Abstract
Objective
To determine the optimal dose of vitamin D supplementation to achieve biochemical vitamin D sufficiency in extremely low gestational age newborns in a masked randomized controlled trial.
Study design
100 infants 23 0/7 to 27 6/7 weeks gestation were randomized to vitamin D intakes of placebo (n=36), 200 IU (n=34), and 800 IU/day (n=30) (approximating 200, 400, or 1000 IU/day, respectively, when vitamin D routinely included in parenteral or enteral nutrition is included). The primary outcomes were serum 25 (OH) vitamin D concentrations on postnatal day 28 and the number of days alive and off respiratory support in the first 28 days.
Results
At birth, 67% of infants had 25(OH) vitamin D < 20 ng/mL suggesting biochemical vitamin D deficiency. Vitamin D concentrations on day 28 were (Median [25th–75th centiles], ng/mL): Placebo: 22 [13–47], 200 IU: 39 [26–57], 800 IU: 84.5 [52–99], p < 0.001. There were no differences in days alive and off respiratory support (Median (25th–75th centiles), days: Placebo: 1 (0–11), 200 IU: 0 (0–8), 800 IU: 0.5 (0–22), p=0.63), or other respiratory outcomes among groups.
Conclusion
At birth, most extremely preterm infants have biochemical vitamin D deficiency. This biochemical deficiency is reduced on day 28 by supplementation with 200 IU/day and prevented by 800 IU/day. Larger trials are required to determine if resolution of biochemical vitamin D deficiency improves clinical outcomes. ClinicalTrials.gov: NCT01600430
Trial registration
ClinicalTrials.gov: NCT01600430
Vitamin D is a fat soluble vitamin involved in multiple physiological processes. Developmental processes regulated by vitamin D include lung development1–4 and maturation of the immune system.5–9 As vitamin D is mostly transferred to the fetus during the third trimester,10, 11 preterm infants are born with lower vitamin D stores. Extremely low gestational age newborns (ELGAN) are at high risk of mortality and multiple morbidities. It is not known if vitamin D insufficiency contributes to mortality or morbidity in these infants.
The American Academy of Pediatrics (AAP) has recommended a dose of 200–400 IU of vitamin D per day for preterm infants,12 and the European Society for Paediatric Gastroenterology Hepatology and Nutrition (ESPGHAN) recommends 800–1000 IU.13 However, evidence is lacking for the magnitude of improvement in serum vitamin D concentrations or clinical outcomes in ELGAN with either target range of vitamin D supplementation. In addition, as vitamin D is critical for many essential physiological processes, it is possible that higher than normal (supra-physiological) but non-toxic concentrations of vitamin D may benefit this vulnerable population similar to vitamin A supplementation.14
This was a pilot trial to identify the optimal dose of vitamin D before embarking on a large multicenter trial using the optimal dose for evaluating clinical outcomes. Our objective was to determine the optimal dose of vitamin D supplementation in ELGAN using a masked randomized controlled trial in which infants were randomized to vitamin D intakes of placebo, 200 IU, and 800 IU/day.
Methods
This multiple-dose, randomized, double-blinded, placebo-controlled trial was approved by the Institutional Review Board at the University of Alabama at Birmingham. Entry criteria were inborn infants with gestational age between 23–27 completed weeks admitted to the neonatal intensive care unit at the University of Alabama Hospital. Exclusion criteria included major congenital or chromosomal anomalies, moribund infant with low likelihood of survival as outborn infants.
Parents or guardians were approached by research staff soon after birth for informed consent. After consent, infants were randomly allocated by the research pharmacy staff using computer-generated stratified randomization codes to one of three groups: (1) placebo (intake of approximately 200 IU/day in parenteral nutrition or feeds), (2) 200 IU supplement (daily intake of approximately 400 IU due to 200 IU supplement + 200 IU in parenteral nutrition or feeds), or (3) 800 IU supplement (daily intake of approximately 1000 IU due to 800 IU supplement +200 IU in parenteral nutrition or feeds). The 200 IU in enteral or parenteral nutrition is an estimate, based on the daily intake of feeds and TPN by infants in our NICU over the first month. As fortified human milk has approximately the same amount of vitamin D as preterm formula, and fortification is started early in our unit, no adjustments were made for daily variations in intake or for unfortified human milk.
Infants received 0.25 mL of study drug (either vitamin D (Enfamil D-Vi-Sol ™) or normal saline (placebo)) every 6 hours through the orogastric tube just before feeds. The medication was dispensed by a research pharmacist in an amber syringe to mask the caregivers. Infants in the placebo group received only saline, and infants in the 200 IU/day group received one dose of vitamin D and three doses of saline and infants in the 800 IU/day group received four doses of vitamin D and none of saline. Each infant was randomized during the first seven days after birth and within 72 hours after initiating enteral feeds. Infants were monitored before every feed for feeding intolerance (abdominal distension or increased gastric residuals). Serum electrolytes, blood gases, and ionized calcium were measured every day while on parenteral nutrition and twice weekly while on enteral feeds during the first month. The intervention was continued until postnatal day 28. Infants were discontinued from study drug administration if they developed necrotizing enterocolitis Bell’s stage II or greater,15 spontaneous intestinal perforation, or if feeds were stopped for more than 24 hrs by the clinical team. Clinical data were collected by a trained research coordinator.
For the estimation of serum 25(OH) vitamin D concentration, whole blood (0.2 mL to 0.25 mL) was collected at birth (cord blood) and at the time of routine sampling on day 14 (± 2 days), and day 28 of life (± 3 days) and centrifuged to collect serum. The samples were stored in −80°C for later analysis. Analysis of the serum 25(OH) vitamin D concentrations was with an enzyme-linked immunosorbent assay (ELISA; Eagle Biosciences, Inc, Nashua, NH) with a dynamic range of 4–120 ng/mL, 100% cross-reactivity between 25(OH)-vitamin D3 and D2, <10% CV for intra-assay and inter-assay variation, and normal range of 30–70 ng/mL for both adults and older children.
