Abstract
Objectives
In population-based studies performed on multiple continents over the past two decades, diabetes mellitus has been negatively associated with the prevalence and progression of abdominal aortic aneurysm (AAA) disease. We investigated the possibility that metformin, the primary oral hypoglycemic agent in use worldwide, may influence the progression of AAA disease
Methods
Pre-operative AAA patients with diabetes were identified from an institutional database. After tabulating individual cardiovascular and demographic risk factors and prescription drug regimens, odds ratios for categorical influences on annual AAA enlargement were calculated via nominal logistical regression. Experimental AAA modeling experiments were subsequently performed in normoglycemic mice to validate the database-derived observations, as well as suggest potential mechanisms of metformin-mediated aneurysm suppression.
Results
Fifty eight patients met criteria for study inclusion. Of 11 distinct classes of medication considered, only metformin usage was negatively associated with AAA enlargement. This association remained significant after controlling for gender, age, cigarette smoking status and obesity. The median enlargement rate in AAA patients not taking oral diabetic medication was 1.5 mm/year; by nominal logistic regression, metformin, hyperlipidemia, and age ≥70 years were associated with below median enlargement, whereas sulfonylurea therapy, initial aortic diameter ≥40 mm and statin usage were associated with above median enlargement. In experimental modeling, metformin dramatically suppressed the formation and progression, with medial elastin and smooth muscle preservation and reduced aortic mural macrophage, CD8 T cell and neovessel density.
Conclusions
Epidemiologic evidence of AAA suppression in diabetes may be attributable to concurrent therapy with the oral hypoglycemic agent metformin.
Introduction
Abdominal aortic aneurysm (AAA) disease remains an important cause of early, preventable death in the 21st century. Despite significant advances in understanding AAA-related genetic, metabolic and environmental risks, and the implementation of population-based disease screening programs worldwide, thousands of patients continue to die prematurely each year from aneurysm-related events1.
While pharmacologic intervention has proven ineffective in limiting aneurysm enlargement or disease progression in numerous clinical trials, the balance of available evidence suggests that patients with diabetes mellitus are less likely to develop AAAs, and when present, AAAs progress or enlarge more slowly in diabetic patients2–5. Investigations into potential anti-aneurysmal implications of diabetes mellitus have focused on both influences of hyperglycemia on the structure and stability of the aortic mural extracellular matrix, including aberrant monocyte-matrix interactions, impaired plasmin generation, impaired aortic mural angiogenesis, or production or consequences of advanced glycation end products6–9.
Alternatively, AAA disease may be limited by the medications used to treat diabetes or its complications, including all hypoglycemic medications as a class or specific agents such as the thiazolidinediones10, 11. The most commonly prescribed medication for diabetes worldwide is metformin (Glucophage). Given its broad influences on metabolism and inflammation, as well as its common usage in management of insulin-resistant (type II) diabetes prevalent in older patients at risk for AAA disease, the potential influence of metformin on aneurysmal aortic degeneration deserves further investigation.
We examined the contemporary relationship between metformin usage and AAA progression in diabetic patients identified in an institution-specific medical record database. In an effort to distinguish between the influences of hyperglycemia and metformin therapy for diabetes management on disease progression, metformin was administered to normoglycemic (e.g. wild type) mice with experimental AAAs.
Methods
Patient cohort and data extraction
The patient cohort was abstracted from STRIDE (Stanford Translational Research Integrated Database Environment), an institution-specific medical informatics platform designed to support clinical and translational research12. For purposes of this project, STRIDE integrates two distinct components: a library of unstructured medical records and a development framework for building queries and research data management applications. As of January 2013, STRIDE included 9 million entries on 1.2 million patients acquired over 18 years13.
Clinical data from de-identified diabetic AAA patients to analyze observed aneurysm enlargement as a function of demographic risk factors for AAA disease, comorbidities and individual cardiovascular and diabetic-related medication regimens. The National Center for Biomedical Ontology (NCBO) Annotator was employed to mine data from unstructured text13. Querying the database for patient records including ICD-9 code 441.4 (abdominal aortic aneurysm without rupture), the word “diabetes”, and the word “aneurysm” mentioned in an imaging report, as well as “mm”, “history”, or “some” in related clinical notes identified a total cohort of 819 unique individuals seen or treated at Stanford Health Care (formerly Stanford Hospital and Clinics) since 2003.
The search was further refined by limiting inclusion to patients with intact aneurysms (no surgical repair) older than 50 years of age, with an infrarenal aortic diameter of ≥3 cm demonstrated on at least two serial measurements separated by ≥ six months using computed tomography (CT). We choose 6 months as the minimal follow up period for two reasons. First, several ongoing clinical trials, including the TEDY trial, have chosen to obtain bi-annual measurements for aneurysm progression14. Second, clinical guidelines generally recommend follow-up intervals of 6 months to one year for larger aneurysms prior to surgical repair. Aortic diameter was determined solely from clinical CT reports to maximize accuracy and reproducibility15. Reliance on CT imaging alone also ensured exclusion of patients with associated distal thoracic aortic diseases (dissection, thoracoabdominal aneurysm or Marfan Syndrome) when present but not otherwise noted in the medical record. Patients with known Ehlers-Danlos Syndrome or Behcet’s Disease were also excluded. The process identified 58 unique diabetic patients with an untreated AAA, with aortic diameter measurements reported on two or more serial CT scans, constituting the final cohort for analysis (Table I, Supplemental Table I and Supplemental Table II).
Table I.
