Abstract
Dysfunctional reward processing has long been considered an important feature of major depressive disorder (MDD). However, depression is a heterogeneous construct and the nature of this heterogeneity may contribute to some of the inconsistent empirical findings on reward dysfunction in MDD. The current study examined one source of heterogeneity, melancholic symptoms, and its association with reward processing. In individuals with MDD (N=141) and MDD-free controls (N=113), EEG alpha asymmetry was measured during a behavioral reward task that probed reward anticipation. Melancholic depression was measured both categorically (DSM diagnosis) and dimensionally (Hamilton Endogenomorphy Scale). Results showed that a dimensional (and not categorical) definition of melancholia predicted reward processing, with higher melancholic symptoms predicting reduced reward anticipation. Importantly, the effects of melancholic symptoms on reduced reward anticipation remained above and beyond overall depression severity. These results suggest that dysfunctional reward processing may only be associated with melancholic symptoms, not depression in general.
Keywords: melancholia, depression, reward processing, EEG asymmetry
Major Depressive Disorder (MDD) is a heterogeneous syndrome and this heterogeneity is problematic because it can lead to inconsistent research on biomarkers, treatments, and prevention efforts. One way to parse heterogeneity is to examine core features of the syndrome as that may identify more homogeneous phenotypes. Numerous theorists and clinicians have proposed that dysfunction in reward processing is a core feature of depression (Clark, Beck, & Beck, 1994; Clark & Watson, 1991). Reviews of both the behavioral and neuroscience literature suggest that individuals with depression demonstrate difficulty with reward-related processing and abnormalities in brain systems implicated in reward processing (Eshel & Roiser, 2010; Foti & Hajcak, 2009; Forbes & Dahl, 2012). Additionally, altered reward functioning has been posited as an early developing risk factor for depression (Davey, Yucel, & Allen, 2008). This hypothesis is supported by studies demonstrating reduced behavioral and neural correlates of reward processing in offspring of depressed parents (Olino et al., 2014; Kujawa, Proudfit, & Klein, 2014; Sharp et al., 2014) as well as longitudinal studies (Bress et al., 2013; Morgan et al., 2013). However, some studies have shown no differences in measures of reward between depressed individuals and controls (Reid, Duke, & Allen, 1998; Pizzagalli et al., 2002). These contrary findings suggest that reward dysfunction may not be a universal feature of depression, but may only be associated with some symptom dimensions or subtypes within MDD.
Melancholic MDD is one subtype that may be particularly associated with reward dysfunction. Melancholic depression is characterized by marked anhedonia, in which individuals almost completely lose interest in, or have practically no reactivity to, pleasurable or rewarding stimuli (APA, 2013). Some researchers have questioned the validity of melancholia as a qualitatively distinct subtype (Joyce et al., 2002), with some positing that melancholia is simply a more severe form of MDD, and not etiologically separate from nonmelancholic MDD (Kendler, 1997). This debate reflects the difficulties in defining melancholia (Rush & Weisenburger, 1994). Indeed, the symptom criteria for melancholia have been revised in nearly all revisions of the DSM since 1980. However, despite these changes, an impaired ability to anticipate or experience pleasure continues today as a central symptom of melancholic MDD in DSM5 (APA, 2013).
Even though anhedonia (and reward processing dysfunction) is a central feature of the diagnostic criteria of melancholia, surprisingly few laboratory studies have directly examined the association between melancholia and reward-related deficits. Day et al. (2015) and Fletcher et al. (2015) used behavioral tasks of positive emotionality and reward learning, respectively, to demonstrate reward deficits specific to melancholia. Studies using psychophysiological methods are also few and have yielded mixed results regarding the association between melancholia and reward processing, with some showing effects for the subtype and others not (Shankman, Sarapas, & Klein, 2011; Foti et al., 2014) - a pattern possibly due to small sample sizes. Thus, the main aim of the current study was to test whether individuals with melancholic MDD demonstrate blunted reward processing compared to non-melancholic MDD in a laboratory reward task using psychophysiology.
One way reward processing has often been measured is with asymmetry of frontal electroencephalogram (EEG) alpha power. Alpha power is the frequency band between 8–13 Hz and is associated with “relaxed wakefulness,” as decreases in alpha tend to emerge when underlying cortical systems engage in active processing (i.e., alpha suppression, Allen, Coan, & Nazarian, 2004). This supports the heuristic that a decrease in alpha power reflects an increase in brain activity (Coan, Allen, & McKnight, 2006; although see Tenke & Kayser, 2005 for a critical discussion of this premise). An asymmetry in frontal EEG alpha power (or “frontal asymmetry”) can therefore be used to reflect relative activity of right and left frontal regions. Most typically, this has been assessed by comparing right versus left frontal alpha power (indicating greater left than right “brain activity”; Allen et al., 2004; Coan et al., 2006).
A greater frontal EEG asymmetry has long been purported to be associated with greater approach motivation tendencies (Davidson, 1998). Consistent with this hypothesis, relative greater frontal EEG asymmetry at rest has been associated with greater self-reported tendencies of approach motivation (e.g., BAS sensitivity, Coan & Allen, 2003; Harmon-Jones & Allen, 1997; Sutton & Davidson, 1997). Additionally, individual differences in frontal EEG asymmetry have predicted reward learning in a behavioral task in a non-clinical sample (Pizzagalli, Sherwood, Henriques, & Davidson, 2005).
Furthermore, frontal EEG asymmetry has been proposed to reflect approach motivation processes in clinical samples such as individuals with MDD. As mentioned above, one of the core features of MDD is a deficit in approach-related tendencies (Henriques & Davidson, 2000; Davidson, 1992). Consistent with this, in comparison to controls, individuals who are at risk for depression (Tomarken, Dichter, Garber, & Simien, 2004), currently experiencing depression (see meta-analysis by Thibodeau, Jorgensen, & Kim, 2006), and in remission from depression (Gotlib, Ranganath, & Rosenfeld, 1998; Stewart, Bismark, Towers, Coan, & Allen, 2010) all demonstrate reduced left relative to right frontal activity at rest. This pattern of asymmetry has also been observed when depression is measured dimensionally (Schaffer, Davidson, & Saron, 1983). Taken together, research on emotional tendencies and emotional responding broadly support EEG alpha asymmetry as an index of approach-related tendencies in both control and clinical samples.
In addition to measuring EEG at rest, researchers have measured EEG alpha activation during behavioral tasks that elicit reward processing (Shankman, Klein, Tenke, & Bruder, 2007, 2013). In fact, according to the capability model of frontal EEG asymmetry (Coan et al., 2006), brain activation elicited during an emotional task may be a stronger indicator of individual differences related to that emotion than activity recorded at rest, as it tests individuals’ capacity to engage neural systems relevant to the emotional construct (in this case, reward anticipation). Frontal EEG asymmetry may therefore be more reflective of reward processing deficits in individuals with depression if measured during an affective challenge rather than at rest (Shankman et al., 2007; Shankman et al., 2013). Thus, in the present study frontal EEG asymmetry will be measured both at rest and during a reward anticipation task in order to examine whether group differences will be found across indices, or will only be elicited during reward anticipation.