Primary Outcomes
The pharmacological outcome was the serum concentration of 25(OH) vitamin D at postnatal day 28, and the primary clinical outcome was the total number of days alive and off respiratory support in the first 28 days. Respiratory support was defined as the need for supplemental oxygen and/or positive pressure ventilation to maintain normal target oxygen saturations (88 – 95%). The number of days on supplemental oxygen is a surrogate of BPD and is directly related with chronic lung disease in preterm infants.
Secondary Outcomes
The secondary outcomes include other respiratory clinical outcomes including retinopathy of prematurity (stages I–III, plus disease, or disease requiring treatment),16, 17 proven necrotizing enterocolitis (Bell’s Stage II and III),15 and intraventricular hemorrhage (Grade I–IV),18 confirmed sepsis and/or meningitis, nutrition, hospitalization, and death. These clinical outcomes were evaluated during the entire initial hospitalization, and the feeding outcomes were cross-sectional at day 14 and day 28 (as most infants are on full feeds by three weeks of age).
Sample Size
A planned sample size of 31 patients per group was estimated to be sufficient to detect a 40% change in serum 25(OH) vitamin D concentration on study day 28 (the primary outcome) with 80% power at an alpha of 0.05 by ANOVA, with the assumption that the standard deviation would be 50% of the mean. A sample size of 20 per group would be sufficient to determine if a difference of 50% in vitamin D concentrations on postnatal day 28 was significant. The total sample size was increased to 100 to account for some attrition in sample size due to death or inadequate sample for analysis. The trial was not powered for clinical outcomes.
A one-way analysis of variance (ANOVA) was used for calculating statistical significance of continuous variables, with the Tukey multiple-comparison test used if p<0.05 by ANOVA. Analysis was by “intention-to-treat”. The chi-square test was used for categorical variables. SigmaPlot Version 12 (Systat Software Inc., San Jose, CA) was used for analysis.
Results
Between June 2012 and October 2014, 100 infants with birth weights ranging from 360 g to 1290 g (mean 770 ± 215 g) were randomized to three vitamin D daily intake groups: 36 infants to the placebo group, 34 to the 200 IU/day, and 30 to the 800 IU/day. Of the 100 infants, 37 did not complete the study: 15 due to death, 12 developed necrotizing enterocolitis or spontaneous intestinal perforation, and 12 were not fed for more than 24 hours (Figure 1; available at www.jpeds.com). Serum 25(OH) vitamin D concentrations were not available for 15, 22 (7 due to death), and 30 (2 due to death) infants at birth, day 14, and 28 respectively. Maternal characteristics did not differ among groups and infant birth weights were not statistically different (Table I). There was a trend towards lower Apgar scores (≤ 3 at 5 minutes) in the 200 IU/day group. The median age at commencement of feeds was 3 days in this population, and vitamin D supplementation was started on median day 5 in all groups.
Figure 1. Revised template of the CONSORT diagram showing the flow of participants through each stage of a randomized trial.
CONSORT diagram illustrates enrollment, allocation, follow-up, and analysis of the randomized controlled trial with two intervention groups (200 IU, 800 IU) and standard supplementation group (placebo) for vitamin D supplementation
IU, international unit; NEC, necrotizing enterocolitis; SIP, spontaneous intestinal perforation; NPO, nil per os.
Table 1.
Demographics and characteristics of infants
| Maternal Characteristics | All (N = 87) | Placebo (N = 33) | 200 IU/day (N = 28) | 800 IU/day (N = 26) |
|---|---|---|---|---|
| Age (years, mean ± SD) | 25.7 ± 5.4 | 24.0 ± 4.0 | 27.3 ± 5.6 | 26.8 ± 6.2 |
| Married | 26(30) | 8 (24) | 9 (32) | 9 (35) |
| ≥ High school education | 69 (79) | 26 (79) | 19 (68) | 24 (92) |
| Medicaid/uninsured | 64 (74) | 25 (76) | 21 (75) | 18 (69) |
| Race | ||||
| White | 38 (44) | 16 (48) | 8 (29) | 14 (54) |
| Black | 48 (55) | 17 (51) | 19 (68) | 12 (46) |
| Other | 1 (1) | 0 (0) | 1 (4) | 0 (0) |
| Gravida (Mean ± SD) | 2.7± 1.8 | 2.2± 1.5 | 3.3± 1.9 | 2.7± 1.8 |
| Parity (Mean ± SD) | 2.0 ± 1.4 | 1.8 ± 1.2 | 2.4 ± 1.4 | 2.0 ± 1.7 |
| Multiple births | 9 (10) | 3 (9) | 3 (11) | 3 (12) |