Main characteristics of patient cohort
| Variable | Result |
|---|---|
| Male, number (% of total) | 48 (82.7) |
| White race, number (% of total) | 44 (75.9) |
| Current smokers, number (% of total) | 32 (55.2) |
| Initial age (years), mean (SE, range) | 72.0 (1.0, 56–90) |
| Final age (years), mean (SE, range) | 74.9 (1.0, 59.4–90.8) |
| Initial diameter (mm), mean (SE, range) | 40.4 (1.2, 30–83) |
| Final diameter (mm), mean (SE, range) | 44.3 (1.4, 30–83) |
| Follow up years, mean (SE, range) | 2.6 (0.3, 0.5–9.0) |
| Annual enlargement rate (mm), mean (SE, range) | 1.3 (0.4, −7.8–9.8) |
The study cohort has 58 patients
All CT images were acquired at Stanford Hospital and Clinics and interpreted by faculty radiologists. For purposes of the study, CT-determined aortic diameters were abstracted from all clinical reports transcribed during the study period; comorbidities, concurrent medical conditions and cardiovascular risk factors were abstracted from unstructured clinical documentation; and medication histories derived from clinical documentation and pharmacy records contained in the STRIDE database. Medication usage was considered to be “present” if identified at the time of any diameter measurement included in the study (first, interval or last), regardless of the duration of therapy. During the period of review, Stanford implemented the EPIC electronic medical record system beginning in 2006, with complete transition to electronic-only records in 2009.
Influence of individual risk factors on aneurysm enlargement
Annual aneurysm enlargement rate was determined by calculating the change in maximal infrarenal aortic diameter (mm) over the period of follow-up available (in years) for each patient. The influences of individual AAA-related risk factors, comorbidities and medications (cardiovascular and diabetic) on aneurysm enlargement were determined by univariate analysis. The presence of a “metformin effect” was further tested by controlling for other known aneurysm risk factors, including age, gender, current smoking status and body mass index (BMI).
Differences in annual aneurysm enlargement rates between groups were determined using the Wilcoxon Rank Test. Odds ratios (ORs) for the association of individual risk factors to annual enlargement above the median rate (obtained from the patients without oral diabetic medication) were determined by nominal logistic regression. Statistical analyses of STRIDE-derived data were performed using Stata 10 software (StataCorp LP, College Station, TX, USA) or JMP10 (SAS JMP, Cary, NC), with significance determined at the P < .05 level.
Data collection and analysis were performed under supervision of the Stanford University Institutional Review Board (IRB). The study cohort was created anonymously via the STRIDE discovery tool under a blanket Stanford University Institutional Review Board (IRB)-approved waiver of informed consent, in full compliance with the requirements of the Health Insurance Portability and Accountability ACT of 199612. Since the subsequent cohort data used for evaluation and statistical analysis was fully de-identified, a project-specific wavier of informed consent for individual participants was also granted (IRB protocol #30669).
Aneurysm modeling system
Male C57BL/6J mice (The Jackson Laboratory, Bar Harbor, ME), 10 to 12 weeks of age, were used for all experiments. Experimental AAAs were created via transient intra-aortic porcine pancreatic elastase (PPE) infusion as previously described16–18. In brief, at laparotomy under inhaled isoflurane anesthesia, 30 μl of type 1 PPE (1.5 U/mL in saline, Catalog # E1250-100MG; Sigma-Aldrich, St. Louis, MO) was infused into a controlled segment of infrarenal aorta for 5 minutes under constant pressure.
Metformin hydrochloride (MP Biomedicals, LLC, Solon, OH) was freshly prepared in phosphate-buffered saline (PBS, pH 7.2) prior to each use. Mice were administered metformin, 250 mg/kg/day in PBS (n=10 mice), or PBS alone (n=7 mice) as vehicle control via oral gavage. The metformin dose was selected based on published rodent modeling system precedents19, comparable to dosing required for insulin-resistant (type 2) diabetes management in humans (up to 35 mg/kg/day) as determined by allometric scaling20. Metformin therapy was initiated 3 days prior to, and terminated 14 days following, PPE infusion.
All murine modeling experiments were conducted in compliance with the Laboratory Animal Care Guidelines of Stanford University, with prior approval of the Administrative Panel on Laboratory Animal Care (protocol #11131).
Outcome analysis
Maximal infrarenal aortic diameter was determined 3 days prior to, and 3, 7, and 14 days following, aneurysm initiation via serial transabdominal ultrasound examinations @ 40 MHz. Diameter determination was performed in parallel by two investigators blinded to study group assignment. An AAA was defined as a 50% or greater increase in infrarenal aortic diameter compared to baseline assessment.
Measurements of body weight and spot (non-fasting) blood glucose concentration were obtained during serial surveillance ultrasound imaging procedures. Blood glucose concentration was determined between 9:00 and 10:00 AM daily using the OneTouch Ultra glucose meter and strip system (LifeScan, Inc., Milpitas, CA). The influences of metformin on body weight and blood glucose were analyzed and reported as a percentage of both values present at baseline. Mice were monitored daily for morbidity and mortality from aneurysm initiation to sacrifice.
Mice were sacrificed via carbon dioxide inhalation at 14 days following PPE infusion. Aortae were harvested, embedded in optimal cutting media, sectioned (6 μm) and fixed with cold acetone for histochemical and immunohistochemical evaluation16–18. Medial elastin integrity was analyzed via use of Verhoeff’s Van Gieson (EVG) stain. Medial SMC, leukocyte and neocapillary density were determined via immunostaining, using standard three-step biotin–streptavidin–peroxidase techniques16–18.
Primary antibodies employed included rabbit anti-mouse SMC α actin polyclonal antibody (Thermo Fisher Scientific Inc, Waltham, MA) and rat monoclonal antibodies (mAb) against mouse CD4 (clone GK1.5), CD8 (clone 53-6.7), CD31 (to detect blood vessels, clone 390), CD45R/B220 (to detect B cells, clone RA3-6B2) and CD68 (to detect macrophages, clone FA-11) (all from Biolegend Inc., San Diego, CA). Additional reagents included biotinylated goat-anti-rabbit or rat IgG and streptavidin-peroxidase conjugate (Jackson ImmunoResearch Laboratories, Inc., West Glove, PA), and AEC peroxidase substrate kits (Vector Laboratories, Inc., Burlingame, CA).
