Abstract
Objective
To determine if the diastolic closing margin (DCM), defined as diastolic blood pressure minus critical closing pressure, is associated with the development of early severe intraventricular hemorrhage (IVH).
Study design
A reanalysis of prospectively collected data was conducted. Premature infants receiving mechanical ventilation (n = 185) with gestational age 23–31 weeks had ~1 hour continuous recordings of umbilical arterial blood pressure, middle cerebral artery cerebral blood flow velocity, and arterial carbon dioxide tension during the first week of life. Models using multivariate generalized linear regression and purposeful selection were used to determine associations with severe IVH.
Results
Severe IVH (grades 3–4) was observed in 14.6% of infants. Apgar score at 5-minutes and DCM were significantly associated with severe IVH, irrespective of the model. A clinically relevant 5-mm Hg increase in DCM was associated with a 1.83–1.89 increased odds of developing severe IVH.
Conclusion
Elevated DCM was associated with severe IVH, consistent with previous animal data showing IVH is associated with hyperperfusion. Measurement of DCM may be more useful than blood pressure in defining cerebral perfusion in premature infants.
Instability of systemic and cerebral hemodynamics during transition and the early neonatal period in premature infants is thought to be associated with developing intraventricular hemorrhage (IVH). The most immature infants have the highest incidence of IVH, and subsequently have worse developmental delay, poorer cognition, and more cerebral palsy, seizure disorders, and visual impairment.1
A delicate balance is required to maintain adequate cerebral perfusion while avoiding too much (hyperperfusion) or too little (ischemia) cerebral blood flow (CBF), both of which may result in injury to the brain. IVH in premature infants originates from thin-walled, poorly supported, immature vessels of the germinal matrix.2,3 Hypotension and low-flow states, as well as reperfusion hyperemia after ischemia have both been implicated in the pathogenesis of IVH in both human premature infants4–6 and in experimental animal models.7,8
Blood flow to the brain of mammals is largely determined by cerebral perfusion pressure, which has been classically defined as arterial blood pressure (ABP) minus intracranial pressure.9 A reasonable goal of hemodynamic management in the neonatal intensive care unit (NICU) is to target a cerebral perfusion pressure that prevents both ischemic and hyperemic states. However, multiple efforts have failed to determine threshold values of mean ABP in premature infants to define either hypotension or hypertension.10,11 Despite the theoretical benefits of maintaining mean ABP in an “optimal range” to maintain intact cerebral autoregulation, there is no evidence that outcomes have been improved by such efforts.12,13 Treatment of hypotension may be deleterious and has been associated with developing IVH.14 We have shown that hypotensive and normotensive premature infants have similar baseline CBF velocity (CBFV) prior to the treatment of hypotension, and that treatment of hypotension with dopamine was associated with CBFV that exceeded the typical values seen in normotensive premature infants.15,16
In this study, we propose an explanation for the lack of clear relationship between ABP, CBF, and IVH in premature infants. We suggest that, in premature infants, ABP is a poor surrogate for cerebral perfusion pressure. The reason for this assertion is that the critical closing pressure (CrCP) of the cerebral vasculature of premature infants is at or near resting mean ABP.17
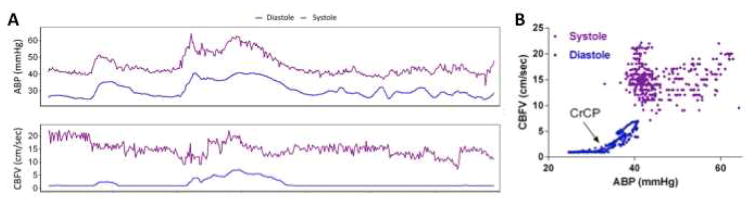
CrCP is the ABP at which blood flow to the brain ceases due to vascular collapse. CrCP is posited to be the sum of vascular wall tension and intracranial pressure.18 Thus, CrCP can be conceptualized as a point of zero reference for converting ABP to an “effective cerebral perfusion pressure” or “closing margin.”19,20 When diastolic ABP is equal to CrCP, CBF occurs only during systole (Figure). The difference between diastolic ABP and CrCP is the diastolic closing margin (DCM).21 The DCM may be a better measure than mean ABP for defining adequacy of brain perfusion pressure in premature infants, as it accounts for intracranial pressure and developmental changes in vascular wall tension.