| Diabetes mellitus | 6 (7) | 0 (0) | 4 (14) | 2 (8) |
| Pregnancy Induced Hypertension | 28 (32) | 10 (30) | 12 (43) | 6 (23) |
| Chronic hypertension | 17 (20) | 5 (15) | 8 (29) | 4 (15) |
| Antepartum Hemorrhage | 13 (15) | 6 (18) | 3 (11) | 4 (15) |
| Clinical chorioamnionitis | 14 (16) | 6 (18) | 4 (14) | 4 (15) |
| Histologic chorioamnionitis | 54 (62) | 21 (64) | 17 (61) | 16 (62) |
| Rupture of Membranes ≥ 18 hrs | 41 (47) | 17 (52) | 14 (50) | 10 (38) |
| Duration of rupture of membranes (hours; Median [range]) | 76 [1–867] | 67 [1–480] | 59 [1–312] | 108 [1–867] |
| Antenatal steroids | 85 (98) | 32 (97) | 28 (100) | 25 (96) |
| Maternal antibiotics | 78 (90) | 31 (94) | 26 (93) | 21 (81) |
| Magnesium sulfate | 83 (95) | 31 (94) | 27 (96) | 25 (96) |
| Vaginal delivery (vertex) | 35 (40) | 12 (36) | 13 (46) | 10 (38) |
| Vaginal delivery (breech) | 6 (7) | 2 (6) | 1 (4) | 3 (12) |
| Cesarean delivery | 46 (53) | 19 (58) | 14 (50) | 13 (50) |
| Infant Characteristics | All (N = 100) | Placebo (N = 36) | 200 IU/day (N = 34) | 800 IU/day (N = 30) |
|---|---|---|---|---|
| Birth Weight (g) Mean ± SD |
770 ± 215 | 774 ± 210 | 744± 210 | 796± 228 |
| Gestational age, wk Mean ± SD |
25.3 ± 1.4 | 25.5 ± 1.4 | 25.3 ± 1.4 | 25.1 ± 1.6 |
| Male sex | 45 (45) | 16 (44) | 15 (44) | 14 (47) |
| Apgar score ≤ 3 | ||||
| At 1 minutes | 74 (74) | 25 (69) | 26 (76) | 23 (77) |
| At 5 minutes | 23 (23) | 4 (11) | 12 (35) | 7 (23) |
| Delivery room care | ||||
| Oxygen | 99 (98) | 35 (97) | 33 (97) | 30 (100) |
| Bag and mask ventilation | 90 (90) | 32 (89) | 32 (94) | 26 (87) |
| CPAP during resuscitation | 29 (29) | 13 (36) | 7 (21) | 9 (30) |
| Intubated during resuscitation | 49 (49) | 16 (44) | 17 (50) | 16 (53) |
| Hypotension | ||||
| ≤ 24 hours of life | 22 (22) | 9 (25) | 5 (15) | 8 (27) |
| Treated with volume therapy | 21 (21) | 7 (19) | 6 (18) | 8 (27) |
| Treated with inotropes | 9 (9) | 4 (11) | 2 (6) | 3 (12) |
| Base deficit, median [mean] | 5 (6.0) | 5 (4.8) | 5 (6.2) | 6 (7.1) |
| Patent ductus arteriosus | ||||
| Diagnosis of patent ductus arteriosus | 14 (14) | 6 (18) | 5 (15) | 3 (12) |
| Treated with indomethacin | 3 (3) | 1 (3) | 2 (6) | 0 (0) |
| Treated with ibuprofen | 1 (1) | 1 (3) | 0 (0) | 0 (0) |
| Treated with surgery | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Intraventricular hemorrhage prophylaxis with indomethacin | 61 (61) | 22 (61) | 24 (71) | 15 (50) |
Data are shown as n (%)
At birth, 67% (57/85) of the infants had 25(OH) vitamin D concentrations < 20 ng/mL, with an average concentration of 17.5 ng/mL (SD 11; 25th–75th centiles: 10–21; Range 3–59) (Figure 2). At postnatal day 14, the placebo and 200 IU groups had serum 25(OH) vitamin D concentrations that were less than that in the 800 IU group (ng/mL; Median (25th–75th centiles): placebo group: 23 (16–30) and 200 IU group: 29 (23–39) vs. 800 IU group: 52 (41–64), p<0.05). At postnatal day 28, the 800 IU/day group had the highest average serum 25(OH) vitamin D concentration (ng/mL; Median (25th–75th centiles): 85 (52–99) vs. placebo group: 22 (13–47) and 200 IU group: 39 (26–57), p<0.05), and the majority of infants in the 800 IU/day group had 25 (OH) vitamin D above the desired range of 20–60 ng/mL. At this time point, the percentage of infants in each group with biochemical vitamin D deficiency (<20 ng/mL) was 41% in the placebo group, 16% in the 200 IU group, and 0% in the 800 IU group (p=0.2). Calcium and phosphate levels were from laboratory studies for clinical purposes closest to postnatal day 28 in 69 and 57 infants, respectively. The mean calcium level was 9.8 mg/dL (SD 0.9) for all infants (n; mean (SD): 200 IU: 9.6 (0.9); 400 IU: 10 (1.2); 1000 IU: 9.9 (0.8)) with a mean phosphate level of 4.5 (SD 1.7) overall (n; mean (SD): 200 IU: 4.4 (1.9); 400 IU: 4.9 (1.8); 1000 IU: 4.5 (1.3)).
Figure 2.
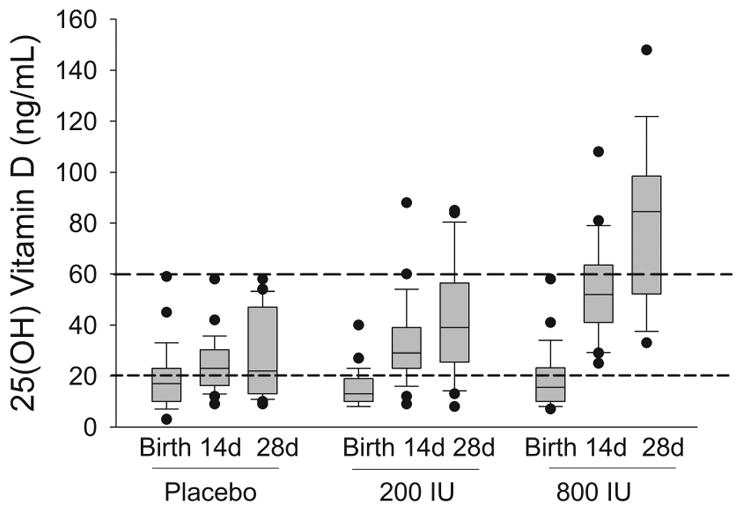
Box Plot illustrating serum 25(OH) vitamin D concentration at birth, day 14, and day 28 in three daily supplementation groups: placebo (total daily intake of approximately 200 IU/day found in formula or TPN), 200 IU/day (total daily intake of approximately 400 IU/day due to 200 IU supplement + 200 IU found in formula or TPN) and 800 IU/day (total daily intake of approximately 1000 IU/day due to 800 IU supplement + 200 IU found in formula or TPN).
IU, international units; d, days.