The present study will also measure melancholia in several ways. While the DSM defines melancholia as a subtype, there have been several studies showing that the categorical diagnosis may not fully capture the construct of melancholia (Kendler, 1997; Parker et al., 2009). Thus, along with a DSM-defined categorical conceptualization of melancholia, the present study will define melancholia dimensionally using the Hamilton Endogenomorphy Scale (HES; Thase, Hersen, Bellack, Himmelhoch, & Kupfer, 1983), an empirically derived subscale of the widely used Hamilton Rating Scale of Depression (HRSD) that captures the melancholic symptoms of depression.
In sum, the present study could help elucidate key mechanisms involved in the development of melancholic depression, a particularly pernicious form of depression. As melancholic depression is characterized by a blunted response to reward, it is hypothesized that melancholic MDD will be associated with reduced left relative to right frontal brain activity compared to non-melancholic MDD and MDD-free control. In contrast, non-melancholic MDD may demonstrate a similar frontal EEG asymmetry profile as controls; however, it is also possible that non-melancholic MDD will show a reduced left relative to right asymmetry in brain activity than controls but not to the same degree as that of melancholic MDD.
Method
Participants
The present study used data drawn from two samples which have been previously described elsewhere (Shankman et al., 2007; 2013). Both samples underwent identical diagnostic assessment procedures and similar reward tasks (the differences are noted below). Participants were over 18 and recruited from the community and area mental health clinics (total N= 254). The MDD groups consisted of 141 individuals with current MDD (38 melancholic, 103 non-melancholic). The control group consisted of 113 non-MDD individuals who were required to have no lifetime diagnoses of MDD or dysthymia. All three groups were allowed to have current or lifetime anxiety disorders (see Table 1). Exclusion criteria for both samples were similar - lifetime diagnosis of a psychotic disorder, bipolar disorder, or dementia; inability to read or write English; history of head trauma with loss of consciousness; and left-handedness. Sample 2 (Shankman et al., 2013) focused on early onset depression and thus excluded individuals whose depression onset was after age 18.
Table 1.
Demographics and clinical characteristics
| Melancholic MDD (N=38) | Non-melancholic MDD (N=103) | Non-MDD controls (N=113) | |
|---|---|---|---|
|
| |||
| Age of onset (SD) | 16.21 (9.17) a | 15.97 (7.39) a | -- |
|
| |||
| Age (SD) | 35.92 (12.86) a | 32.79 (11.66) a | 32.56 (12.50) a |
|
| |||
| Sex (% female) | 60.53% a | 68.93% a | 66.37% a |
|
| |||
| Ethnicity (% white) | 55.3% a | 64.1% a | 50.4% a |
|
| |||
| Lifetime anxiety disorder | 52.63% a | 44.66% a | 21.24% b |
|
| |||
| Lifetime substance use disorder | 23.68% a | 30.10% a | 5.31% b |
|
| |||
| Psychiatric medication | 36.84% a | 43.69% a | 7.08% b |
| Multiple drugs | 7.89% a | 10.68% a | 1.77% b |
| Antidepressants | 23.68% a | 27.18% a | 5.31% b |
| Benzodiazepines | 5.26% a | 4.85% a | 0% |
| Mood stabilizers | 0% a | 0% a | 0% a |
| Antipsychotics | 0% a | 0% a | 0% a |
| Other (stimulant) | 0% a | 0.97% a | 0% a |
|
| |||
| HRSD severity (SD) | 29.00 (8.12) a | 25.35 (7.38) b | 3.21 (4.90) c |
|
| |||
| HES Melancholia subscale (SD) | 9.00 (3.08) a | 7.49 (2.42) b | 1.11 (1.72) c |
|
| |||
| GAF (SD) | 51.34 (6.70) a | 53.44 (7.09) a | 81.50 (14.51) b |
Note. Different superscripts represent significant (p < .05) group differences
Measures
Clinical diagnoses (including the categorical melancholic subtype) were determined using the Structured Clinical Interview for DSM–IV (SCID; First, Spitzer, Gibbon, & Williams, 1996). Diagnosticians were trained to criterion on the SCID and supervised by a licensed clinical psychologist. Twenty SCIDs were audio recorded and scored by a second rater blind to original diagnoses to determine reliability of diagnoses. Interrater reliability indicated perfect agreement for PD and MDD diagnoses (kappas = 1.00).
Depression severity was measured using the Hamilton Rating Scale for Depression (HRSD; Hamilton, 1960). The HRSD is a 24-item, clinician-administered rating scale probing depressive symptoms over the previous week on a 0 to 4 scale (absent, mild, moderate, or severe). The HRSD is widely used as a measure of depression severity and has good psychometric properties (Trajkovic et al., 2011). In the present sample, Cronbach’s alpha for the HRSD was .92.
Melancholic symptoms were measured dimensionally through the Hamilton Endogenomorphy Subscale (HES; Thase et al., 1983). The HES is a subset of items from the HRSD and was designed to capture the symptoms of melancholic or endogenous MDD. These items are Middle Insomnia, Late Insomnia, Work and Activities (measure of anhedonia), Psychomotor Retardation, Psychomotor Agitation, Loss of Weight, Diurnal Variation (AM), and Hopelessness. The HES has been shown to validly discriminate melancholic (i.e. endogenomorphic) depression from nonmelancholic depression (Thase et al., 1983). HES scores also correlate highly with ICD-9 criteria of endogenous depression, another measure of melancholia (Maier & Philipp, 1986). Some researchers have questioned the use of HES for severe (and inpatient) populations; however, the HES has demonstrated moderate associations with both RDC and DSM diagnoses of melancholic MDD, as well as an association with dexamethasone test response (a putative marker of melancholia; Zimmerman et al., 1986). Additionally, the present study only included non-inpatients – a population for which the HES has been validated (Thase et al., 1983). Nevertheless, given that relatively few studies that have validated the HES as a measure of melancholia, we examined the HES’s ability to discriminate those with and without a categorical diagnosis of melancholia and HES’s association with trait measures positive emotionality. Cronbach’s alpha for the HES was .75 in the current sample.
In both samples, participants also completed the General Temperament Survey (GTS; Clark & Watson, 1990), a self-report measure of trait positive and negative emotionality. The GTS is a 90-item true-or-false questionnaire developed to assess general aspects of temperament; it contains factor analytically derived scales of Negative Temperament (GTS-NT) and Positive Temperament (GTS-PT). High scores on the GTS-NT indicate a tendency to experience emotions such as anger, guilt, and sadness. High scores on the GTS-PT indicate a tendency to experience emotions such as cheerfulness, enthusiasm, and excitement. Cronbach’s alphas for the GTS-PT and GTS-NT were .89 and .92, respectively.
In sample 2, the Temporal Experience of Pleasure Scale (TEPS; Gard, Gard, Kring & John, 2006) was used to measure anticipatory positive affective tendencies (10 items, e.g., “Looking forward to a pleasurable experience is in itself pleasurable.”) and consummatory positive affective tendencies (8 items, e.g., “I enjoy taking a deep breath of fresh air when I walk outside.”). Participants rated each item from 1 (very false for me) to 6 (very true for me), and their responses were summed to determine their TEPS-Anticipatory, TEPS-Consummatory, and TEPS-Overall indices. Consistent with previous research (Gard et al., 2006), this scale had adequate reliability (TEPS-Ant: α= .80, TEPS-Con: α = .75, TEPS-Total: α = .87).
