Abstract
Since 3/26/2012, the Kidney Donor Profile Index (KDPI) has been provided with all deceased-donor kidney offers, with the goal of improving the ECD indicator. Although an improved risk index may facilitate identification and transplantation of marginal yet viable kidneys, a granular percentile system may reduce provider-patient communication flexibility, paradoxically leading to more discards (“labeling effect”). We studied the discard rates of the kidneys recovered for transplantation between 3/26/2010-3/25/2012 (“ECD era”, N=28,636) and 3/26/2012-3/25/2014 (“KDPI era”, N=29,021) using SRTR data. There was no significant change in discard rate from ECD era (18.1%) to KDPI era (18.3%) among the entire population (aOR=0.971.041.10, p=0.3), or in any KDPI stratum. However, among kidneys in which ECD and KDPI indicators were discordant, “high risk” SCD kidneys (with KDPI>85) were at increased risk of discard in the KDPI era (aOR=1.071.421.89, p=0.02). Yet, recipients of these kidneys were at much lower risk of death (aRR=0.560.770.94 at 2 years post-transplant) compared to those remaining on dialysis waiting for low-KDPI kidneys. Our findings suggest that there might be an unexpected, harmful labeling effect of reporting a high KDPI for SCD kidneys, without the expected advantage of providing a more granular risk index.
INTRODUCTION
Kidney Donor Profile Index (KDPI) (1,2) is a percentile measure designed to characterize donor-associated risks of deceased donor kidneys. Compared with the previous dichotomous classification of standard criteria donor (SCD) versus expanded criteria donor (ECD) (3), KDPI is a continuous scale based on more donor characteristics (10 for KDPI vs 4 for ECD). However, despite being an improved prediction tool from ECD, KDPI still has only moderate predictive power (c=0.60) and lacks the ability to differentiate kidneys of similar KDPI values with high confidence (1,2,4,5). Additionally, KDPI is solely based on pre-retrieval factors and calculated by comparing the kidney being considered to kidneys transplanted in the previous calendar year.
On March 26, 2012, the Organ Procurement and Transplantation Network (OPTN) began providing KDPI with all deceased-donor kidney offers. On one hand, the implementation of a superior prediction tool could have been expected to increase the utilization of marginal yet viable kidneys that would have been unnecessarily discarded under the crude SCD/ECD dichotomy. A good example might be healthy donors aged over 60; while they are universally labeled as ECD, the particularly high quality grafts from such donors (6) would be better characterized by KDPI, increasing the utilization of these kidneys.
In contrast, concerns have also been raised that providing KDPI may lead to unnecessary discard of some high-KDPI kidneys (7,8), possibly through a “labeling effect.” Compared to the binary nature of SCD versus ECD, the granularity of KDPI may imply high precision and allow minimal flexibility in interpretation and communication. For example, an ECD kidney with a KDPI of 90 would have to be, by definition, described to a patient as “worse than 90% of offered kidneys” in the KDPI era, while it could have been reasonably described as a “good enough to provide you survival benefit although not the best” kidney in the ECD era. In other words, there is less flexibility in provider-patient communication when a number is assigned to the kidney, regardless of the actual ability of that number to predict outcomes.
These two competing scenarios suggest that providing KDPI could feasibly have increased or decreased the discard of deceased donor kidneys. Furthermore, the impact of providing KDPI might have been more pronounced on the subset of kidneys where the KDPI score was discordant from SCD/ECD; for example, high-KDPI SCD kidneys or low-KDPI ECD kidneys. The goals of this study were to better understand the impact of providing KDPI by characterizing the changes in discard rates after the KDPI implementation among 1) all kidneys recovered for transplantation, 2) kidneys in different KDPI strata; and 3) discordant kidneys by ECD and KDPI indexes.
METHODS
Study design and data collection
We compared deceased-donor kidneys recovered for transplantation in the United States between March 26, 2010 – March 25, 2012 (which we termed the “ECD era”) with those recovered between March 26, 2012 – March 25, 2014 (the “KDPI era”). These dates were chosen so that the 2-year period preceding the implementation of KDPI would be comparable to the same period of time following the implementation. The unit of analysis was the transplantable kidney; in other words, the two kidneys recovered from each donor were analyzed separately, except for those recovered for en-bloc transplantation which were treated as a single entity.