Figure.
Critical closing pressure (CrCP) is a point of zero reference for determining effective cerebral perfusion pressure from arterial blood pressure (ABP). A, Single patient recording over a one-hour session. During increases of systolic ABP from the baseline of 40 – 45 mm Hg the systolic CBFV is constrained, demonstrating pressure autoregulation. By contrast, diastolic CBFV is zero (blood flow limited to systolic events) until diastolic ABP is increased above a threshold of 32 mm Hg. B) The lack of correlation between systolic CBFV and ABP again demonstrates pressure autoregulation of systolic CBF. The diastolic data demonstrates the CrCP at 32 mm Hg, the minimum vascular pressure required to sustain CBF.
Our objective was to measure CrCP and calculate DCM in a cohort of premature infants during the first week of life and evaluate the association of DCM and severe IVH. We hypothesized that higher effective cerebral perfusion pressure (i.e., higher DCM) is associated with IVH in premature infants. CrCP can be calculated from the interaction between CBFV measured with transcranial Doppler and ABP at the frequency of the cardiac cycle. To test the hypothesis that DCM is associated with severe IVH, we reanalyzed a historical data set containing high-resolution recordings of both CBFV and umbilical artery ABP tracings, combined with the short-term outcome of abnormal cranial ultrasound findings during the first week of life.
Methods
The present study is a reanalysis of previously published data prospectively collected from infants born from July 2002 to April 2008.15,22–25 Approval was obtained by the University of Arkansas for Medical Sciences IRB, and informed parental consent was obtained before enrollment. Very low birth weight (<1500 grams) infants who were mechanically ventilated and had an umbilical artery catheter in place were potential subjects. Those with major congenital anomalies and chromosomal abnormalities, and those in extremis were excluded. Cranial ultrasounds were performed as close to birth as possible (usually within 1–2 hours) to assess for early and preexisting brain injury, between days of life 2–4, and finally at day of life 7. All infants had ABP measurement with an umbilical artery catheter placed for clinical monitoring. Middle cerebral artery CBFV was recorded with transcranial Doppler (Nicolet Vascular/Natus Medical Incorporated, San Carlos, CA). Arterial carbon dioxide tension (PaCO2) was continuously recorded with a Neotrend-L fiber-optic sensor (Diametrics Medical Ltd, St. Paul, MN). Analog data were collected simultaneously using a PowerLab 8 Channel data acquisition system (AD Instruments, Mountain View, CA).
Digitized files were analyzed using ICM+ software (ICM+, Cambridge Enterprise, UK). Artifact was removed using valid values range filters and by visual inspection of each individual recording. For all subjects, there were two 1-hour recording sessions on days of life 1 to 3 and then a daily 1-hour session on days 4 to 7. Of 1,042 subject sessions, 688 subject sessions from 185 subjects had sufficient data to render a CrCP (median 4 recording sessions per subject). A single mean CrCP was reported for each infant averaged across all subject sessions. We chose to collapse the data to one-averaged session per subject as we felt that the multiple subject sessions would create confusion because the number of sessions was inconsistent from subject to subject, and there was only a small degree of variability between individual subject sessions. Trends of systolic and diastolic CBFV and ABP were made by sequential analysis of transformed 10-second segments. The algorithm implemented in ICM+ detects systolic peaks and diastolic valleys using a modified Pan-Tompkins method, rendering a single vector for systole and diastole covering the specified (10-second) buffer. Trends of mean CBFV and ABP were made from consecutive 10-second averages of the 200 Hz primary signals.
CrCP was calculated from ABP and CBFV tracings using equations derived from a model of the cerebral vasculature with resistance and compliance in a parallel circuit. In this model, CBFV is described by an alternating flow velocity at the frequency of the cardiac cycle, and CrCP is derived from an equation of impedance to flow velocity. The full derivation of this method has been previously described in detail and validated.17,21 In brief, CrCP is first estimated from the model by the equation:
where CVR is cerebral vascular resistance and Ca is cerebral arterial compliance, which are not directly measureable, but can be calculated using CBFV and ABP waveforms. The product of resistance and compliance is termed the time constant τ.26 For each subject-session, DCM was calculated as diastolic ABP minus CrCP.