There were no differences in days alive and off respiratory support as days alive and off mechanical ventilation in the first 28 days. The rates of early-onset sepsis among the three groups were similar (Table II). The rate of late-onset sepsis in the 800 IU/day vitamin D group was not statistically different compared with the other two groups. Death at ≤36w PMA was increased and hospitalization at 36 w PMA was decreased in the 200 IU/day group, although mortality or bronchopulmonary dysplasia (BPD) by 36 weeks PMA did not differ among the groups. There were fifteen deaths overall, with two, nine, and four occurring in the placebo, 200 IU/day, and 800 IU/day groups, respectively. Of these, seven deaths occurred during the first 14 days, two between day 15 and 28, and the remaining six after 28 days. The most common cause of death was respiratory distress syndrome (7), four with associated pulmonary hemorrhage and one with severe intraventricular hemorrhage. Bronchopulmonary dysplasia was the cause of death in five infants. The cause of death in the other four infants was necrotizing enterocolitis, herpes simplex viral meningitis, pulmonary vein stenosis, and sepsis. Other clinical outcomes during the entire hospitalization, including retinopathy of prematurity (stages I–III, plus disease, or disease requiring treatment),16, 17 rate of weight gain, proven necrotizing enterocolitis (Bell’s Stage II and III)15, and intraventricular hemorrhage (grade I–IV)19 did not differ between the groups.
Table 2.
Clinical outcomes of enrolled infants
| Clinical Outcomes | All Infants (N = 100) | Placebo (N = 36) | 200 IU/day (N = 34) | 800 IU/day (N = 30) | p |
|---|---|---|---|---|---|
| Respiratory Disease, n (%) | |||||
| Respiratory distress syndrome | 90 (90) | 31 (86) | 32 (94) | 27 (90) | 0.71 |
| Received Surfactant < 72 hrs | 80 (80) | 26 (72) | 30 (88) | 24 (80) | 0.25 |
| Received Nitric oxide | 19 (19) | 11 (31) | 4 (12) | 4 (13) | 0.09 |
| Pneumothorax | 2 (2) | 1 (3) | 1 (3) | 0 (0) | 0.65 |
| Pulmonary hemorrhage | 8 (8) | 1 (3) | 5 (15) | 2 (7) | 0.18 |
| Respiratory support, n (%) | |||||
| On > 21% FiO2 at 28 days | 63 (63) | 25 (69) | 21 (62) | 17 (57) | 0.55 |
| On > 21% FiO2 at 36 wks | 36 (36) | 15 (42) | 12 (35) | 9 (30) | 0.61 |
| BPD (> 30% FiO2) at 28 days | 45 (45) | 21 (58) | 12 (35) | 12 (40) | 0.12 |
| Severe BPD (IMV or CPAP or ≥30% FiO2) at 36 wks | 29 (35) | 12 (36) | 10 (43) | 8 (31) | 0.84 |
| Steroids for BPD | 27 (27) | 13 (36) | 8 (24) | 6 (20) | 0.29 |
| Days alive and off respiratory support at ≤ 28 d median (25th–75th Centiles) mean ± SD |
0.5 (0–22) 6.53 ± 9.5 |
1 (0–11) 6.8 ± 9.5 |
0 (0–8) 5.5± 9.1 |
0.5 (0–22) 7.0 ± 10.3 |
0.63 0.78 |
| Death or BPD at 36w | 46 (46%) | 17 (47) | 19 (56) | 11 (37) | 0.63 |
| Oxygen requirement, median (25th–75th Centiles) | |||||
| Highest FiO2 on day 28 | 30.5 (39.5) | 35 (21–50) | 29 (22–39) | 30 (21–50) | 0.55 |
| Highest FiO2 on day 36 | 21 (32.1) | 21 (21–38) | 23 (21–35) | 21 (21–30) | 0.62 |
| Necrotizing enterocolitis, n (%) | |||||
| All NEC | 6 (6) | 1 (3) | 4 (12) | 1 (3) | 0.22 |
| NEC Stage I–II | 2 (2) | 0 (0) | 2 (6) | 0 (0) | 0.14 |
| NEC Stage III | 4 (4) | 1 (3) | 2 (6) | 1 (3) | 0.78 |
| Spontaneous intestinal perforation, n (%) | 6 (6) | 1 (3) | 2 (6) | 3 (10) | 0.47 |
| Neurological disease, n (%) | |||||
| Head imaging < 10 days | 58 (58) | 21 (58) | 18 (53) | 19 (63) | 0.70 |
| IVH Grade I | 29 (29) | 13 (36) | 8 (24) | 8 (27) | 0.48 |
| IVH Grade II | 21 (21) | 7 (19) | 10 (29) | 4 (13) | 0.28 |
| IVH Grade IIII | 12 (12) | 3 (8) | 6 (18) | 3 (10) | 0.45 |
| IVH Grade IV | 7 (7) | 1 (3) | 4 (12) | 2 (7) | 0.34 |
| Seizures > 72 hrs | 4 (4) | 0 (0) | 3 (9) | 1 (3) | 0.17 |
| Head imaging normal > 28 days | 62 (62) | 24 (67) | 18 (53) | 20 (67) | 0.41 |
| Retinopathy of prematurity (L or R), n (%) | |||||
| ROP diagnosed | 43 (43) | 18 (50) | 10 (29) | 15 (50) | 0.14 |
| Immature vessels zone I and II | 26 (26) | 9 (25) | 10 (29) | 7 (23) | 0.85 |
| Immature vessels zone III | 11 (11) | 4 (11) | 6 (18) | 1 (3) | 0.19 |
| ROP in zone I and II | 20 (20) | 10 (28 | 6 (18) | 4 (13) | 0.32 |
| Stage I or II; zone III | 5 (5) | 3 (8) | 0 (0) | 2 (7) | 0.25 |
| ROP stage III or higher | 7 (7) | 3 (8) | 2 (6) | 2 (7) | 0.92 |
| Plus disease | 3 (3) | 2 (6) | 0 (0) | 1 (3) | 0.39 |