This study used data from the Scientific Registry of Transplant Recipients (SRTR). The SRTR data system includes data on all donors, waitlisted candidates, and transplant recipients in the US, submitted by the members of the Organ Procurement and Transplantation Network (OPTN). The Health Resources and Services Administration (HRSA), US Department of Health and Human Services, provides oversight to the activities of the OPTN and SRTR contractors.
Kidney Donor Profile Index
KDPI was calculated from donor variables including age, race, diabetes, hypertension, serum creatinine, height, weight, hepatitis C seropositivity, and cause of death, using the method described by the OPTN (2,9).
Comparison of ECD versus KDPI as a predictor of discard
To investigate whether ECD and KDPI were independently associated with discard (when holding the other variables constant), we used a bivariate generalized estimating equation (GEE) (10). In other words, this analysis (where discard was the outcome and ECD and KDPI were exposures) allowed us to compare the discard rates between SCD and ECD kidneys of the same KDPI value as well as those between lower- and higher-KDPI kidneys of the same ECD status. This analysis was performed separately on data from the ECD and KDPI eras. We also assessed the predictive accuracy of ECD and KDPI by comparing the area under the receiver operating characteristic curve (AUC) among all KDPI-era kidneys. We tested the statistical significance of the difference in AUC using the methods described by DeLong et al (11).
Changes in discard rates
We compared the odds of discard between ECD and KDPI eras using a GEE to account for clustering. Odds ratios were adjusted for donor variables including age, sex, race, diabetes, hypertension, ECD status, donation after cardiac death (DCD), serum creatinine, cause of death, and blood type. Age and serum creatinine were treated as continuous variables with empirically derived splines at 25 years for age and at 0.9 and 1.3 mg/dl for serum creatinine. All other covariates were treated as categorical variables. Observations with missing information about hypertension or diabetes were treated with missingness indicators. Those with missing creatinine or ABO were excluded from the analyses (N=61; 0.1%).
KDPI strata
To investigate whether providing KDPI differentially affected specific subgroups of the population, we stratified the population by KDPI quintiles (0-20, 21-40, 41-60, 61-80 and 81-100) and top and bottom 5 percentiles (0-5 and 96-100), and compared the discard rates between eras among kidneys within each stratum.
ECD-KDPI discordant subgroups
Discordance was defined as ECD kidneys with KDPI≤85 (which we termed “ECD/low-KDPI”) or SCD kidneys with KDPI>85 (termed “SCD/high-KDPI”). The threshold of 85 was adopted from the new Kidney Allocation System that replaced the ECD label with KDPI>85 (12–14); in other words, in the new allocation system, KDPI>85 kidneys are allocated according to a separate set of rules, akin to the previous allocation system where ECD kidneys were allocated according to a separate set of rules.
Survival benefit
We investigated the survival benefit of kidney transplantation within the subgroups in which differences in discard rates between the two eras were observed. Survival benefit was evaluated by comparing the cumulative incidence of death among transplant recipients to that among those remained on dialysis awaiting kidneys with lower KDPI (thereby receiving lower-KDPI kidneys after the national average waiting time). We treated transplantation as a time-varying exposure which moves the patients from the waitlist risk set to the transplant risk set, as previously described (15).
Statistical analysis
Population characteristics were compared between eras using a t-test for a continuous variable and chi-square tests for categorical variables. 95% confidence intervals were reported as per the method of Louis and Zeger (16). All analyses were performed using Stata 14.0 for Linux (StataCorp LP, College Station, Texas).