Statistical analyses
A multivariate logistic model was built including univariate covariates with a threshold of p<0.1. A purposeful selection modeling approach guided the building of an additional multivariate logistic regression model to determine associations of covariates with severe IVH (grades 3–4).27 Variables and characteristics potentially related to severe IVH (vs 0–2) were tested in univariate analyses (t-test, Wilcoxon rank-sum test, or chi-square test, as appropriate) and included: estimated gestation age (EGA), sex, mode of delivery, multiple gestation, 5-minute Apgar score, use of vasopressors, mean ABP, mean CBFV, mean DCM, and mean PaCO2. Multicollinearity between mean ABP, mean CBFV, and mean DCM was ruled out using the REG procedure in SAS (SAS Institute, Cary, NC).28
Results
The EGA of the cohort was 26.2 ± 1.97 weeks (mean ± SD, range 23–31 weeks) and birth weight was 822 ± 236 g. In this cohort, 96 (52%) were female, 129 (70%) were born by cesarean delivery, 163 (88%) were exposed to antenatal steroids, 47 (25%) were from multiple gestation pregnancies, 62 (34%) received vasopressor support, and all were mechanically ventilated during the monitoring sessions. The Apgar scores at 1 and 5 minutes were 4 (2–6) and 6 (5–7), respectively. A total of 172 (93%) infants survived to hospital discharge, with 3 deaths occurring outside of the first week of life. For the severe IVH group, the median (IQR) number of recording sessions was 4 (2–6) compared with the non-severe IVH/no IVH group where the median number of recording sessions was 4 (2.8–6). The median (IQR) DCM was 5.6 mm Hg (4.2–6.9). DCM decreased with GA from 6.0 mm Hg (4.3–7.2) at 23 wk to 2.9 mm Hg (2.6–4.5) at 31 weeks’ gestation. The full spectrum of DCM is summarized across GA in Table I.
Table 1.
Diastolic closing margin (DCM) in premature newborns 23 weeks–31 weeks.
| GA (weeks) | n | Mean | Median | IQR |
|---|---|---|---|---|
| 23 | 12 | 5.9 | 6.0 | 4.3 – 7.2 |
| 24 | 34 | 6.1 | 6.1 | 4.7 – 7.1 |
| 25 | 22 | 6.7 | 6.4 | 4.6 – 8.3 |
| 26 | 29 | 5.6 | 5.6 | 4.6 – 6.9 |
| 27 | 29 | 5.4 | 4.6 | 3.9 – 6.3 |
| 28 | 29 | 5.2 | 4.8 | 3.7 – 6.6 |
| 29 | 18 | 6.0 | 5.6 | 4.5 – 6.7 |
| 30 | 4 | 3.3 | 3.3 | 2.7 – 3.8 |
| 31 | 2 | 3.8 | 2.9 | 2.6 – 4.5 |
IQR – interquartile range.
Univariate analysis
The results of univariate analysis of factors related to severe IVH are shown in Table II. EGA (p=0.011), 5-minute Apgar score (p=0.0038), use of vasopressors (p=0.081), mean ABP (p=0.089), and mean DCM (p=0.0063) were associated with severe IVH.
Table 2.
Univariate analysis of variables related to severe IVH
| Characteristics | Non-severe IVH (0, 1, 2) (N=158) | Severe IVH (3, 4) (N=27) | p–value |
|---|---|---|---|
| EGA | 26.3 (1.99) | 25.3 (1.58) | 0.011 |
| Female | 53.2% | 44.4% | 0.40 |
| Vaginal delivery | 30.4% | 29.6% | 0.94 |
| Multiple gestation | 23.4% | 37.0% | 0.13 |
| Apgar 5 min | 6 (5 – 7) | 5 (3 – 6) | 0.0038 |
| Vasopressor use | 31.0% | 48.1% | 0.081 |
| Mean ABP | 37.0 (5.07) | 35.3 (4.29) | 0.089 |
| Mean CBFV | 16.6 (3.51) | 17.4 (3.76) | 0.29 |
| Mean DCM | 8.5 (3.77) | 10.7 (4.26) | 0.0063 |
| Mean PaCO2 | 47.5 (4.61) | 48.8 (5.34) | 0.22 |
EGA – mean estimated gestational age; Apgar 5 min – median 5-minute Apgar score (IQR); ABP – arterial blood pressure; CBFV – middle cerebral blood flow velocity; DCM – diastolic closing margin; PaCO2 – arterial carbon dioxide tension.