| Treatment with Avastin | 6 (6) | 2 (6) | 2 (6) | 2 (7) | 0.98 |
| Other treatments | 1 (1) | 1 (3) | 0 (0) | 0 (0) | 0.41 |
| Infection, n (%) | |||||
| Early onset sepsis (≤ 72 hrs) | 6 (6) | 1 (3) | 4 (12) | 1 (3) | 0.22 |
| Antibiotics > 5 days starting < 72hrs | 29 (29) | 11 (31) | 4 (12) | 7 (23) | 0.16 |
| Late onset sepsis (> 72 hrs) | 29 (29) | 13 (36) | 11 (32) | 5 (17) | 0.19 |
| Coagulase negative staphylococci | 12 (12) | 7 (19) | 4 (12) | 1 (3) | 0.13 |
| Escherichia coli | 3 (3) | 1 (3) | 2 (6) | 0 (0) | 0.39 |
| Methicillin-resistant Staphylococcus aureus | 4 (4) | 1 (3) | 2 (6) | 1 (3) | 0.78 |
| Other organisms | 10 (10) | 5 (14) | 2 (6) | 3 (10) | 0.54 |
| Lumbar puncture | 62 (62) | 24 (67) | 20 (59) | 18 (60) | 0.77 |
| Meningitis, culture positive | 7 (7) | 3 (8) | 1 (3) | 3 (10) | 0.50 |
| Nutrition | |||||
| First feed with human milk, n (%) | 91 (91) | 34 (94) | 30 (88) | 27 (90) | 0.65 |
| At day 14 on human milk, n (%) | 50 (50) | 21 (58) | 12 (35) | 17 (57) | 0.11 |
| At day 14 on human milk or formula, n (%) | 14 (14) | 7 (19) | 5 (15) | 2 (7) | 0.33 |
| At day 28 on human milk, n (%) | 37 (37) | 17 (47) | 8 (24) | 12 (40) | 0.11 |
| At day 28 on human milk or formula, n (%) | 7 (7) | 2 (6) | 1 (3) | 4 (13) | 0.24 |
| At day 14, total feeding volumes, mean±SD | 113± 50 | 113 ± 48 | 102± 58 | 127± 39 | 0.22 |
| At day 28, total feeding volumes, mean±SD | 131 ± 35 | 131 ± 34 | 137± 30 | 124 ± 41 | 0.71 |
| Days until BW regained, median (25th–75th Centiles) | 14 (10–19) | 14 (11–18) | 16 (12–19) | 11 (9–20) | 0.39 |
| Days to 120 ml/kg/day, median (25th–75th Centiles) | 13 (10–18) | 14 (11–18) | 12 (11–20) | 12 (9–15) | 0.53 |
| Days on TPN, median (25th–75th Centiles) | 10 (6–14) | 10 (5–13) | 11 (7–17) | 9 (5–14) | 0.46 |
| Hospitalization, n (%) | |||||
| In hospital at 36 wks | 82 (82) | 33 (92) | 23 (68) | 26 (87) | 0.02 |
| Still in hospital at 120 days | 32 (32) | 13 (36) | 10 (29) | 9 (30) | 0.80 |
| Death ≤ 36 wks, n (%) | 9 (9) | 1 (3) | 7 (21) | 1 (3) | 0.02 |
Discussion
The majority of ELGAN had biochemical evidence of vitamin D deficiency at birth. The AAP recommended intake of 400 IU/day12 (200 IU/day supplement + 200 IU in parenteral and enteral nutrition) did not significantly increase the serum 25(OH) vitamin D concentration at 14 days of age although most infants had adequate concentrations by 28 days of age. Use of the ESPGHAN recommended intake of 800–1000 IU/day13 led to adequate concentrations at 14 days of age but higher than desired concentrations of serum 25(OH) vitamin D at 28 days of age without observed toxicity. No significant differences were noted in clinical outcomes among the groups, although the trial was not powered to determine an effect on clinical outcomes.
The strengths of this study, a double-blinded, randomized, multi-dose trial evaluating the effect of vitamin D supplementation in ELGAN, include masking of the intervention and assessment of outcomes, the multi-dose regimen used, and the early initiation of enteral vitamin D supplementation within the first week after birth. Data were analyzed by intent to treat, which included infants who were discontinued from study intervention because of feeding intolerance. The sample size, which was larger than previous studies of vitamin D supplementation in preterm infants,20–23 was sufficient to determine pharmacologic outcome. Infants in this trial were also more preterm and at lower birth weight and at higher risk of mortality and morbidity as compared with previous trials. Limitations were that adequate blood samples were not collected on all infants as sampling was limited to clinical indications, and these missing values limit the validity of the results to some extent. The sample size was adequate for the biochemical outcome but was inadequate for assessment of clinical outcomes as well as differences in baseline patient characteristics between infant groups. Additionally, bone mineral content or calcium and phosphate intake were not measured in this study. It is important to note that multiple methods are used for quantitative analysis of 25 (OH) vitamin D concentrations (ELISA, Radio-Immuno Assay, High-Performance Liquid Chromatography etc), and differences in methods of analysis may lead to variation in results.