Tasks and Materials
Resting EEG was recorded in six one-minute blocks, with each block consisting of either an eyes-open (O) or an eyes-closed (C) condition, in one of two counterbalanced orders (COOCCO or OCCOOC).
As described elsewhere (Shankman et al., 2007; 2013), a computerized slot machine paradigm was used to measure reward sensitivity. In this paradigm, three reels of numbers and fruits appeared on the screen and “spun” simultaneously for 11 s before they “landed” on a result. The task included two conditions with 30 trials each (60 total): a reward condition (R) and a no-incentive condition (NI). During R, participants won money if the reels landed on three fruits. During NI, participants did not win money regardless of the outcome. Thus, R was designed to elicit reward anticipation, whereas NI served as control for several aspects of R (e.g., visual input, anticipating an outcome). The amount of potential winnings during each R trial ranged from $0.30 to $0.45 in sample 1 and $0.50 to $3.00 in sample 2. Notably, participants never lost money in either condition if the reels did not land on three pieces of fruit.1 Trials were divided into three blocks and presented in a pseudorandom order. There were never more than two consecutive trials of similar type or outcome. Participants began the task with $5.00 in sample 1 and $2.00 in sample 2 and were told the specific condition (R or NI) prior to each trial. The potential amount of winnings in each R trial was not disclosed. Twice during the slot machine game, participants retrospectively rated their feelings during each portion of the task. Participants were asked how much they “looked forward to three pieces of fruit” during both the R and NI conditions on a Likert scale ranging from 1 (not at all) to 7 (extremely). Self-reported ratings of reward anticipation were calculated subtracting the NI rating from the R rating.
Data Recording and Processing
In sample 1, EEG was recorded during the reward task from two homologous pairs of electrodes overlapping frontal (F3/F4; F7/F8), central (C3/C4; T7/T8), and posterior (P3/P4; P7/P8) brain regions over both left and right hemispheres and from one midline electrode (Cz) with a stretch-lycra electrode cap (Electro-Cap International, Inc., Eaton, OH; tin electrodes). The ground electrode was at the frontal pole (Fpz). In sample 2, data were recorded during the reward task using Ag/AgCl electrodes in a 64-channel stretch-lycra electrode cap. The ground electrode was at the frontal pole (AFZ) and the online reference was near the vertex (between CZ and CPZ). In both samples, electrodes placed at the right supra- and infraorbital sites were used to monitor vertical eye movements (VEOG) and electrodes placed at the right and left outer canthi were used to monitor horizontal eye movements (HEOG). Electrode impedances were under 5,000 ohms, and homologous sites (e.g., F3/F4) were within 1,500 ohms of each other in both samples.
In sample 1, data were recorded through a Grass Neurodata acquisition system at a gain of 10 K (5 K for eye channels) with a bandpass of 1–30 Hz. In sample 2, data were recorded through a Neuroscan Synamp2 data acquisition system at a gain of 10K (5K for eye channels) with a bandpass of DC-200 Hz. In both samples, data were acquired and digitized using an EEG acquisition system (Neuroscan 4.5) continuously at a rate of 1000 Hz. EEG data were re-referenced offline by computing a digitally derived “linked mastoids” reference using data from the left and right mastoid.
Neuroscan 4.5 was used to process all EEG data. EEG data from the 11-s period while the slot machine reels were spinning were segmented into consecutive 1.024-s epochs every 0.512 s (50% overlap). After referencing to a linked mastoid reference offline and then applying a baseline correction, data contaminated by blinks, eye movements, and movement-related artifacts were manually excluded by direct visual inspection of the data. The EEG was tapered over the entire 1.024-s epoch by a Hanning window to suppress spectral side lobes. Artifact-free data were recovered in adjacent (overlapping) epochs and power spectra were computed offline from EEG data by using a fast Fourier transform. Subsequently, the average absolute alpha power was computed for each electrode site and then natural log transformed to normalize the data. Consistent with previous studies (e.g., Bruder et al., 1997), the alpha band was defined as 7.81–12.70 Hz and used as an inverse measure of regional brain activity (note: this range was not exactly the typical 8–13 Hz because the epoch length was not exactly a second).
Frontal resting EEG asymmetry scores were computed by subtracting alpha power of the average of left frontal electrodes from alpha power of the average of homologous right electrodes (e.g., average of F4 & F8, subtracting the average of F3 & F7) such that higher values on this asymmetry difference score reflected greater activity in left relative to right frontal regions at rest. When eyes-closed vs. eyes-open conditions were included as an additional within-subjects factor, this factor did not interact with the results. The two conditions (open and closed) were therefore averaged for each participant prior to calculating asymmetry scores.
For the reward task, frontal EEG asymmetry scores were computed for the R and NI conditions by subtracting alpha power of the average of left frontal electrodes from alpha power of the average of homologous right electrodes (e.g., average of F4 & F8, subtracting the average of F3 & F7) such that higher values on this asymmetry difference score reflected greater activity in left relative to right frontal regions during reward anticipation. Averaging power from neighboring electrodes helps yield a more representative frontal asymmetry score. These specific frontal channels were chosen as they were common to both samples. The frontal asymmetry scores for NI were then subtracted from that of R to create a variable reflecting how much frontal asymmetry ‘potentiates’ (or increases) during the R compared to NI condition (analogous to an ERP difference waveform).
Statistical Analysis
All analyses were performed with SPSS version 22. To test the aim of this study using the categorical definition of melancholia, ANCOVAs were conducted to examine whether the three groups (controls, nonmelancholic MDD, melancholic MDD) exhibited different frontal EEG asymmetry scores during reward anticipation. To test the aim of this study using the dimensional definition of melancholia, multiple regressions were conducted with frontal EEG asymmetry score from the reward task as the dependent variable and HES symptoms as the main predictor. Covariates in both analyses were overall depression severity (total HRSD score), sample (1 vs. 2), medication status (yes vs. no), age and gender. As total HRSD score includes the HES items, we also ran analyses using only the nonmelancholic HRSD items as the covariate instead of total HRSD score.
In both analyses, diagnosis of an anxiety disorder and the study sample were each tested for moderating effects on the relationship between melancholic symptoms and frontal EEG asymmetry.
To examine the effect of melancholia on frontal asymmetry at rest, multiple regressions were conducted with frontal resting EEG asymmetry score as the dependent variable and HES symptoms as the main predictor, along with the same covariates from the main analyses.