Multivariate analyses
A multivariate model that included univariate analysis covariates with p-values <0.1 is shown in Table III. After controlling for covariates, lower 5-minute Apgar score (p=0.02) and increased mean DCM (p=0.02) were significantly associated with severe IVH. A 5-mm Hg increase in mean DCM was associated with 1.89-increased odds of developing severe IVH.
Table 3.
Multiple logistic regression model (using covariates with univariate p-values <0.1) for factors significantly associated with severe IVH
| Metric | Unit Increase | Odds Ratio | 95% CI | p-value |
|---|---|---|---|---|
| Apgar 5 min | 1 score unit | 0.76 | 0.60 – 0.96 | 0.02 |
| Mean ABP | 1 mmHg | 0.96 | 0.86 – 1.07 | 0.44 |
| EGA | 1 week | 0.95 | 0.70 – 1.29 | 0.76 |
| Mean DCM | 1 mmHg | 1.14 | 1.02 – 1.26 | 0.02 |
| 5 mmHg | 1.89 | 1.10 – 3.23 | ||
| Vasopressor use | 1.62 | 0.65 – 4.06 | 0.30 |
Apgar 5 min – 5-minute Apgar score; ABP – arterial blood pressure; EGA – estimated gestational age; DCM – diastolic closing margin, shown for 1-mmHg unit effect size and a more clinically relevant effect size of 5-mmHg.
The final multivariate model was judged to be the best balance of significance and variable inclusion by the purposeful selection procedure. After controlling for one another, lower 5-minute Apgar score by 1 score unit (odds ratio (OR)=0.72, 95% CI of [0.58 – 0.90]; p=0.003) and increased mean DCM by 1 mm Hg (OR=1.13, 95% CI of [1.02 – 1.25]; p=0.02) and by 5 mm Hg (OR=1.83, 95% CI of [1.11 – 3.01]) were significantly associated with severe IVH. The odds ratio of Apgar score at 5-minutes and mean DCM were very similar between the regression models.
Discussion
CrCP is the sum of vascular wall tension and intracranial pressure.29 Intracranial pressure alone is a more familiar zero point reference for calculating cerebral perfusion pressure.30 This is valid when small differences in vascular wall tension are not significant relative to ABP. During transition and the early newborn period, ABP in premature infants is low enough that small variances of either vascular wall tension or intracranial pressure can substantively impact the effective cerebral perfusion pressure. In theory then, by adjusting for changes in both vascular wall tension and intracranial pressure, CrCP is a more reliable zero point reference for calculating perfusion pressure than intracranial pressure alone in premature infants.
Beginning at 23–24 weeks’ gestation, as ABP is increasing, CrCP is also increasing at a rate of 1.4 mm Hg per week, maintaining a low DCM.17 At term gestation and into adulthood, ABP continues to increase but CrCP plateaus at ~30 mmHg, rendering a progressive increase in the DCM. The wide DCM in term infants and adult patients minimizes the effect of relatively small vascular wall tension and intracranial pressure changes on cerebral perfusion pressure.
Our observation that DCM is associated with severe IVH when mean ABP is not supports our assertion that mean ABP alone is not informative of cerebral perfusion pressure in premature infants. This association between high DCM and severe IVH does not necessarily indicate causation. The mechanistic link between the two may be explained as a reduction in CrCP due to IVH, or an unmeasured phenomenon such as post-ischemic hyperemia leading to both high DCM and IVH. Although the results of this study cannot inform hemodynamic management of premature infants, it does however suggest that DCM may be more informative than standard ABP as a proxy of cerebral perfusion pressure. To test this hypothesis more widely would require routine bedside transcranial Doppler monitoring for calculating the DCM. Continuous transcranial Doppler ultrasound, however, is a research technique that is not routinely clinically used in the NICU. Further, there are limitations to its use as it is technically difficult to apply and maintain accurate CBFV recordings (i.e., there is a long learning curve), and it can potentially heat the fragile skin and underlying tissues of premature infants if left in place for a long duration. Due to its limitations, intermittent rather than continuous DCM determination could eventually be used at the bedside to guide hemodynamic management of premature infants.