Aortic medial elastin degradation and SMC depletion, as determined on the basis of EVG and anti-SMC α-actin antibody staining, respectively, were analyzed on a qualitative scale (I (mild) to IV (severe)) as previously described18. Aoritc macrophage accumulation was graded on a similar I to IV scale according to mural infiltration patterns and proportionality as a function of aortic circumference (AC): (I) diffuse infiltration, or aggregate infiltration <25% of AC; (II) aggregate infiltration >25% but < 50% of AC; (III) aggregate infiltration >50% but <75% AC; and (IV) aggregate infiltration >75% AC. Mural lymphocyte infiltration and angiogenesis were defined as the number of positively stained lymphocytes or CD31-expressing blood vessels, respectively, per aortic cross-section (ACS) at 200x magnification.
Determination of significance
Outcome measurements were expressed as the mean and standard error (SE), unless otherwise defined. Differences between groups were determined by either two-way ANOVA, non-parametric Mann-Whitney or Kaplan-Meier tests (the latter used to compare aneurysm incidence between groups) using GraphPad Prism (Version 5c; GraphPad Software, La Jolla, CA). Significance was assumed at P < .05 level.
Results
Metformin regimen is negatively correlated with clinical aneurysm progression
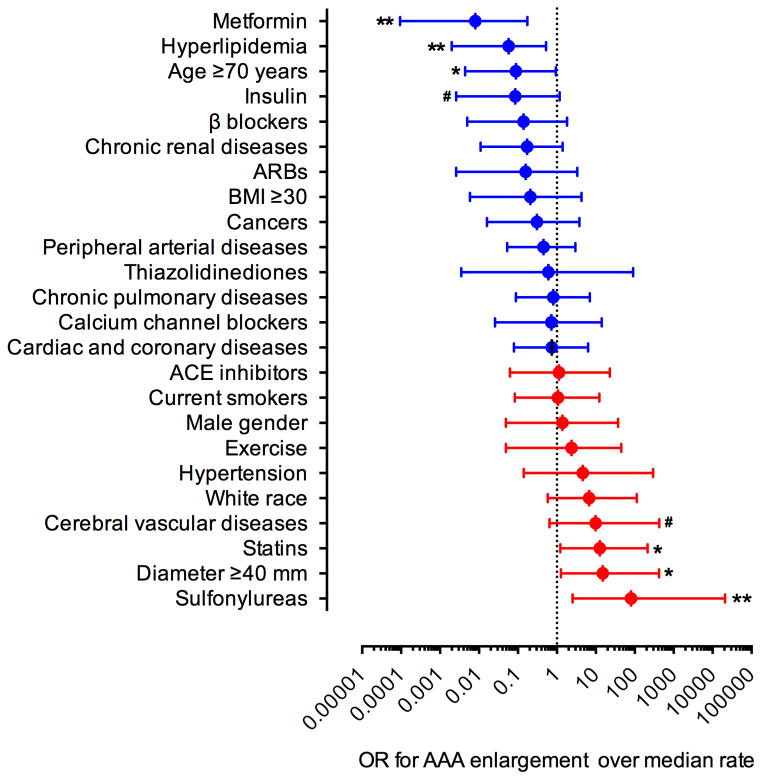
The average length of follow-up was 2.6 years (range 6 months to 9 years). Fourteen patients (24%) were followed for < 1 year, 37 patients (64%) were followed for > 1 but < 5 years, and 7 (12%) patients were followed for ≥ 5 years. The average rate of AAA enlargement was 1.3 mm/year. Consistent with published studies21, increased rates of enlargement (up to 9.8 mm/year), quiescence (no change) or reductions in AAA diameter (up to −7.8 mm/year) were seen in 40, 7 and 11 patients, respectively. There was no significant correlation between the rate of aneurysm enlargement and length of follow up (Supplemental Figure 1, r=0.05, p=0.70). Table II summarizes the relationship of each AAA risk factor, comorbidity, and prescribed diabetic or cardiovascular medication to change in aortic diameter over time. Of the 11 classes of medication considered, only metformin therapy demonstrated a negative association with aneurysm enlargement. No risk factor or comorbidity tabulated in Table II was significantly associated with aneurysm enlargement. Metformin use was subsequently controlled for each variable considered in Table II to account for potential interactions between variables; in each paired comparison, metformin usage remained significant (Supplemental Figures 2–6). The odds ratios for the effect of each variable considered in Table II on the rate of aneurysm enlargement above the median (1.5 mm/year) (obtained from the patients not receiving oral diabetic medication in our patient cohort) is demonstrated in Figure 1. Metformin therapy, advanced age, and a concomitant history of hyperlipidemia were associated with AAA enlargement below the median rate, while statin therapy, greater baseline aneurysm diameter, and sulfonylurea therapy were associated with enlargement above the median rate. Together, these results suggest a negative association between metformin use and progression of AAA disease.
Table II.