Results
Demographics and clinical characteristics
Participants in the three groups (two MDD groups and non-MDD controls) did not significantly differ on age, F(2,253) = 1.15, p = .32, ηp2 = .01, gender, χ2 (2, N=254) = .88, p = .64, C = .06, or ethnicity, χ2 (2, N=254) = 4.12, p = .13, C = .13. As expected, the control and two MDD groups differed on comorbid anxiety, χ2 (2, N=254) = 18.70, p < .001, C = .26. MDD severity, F(2,253) = 398.53, p < .001, ηp2 = .76, melancholic symptoms, F(2,253) = 289.48, p < .001, ηp2 = .70, psychiatric medication, χ2 (2, N=254) = 39.71, p < .001, C = 0.37, and current functioning, F(2,253) = 212.90, p < .001, ηp2 = .63. Melancholic and non-melancholic MDD groups did not differ on age of onset2 of a depressive disorder, t(139) = .16, p = .87, d = .03, comorbid anxiety status, χ2 (1, N=141) = .71, p = .40, C = 0.05, psychiatric medication, χ2 (1, N=141) = .54, p = .47, C = .05, or current functioning t(139) = −1.58, p = .12, d = .30. But melancholic MDD did present higher overall MDD severity, t(139) = 2.54, p = .01, d = .48, and, as expected, higher melancholic symptoms, t(139) = 3.05, p = .003, d = .58, than non-melancholic MDD. The two study locations did not differ in EEG alpha asymmetry during reward (t = .51, p = .61, d = .06) but did differ in EEG alpha asymmetry at rest (t = −2.62, p = .01, d = .36).
HES validation
As mentioned above, given that the HES has not been used extensively, we conducted several analyses to validate the HES as a measure of melancholia. Individuals with a categorical diagnosis of melancholic depression scored higher on the HES than individuals with non-melancholic depression (t(139) = 3.05, p = .003, d = .58). These results remained after controlling for nonmelancholic symptoms from the HRSD (β = −.19, t(140) = −2.50, p = .01). HES was also significantly correlated with trait measures of positive emotionality (GTS-PE; r = −.61, p < .001), TEPS-Anticipatory (r = −.54, p < .001), TEPS-Consummatory (r = −.44, p < .001), and TEPS-Overall (r = −.53, p < .001). Importantly, the correlation between HES and trait positive emotionality remained significant independent of the effects of negative emotionality (β = −.44, t(253) = −6.67, p < .001) or nonmelancholic symptoms from the HRSD (β = −.40, t(246) = −4.27, p < .001). Similarly, independent of the effects of nonmelancholic symptoms from the HRSD, correlations remained significant between HES and TEPS-Anticipatory (β = −.46, t(147) = −3.51, p = .001), TEPS-Consummatory (β = −.31, t(147) = −2.18, p = .03), and TEPS-Overall (β = −.43, t(147) = −3.29, p = .001).
Categorical analyses
When melancholia was defined categorically, no significant effect of group was found on EEG asymmetry during the reward anticipation task, F(2,253) = .84, p = .43, ηp2 = .01 or during resting EEG F(2, 252) = .77, p = .47, ηp2 = .01. Effects of group on EEG asymmetry during reward anticipation were not moderated by study location, F(2,253) = .76, p = .47, ηp2 = .01, or diagnosis of an anxiety disorder, F(2,253) = .05, p = .96, ηp2 < .001.
Dimensional analyses
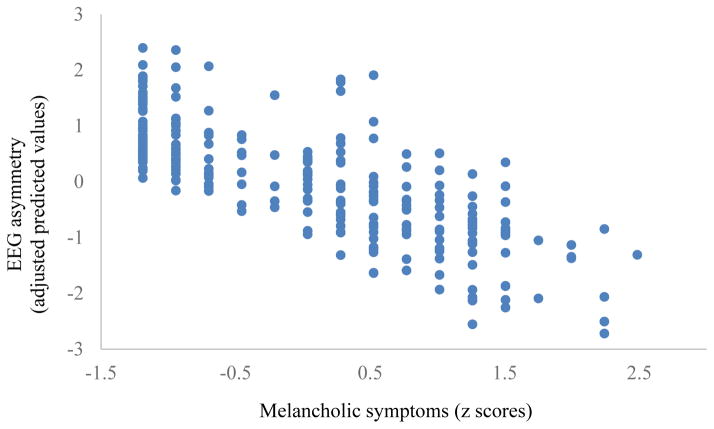
When melancholia was defined continuously, as hypothesized, melancholic symptoms predicted frontal EEG asymmetry during reward anticipation, β = −.20, t(253) = −3.01, p = .003. Overall depression severity also predicted frontal EEG asymmetry, β = −.15, t(253) = −2.19, p = .03. Importantly, when both melancholic symptoms and overall depression severity were entered in the same regression model, melancholic symptoms remained a significant predictor, β = −.38, t(253) = −2.42, p = .023 (see Figure 1) but depression severity dropped to non-significance, β = .21, t(253) = 1.29, p = .20 (see Table 2). Effects were not moderated by study location, β = .03, t(253) = .34, p = .73, or diagnosis of an anxiety disorder, β = .12, t(253) = 1.52, p = .13. Melancholic symptoms did not predict resting EEG asymmetry, β = .11, t(251) = .67, p = .51. Melancholic symptoms also did not predict self-reported ratings of reward anticipation, β = .22, t(152) = 1.06, p = .29.
Figure 1.
Association between melancholic symptoms and EEG asymmetry
Table 2.
Effect of melancholia on EEG asymmetry
| Variable | β | t | p-value |
|---|---|---|---|
| Block 1 | |||
|
| |||
| Age | 0.14* | 2.12 | 0.04 |
| Gender | 0.01 | 0.21 | 0.83 |
| Study location | −0.02 | −0.26 | 0.79 |
| Psychiatric medication | −0.05 | −0.68 | 0.50 |
| HRSD total | −0.15* | −2.19 | 0.03 |
|
| |||
| Block 2 | |||
|
| |||
| Age | 0.16* | 2.40 | 0.02 |
| Gender | <0.01 | 0.03 | 0.97 |
| Study location | −0.02 | −0.39 | 0.70 |
| Psychiatric medication | −0.06 | −0.86 | 0.39 |
| HRSD total | 0.21 | 1.29 | 0.20 |
| HES | −0.38* | −2.42 | 0.02 |
When the same analysis was conducted examining the effect of melancholic symptoms on frontal EEG asymmetry in the two depressed groups alone, (i.e., excluding depression-free controls), the effect remained significant: melancholic symptoms was associated with frontal EEG asymmetry during reward anticipation independent of depression severity, β = −.24, t(140) = −2.08, p = .04. In a separate analysis, we excluded depression-free individuals using psychiatric medication and found that melancholia continued to predict EEG asymmetry during reward anticipation, β = −.36, t(245) = −2.24, p = .03.
When non-melancholic items were used as the covariate instead of overall depression severity, nonmelancholic symptoms alone did not predict frontal EEG asymmetry, β = −.12, t(253) = −1.72, p = .09. Melancholic symptoms remained a significant predictor when both melancholic and nonmelancholic symptoms were entered in the same regression model, β = −.32, t(253) = −2.78, p = .006. Effects of melancholia remained when positive emotionality scores from the GTS-PT were included in the model, β = −.40, t(253) = −2.44, p = .02. The effect of melancholic symptom on frontal EEG asymmetry also remained significant when lifetime substance abuse or dependence was included as a covariate in the model, β = −.38, t(45) = −2.41, p = .02.