During the early postnatal period, premature infants are exposed to a variety of hemodynamic stressors and are at risk for both high- and low-perfusion injuries. In particular, premature infants are at risk of myocardial stun during the first 48 hours of life and low flow states that may play a role in brain injury.31,32 Although we found an association between elevated cerebral perfusion pressure and severe IVH, we did not evaluate low perfusion pressure as a risk factor for ischemic injury, such as periventricular white matter injury. This is because our study used cranial ultrasound instead of MRI, which is insensitive for diagnosing ischemic white matter damage. Until such data are available from our ongoing studies using MRI, we would discourage any suggestion that lower perfusion pressures render overall more favorable outcomes. This study is the first examination of the relation between effective perfusion pressure and severe IVH in premature infants, and is best suited as preliminary data for further investigation.
One limitation of the study is that previously published data were reanalyzed for the new variables of CrCP and DCM. The need for high-quality resolution of systolic and diastolic ABP and CBFV resulted in some data dropout, which could have introduced unexpected bias. In addition, due to the observational nature of this study, we were unable to determine the exact timing of the IVH, so we used all sessions in the analysis irrespective of if they occurred before or after the IVH. Another limitation was an inability to determine the impact of antenatal steroids on the development of IVH as both the severe IVH and non-severe/no IVH groups had nearly 90% exposure to antenatal steroids.
Uncertainty remains with respect to which area of the premature brain is most important for long-term neurodevelopment. The cerebral cortex, a vital organ later in life, is the area of the brain most readily and easily monitored in premature infants. However, it is possible that the cerebral cortex is actually not vital during the early newborn period in premature infants and may only require CBF during a portion of the cardiac cycle (i.e., systole).33 Thus, the treatment of hypotension and its impact on the DCM and risk for brain injury warrants further investigation in future clinical trials.
Elevated effective perfusion pressure estimated by the DCM was associated with severe IVH in this cohort of premature infants. DCM, a Doppler-ultrasound based assessment of cerebral perfusion, may be more useful than mean ABP in guiding individualized hemodynamic management and mitigate the risk for severe IVH in this vulnerable population.
Acknowledgments
J.K. was supported by the National Institutes of Health (1K23NS43185, RR20146, and 1R01NS060674) and the University of Arkansas for Medical Sciences Translational Research Institute (1UL1RR029884). C.R. is supported by the Chao Physician Scientist Award (Baylor College of Medicine; Houston, TX) and by the National Institutes of Health (1K23NS091382-01A1).
We thank Natalie C. Sikes and Melanie J. Mason for technical assistance, and appreciate the support of the University of Arkansas for Medical Sciences neonatologists, NICU nurses, respiratory therapists, and ultrasound technicians.