Univariate analysis of aneurysm enlargement rate in diabetic patients
| Aneurysm risk factor | Comorbidity | Medications |
|---|---|---|
| Gender | Cardiac & coronary diseases | Metformin |
| Male (48) 1.1 ± 0.4 | No (22) 1.8 ± 0.7 | No (43) 1.7 ± 0.5 |
| Female (10) 2.7 ± 1.1 | Yes (36) 1.1 ± 0.5 | Yes (15) 0.4 ± 0.6* |
| Initial age (years) | Peripheral arterial diseases | Sulfonylureas |
| <70 (23) 1.0 ± 0.6 | No (30) 1.8 ± 0.6 | No (45) 1.4 ± 0.5 |
| ≥70 (35) 1.6 ± 0.6 | Yes (28) 0.9 ± 0.5 | Yes (13) 1.3 ± 0.5 |
| Race | Cerebral vascular diseases | Thiazolidinediones |
| White (44) 1.4 ± 0.5 | No (48) 1.2 ± 0.5 | No (56) 1.3 ± 0.4 |
| Black (1) 1.6 | Yes (10) 1.9 ± 0.8 | Yes (2) 1.7 ± 0.9 |
| Asian (9) 0.9 ± 1.2 | Hypertension | DPP4 inhibitors |
| Other (2) 1.8 ± 1.0 | No (9) 1.9 ± 0.9 | No (56) 1.4 ± 0.4 |
| Unknown (2) 2.6 ± 2.1 | Yes (49) 1.3 ± 0.5 | Yes (2) −1.0 ± 1.7 |
| Family history | Hyperlipidemia | Meglitinides |
| No (44) 1.6 ± 0.5 | No (29) 1.8 ± 0.6 | No (57) 1.4 ± 0.4 |
| Yes (10) 0.3 ± 0.5 | Yes (29) 0.9 ± 0.5 | Yes (1) 1.5 |
| Unknown (4) 0.8 ± 2.2 | Chronic pulmonary diseases | Insulin |
| Smoking status | No (35) 1.6 ± 0.5 | No (45) 1.5 ± 0.5 |
| Non (9) 0.6 ± 1.3 | Yes (23) 1.0 ± 0.7 | Yes (13) 0.8 ± 0.5 |
| Previous (7) −0.1 ± 1.4 | Chronic renal diseases | ARBs |
| Current (32) 1.8 ± 0.6 | No (41) 1.6 ± 0.4 | No (43) 1.6 ± 0.5 |
| Unknown (10) 1.6 ± 0.5 | Yes (17) 0.8 ± 1.0 | Yes (15) 0.7 ± 0.7 |
| BMI | Cancers | ACE inhibitors |
| <30 (38) 1.4 ± 0.6 | No (44) 1.8 ± 0.5 | No (33) 1.1 ± 0.6 |
| ≥30 (13) 1.0 ± 0.8 | Yes (14) 0.1 ± 0.9 | Yes (25) 1.7 ± 0.6 |
| Unknown (7) 2.0 ± 0.7 | Calcium channel blockers | |
| Initial diameter (mm) | No (35) 0.8 ± 0.5 | |
| <40 (33) 1.3 ± 0.4 | Yes (23) 2.1 ± 0.7# | |
| ≥40 (25) 1.5 ± 0.8 | β blockers | |
| Exercise | No (26) 1.4 ± 0.6 | |
| No (39) 1.3 ± 0.6 | Yes (32) 1.3 ± 0.6 | |
| Yes (19) 1.5 ± 0.5 | Statins | |
| No (21) 1.1 ± 0.8 | ||
| Yes (37) 1.5 ± 0.5 |
Annual aneurysm enlargement rates in all groups are expressed as mean ± standard error (mm). Numbers in individual parentheses represent the cases of patients. ARBs: angiotensin II type 1 receptor blockers. ACE: angiotensin-converting enzyme. BMI: body mass index. DPP4: dipeptidyl peptidase 4. Nonparametric Wilcoxon test, 0.05<#P<0.1 and *P<0.05 compared to no metformin treatment group.
Figure 1. AAA enlargement as a function of demographic and environmental risk factors.
The median rate of AAA enlargement for diabetic patients not receiving oral hypoglycemic therapy was 1.5 mm/year. Nominal logistic regression was used to calculate odds ratios (OR) for individual variables. All data are ORs with the 95% conference interval (CI). Likelihood-ratio test, .05 < #P< .1, *P < .05 and **P < .01.
Metformin inhibits experimental aneurysms
To help differentiate the relative influence of hyperglycemia itself vs. metformin therapy on AAA progression, experimental AAAs were created in C57BL/6J (wild type), normoglycemic mice. Metformin treatment did not influence spot non-fasting blood glucose levels in these mice, however, a slight but significant reduction in body weight was noted throughout the period of metformin administration (Supplemental Figure 7).
As expected, infrarenal aortic diameters increased substantially following PPE infusion; all control mice formed aneurysms within seven days. In the metformin-treated mice, however, AAA enlargement was remarkably attenuated and, of the 10 receiving metformin, only 4 (40%) developed AAAs (Figure 2). Thus metformin, when administered throughout the period of aneurysm induction and enlargement, limits both AAA initiation and progression in normoglycemic mice.
Figure 2. Effect of metformin treatment on experimental AAAs.
AAAs were created via intra-aortic PPE infusion in 10–12 week old male C57BL/6J mice. These mice were treated with either metformin in vehicle (250 mg/kg/day, n=10 mice) or vehicle alone (PBS, n=7 mice) beginning 3 days prior to PPE infusion, for a total of 17 days. Aneurysm formation and progression were monitored via ultrasound in vivo. (A) Representative ultrasound images prior to, and 14 days following, PPE infusion in metformin- and vehicle-treated mice. (B) Mean and SE of aortic diameters prior to, and indicated days following, AAA creation in both treatment groups. Two-way ANOVA, *P < .05 and **P < .01 compared to vehicle group. (C) Influence of metformin treatment on AAA incidence. An AAA is defined as a 50% or great increase over the baseline diameter. Kaplan-Meier test, *P < .05 compared to vehicle group.