Lastly, we explored which of the HES melancholia items were associated with frontal EEG asymmetry during the task. Out of the eight individual items of the HES, middle insomnia (r = −.14, p = .03), late insomnia (r = −.13, p = .04), anhedonia (r = −.14, p = .03), and hopelessness (r = −.13, p = .04) each negatively predicted frontal EEG asymmetry. These small effects were no longer significant when these items were placed in the same model. In other words, when controlling for the two aspects of insomnia and hopelessness, the association between anhedonia and reduced reward anticipation diminished. Additionally, psychomotor disturbances (retardation and agitation), loss of weight, and diurnal variation did not have an effect on frontal EEG asymmetry during reward anticipation.
Discussion
Consistent with our hypothesis, results demonstrated that melancholic depression when defined dimensionally (but not categorically) predicted reduced left relative to right frontal EEG activation during a reward anticipation task. These findings remained, even when adjusting for covariates such as age, gender, study location, medication status, and comorbid anxiety disorders. Higher overall depression severity and non-melancholic depression severity were also associated with reduced left relative to right frontal EEG activity. However, the effect of melancholia was not due to these two dimensions as melancholia remained significant even when each of them were included as covariates (and, of note, overall depression severity became non-significant after controlling for melancholic symptoms). That is, rather than being a general feature of all forms of MDD, reward dysfunction, in particular reward anticipation deficits, may be specifically related to the melancholia.
At its face, showing that a subtype typically characterized by pleasure deficits does indeed predict reduced reward anticipation may seem intuitive. However, pleasure deficits (i.e., anhedonia) are multidimensional (Shankman et al., 2014) and few studies of melancholia have specifically focused on the dimension of reward anticipation. Several other studies have examined the association between melancholia and deficits in other positive valence domains (Leventhal & Rehm, 2005). Day et al. (2015) used a facial recognition task and found that melancholic MDD was characterized by a loss of sensitivity to positive facial expressions compared to non-melancholic MDD and healthy controls, even when controlling for MDD severity. Notably, these differences were specific to impairments in processing positive emotion and not problems in general emotion processing. Fletcher et al. (2015) used a probabilistic reward learning task to demonstrate that individuals with melancholic MDD had reduced response bias, suggesting blunted reward learning. Melancholia is also distinctive in its impairments in motivation and effort related to goal-pursuit. Rogers, Bellgrove, Chiu, Mileshkin, and Bradshaw (2004) found that melancholic and non-melancholic groups performed similarly on cognitive tasks such as the stroop task but the performance of those with melancholia suffered when faced with increased cognitive load of additional tasks. These findings suggest that the psychological profile of melancholic MDD is characterized by deficits in specific positive valence behavioral constructs, such as reward learning and motivation. The current study adds to this literature and further identifies anticipatory deficits in reward processing as a feature of the melancholic subtype.
On the other hand, the present results may suggest that other features of melancholia (besides anhedonia) are predictive of reward anticipation deficits. Melancholia’s effect on reward anticipation was not solely due to the HES symptom of anhedonia as anhedonia only had a small effect size and several other melancholic symptoms had comparable effects (middle insomnia, late insomnia, hopelessness). Similarly, the effect of melancholia on EEG asymmetry held independent of the effect of trait positive emotionality. However, the trait positive emotionality and HES anhedonia items are broad measures of anhedonia (e.g., HES anhedonia collapses multiple components and correlates of anhedonia into one item - interest, motivation, time spent doing activities, etc.). Thus, it is possible that specific features of anhedonia may be driving the association between melancholia and EEG asymmetry during reward anticipation.
In addition to the behavioral correlates of melancholia, melancholia is also characterized by unique biological variables compared to other forms of MDD. Melancholia has long been viewed as a type of MDD with a more biological (rather than environmental) etiology (Klein, 1974; Taylor & Fink, 2006). Bracht et al. (2014) recently found that relative to controls and those with nonmelancholic MDD, melancholic MDD was associated with reduced white matter integrity in the medial forebrain bundle, a key white matter track for reward processing and dopamine transmission. Relatedly, dopamine plays an important role in the pursuit of rewards (Treadway & Zald, 2011) and dopaminergic abnormalities appear to be particularly prevalent in those with melancholic depression (Parker, 2007; Parker et al., 1995; Weinberg, Liu, & Shankman, 2016). Interestingly, asymmetry of D2 receptor availability within the striatum was found to be associated with motivation and distinction between reward and punishment such that greater motivation was associated with higher dopamine receptor availability in the left relative to right striatum (Tomer et al, 2008; 2014). Taken together, along with the role of dopamine in reward pursuit, it is possible that asymmetry and abnormalities in the dopamine system may contribute to EEG frontal asymmetry during reward anticipation. The present frontal EEG asymmetry study supports these studies on the neurobiological correlates of melancholia and, more broadly, supports other neuroimaging studies showing PFC abnormalities in melancholia (Korgaonkar et al., 2011; Pizzagalli et al., 2004).
Given that the majority of our sample included individuals with early onset depression (before age 18), reward anticipation deficits may be particularly important as a risk factor for early onset melancholic depression. Early onset depression has a more severe and chronic course and is associated with greater comorbidity (Klein, Shankman, & McFarland, 2006). Additionally, depression that begins in childhood or adolescence may be associated with reward circuitry dysfunction (Forbes & Dahl, 2012) and, in particular, regions involved in reward anticipation (Forbes et al., 2009; Davey et al., 2008). Our findings further extend this line of research and suggest that reduced left relative to right frontal brain activity during reward anticipation may also be important to examine in adolescence as an important target of prevention efforts for melancholic depression.
The current study did not find a significant relationship between melancholia (whether defined categorically or dimensionally) and resting EEG asymmetry. This finding is not wholly unexpected given the prior findings from the limited literature on melancholia and resting EEG. In one resting EEG study, Quinn, Rennie, Harris, and Kemp (2014) found that melancholic MDD and controls did not differ whereas the non-melancholic MDD group exhibited greater relative left frontal activation. This finding was contrary to their hypothesis and somewhat unexpected. These puzzling results could be due to the limitations of the resting EEG design as it is a less powerful indicator of individual differences of “affective style” compared to EEG measured during an affective task (Coan et al., 2006). The capability model of frontal EEG asymmetry further states that brain activation elicited during an emotional task may be a stronger indicator of individual differences related to that emotion than activity recorded at rest, as it tests individuals’ capacity to engage neural systems relevant to the emotional tendency (Coan et al., 2006). Thus, it is possible that the reward task was a stronger measure of individual differences than EEG asymmetry measured at rest.
Extending the work of Quinn et al. (2014), the current study examined EEG activation during a reward task rather than at rest and thus may better capture group differences as it represents a ‘probe’ of the reward system. A prior EEG study from our group using a subsample of the current report found that those with melancholic depression exhibited a different EEG asymmetry than those with nonmelancholic depression and controls (although only at a trend level) in posterior electrodes (Shankman et al., 2011). These effects were not found in the present study with a larger sample, although this may have been due to the fact that the prior report had a small number of individuals with melancholic MDD (N=17).