Preterm infants are at higher risk of lower 25 (OH) vitamin D levels, compared with more mature infants.24–26 Preterm infants with lower 25(OH) vitamin D are also at higher risk of acute respiratory morbidity 26, 27 and BPD.28 ELGAN are at high risk for low serum vitamin D concentrations not only because transfer of vitamin D occurs later in gestation,10, 11 but perhaps also because of maternal insufficiency.29 In a study of 200 pregnant African American women, 29.2% had serum 25(OH) vitamin D concentrations of < 15 ng/mL immediately prior to delivery, and 47% of their infants had 25(OH) vitamin D concentrations at birth of < 15 ng/mL.30 This vitamin D deficiency may have important consequences in ELGAN. Vitamin D is necessary for bone health31, 32 and it also is important for the saccular and alveolar stages of lung development,1, 2 the dysregulation of which contributes to bronchopulmonary dysplasia in ELGAN. Pups of vitamin D deficient mice have reduced lung volumes and fewer alveoli compared with pups of vitamin D sufficient dams,1, 2 a phenotype similar to the “new BPD.”33
Observational studies have suggested that current nutritional practices are effective in reducing biochemical evidence of vitamin D deficiency in larger preterm infants25, 43 but there has been a lack of data in ELGAN. Four trials compared the vitamin D status in premature infants following random assignment to vitamin D supplementation.20–23 Pittard et al evaluated 27 low-birth-weight and 25 full-term well infants from birth to 16 weeks after delivery and found that 400 IU of vitamin D was a sufficient daily intake for both premature and full-term well infants.23 In the study by Koo et al,22 who evaluated supplementation with three dose regimens (200, 400, and 800 IU/day) of vitamin D in very low birth weight infants, infants in the higher dose group had a 25(OH) vitamin D achieved concentration of only around 31 ng/mL. Koo et al22 found growth and metabolic outcomes, duration of mechanical ventilation (2–3 days), and oxygen supplementation (8–9 days) did not differ between groups22. It is not clear why ELGAN in our trial receiving 800 IU/day of vitamin D had 25(OH) vitamin D concentrations substantially higher than the VLBW infants receiving a similar dose in the study by Koo et al.22 It is possible that a standard weight-independent dose for all premature infants may not be appropriate, and a weight-based dosing (IU/kg) may improve vitamin D levels. However, we did not notice an inverse correlation between the serum vitamin D concentration and birth weight (data not shown) suggesting that smaller infants are not more likely to have higher concentrations with a given magnitude of supplementation. It is possible that differences in study participants (infants in our trial were substantially smaller and more immature), in form of supplementation (infants in the study by Koo et al had formula supplemented with vitamin D), or timing (were enrolled when clinically stable and recovering from respiratory illness and on feeds of at least 75 kcal/kg/day) may account for these differences22. Two small clinical trials evaluating dose-response effect of oral vitamin D after establishment of full enteral feeding had no significant differences in vitamin D status after supplementation in preterm infants. 20, 21 Hanson et al randomized formula-fed late preterm infants born ≥ 32 weeks gestational age to receive 400 IU/day or placebo, and both the placebo and supplemented groups had levels considered insufficient at discharge21. Backstrom et al studied 39 infants < 33 weeks of gestational age who were randomly allocated to receive vitamin D 200 IU/kg of body weight/day up to a maximum of 400 IU/day or 960 IU/day until 3 months old.20 Preterm infants in the low vitamin D group received assisted ventilation longer and had a trend for longer duration of oxygen supplementation. These previous studies evaluated VLBW infants, and not ELGAN who are at much higher risk of respiratory morbidity and mortality, and may have much lower vitamin D stores due to birth at an earlier gestational age and a greater impairment in nutrition.44
In our study, vitamin D supplementation at 800 IU/day led to many infants with 25(OH) vitamin D concentrations beyond those generally considered optimal (>60ng/mL).45, 46 Vitamin D toxicity, usually presenting as emesis, paresthesias, and hypercalcemia, is generally not seen until levels of 250–300 ng/mL are reached.45, 47 Higher 25(OH) vitamin D concentrations are generally well tolerated as its active metabolite (1,25 (OH) vitamin D) is tightly regulated.48, 49 We did not notice any evidence of biochemical (vitamin D concentrations > 200 ng/mL) or overt clinical toxicity (higher serum calcium or lower phosphate) in the higher dose group, and in fact observed a trend toward fewer infants with late onset sepsis in this group, as well as a trend for fewer infants on oxygen at 28 days or receiving steroids for BPD. Similar to vitamin A supplementation, where a large randomized controlled trial used higher doses than those recommended in pilot studies and found statistical improvement in chronic lung disease in ELGAN.50 It is plausible that higher doses of vitamin D may be well tolerated in premature infants, and in fact may improve clinical outcomes. The higher dose given early corrects the biochemical vitamin D deficiency by two weeks of age, but leads to higher than “normal” concentrations by four weeks.
The results of this pilot trial suggest that initial higher doses (800 IU/day) for a shorter duration (1–2 weeks) to restore serum vitamin D concentrations to the normal range followed by a lower dosage (200 IU/day) may be an ideal dosing regimen for ELGAN. A large randomized trial evaluating multiple important clinical outcomes is required to determine if such dosing of vitamin D decreases the risk of lung disease or late onset sepsis in the extremely preterm infant.
Acknowledgments
Supported by the National Institutes of Health (Kaul Pediatric Research Institute Senior Investigator Award U10 HD34216).
Abbreviations
- ELGAN
Extremely low gestational age newborns
- AAP
American Academy of Pediatrics
- ESPGHAN
European Society for Paediatric Gastroenterology Hepatology and Nutrition
- BPD
Bronchopulmonary dysplasia
Footnotes