Abbreviations
- ABP
arterial blood pressure
- CBF
cerebral blood flow
- CBFV
cerebral blood flow velocity
- PaCO2
arterial carbon dioxide tension
- CrCP
critical closing pressure
- DCM
diastolic closing margin
- EGA
estimated gestational age
- IVH
intraventricular hemorrhage
- NICU
neonatal intensive care unit
Footnotes
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hack M. Young adult outcomes of very-low-birth-weight children. Sem Fetal Neonatal Med. 2006;11:127–137. doi: 10.1016/j.siny.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 2.Volpe JJ. Intraventricular hemorrhage in the premature infant--current concepts. Part II. Ann Neurol. 1989;25:109–116. doi: 10.1002/ana.410250202. [DOI] [PubMed] [Google Scholar]
- 3.Volpe JJ. Intraventricular hemorrhage in the premature infant--current concepts. Part I. Ann Neurol. 1989;25:3–11. doi: 10.1002/ana.410250103. [DOI] [PubMed] [Google Scholar]
- 4.Osborn DA, Evans N, Kluckow M. Clinical detection of low upper body blood flow in very premature infants using blood pressure, capillary refill time, and central-peripheral temperature difference. Arch Dis Child Fetal Neonatal Ed. 2004;89:F168–173. doi: 10.1136/adc.2002.023796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Watkins AM, West CR, Cooke RW. Blood pressure and cerebral haemorrhage and ischaemia in very low birthweight infants. Early Hum Devel. 1989;19:103–110. doi: 10.1016/0378-3782(89)90120-5. [DOI] [PubMed] [Google Scholar]
- 6.Perlman J, Volpe JJ. Intraventricular hemorrhage in extremely small premature infants. Am J Dis Child. 1986;140:1122–1124. doi: 10.1001/archpedi.1986.02140250048034. [DOI] [PubMed] [Google Scholar]
- 7.Ment LR, Stewart WB, Duncan CC, Pitt BR, Rescigno A, Cole J. Beagle puppy model of perinatal cerebral infarction. J Neurosurg. 1985;63:441–447. doi: 10.3171/jns.1985.63.3.0441. [DOI] [PubMed] [Google Scholar]
- 8.Ment LR, Stewart WB, Duncan CC, Pitt BR. Beagle puppy model of perinatal cerebral insults. J Neurosurg. 1986;65:847–850. doi: 10.3171/jns.1986.65.6.0847. [DOI] [PubMed] [Google Scholar]
- 9.Schwartz AE, Sandhu AA, Kaplon RJ, Young WL, Jonassen AE, Adams DC, et al. Cerebral blood flow is determined by arterial pressure and not cardiopulmonary bypass flow rate. Ann Thorac Surg. 1995;60:165–169. discussion 169–170. [PubMed] [Google Scholar]
- 10.Laughon M, Bose C, Allred E, O’Shea TM, Marter LJ, Bednarek F, et al. Factors associated with treatment for hypotension in extremely low gestational age newborns during the first postnatal week. Pediatrics. 2007;119:273–280. doi: 10.1542/peds.2006-1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al-Aweel I, Pursley D, Rubin L, Sharh B, Weisberger S, Richardson D. Variations in prevalence of hypotension, hypertension, and vasopressor use in NICUs. J Perinatol. 2001;21:272–278. doi: 10.1038/sj.jp.7210563. [DOI] [PubMed] [Google Scholar]
- 12.da Costa CS, Czosnyka M, Smielewski P, Mitra S, Stevenson GN, Austin T. Monitoring of Cerebrovascular Reactivity for Determination of Optimal Blood Pressure in Preterm Infants. J Pediatr. 2015;167:86–91. doi: 10.1016/j.jpeds.2015.03.041. [DOI] [PubMed] [Google Scholar]
- 13.Pellicer A, del Carmen Bravo M, Madero R, Salas S, Quero J, Cabañas F. Early Systemic Hypotension and Vasopressor Support in Low Birth Weight Infants: Impact on Neurodevelopment. Pediatrics. 2009;123:1369–1376. doi: 10.1542/peds.2008-0673. [DOI] [PubMed] [Google Scholar]
- 14.Dempsey E, Al Hazzani F, Barrington K. Permissive hypotension in the extremely low birthweight infant with signs of good perfusion. Arch Dis Child Fetal Neonatal Ed. 2009;94:F241–F244. doi: 10.1136/adc.2007.124263. [DOI] [PubMed] [Google Scholar]
- 15.Lightburn MH, Gauss CH, Williams DK, Kaiser JR. Cerebral blood flow velocities in extremely low birth weight infants with hypotension and infants with normal blood pressure. J Pediatr. 2009;154:824–828. doi: 10.1016/j.jpeds.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lightburn MH, Gauss CH, Williams DK, Kaiser JR. Observational study of cerebral hemodynamics during dopamine treatment in hypotensive ELBW infants on the first day of life. J Perinatol. 2013;33:698–702. doi: 10.1038/jp.2013.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rhee CJ, Fraser CD, III, Kibler KK, Easley RB, Andropoulos DB, Czosnyka M, et al. Ontogeny of critical closing pressure. Pediatr Res. 2015;78:71–75. doi: 10.1038/pr.2015.67. [DOI] [PubMed] [Google Scholar]