The present study found significant effects when melancholia was defined dimensionally but not when it was defined categorically. One reason for this finding may be that the current categorical criteria for melancholic depression do not adequately capture the melancholia subtype (Parker et al., 2010). Foti, Carlson, Sauduer, & Proudfit (2014) examined the effect of MDD heterogeneity on monetary reward sensitivity and found blunted reward sensitivity only in the MDD subgroup characterized by the single symptom of impaired mood reactivity but also failed to find significant effects using the full DSM defined melancholic subtype. Along with Foti et al. (2014), the current study further supports that a continuous dimension might better characterize melancholia than the DSM defined category (Parker et al., 2010).
There were several notable strengths of the paper. Specifically, the present study included a heterogeneous population with comorbid anxiety and a wide age range. Allowing comorbid anxiety in the sample provided better external validity than excluding comorbid anxiety, given the high co-occurrence of the two types of internalizing conditions (Shankman & Klein, 2003). Furthermore, this approach allowed us to test whether comorbid anxiety moderated the effects, an approach that is better than including anxiety as a covariate (Miller & Chapman, 2001). The present study also utilized a large sample (for psychophysiological studies) and examined both categorical and dimensional measures of melancholia. However, there were also several limitations. First, many in the current sample used varied psychiatric medications. Although the analyses adjusted for the effect of medication use, it was not possible to examine specific types of medication given the heterogeneous regimens of the sample and small group sizes of each class of medication. Second, although we previously documented excellent inter-rater reliability for a diagnosis of MDD (Shankman et al., 2013), reliability for the melancholia diagnosis was not obtained. Also, we were unable to use the same instrument to measure both categorical and dimensional aspects of melancholia (as we used the SCID and HRSD, respectively). Third, although the effects of melancholia were moderate (β = −.38) it only accounted for partial variance in EEG alpha asymmetry. Thus, there are other factors that likely account for EEG frontal asymmetry during reward anticipation. Lastly, we examined the effect of similar constructs to melancholia on frontal EEG asymmetry, such as anticipatory and consummatory affect (TEPS) and positive and negative emotionality (GTS) and found no significant associations. However, this could be due to the trait nature of the TEPS and GTS whereas HES measured state-like symptoms. Examination of the role of state positive affect variables may have yielded different results.
In sum, the present results support the premise that melancholia is characterized by deficits in reward anticipation and that this association is not simply due to the fact that melancholia is a more severe form of MDD. The current study expands previous literature on reward dysfunction in melancholia by examining the role of melancholic symptoms in reward anticipation deficits. Future studies could explore the association between melancholia and other facets of reward processing, such as reward learning (Fletcher et al., 2015) or willingness to work for rewards to determine which specific positive valence domains are abnormal in melancholia.
GENERAL SCIENTIFIC SUMMARY.
The results of this study suggest that reward anticipation deficits may be particularly related to melancholic features of depression. This effect appears to be not just due to depression severity, but reflect melancholic symptoms specifically.
Acknowledgments
This work was supported by National Institute of Mental Health under Grants R01MH098093, R21 MH080689 and F31MH67309 awarded to Dr. Stewart Shankman.
Footnotes
There were also loss trials included (12 in sample 1, 18 in sample 2) in which participants lost money if the reels landed on three fruits. Loss trials were included because pilot testing suggested that the slot task was less engaging if there were only NI and R trials, and interspersing loss trials during the game made the R trials feel “more exciting.”
In our previous report (Shankman et al., 2007), an earlier age of onset was associated with EEG asymmetry. Of note, in the present study, melancholia was associated with EEG asymmetry independent of age of onset, although this may have been due to the fact that the inclusion criteria for study 2 was an onset before 18, hence restricting the onset range.
Effects of melancholia on frontal EEG asymmetry were also examined in the loss trials and were not significant, β = −.09, t(253) =−.53, p = .59. Although there were significantly fewer loss trials compared to the other conditions, this result suggests that the effects of melancholia may be specific to reward anticipation (rather than a general effect of anticipation or uncertainty).
References
- Allen JJB, Coan JA, Nazarian M. Issues and assumptions on the road from raw signal to metrics of frontal EEG asymmetry in emotion. Biological Psychology. 2004;67:183– 218. doi: 10.1016/j.biopsycho.2004.03.007. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5. Arlington, VA: American Psychiatric Association; 2013. [Google Scholar]
- Bracht T, Horn H, Strik W, Federspiel A, Schnell S, … Walther S. White matter microstructure alteractions of the medial forebrain bundle in melancholic depression. Journal of Affective Disorders. 2014;155:186–193. doi: 10.1016/j.jad.2013.10.048. [DOI] [PubMed] [Google Scholar]
- Bress JN, Foti D, Kotov R, Klein DN, Hajcak G. Blunted neural response to rewards prospectively predicts depression in adolescent girls. Psychophysiology. 2013;50:74–81. doi: 10.1111/j.1469-8986.2012.01485.x. [DOI] [PubMed] [Google Scholar]
- Bruder GE, Fong R, Tenke CE, Leite P, Towey JP, Stewart JE, … Quitkin FM. Regional brain asymmetries in major depression with or without an anxiety disorder: A quantitative electroencephalographic study. Biological Psychiatry. 1997;41:939– 948. doi: 10.1016/S0006-3223(96)00260-0. [DOI] [PubMed] [Google Scholar]
- Clark DA, Beck AT, Beck JS. Symptom differences in major depression, dysthymia, panic disorder, and generalized anxiety disorder. American Journal of Psychiatry. 1994;151:205–209. doi: 10.1176/ajp.151.2.205. [DOI] [PubMed] [Google Scholar]
- Clark LA, Watson D. The General Temperament Survey. Southern Methodist University; Dallas, TX: 1990. Unpublished manuscript. [Google Scholar]
- Clark LA, Watson D. Tripartite model of anxiety and depression: Psychometric evidence and taxonomic implications. Journal of Abnormal Psychology. 1991;100(3):316–336. doi: 10.1037//0021-843x.100.3.316. [DOI] [PubMed] [Google Scholar]
- Coan JA, Allen JJB. Frontal EEG asymmetry and the behavioral activation and inhibition systems. Psychophysiology. 2003;40(1):106–114. doi: 10.1111/1469-8986.00011. [DOI] [PubMed] [Google Scholar]