RESULTS
Study population
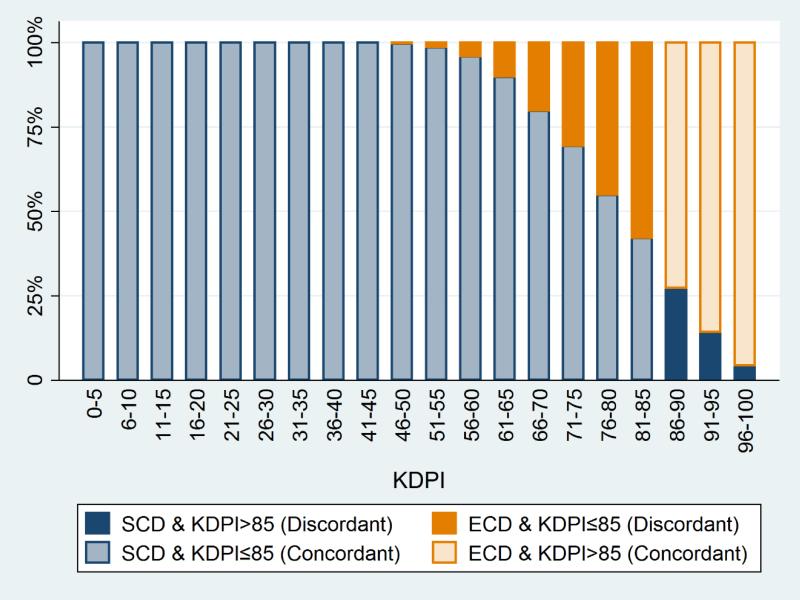
A total of 28,636 kidneys were recovered during the 2-year ECD era and 29,021 during the 2-year KDPI era (Table 1). Figure 1 illustrates the distribution of ECD status by KDPI. Across both eras, there were 11,957 (20.7%) ECD kidneys, which included 4,811 (8.3%) discordant ECD/low-KDPI kidneys. SCD kidneys included 1,270 (2.2%) discordant SCD/high-KDPI kidneys. In the KDPI era, there were slightly fewer kidneys from ECD donors (20.1% vs. 21.4%), more from DCD donors (15.4% vs. 13.7%), more kidneys from donors who died from anoxia (30.2% vs. 26.1%), and fewer from donors who died from cerebrovascular diseases (32.7% vs. 35.8%) or head trauma (34.2% vs. 35.2%; p<0.001 for all comparisons). Other variables, such as donor age, sex, hypertension, and diabetes, were not significantly different between eras.
Table 1.
Deceased donor kidney offers between 2010-2014, before and after the introduction of KDPI. P-values were calculated using a t-test for age and chi-square tests for all other variables.
| ECD era (2010-2012) (n=28,636) | KDPI era (2012-2014) (n=29,021) | p-value | |
|---|---|---|---|
| Age (y, median (IQR)) | 42 (25-53) | 41 (25-53) | 0.4 |
| Female sex | 40.7% | 40.3% | 0.3 |
| ECD | 21.4% | 20.1% | <0.001 |
| DCD | 13.7% | 15.4% | <0.001 |
| Hypertension | 32.2% | 31.7% | 0.1 |
| Diabetes | 9.8% | 10.0% | 0.7 |
| Cause of Death | |||
| Anoxia | 26.1% | 30.2% | <0.001 |
| Cerebrovascular | 35.8% | 32.7% | |
| Head trauma | 35.2% | 34.2% | |
| Others | 2.8% | 2.9% |
DCD, donation after cardiac death; ECD, expanded criteria donor; KDPI, Kidney Donor Profile Index.
Figure 1. Proportion of ECD kidneys by KDPI strata.
ECD kidneys with KDPI≤85 and SCD kidneys with KDPI>85 were defined as discordant.
ECD, expanded criteria donor; KDPI, Kidney Donor Profile Index; SCD, standard criteria donor.
