] [PubMed] [Google Scholar]
- 17.Low E, Boylan GB, Mathieson SR, Murray DM, Korotchikova I, Stevenson NJ, et al. Cooling and seizure burden in term neonates: an observational study. Arch Dis Child Fetal Neonatal Ed. 2012;97:F267–72. doi: 10.1136/archdischild-2011-300716. [DOI] [PubMed] [Google Scholar]
- 18.Orbach SA, Bonifacio SL, Kuzniewicz MW, Glass HC. Lower incidence of seizure among neonates treated with therapeutic hypothermia. J Child Neurol. 2014;29:1502–7. doi: 10.1177/0883073813507978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Srinivasakumar P, Zempel J, Wallendorf M, Lawrence R, Inder T, Mathur A. Therapeutic hypothermia in neonatal hypoxic ischemic encephalopathy: electrographic seizures and magnetic resonance imaging evidence of injury. J Pediatr. 2013;163:465–70. doi: 10.1016/j.jpeds.2013.01.041. [DOI] [PubMed] [Google Scholar]
- 20.Boylan GB, Rennie JM, Chorley G, Pressler RM, Fox GF, Farrer K, et al. Second-line anticonvulsant treatment of neonatal seizures: a video-EEG monitoring study. Neurology. 2004;62:486–8. doi: 10.1212/01.wnl.0000106944.59990.e6. [DOI] [PubMed] [Google Scholar]