- Coan JA, Allen JJB, McKnight PE. A capability model of individual differences in frontal EEG asymmetry. Biological Psychology. 2006;72:198–207. doi: 10.1016/j.biopsycho.2005.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey CG, Yucel M, Allen NB. The emergence of depression in adolescenece: Development of the prefrontal cortex and the representation of reward. Neuroscience & Biobehavioral Reviews. 2008;32(1):1–19. doi: 10.1016/j.neubiorev.2007.04.016. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Anterior cerebral asymmetry and the nature of emotion. Brain and Cognition. 1992;20:125–151. doi: 10.1016/0278-2626(92)90065-t. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Affective style and affective disorders: Perspectives from affective neuroscience. Cognition and Emotion. 1998;12:307–330. [Google Scholar]
- Day CV, Gatt JM, Etkin A, DeBattista C, Schatzberg AF, Williams LM. Cognitive and emotional biomarkers of melancholic depression: An iSPOT-D report. Journal of Affective Disorders. 2015;176:141–150. doi: 10.1016/j.jad.2015.01.061. [DOI] [PubMed] [Google Scholar]
- Eshel N, Roiser JP. Reward and punishment processing in depression. Biological Psychiatry. 2010;68:118–124. doi: 10.1016/j.biopsych.2010.01.027. [DOI] [PubMed] [Google Scholar]
- First MG, Spitzer RL, Gibbon M, Williams JB. Structured clinical interview for DSM-IV Axis I disorders, Clinical version (SCID-CV) Washington, D. C: American Psychiatric Press; 1996. [Google Scholar]
- Fletcher K, Parker G, Paterson A, Fava M, Iosifescu D, Pizzagalli DA. Anhedonia in melancholic and non-melancholic depressive disorders. Journal of Affective Disorders. 2015;184:81–88. doi: 10.1016/j.jad.2015.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Dahl RE. Research review: Altered reward function in adolescent depression: what, when and how? Journal of Child Psychology and Psychiatry. 2012;53(1):3–15. doi: 10.1111/j.1469-7610.2011.02477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Hariri AR, Martin SL, Silk JS, Moyles DL, Fisher PM, … Dahl RE. Altered striatal activation predicting real-world positive affect in adolescent major depressive disorder. The American Journal of Psychiatry. 2009;166:64–73. doi: 10.1176/appi.ajp.2008.07081336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foti D, Carlson JM, Sauder CL, Proudfit GH. Reward dysfunction in major depression: Multimodal neuroimaging evidence for refining the melancholic phenotype. NeuroImage. 2014;101:50–58. doi: 10.1016/j.neuroimage.2014.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foti D, Hajcak G. Depression and reduced sensitivity to non-rewards versus rewards: Evidence from event-related potentials. Biological Psychology. 2009;81:1–8. doi: 10.1016/j.biopsycho.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Gard DE, Gard MG, Kring AM, John OP. Anticipatory and consummatory components of the experience of pleasure: A scale development study. Journal of Research on Personality. 2006;40:1086–1102. [Google Scholar]
- Gotlib IH, Ranganath C, Rosenfeld JP. Frontal EEG alpha asymmetry, depression and cognitive functioning. Cognition and Emotion. 1998;12:449–478. [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery & Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon-Jones E, Allen JJB. Behavioral activation sensitivity and resting frontal EEG asymmetry: Covariation of putative indicators related to risk for mood disorders. Journal of Abnormal Psychology. 1997;106(1):159–163. doi: 10.1037//0021-843x.106.1.159. [DOI] [PubMed] [Google Scholar]
- Henriques JB, Davidson RJ. Decreased responsiveness to reward in depression. Cognition and Emotion. 2000;14(5):711–724. [Google Scholar]
- Joyce PR, Mulder RT, Luty SE, McKenzie JM, Sullivan PF, Abbott RM, Stevens IF. Melancholia: Definitions, risk factors, personality, neuroendocrine markers and differential antidepressant response. Australian and New Zealand Journal of Psychiatry. 2002;36:376–383. doi: 10.1046/j.1440-1614.2001.01025.x. [DOI] [PubMed] [Google Scholar]
- Kendler K. The Diagnostic Validity of Melancholic Major Depression in a Population- Based Sample of Female Twins. Archives of General Psychiatry. 1997;54:299–304. doi: 10.1001/archpsyc.1997.01830160013002. [DOI] [PubMed] [Google Scholar]
- Klein DF. Endogenomorphic depression: A conceptual and terminological revision. Archives of General Psychiatry. 1974;31:447–454. doi: 10.1001/archpsyc.1974.01760160005001. [DOI] [PubMed] [Google Scholar]
- Klein DN, Shankman SA, McFarland B. Classification of mood disorders. In: Stein DJ, Kupfer DJ, Schatzberg AF, editors. The American Psychiatric Publishing Textbook of Mood Disorders. Washington, DC: American Psychiatric Publishing, Inc; 2006. pp. 17–32. [Google Scholar]
- Korgaonkar MS, Grieve SM, Koslow SH, Gabrieli JD, Gordon E, Williams LM. Loss of white matter integrity in major depressive disorder: evidence using a tract-based spatial statistical analysis of diffusion tensor imaging. Human Brain Mapping. 2011;32:2161–2171. doi: 10.1002/hbm.21178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa A, Proudfit GH, Klein DN. Neural reactivity to rewards and losses in offspring of mothers and fathers with histories of depressive and anxiety disorders. Journal of Abnormal Psychology. 2014;123(2):287–297. doi: 10.1037/a0036285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, Rehm LP. The empirical status of melancholia: Implications for psychology. Clinical Psychology Review. 2005;25:25–44. doi: 10.1016/j.cpr.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Maier W, Philipp M. A polydiagnostic scale for dimensional classification of endogenous depression derivation and validation. Acta Psychiatrica Scandinavica. 1986;74(2):152–160. doi: 10.1111/j.1600-0447.1986.tb10599.x. [DOI] [PubMed] [Google Scholar]
- Miller GA, Chapman JP. Misunderstanding analysis of covariance. Journal of Abnormal Psychology. 2001;110(1):40–48. doi: 10.1037//0021-843x.110.1.40. [DOI] [PubMed] [Google Scholar]
- Morgan JK, Olino TM, McMakin DL, Ryan ND, Forbes EE. Neural response to reward as a predictor of increases in depressive symptoms in adolescence. Neurobiology of Disease. 2013;52:66–74. doi: 10.1016/j.nbd.2012.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olino TM, McMakin DL, Morgan JK, Silk JS, Birmaher B, … Forbes EE. Reduced reward anticipation in youth at high risk for unipolar depression: A preliminary study. Developmental Cognitive Neuroscience. 2014;8:55–64. doi: 10.1016/j.dcn.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker G. Defining melancholia: the primacy of psychomotor disturbance. Acta Psychiatrica Scandinavica. 2007;115:21–30. doi: 10.1111/j.1600-0447.2007.00959.x. [DOI] [PubMed] [Google Scholar]
- Parker G, Fletcher K, Hyett M, Hadzi-Pavlovic D, Barrett M, Synnott H. Measuring melancholia: the utility of a prototypic symptom approach. Psychological Medicine. 2009;39:989–998. doi: 10.1017/S0033291708004339. [DOI] [PubMed] [Google Scholar]