Associations of ECD versus KDPI with discard
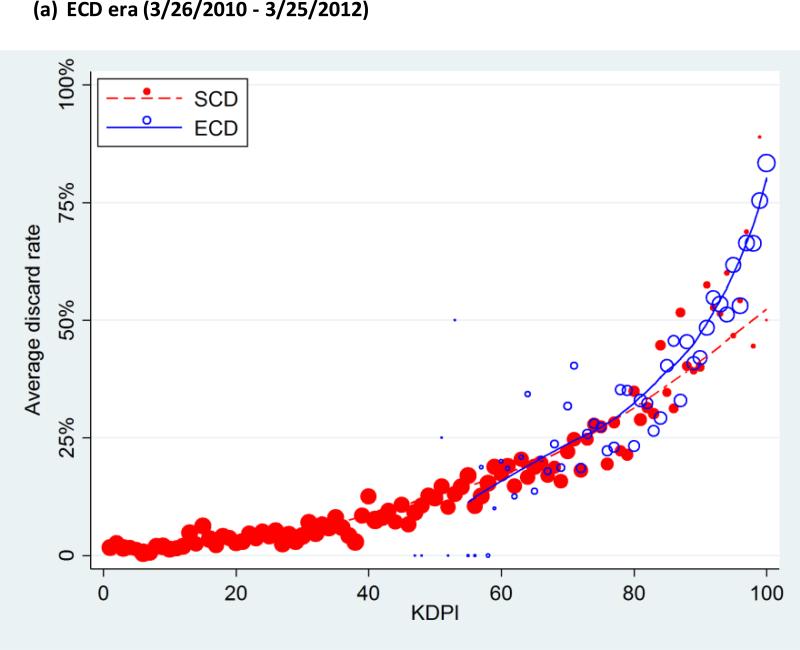
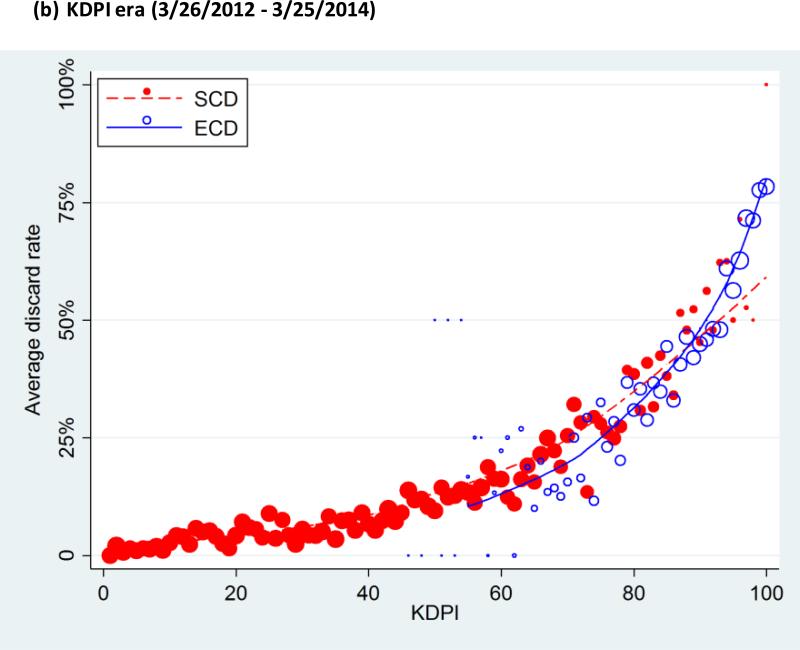
There was an overall trend in which the subgroups with higher KDPI showed higher discard rates (Figure 2). In the ECD era, the discard rates were 2.4%, 5.2%, 11.9%, 22.2%, and 49.6%, from the lowest to the highest quintile of KDPI, and 69.0% in the highest-risk group (KDPI 96-100). Similarly, in the KDPI era, the discard rates were 2.6%, 5.8%, 12.0%, 22.8%, and 50.6%, from the lowest to the highest quintile, and 71.6% in the highest-risk group (Table 2). Across both eras, ECD kidneys were discarded at higher rates than SCD kidneys: the discard rates were 10.9% among SCD and 44.9% among ECD kidneys in the ECD era, and 11.7% among SCD and 44.8% among ECD kidneys in the KDPI era.
Figure 2. Average discard rate, by Kidney Donor Profile Index (KDPI).
Both KDPI and ECD were associated with discard rate in ECD era (a) as well as KDPI era (b). The lines indicate lowess plots. The markers were sized proportionally to the number of kidneys each dot represents.
ECD, expanded criteria donor; KDPI, Kidney Donor Profile Index.
Table 2.