- 18.Nichol J, Girling F, Jerrard W, Claxton E, Burton A. Fundamental instability of the small blood vessels and critical closing pressures in vascular beds. Am J Physiol. 1951;164:330–344. doi: 10.1152/ajplegacy.1951.164.2.330. [DOI] [PubMed] [Google Scholar]
- 19.Jagersberg M, Schaller C, Bostrom J, Schatlo B, Kotowski M, Thees C. Simultaneous bedside assessment of global cerebral blood flow and effective cerebral perfusion pressure in patients with intracranial hypertension. Neurocrit Care. 2010;12:225–233. doi: 10.1007/s12028-009-9300-2. [DOI] [PubMed] [Google Scholar]
- 20.Varsos GV, Richards H, Kasprowicz M, Budohoski KP, Brady KM, Reinhard M, et al. Critical closing pressure determined with a model of cerebrovascular impedance. J Cereb Blood Flow Metab. 2013;33:235–243. doi: 10.1038/jcbfm.2012.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Varsos G, Richards H, Kasprowicz M, Reinhard M, Smielewski P, Brady KM, et al. Cessation of diastolic cerebral blood flow velocity: the role of critical closing pressure. Neurocrit Care. 2013:1–9. doi: 10.1007/s12028-013-9913-3. [DOI] [PubMed] [Google Scholar]
- 22.Kaiser JR, Gauss CH, Williams DK. Surfactant administration acutely affects cerebral and systemic hemodynamics and gas exchange in very-low-birth-weight infants. J Pediatr. 2004;144:809–814. doi: 10.1016/j.jpeds.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 23.Kaiser JR, Gauss CH, Williams DK. The effects of hypercapnia on cerebral autoregulation in ventilated very low birth weight infants. Pediatr Res. 2005;58:931–935. doi: 10.1203/01.pdr.0000182180.80645.0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaiser J, Gauss C, Williams D. Tracheal suctioning is associated with prolonged disturbances of cerebral hemodynamics in very low birth weight infants. J Perinatol. 2007;28:34–41. doi: 10.1038/sj.jp.7211848. [DOI] [PubMed] [Google Scholar]
- 25.Kaiser J, Gauss C, Williams D. The effects of closed tracheal suctioning plus volume guarantee on cerebral hemodynamics. J Perinatol. 2011;31:671–676. doi: 10.1038/jp.2011.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kasprowicz M, Diedler J, Reinhard M, Carrera E, Steiner LA, Smielewski P, et al. Time constant of the cerebral arterial bed in normal subjects. Ultrasound Med Biol. 2012;38:1129–1137. doi: 10.1016/j.ultrasmedbio.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 27.Bursac Z, Gauss CH, Williams DK, Hosmer DW. Purposeful selection of variables in logistic regression. Source Code Biol Med. 2008;3:17. doi: 10.1186/1751-0473-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Belsley D, Kuh E, Welsch R. Regression Diagnostics: Identifying influential data and sources of collinearity. New York: John Wiley and Sons; 1980. [Google Scholar]
- 29.Nichol J, Girling F, Jerrard W, Claxton EB, Burton AC. Fundamental instability of the small blood vessels and critical closing pressures in vascular beds. Am J Physiol. 1951;164:330–344. doi: 10.1152/ajplegacy.1951.164.2.330. [DOI] [PubMed] [Google Scholar]
- 30.The Brain Trauma Foundation. The American Association of Neurological Surgeons. The Joint Section on Neurotrauma and Critical Care. Guidelines for cerebral perfusion pressure. J Neurotrauma. 2000;17:507–511. doi: 10.1089/neu.2000.17.507. [DOI] [PubMed] [Google Scholar]
- 31.Evans N, Kluckow M. Early determinants of right and left ventricular output in ventilated preterm infants. Arch Dis Child Fetal Neonatal Ed. 1996;75:F183–186. doi: 10.1136/fn.74.2.f88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kluckow M, Evans N. Superior vena cava flow in newborn infants: a novel marker of systemic blood flow. Arch Dis Child Fetal Neonatal Ed. 2000;82:F182–187. doi: 10.1136/fn.82.3.F182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seri I. Low superior vena cava flow during the first postnatal day and neurodevelopment in preterm neonates. J Pediatr. 2004;145:573–575. doi: 10.1016/j.jpeds.2004.08.064. [DOI] [PubMed] [Google Scholar]