Histopathologic features associated with metformin administration
To identify potential mechanisms of metformin-mediated experimental aneurysm inhibition, aortic frozen sections were prepared for histopathologic analyses at 14 days following PPE infusion. Tissue analysis was performed at 14 days due to the relative magnitude of AAA inhibition evident at that timepoint (Figure 2A). As demonstrated in Figures 3 and 4, substantial aortic mural elastin degradation and SMC depletion were present in vehicle-treated mice. Metformin treatment, by comparison, was associated with relative aortic medial elastin and SMC preservation, reduced B cell, macrophage, CD4 and CD8 T cell accumulation, and reduced mural neovessel density. These experimental results suggest that metformin-mediated AAA resistance in normoglycemic mice is associated with reduced aortic inflammation, elastin degradation and SMC depletion
Figure 3. Representative histology from metformin- and vehicle-treated, PPE-infused mice.
Aortic frozen sections were prepared 14 days following AAA creation. Elastin integrity was assessed via the EVG stain. Mural macrophages and angiogenesis were stained with mAbs against CD68 and CD31, respectively, via immunohistochemical staining. Representative images shown. n=7 and 10 mice in vehicle and metformin groups, respectively. Original magnification: 200X.
Figure 4. Effects of metformin treatment on medial elastin and SMC integrity and mural inflammation in experimental AAA.
Aortic frozen sections from metformin- (n=10) and vehicle- (n=7) treated mice 14 days following PPE infusion were stained for elastin (EVG), SMC (SMC α-actin) and leukocyte subsets (CD68 for macrophages, CD4 for CD4 T cells, CD8 for CD8 T cells, B220 for B cells, CD31 for angiogenesis). Elastin degradation, SMC depletion and mural macrophage infiltration were graded as score I (mild) to score IV (severe). Mural CD4 T cells, CD8 T cells, B cells and angiogenesis were quantified as positive cells or blood vessels per ACS. Non-parametric Mann-Whitney test, *P < .05 and **P < .01 compared to vehicle treatment group.
Discussion
A search algorithm was created to identify a cohort of consecutive patients with both diabetes mellitus and abdominal aortic aneurysm disease within an institution-specific electronic data repository12. Refinements to the search process identified all recent diabetic AAA patients with intact, unrepaired aneurysms monitored by serial CT scans separated by intervals of up to nine years. Logistic regression, incorporating a broad range of demographic and clinical risk factors associated with AAA disease, identified metformin as the single variable most significantly associated with reduced aneurysm enlargement. The ability of metformin to suppress experimental aneurysm progression, independent of the influences of hyperglycemia alone, was subsequently confirmed in a well-established murine modeling system. Together, these results suggest that at least some of the observed attenuation of AAA risk in diabetic patient populations may be attributable to metformin-mediated effects on aneurysm pathogenesis and progression
Despite worldwide acceptance as the drug of choice for type 2 diabetes management, the precise mechanism(s) by which metformin reduces insulin resistance in type 2 (adult onset) diabetes remain incompletely understood22. Additional “off target” benefits from metformin therapy have been recognized or predicted in the treatment of polycystic ovarian disease and cancer23, 24. Indeed, data from multiple recent retrospective cohort studies suggests that diabetic patients on metformin are less likely to develop some forms of cancer, and when cancer is recognized, respond to treatment more favorably than patients not receiving metformin therapy prior to diagnosis23. In aggregate, cancer burden in diabetic patients on metformin may be reduced by as much as 40%25.
The metformin-mediated mechanisms proposed to reduce cancer burden may be relevant to those associated with improved outcomes in patients with cardiovascular disease and polycystic ovary syndrome patients. As summarized recently by Kinaan and colleagues26, metformin effects potentially relevant to cardiovascular health include suppression of mitochondrial oxidative phosphorylation and reactive oxygen species in infiltrative mural macrophages in atherosclerotic or aneurysmal arterial diseases, reduction of NF-kB activity, inhibition of the mammalian target of rapamycin pathway and autophagy, inhibition of mural angiogenesis, potential anti-inflammatory changes to the gastrointestinal microbiome, and up-regulation of the silent information regulator-2 (SIRT) family of proteins, or sirtulins24, 26–28.
Sirtulins are histone deacetylases believed to promote the longevity that is achieved through caloric restriction, among other salutary metabolic and cardiovascular target effects29. In experimental models of chronic diabetes, metformin administration increased SIRT1 protein expression and activity in the aortae of older mice28. Potential anti-aneurysmal effects of SIRT activity include reduction of plasminogen activator inhibitor-1 activity and modulation of the atheroprotective effects of angiotensin-converting enzyme inhibitors30. Interestingly, vascular SIRT1 expression is reduced in cigarette smoking, increased in response to increased pulsatile luminal blood flow, and augmented by exposure to resveratrol, the putative atheroprotective component found in red wine – all environmental and hemodynamic conditions relevant to the pathogenesis and progression of aneurysmal and occlusive arterial diseases31–33.
The idea that hyperglycemia, or hormonal or metabolic consequences associated with diabetes (such as obesity) may reduce AAA disease risk remains a counterintuitive and controversial proposition. Although solidly supported by epidemiologic analyses in a variety of populations over the last two decades4, 34, identification of the mechanism(s) responsible for the apparent negative association has proven elusive. In addition to metformin, hyperglycemia alone has suppressive effect on AAAs, at least in some experimental models9. Since neither fasting glucose nor glycosylated hemoglobin levels were routinely acquired at Stanford for these patients, the STRIDE-derived data did not allow for analysis of medication effects on aneurysm enlargement independent of concurrent glycemic control. As a class, the thiazolidinediones (e.g. rosiglitazone, or Avandia™) may also suppress aneurysms and, conversely, the sulfonylureas apparently promote some forms of cardiovascular disease35.
Additional limitations of the clinical study include the relatively small size of the diabetic patient cohort (offset to a certain degree by the precision inherent in CT vs. ultrasound aortic diameter measurement), the retrospective nature of cohort identification (including both insulin-resistant and insulin-deficient patients) and data analysis, variability in surveillance intervals between CT scans, uncertainty regarding the duration of, or compliance with, prescribed medical regimens, and analytic risks inherent in considering the effects of a large number of variables on a small patient cohort. This latter instance also prevented us from robustly eliminating confounding effects and covariates from all the variables considered on Table II, although the influence of metformin use on progression was controlled for the most common AAA demographic and clinical risk factors as noted in Supplementary materials.