The author declares no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lange NE, Litonjua A, Hawrylowicz CM, Weiss S. Vitamin D, the immune system and asthma. Expert review of clinical immunology. 2009;5:693–702. doi: 10.1586/eci.09.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zosky GR, Berry LJ, Elliot JG, James AL, Gorman S, Hart PH. Vitamin D deficiency causes deficits in lung function and alters lung structure. American journal of respiratory and critical care medicine. 2011;183:1336–43. doi: 10.1164/rccm.201010-1596OC. [DOI] [PubMed] [Google Scholar]
- 3.Nguyen T, Guillozo H, Marin L, Tordet C, Koite S, Garabedian M. Evidence for a vitamin D paracrine system regulating maturation of developing rat lung epithelium. cell differentiation. 1996;28:36. doi: 10.1152/ajplung.1996.271.3.L392. [DOI] [PubMed] [Google Scholar]
- 4.Phokela SS, Peleg S, Moya FR, Alcorn JL. Regulation of human pulmonary surfactant protein gene expression by 1α, 25-dihydroxyvitamin D3. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2005;289:L617–L26. doi: 10.1152/ajplung.00129.2004. [DOI] [PubMed] [Google Scholar]
- 5.Walker VP, Modlin RL. The vitamin D connection to pediatric infections and immune function. Pediatric research. 2009;65:106R–13R. doi: 10.1203/PDR.0b013e31819dba91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chun RF, Liu PT, Modlin RL, Adams JS, Hewison M. Impact of vitamin D on immune function: lessons learned from genome-wide analysis. Frontiers in physiology. 2014;5:151. doi: 10.3389/fphys.2014.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.White JH. Vitamin D metabolism and signaling in the immune system. Reviews in endocrine & metabolic disorders. 2012;13:21–9. doi: 10.1007/s11154-011-9195-z. [DOI] [PubMed] [Google Scholar]
- 8.Hewison M. Vitamin D and innate and adaptive immunity. Vitamins and hormones. 2011;86:23–62. doi: 10.1016/B978-0-12-386960-9.00002-2. [DOI] [PubMed] [Google Scholar]
- 9.Lagishetty V, Liu NQ, Hewison M. Vitamin D metabolism and innate immunity. Molecular and cellular endocrinology. 2011;347:97–105. doi: 10.1016/j.mce.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dror DK, Allen LH. Vitamin D inadequacy in pregnancy: biology, outcomes, and interventions. Nutrition reviews. 2010;68:465–77. doi: 10.1111/j.1753-4887.2010.00306.x. [DOI] [PubMed] [Google Scholar]
- 11.Kovacs CS. Vitamin D in pregnancy and lactation: maternal, fetal, and neonatal outcomes from human and animal studies. The American journal of clinical nutrition. 2008;88:520S–8S. doi: 10.1093/ajcn/88.2.520S. [DOI] [PubMed] [Google Scholar]
- 12.Abrams SA Committee on N. Calcium and vitamin d requirements of enterally fed preterm infants. Pediatrics. 2013;131:e1676–83. doi: 10.1542/peds.2013-0420. [DOI] [PubMed] [Google Scholar]
- 13.Agostoni C, Buonocore G, Carnielli V, De Curtis M, Darmaun D, Decsi T, et al. Enteral nutrient supply for preterm infants: commentary from the European Society of Paediatric Gastroenterology, Hepatology and Nutrition Committee on Nutrition. Journal of pediatric gastroenterology and nutrition. 2010;50:85–91. doi: 10.1097/MPG.0b013e3181adaee0. [DOI] [PubMed] [Google Scholar]
- 14.Darlow BA, Graham PJ. Vitamin A supplementation to prevent mortality and short- and long-term morbidity in very low birthweight infants. Cochrane Database Syst Rev. 2011:CD000501. doi: 10.1002/14651858.CD000501.pub3. [DOI] [PubMed] [Google Scholar]
- 15.Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L, et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Annals of surgery. 1978;187:1–7. doi: 10.1097/00000658-197801000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.MacDorman MF, Martin JA, Mathews TJ, Hoyert DL, Ventura SJ. Explaining the 2001–2002 infant mortality increase in the United States: data from the linked birth/infant death data set. International journal of health services : planning, administration, evaluation. 2005;35:415–42. doi: 10.2190/TJ2N-DADV-1EP5-5C7F. [DOI] [PubMed] [Google Scholar]
- 17.Davitt BV, Wallace DK. Plus disease. Survey of ophthalmology. 2009;54:663–70. doi: 10.1016/j.survophthal.2009.02.021. [DOI] [PubMed] [Google Scholar]
- 18.Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 1978;92:529–34. doi: 10.1016/s0022-3476(78)80282-0. [DOI] [PubMed] [Google Scholar]
- 19.Burstein J, Papile L, Burstein R. Intraventricular hemorrhage and hydrocephalus in premature newborns: a prospective study with CT. American Journal of Roentgenology. 1979;132:631–5. doi: 10.2214/ajr.132.4.631. [DOI] [PubMed] [Google Scholar]
- 20.Backström M, Mäki R, Kuusela A, Sievänen H, Koivisto A, Ikonen R, et al. Randomised controlled trial of vitamin D supplementation on bone density and biochemical indices in preterm infants. Archives of Disease in Childhood-Fetal and Neonatal Edition. 1999;80:F161–F6. doi: 10.1136/fn.80.3.f161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hanson CK. Late Preterm Infants During Initial Hospitalization. University of Nebraska Medical Center; 2010. Response to Supplemental Vitamin D in Formula Fed. [Google Scholar]
- 22.Koo WW, Krug-Wispe S, Neylan M, Succop P, Oestreich AE, Tsang RC. Effect of three levels of vitamin D intake in preterm infants receiving high mineral-containing milk. Journal of pediatric gastroenterology and nutrition. 1995;21:182–9. doi: 10.1097/00005176-199508000-00010. [DOI] [PubMed] [Google Scholar]
- 23.Pittard WB, Geddes KM, Hulsey TC, Hollis BW. How much vitamin D for neonates? American Journal of Diseases of Children. 1991;145:1147–9. doi: 10.1001/archpedi.1991.02160100079027. [DOI] [PubMed] [Google Scholar]
- 24.Burris HH, Van Marter LJ, McElrath TF, Tabatabai P, Litonjua AA, Weiss ST, et al. Vitamin D status among preterm and full-term infants at birth. Pediatr Res. 2014;75:75–80. doi: 10.1038/pr.2013.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCarthy RA, McKenna MJ, Oyefeso O, Uduma O, Murray BF, Brady JJ, et al. Vitamin D nutritional status in preterm infants and response to supplementation. The British journal of nutrition. 2013;110:156–63. doi: 10.1017/S0007114512004722. [DOI] [PubMed] [Google Scholar]
- 26.Ataseven F, Aygun C, Okuyucu A, Bedir A, Kucuk Y, Kucukoduk S. Is vitamin d deficiency a risk factor for respiratory distress syndrome? International journal for vitamin and nutrition research Internationale Zeitschrift fur Vitamin- und Ernahrungsforschung Journal international de vitaminologie et de nutrition. 2013;83:232–7. doi: 10.1024/0300-9831/a000165. [DOI] [PubMed] [Google Scholar]