- Parker G, Fletcher K, Barrett M, Synnott H, Breakspear M, … Hadzi-Pavlovic D. Inching toward Bethlehem: Mapping melancholia. Journal of Affective Disorders. 2010;123:291–298. doi: 10.1016/j.jad.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Parker G, Hadzi-Pavlovic D, Austin M, Mitchell P, Wilhelm K, Hickie I, … Eyers K. Sub-typing depression: I. is psychomotor disturbance necessary and sufficient to the definition of melancholia? Psychological Medicine. 1995;25(4):815–823. doi: 10.1017/s0033291700035066. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA, Nitschke JB, Oakes TR, Hendrick AM, Horras KA, Larson CL, … Davidson RJ. Brain electrical tomography in depression: The importance of symptom severity, anxiety, and melancholic features. Biological Psychiatry. 2002;52:73–85. doi: 10.1016/s0006-3223(02)01313-6. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA, Oakes TR, Fox AS, Chung MK, Larson CL, … Davidson RJ. Functional but not structural subgenual prefrontal cortex abnormalities in melancholia. Molecular Psychiatry. 2004;9:393–405. doi: 10.1038/sj.mp.4001501. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA, Sherwood RJ, Henriques JB, Davidson RJ. Frontal brain asymmetry and reward responsiveness. Psychological Science. 2005;16(10):805–813. doi: 10.1111/j.1467-9280.2005.01618.x. [DOI] [PubMed] [Google Scholar]
- Quinn CR, Rennie CJ, Harris AW, Kemp AH. The impact of melancholia versus non-melancholia on resting-state, EEG alpha asymmetry: electrophysiological evidence for depression heterogeneity. Psychiatry Research. 2014;215:614–617. doi: 10.1016/j.psychres.2013.12.049. [DOI] [PubMed] [Google Scholar]
- Reid SA, Duke LM, Allen JJB. Resting frontal electroencephalographic asymmetry in depression: Inconsistencies suggest the need to identify mediating factors. Psychophysiology. 1998;1998:389–404. [PubMed] [Google Scholar]
- Rogers MA, Bellgrove MA, Chiu E, Mileshkin C, Bradshaw JL. Response selection deficits in melancholic but not nonmelancholic unipolar major depression. Journal of Clinical and Experimental Neuropsychology. 2004;26(2):169–179. doi: 10.1076/jcen.26.2.169.28086. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Weisenburger JE. Melancholic symptom features and DSM-IV. American Journal of Psychiatry. 1994;151:489–498. doi: 10.1176/ajp.151.4.489. [DOI] [PubMed] [Google Scholar]
- Schaffer CE, Davidson RJ, Saron C. Frontal and parietal electroencephalogram asymmetry in depressed and nondepressed subjects. Biological Psychiatry. 1983;18(7):753– 762. [PubMed] [Google Scholar]
- Shankman SA, Katz AC, DeLizza AA, Sarapas C, Gorka SM, Campbell ML. The different facets of anhedonia and their associations with different psychopathologies. In: Ritsner MS, editor. Anhedonia: A Comprehensive Handbook Volume I: Conceptual Issues And Neurobiological Advances. Dordrecht: Springer; 2014. pp. 3–22. [Google Scholar]
- Shankman SA, Klein DN. The relation between depression and anxiety: An evaluation of the tripartite, approach-withdrawal and valence-arousal models. Clinical Psychology Review. 2003;23(4):605–637. doi: 10.1016/s0272-7358(03)00038-2. [DOI] [PubMed] [Google Scholar]
- Shankman SA, Klein DN, Tenke CE, Bruder GE. Reward sensitivity in depression: A biobehavioral study. Journal of Abnormal Psychology. 2007;116:95–104. doi: 10.1037/0021-843X.116.1.95. [DOI] [PubMed] [Google Scholar]
- Shankman SA, Sarapas C, Klein DN. The effect of pre- vs. post-reward attainment on EEG asymmetry in melancholic depression. International Journal of Psychophysiology. 2011;79:287–295. doi: 10.1016/j.ijpsycho.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankman SA, Nelson BD, Sarapas C, Robison-Andrew EJ, Campbell ML, Altman SE, … Gorka SM. A psychophysiological investigation of threat and reward sensitivity in individuals with panic disorder and/or major depressive disorder. Journal of Abnormal Psychology. 2013;112:322–338. doi: 10.1037/a0030747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp C, Kim S, Herman L, Pane H, Reuter T, Strathearn L. Major depression in mothers predicts reduced ventral striatum activation in adolescent female offspring with and without depression. Journal of Abnormal Psychology. 2014;123(2):298–309. doi: 10.1037/a0036191. [DOI] [PubMed] [Google Scholar]
- Stewart JL, Bismark AW, Towers DN, Coan JA, Allen JJB. Resting frontal EEG asymmetry as an endophenotype for depression risk: Sex-specific patterns of frontal brain asymmetry. Journal of Abnormal Psychology. 2010;119(3):502–512. doi: 10.1037/a0019196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton SK, Davidson RJ. Prefrontal brain asymmetry: A biological substrate of the behavioral approach and inhibition systems. Psychological Science. 1997;8(3):204–210. [Google Scholar]
- Taylor MA, Fink M. Restoring melancholia in the classification of mood disorders. Journal of Affective Disorders. 2008;105:1–14. doi: 10.1016/j.jad.2007.05.023. [DOI] [PubMed] [Google Scholar]
- Tenke CE, Kayser J. Reference-free quantification of EEG spectra: combining current source density (CSD) and frequency principal components analysis (fPCA) Clinical Neurophysiology. 2005;116(12):2826–2846. doi: 10.1016/j.clinph.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Thase ME, Hersen M, Bellack AS, Himmelhoch JM, Kupfer DJ. Validation of a Hamilton subscale for endogenomorphic depression. Journal of Affective Disorder. 1983;5:267–278. doi: 10.1016/0165-0327(83)90050-2. [DOI] [PubMed] [Google Scholar]
- Thibodeau R, Jorgensen RS, Kim S. Depression, anxiety, and resting frontal EEG asymmetry: A meta-analytic review. Journal of Abnormal Psychology. 2006;115(4):715–729. doi: 10.1037/0021-843X.115.4.715. [DOI] [PubMed] [Google Scholar]
- Tomarken AJ, Dichter GS, Garber J, Simien C. Resting frontal brain activity: Linkages to maternal depression and socio-economic status among adolescents. Biological Psychology. 2004;67(1–2):77–102. doi: 10.1016/j.biopsycho.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Tomer R, Goldstein RZ, Wang GJ, Wong C, Volkow ND. Incentive motivation is associated with striatal dopamine asymmetry. Biological Psychology. 2008;77:98–101. doi: 10.1016/j.biopsycho.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomer R, Slagter HA, Christian BT, Fox AS, King CR, … Davidson RJ. Love to win or hate to lose? Asymmetry of dopamine D2 receptor binding predicts sensitivity to reward versus punishment. Journal of Cognitive Neuroscience. 2014;26(5):1039–1048. doi: 10.1162/jocn_a_00544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trajković G, Starčević V, Latas M, Leštarević M, Ille T, … Marinković J. Reliability of the Hamilton Rating Scale for Depression: A meta-analysis over a period of 49 years. Psychiatry Research. 2011;189:1–9. doi: 10.1016/j.psychres.2010.12.007. [DOI] [PubMed] [Google Scholar]
- Treadway MT, Zald DH. Reconsidering anhedonia in depression: Lessons from translational neuroscience. Neuroscience and Beiobehavioral Reviews. 2011;35:537–555. doi: 10.1016/j.neubiorev.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg A, Liu H, Shankman SA. Blunted neural response to errors as a trait marker of melancholic depression. Biological Psychology. 2016;113:100–107. doi: 10.1016/j.biopsycho.2015.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman M, Coryell W, Pfohl B, Stangl D. Validity of the Hamilton Endogenous Subscale: An independent replication. Psychiatry Research. 1986;18:209–215. doi: 10.1016/0165-1781(86)90108-3. [DOI] [PubMed] [Google Scholar]