Experimental AAA modeling was performed to both complement and confirm the clinical study results. The murine modeling experiments allowed for analysis of metformin effects on experimental aneurysm progression independent of additional confounding influences associated with the diabetic state. Limitations to the murine modeling include uncertain relevance to the human condition (despite widespread use and acceptance of the PPE infusion model in the aneurysm literature), the relatively young age of the mice involved, initiation of metformin therapy prior to aneurysm creation (potentially diminishing translational value), and the absence of additional experimental groups (e.g. aneurysm creation in diabetic mice) or treatment regimens (eg. dose-ranging experiments) to further characterize the metformin effect. As previously noted, the modeling experiments were planned and performed to validate and provide context to the observed clinical data; further experiments with more experimental groups incorporating complementary experimental models (such as the angiotensin infusion model in hyperlipidemic mice) will be necessary to determine the translational potential of metformin in medical AAA disease management, The fact that hyperglycemia itself limits experimental AAAs would make analysis of outcome data in chronic diabetic mouse models (e.g. db/db mice) difficult in terms of isolating the effect of metformin alone9. Importantly, in a very recent study performed in complementary murine AAA models, one in which aortic aneurysms develop following subcutaneous delivery of angiotensin II in apolipoprotein E-deficient mice, metformin therapy was also noted to reduce AAA diameter enlargement and histologic disease progression, reducing concern that the present findings are model-specific and increasing the generalizability of the current results27. Aside from AAA modeling experiments, no opportunity exists to examine a “metformin” effect in non-diabetic AAA patients, as additional therapeutic applications are currently limited to relatively uncommon conditions occurring in patients not otherwise considered at risk for AAA disease23, 24.
Additionally and somewhat unexpectedly, the present study also identified a positive association between statin usage and AAA enlargement. This finding contrasts with prior results from AAA patients, with and without diabetes, in whom brief periods of statin therapy were noted to reduce aortic mural inflammation and pro-inflammatory mediator expression in aneurysmal aortic tissue when subsequently harvested during surgical repair36. Although perioperative mortality and cardiovascular morbidity is clearly improved by statin therapy in all AAA patients undergoing surgical repair, a prior meta-analysis of the existing literature failed to demonstrate an effect, positive or negative, of statin therapy on AAA enlargement or disease progression prior to surgical repair in patient populations not stratified by the presence or absence of diabetes36–38.
In the present analysis of diabetic AAA patients, the positive statin effect remained significant after being controlled for other conditions known to influence AAA enlargement (Supplemental Figure 6); the overall results were strongly influenced by positive effects in non-smokers and patients with an initial aortic diameter <40 mm. Interestingly, prior studies have suggested that in patients with familial AAA disease, defined as being present in first-degree relatives and siblings, traditional atherosclerotic vascular disease risk factors, including smoking status, appear to play a less significant role39 in promoting AAA disease and therefore may be less amenable to amelioration through statin use. Familial AAA disease history was not consistently available through the STRIDE database, and thus not analyzed as a study variable. Also, long-term statin use has been consistently associated with increasing the risk of diabetes and, recently, the potential for diabetic-related cardiovascular disease outcomes such as peripheral vascular disease events40. Thus the unexpected statin influence on AAA progression identified in this small series deserves further investigation in a larger cohort of AAA patients with and without diabetes.
Conclusion
Retrospective analysis of a large university health system-based clinical data repository, complemented by experimental data derived from a commonly used murine AAA modeling system, suggests that metformin may limit the progression of abdominal aortic aneurysm disease. Further studies should be undertaken to confirm the significance and generalizability of these results.
Supplementary Material
Acknowledgments
This work was supported in part by the grants from the National Heart, Lung and Blood Institute (1R21HL109750-03 and 1R21HL112122-03). The STRIDE project was supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences (UL1 RR025744). Additional support included individual fellowships from the China Scholarship Council (JX) and Keio University (NF), and Medical Scholar (KJG) and Major Undergraduate (EBK) research awards from Stanford University. We thank Stanford Pathology Professor Sara A. Michie, MD for her help in histology interpretation and manuscript review, and Mary Gerritsen, PhD for guidance with allometric scaling of metformin dosing between species.