- 27.Onwuneme C, Martin F, McCarthy R, Carroll A, Segurado R, Murphy J, et al. The Association of Vitamin D Status with Acute Respiratory Morbidity in Preterm Infants. J Pediatr. 2015;166:1175–80. e1. doi: 10.1016/j.jpeds.2015.01.055. [DOI] [PubMed] [Google Scholar]
- 28.Cetinkaya M, Cekmez F, Erener-Ercan T, Buyukkale G, Demirhan A, Aydemir G, et al. Maternal/neonatal vitamin D deficiency: a risk factor for bronchopulmonary dysplasia in preterms? J Perinatol. 2015;35:813–7. doi: 10.1038/jp.2015.88. [DOI] [PubMed] [Google Scholar]
- 29.Harvey NC, Holroyd C, Ntani G, Javaid K, Cooper P, Moon R, et al. Vitamin D supplementation in pregnancy: a systematic review. Health technology assessment (Winchester, England) 2014;18:1–190. doi: 10.3310/hta18450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sachan A, Gupta R, Das V, Agarwal A, Awasthi PK, Bhatia V. High prevalence of vitamin D deficiency among pregnant women and their newborns in northern India. The American journal of clinical nutrition. 2005;81:1060–4. doi: 10.1093/ajcn/81.5.1060. [DOI] [PubMed] [Google Scholar]
- 31.McCollum EV, Simmonds N, BeckerJE SP. Studies on experimental rickets. XXI. An experimental demonstration of the existence of a vitamin which promotes calcium deposition. J Biol Chem. 1922;53 [PubMed] [Google Scholar]
- 32.MOZOÅOWSKI W. Jäccaron; drzej Sniadecki (1768–1838) on the Cure of Rickets. Nature. 1939;143:121. [Google Scholar]
- 33.Coalson JJ. Seminars in perinatology. Elsevier; 2006. Pathology of bronchopulmonary dysplasia; pp. 179–84. [DOI] [PubMed] [Google Scholar]
- 34.Lange N, Litonjua AA, Gibbons FK, Giovannucci E, Christopher KB. Pre-hospital vitamin D concentration, mortality, and bloodstream infection in a hospitalized patient population. The American journal of medicine. 2013;126:640.e19–.e27. doi: 10.1016/j.amjmed.2012.12.014. [DOI] [PubMed] [Google Scholar]
- 35.Moromizato T, Litonjua AA, Braun AB, Gibbons FK, Giovannucci E, Christopher KB. Association of low serum 25-hydroxyvitamin D levels and sepsis in the critically ill. Critical care medicine. 2014;42:97–107. doi: 10.1097/CCM.0b013e31829eb7af. [DOI] [PubMed] [Google Scholar]
- 36.Ginde AA, Camargo CA, Shapiro NI. Vitamin D insufficiency and sepsis severity in emergency department patients with suspected infection. Academic Emergency Medicine. 2011;18:551–4. doi: 10.1111/j.1553-2712.2011.01047.x. [DOI] [PubMed] [Google Scholar]
- 37.Braun A, Chang D, Mahadevappa K, Gibbons FK, Liu Y, Giovannucci E, et al. Association of low serum 25-hydroxyvitamin D levels and mortality in the critically ill. Critical care medicine. 2011;39:671. doi: 10.1097/CCM.0b013e318206ccdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matthews LR, Ahmed Y, Wilson KL, Griggs DD, Danner OK. Worsening severity of vitamin D deficiency is associated with increased length of stay, surgical intensive care unit cost, and mortality rate in surgical intensive care unit patients. The American Journal of Surgery. 2012;204:37–43. doi: 10.1016/j.amjsurg.2011.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McNally JD, Leis K, Matheson LA, Karuananyake C, Sankaran K, Rosenberg AM. Vitamin D deficiency in young children with severe acute lower respiratory infection. Pediatric pulmonology. 2009;44:981–8. doi: 10.1002/ppul.21089. [DOI] [PubMed] [Google Scholar]
- 40.Inamo Y, Hasegawa M, Saito K, Hayashi R, Ishikawa T, Yoshino Y, et al. Serum vitamin D concentrations and associated severity of acute lower respiratory tract infections in Japanese hospitalized children. Pediatrics international : official journal of the Japan Pediatric Society. 2011;53:199–201. doi: 10.1111/j.1442-200x.2010.03224.x. [DOI] [PubMed] [Google Scholar]
- 41.Karatekin G, Kaya A, Salihoglu O, Balci H, Nuhoglu A. Association of subclinical vitamin D deficiency in newborns with acute lower respiratory infection and their mothers. European journal of clinical nutrition. 2009;63:473–7. doi: 10.1038/sj.ejcn.1602960. [DOI] [PubMed] [Google Scholar]
- 42.Cetinkaya M, Cekmez F, Buyukkale G, Erener-Ercan T, Demir F, Tunc T, et al. Lower vitamin D levels are associated with increased risk of early-onset neonatal sepsis in term infants. Journal of perinatology : official journal of the California Perinatal Association. 2015;35:39–45. doi: 10.1038/jp.2014.146. [DOI] [PubMed] [Google Scholar]
- 43.Pinto K, Collins CT, Gibson RA, Andersen CC. Vitamin D in preterm infants: A prospective observational study. Journal of paediatrics and child health. 2015;51:679–81. doi: 10.1111/jpc.12847. [DOI] [PubMed] [Google Scholar]
- 44.Ehrenkranz RA, Das A, Wrage LA, Poindexter BB, Higgins RD, Stoll BJ, et al. Early nutrition mediates the influence of severity of illness on extremely LBW infants. Pediatric research. 2011;69:522–9. doi: 10.1203/PDR.0b013e318217f4f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jones G. Pharmacokinetics of vitamin D toxicity. The American journal of clinical nutrition. 2008;88:582S–6S. doi: 10.1093/ajcn/88.2.582S. [DOI] [PubMed] [Google Scholar]
- 46.Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. The Journal of Clinical Endocrinology & Metabolism. 2011;96:53–8. doi: 10.1210/jc.2010-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hathcock JN, Shao A, Vieth R, Heaney R. Risk assessment for vitamin D. The American journal of clinical nutrition. 2007;85:6–18. doi: 10.1093/ajcn/85.1.6. [DOI] [PubMed] [Google Scholar]
- 48.Blank S, Scanlon KS, Sinks TH, Lett S, Falk H. An outbreak of hypervitaminosis D associated with the overfortification of milk from a home-delivery dairy. American Journal of Public Health. 1995;85:656–9. doi: 10.2105/ajph.85.5.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pettifor JM, Bikle DD, Cavaleros M, Zachen D, Kamdar MC, Ross FP. Serum levels of free 1, 25-dihydroxyvitamin D in vitamin D toxicity. Annals of internal medicine. 1995;122:511–3. doi: 10.7326/0003-4819-122-7-199504010-00006. [DOI] [PubMed] [Google Scholar]
- 50.Tyson JE, Wright LL, Oh W, Kennedy KA, Mele L, Ehrenkranz RA, et al. Vitamin A supplementation for extremely-low-birth-weight infants. National Institute of Child Health and Human Development Neonatal Research Network. N Engl J Med. 1999;340:1962–8. doi: 10.1056/NEJM199906243402505. [DOI] [PubMed] [Google Scholar]