Footnotes
Other
Presented at 2015 Western Vascular Society Meeting at Maui, Hawaii, USA.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stather PW, Sidloff DA, Rhema IA, Choke E, Bown MJ, Sayers RD. A review of current reporting of abdominal aortic aneurysm mortality and prevalence in the literature. Eur J Vasc Endovasc Surg. 2014;47(3):240–2. doi: 10.1016/j.ejvs.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 2.Lederle FA, Johnson GR, Wilson SE, Chute EP, Littooy FN, Bandyk D, et al. Prevalence and associations of abdominal aortic aneurysm detected through screening. Aneurysm Detection and Management (ADAM) Veterans Affairs Cooperative Study Group. Ann Intern Med. 1997;126(6):441–9. doi: 10.7326/0003-4819-126-6-199703150-00004. [DOI] [PubMed] [Google Scholar]
- 3.Lederle FA. The strange relationship between diabetes and abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. 2012;43(3):254–6. doi: 10.1016/j.ejvs.2011.12.026. [DOI] [PubMed] [Google Scholar]
- 4.De Rango P, Farchioni L, Fiorucci B, Lenti M. Diabetes and abdominal aortic aneurysms. Eur J Vasc Endovasc Surg. 2014;47(3):243–61. doi: 10.1016/j.ejvs.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 5.Sweeting MJ, Thompson SG, Brown LC, Powell JT. Meta-analysis of individual patient data to examine factors affecting growth and rupture of small abdominal aortic aneurysms. Br J Surg. 2012;99(5):655–65. doi: 10.1002/bjs.8707. [DOI] [PubMed] [Google Scholar]
- 6.Zhang F, Kent KC, Yamanouchi D, Zhang Y, Kato K, Tsai S, et al. Anti-receptor for advanced glycation end products therapies as novel treatment for abdominal aortic aneurysm. Ann Surg. 2009;250(3):416–23. doi: 10.1097/SLA.0b013e3181b41a18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Golledge J, Karan M, Moran CS, Muller J, Clancy P, Dear AE, et al. Reduced expansion rate of abdominal aortic aneurysms in patients with diabetes may be related to aberrant monocyte-matrix interactions. Eur Heart J. 2008;29(5):665–72. doi: 10.1093/eurheartj/ehm557. [DOI] [PubMed] [Google Scholar]
- 8.Dua MM, Miyama N, Azuma J, Schultz GM, Sho M, Morser J, et al. Hyperglycemia modulates plasminogen activator inhibitor-1 expression and aortic diameter in experimental aortic aneurysm disease. Surgery. 2010;148(2):429–35. doi: 10.1016/j.surg.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miyama N, Dua MM, Yeung JJ, Schultz GM, Asagami T, Sho E, et al. Hyperglycemia limits experimental aortic aneurysm progression. J Vasc Surg. 2010;52(4):975–83. doi: 10.1016/j.jvs.2010.05.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Torsney E, Pirianov G, Cockerill GW. Diabetes as a negative risk factor for abdominal aortic aneurysm - does the disease aetiology or the treatment provide the mechanism of protection? Curr Vasc Pharmacol. 2013;11(3):293–8. doi: 10.2174/1570161111311030003. [DOI] [PubMed] [Google Scholar]
- 11.Thompson A, Cooper JA, Fabricius M, Humphries SE, Ashton HA, Hafez H. An analysis of drug modulation of abdominal aortic aneurysm growth through 25 years of surveillance. J Vasc Surg. 2010;52(1):55–61. e2. doi: 10.1016/j.jvs.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 12.Lowe HJ, Ferris TA, Hernandez PM, Weber SC. STRIDE--An integrated standards-based translational research informatics platform. AMIA Annu Symp Proc. 2009;2009:391–5. [PMC free article] [PubMed] [Google Scholar]
- 13.Jung K, LePendu P, Iyer S, Bauer-Mehren A, Percha B, Shah NH. Functional evaluation of out-of-the-box text-mining tools for data-mining tasks. J Am Med Inform Assoc. 2015;22(1):121–31. doi: 10.1136/amiajnl-2014-002902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morris DR, Cunningham MA, Ahimastos AA, Kingwell BA, Pappas E, Bourke M, et al. TElmisartan in the management of abDominal aortic aneurYsm (TEDY): The study protocol for a randomized controlled trial. Trials. 2015;16:274. doi: 10.1186/s13063-015-0793-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang XL, Thompson MM, Dole WP, Dalman RL, Zalewski A. Standardization of outcome measures in clinical trials of pharmacological treatment for abdominal aortic aneurysm. Expert Rev Cardiovasc Ther. 2012;10(10):1251–60. doi: 10.1586/erc.12.128. [DOI] [PubMed] [Google Scholar]
- 16.Iida Y, Xu B, Xuan H, Glover KJ, Tanaka H, Hu X, et al. Peptide inhibitor of CXCL4-CCL5 heterodimer formation, MKEY, inhibits experimental aortic aneurysm initiation and progression. Arterioscler Thromb Vasc Biol. 2013;33(4):718–26. doi: 10.1161/ATVBAHA.112.300329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iida Y, Xu B, Schultz GM, Chow V, White JJ, Sulaimon S, et al. Efficacy and mechanism of angiotensin II receptor blocker treatment in experimental abdominal aortic aneurysms. PLoS One. 2012;7(12):e49642. doi: 10.1371/journal.pone.0049642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rouer M, Xu BH, Xuan HJ, Tanaka H, Fujimura N, Glover KJ, et al. Rapamycin limits the growth of established experimental abdominal aortic aneurysms. Eur J Vasc Endovasc Surg. 2014;47(5):493–500. doi: 10.1016/j.ejvs.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 19.Tajima K, Nakamura A, Shirakawa J, Togashi Y, Orime K, Sato K, et al. Metformin prevents liver tumorigenesis induced by high-fat diet in C57Bl/6 mice. Am J Physiol Endocrinol Metab. 2013;305(8):E987–98. doi: 10.1152/ajpendo.00133.2013. [DOI] [PubMed] [Google Scholar]
- 20.West GB, Brown JH. The origin of allometric scaling laws in biology from genomes to ecosystems: towards a quantitative unifying theory of biological structure and organization. J Exp Biol. 2005;208(Pt 9):1575–92. doi: 10.1242/jeb.01589. [DOI] [PubMed] [Google Scholar]
- 21.Brady AR, Thompson SG, Fowkes FG, Greenhalgh RM, Powell JT. Abdominal aortic aneurysm expansion: risk factors and time intervals for surveillance. Circulation. 2004;110(1):16–21. doi: 10.1161/01.CIR.0000133279.07468.9F. [DOI] [PubMed] [Google Scholar]
- 22.Pernicova I, Korbonits M. Metformin--mode of action and clinical implications for diabetes and cancer. Nat Rev Endocrinol. 2014;10(3):143–56. doi: 10.1038/nrendo.2013.256. [DOI] [PubMed] [Google Scholar]
- 23.Pierotti MA, Berrino F, Gariboldi M, Melani C, Mogavero A, Negri T, et al. Targeting metabolism for cancer treatment and prevention: metformin, an old drug with multi-faceted effects. Oncogene. 2013;32(12):1475–87. doi: 10.1038/onc.2012.181. [DOI] [PubMed] [Google Scholar]
- 24.Victor VM, Rovira-Llopis S, Banuls C, Diaz-Morales N, Lopez-Domenech S, Escribano-Lopez I, et al. Metformin modulates human leukocyte/endothelial cell interactions and proinflammatory cytokines in polycystic ovary syndrome patients. Atherosclerosis. 2015;242(1):167–73. doi: 10.1016/j.atherosclerosis.2015.07.017. [DOI] [PubMed] [Google Scholar]
- 25.Giovannucci E, Harlan DM, Archer MC, Bergenstal RM, Gapstur SM, Habel LA, et al. Diabetes and cancer: a consensus report. Diabetes Care. 2010;33(7):1674–85. doi: 10.2337/dc10-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kinaan M, Ding H, Triggle CR. Metformin: An Old Drug for the Treatment of Diabetes but a New Drug for the Protection of the Endothelium. Med Princ Pract. 2015;24(5):401–15. doi: 10.1159/000381643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vasamsetti SB, Karnewar S, Kanugula AK, Thatipalli AR, Kumar JM, Kotamraju S. Metformin inhibits monocyte-to-macrophage differentiation via AMPK-mediated inhibition of STAT3 activation: potential role in atherosclerosis. Diabetes. 2015;64(6):2028–41. doi: 10.2337/db14-1225. [DOI] [PubMed] [Google Scholar]
- 28.Caton PW, Nayuni NK, Kieswich J, Khan NQ, Yaqoob MM, Corder R. Metformin suppresses hepatic gluconeogenesis through induction of SIRT1 and GCN5. J Endocrinol. 2010;205(1):97–106. doi: 10.1677/JOE-09-0345. [DOI] [PubMed] [Google Scholar]
- 29.D’Onofrio N, Vitiello M, Casale R, Servillo L, Giovane A, Balestrieri ML. Sirtuins in vascular diseases: Emerging roles and therapeutic potential. Biochim Biophys Acta. 2015;1852(7):1311–22. doi: 10.1016/j.bbadis.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 30.Marampon F, Gravina GL, Scarsella L, Festuccia C, Lovat F, Ciccarelli C, et al. Angiotensin-converting-enzyme inhibition counteracts angiotensin II-mediated endothelial cell dysfunction by modulating the p38/SirT1 axis. J Hypertens. 2013;31(10):1972–83. doi: 10.1097/HJH.0b013e3283638b32. [DOI] [PubMed] [Google Scholar]
- 31.Csiszar A, Labinskyy N, Podlutsky A, Kaminski PM, Wolin MS, Zhang C, et al. Vasoprotective effects of resveratrol and SIRT1: attenuation of cigarette smoke-induced oxidative stress and proinflammatory phenotypic alterations. Am J Physiol Heart Circ Physiol. 2008;294(6):H2721–35. doi: 10.1152/ajpheart.00235.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Napoli C, Balestrieri ML, Sica V, Lerman LO, Crimi E, De Rosa G, et al. Beneficial effects of low doses of red wine consumption on perturbed shear stress-induced atherogenesis. Heart Vessels. 2008;23(2):124–33. doi: 10.1007/s00380-007-1015-8. [DOI] [PubMed] [Google Scholar]
- 33.Liu J, Bi X, Chen T, Zhang Q, Wang SX, Chiu JJ, et al. Shear stress regulates endothelial cell autophagy via redox regulation and Sirt1 expression. Cell Death Dis. 2015;6:e1827. doi: 10.1038/cddis.2015.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Rango P, Cao P, Cieri E, Parlani G, Lenti M, Simonte G, et al. Effects of diabetes on small aortic aneurysms under surveillance according to a subgroup analysis from a randomized trial. J Vasc Surg. 2012;56(6):1555–63. doi: 10.1016/j.jvs.2012.05.078. [DOI] [PubMed] [Google Scholar]
- 35.Thule PM, Umpierrez G. Sulfonylureas: a new look at old therapy. Curr Diab Rep. 2014;14(4):473. doi: 10.1007/s11892-014-0473-5. [DOI] [PubMed] [Google Scholar]
- 36.van der Meij E, Koning GG, Vriens PW, Peeters MF, Meijer CA, Kortekaas KE, et al. A clinical evaluation of statin pleiotropy: statins selectively and dose-dependently reduce vascular inflammation. PLoS One. 2013;8(1):e53882. doi: 10.1371/journal.pone.0053882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takagi H, Yamamoto H, Iwata K, Goto S, Umemoto T. Effects of statin therapy on abdominal aortic aneurysm growth: a meta-analysis and meta-regression of observational comparative studies. Eur J Vasc Endovasc Surg. 2012;44(3):287–92. doi: 10.1016/j.ejvs.2012.06.021. [DOI] [PubMed] [Google Scholar]
- 38.Twine CP, Williams IM. Systematic review and meta-analysis of the effects of statin therapy on abdominal aortic aneurysms. Br J Surg. 2011;98(3):346–53. doi: 10.1002/bjs.7343. [DOI] [PubMed] [Google Scholar]
- 39.van de Luijtgaarden KM, Bastos Goncalves F, Hoeks SE, Valentijn TM, Stolker RJ, Majoor-Krakauer D, et al. Lower atherosclerotic burden in familial abdominal aortic aneurysm. J Vasc Surg. 2014;59(3):589–93. doi: 10.1016/j.jvs.2013.08.096. [DOI] [PubMed] [Google Scholar]
- 40.Mansi I, Frei CR, Wang CP, Mortensen EM. Statins and New-Onset Diabetes Mellitus and Diabetic Complications: A Retrospective Cohort Study of US Healthy Adults. J Gen Intern Med. 2015 doi: 10.1007/s11606-015-3335-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.