Odds of discard by KDPI strata and by ECD-KDPI discordance. Odds ratios adjusted for donor age, sex, race, diabetes, hypertension, ECD, DCD, serum creatinine, cause of death and blood type. Each row represents a separate, stratified analysis.
| ECD era (2010-2012) | KDPI era (2012-2014) | |||||
|---|---|---|---|---|---|---|
| N | Discard | N | Discard | aOR | p-value | |
| Overall | 28636 | 18.1% | 29021 | 18.3% | 0.971.041.10 | 0.3 |
| Quintiles | ||||||
| KDPI 0-20 | 5826 | 2.4% | 5895 | 2.6% | 0.771.021.36 | 0.9 |
| KDPI 21-40 | 5846 | 5.2% | 5848 | 5.8% | 0.871.061.30 | 0.6 |
| KDPI 41-60 | 5668 | 11.9% | 5973 | 12.0% | 0.840.971.12 | 0.6 |
| KDPI 61-80 | 5593 | 22.2% | 5771 | 22.8% | 0.921.031.16 | 0.6 |
| KDPI 81-100 | 5703 | 49.6% | 5534 | 50.6% | 0.961.071.17 | 0.2 |
| Highest- & lowest-risk | ||||||
| KDPI 0-5 | 1360 | 1.8% | 1492 | 1.1% | 0.250.541.14 | 0.1 |
| KDPI 6-95 | 25780 | 16.0% | 26178 | 16.6% | 0.971.041.11 | 0.3 |
| KDPI 96-100 | 1496 | 69.0% | 1351 | 71.6% | 0.861.061.31 | 0.6 |
| ECD-KDPI discordance | ||||||
| SCD & KDPI>85 | 643 | 46.2% | 627 | 50.7% | 1.071.421.89 | 0.02 |
| ECD & KDPI≤85 | 2463 | 27.5% | 2348 | 27.4% | 0.820.981.16 | 0.8 |
| Concordant | 25530 | 16.5% | 26046 | 16.7% | 0.971.031.11 | 0.3 |
DCD, donation after cardiac death; ECD, expanded criteria donor; KDPI, Kidney Donor Profile Index.
In the KDPI era, KDPI values of SCD kidneys ranged from 1 to 100 and those of ECD ranged from 46 to 100. Among all kidneys with KDPI 46-100 in the KDPI era, univariate analyses showed a strong association of discard with ECD status (OR=2.763.023.30; p<0.001) as well as with KDPI (OR=1.301.331.35; p<0.001; per 5-percentile increase). However, in a bivariate analysis that included both ECD and KDPI, ECD status was not associated with discard (OR=0.820.931.04; p=0.2) while KDPI still was (OR=1.311.341.37; p<0.001; per 5-percentile increase). The same pattern was also observed among the ECD era kidneys: both ECD (OR=2.913.193.49; p<0.001) and KDPI (OR=1.301.321.35; p<0.001; per 5-percentile increase) were significantly associated with discard in univariate analyses, but only KDPI was associated with discard in bivariate analysis (OR=1.281.311.34; p<0.001; per 5-percentile increase) while ECD was not (OR=0.981.101.24; p=0.1). In other words, discard rates did not differ by ECD status among kidneys with the same KDPI (Figure 2). Furthermore, KDPI displayed a greater AUC than ECD across both eras (0.82 versus 0.68; p<0.001).
Changes in discard rates
The overall discard rate was 18.1% in the ECD era and 18.3% in the KDPI era, with no significant change between eras among the entire population (aOR=0.971.041.10; p=0.3) or in any of the KDPI subgroups (Table 2). Among discordant kidneys, the discard rate was virtually unchanged in the ECD/low-KDPI subgroup (27.5% to 27.4%; aOR=0.820.981.16; p=0.8) but statistically significantly increased among SCD/high-KDPI kidneys from 46.2% to 50.7% (aOR=1.071.421.89; p=0.02).
Characteristics of discordant SCD/high-KDPI donors
The donors of discordant SCD/high-KDPI kidneys had several distinctively different characteristics compared to those of concordant SCD/low-KDPI or ECD/high-KDPI kidneys. SCD/high-KDPI donors were older than SCD/low-KDPI but younger than ECD/high-KDPI donors (median age; 42.9, 33.7, and 63.1 years, respectively). SCD/high-KDPI donors were more likely to be African American than SCD/low-KDPI and ECD/high-KDPI donors (61.1%, 14.2%, and 26.6%, respectively). SCD/high-KDPI donors were much more likely to be hepatitis C virus positive and have donated after cardiac death than SCD/low-KDPI and ECD/high-KDPI donors (HCV positive: 22.0%, 3.3%, and 4.6%; DCD: 25.2%, 16.2%, and 6.9%, respectively). P-values were lower than 0.001 for all comparisons.
Survival benefit of SCD/high-KDPI kidneys
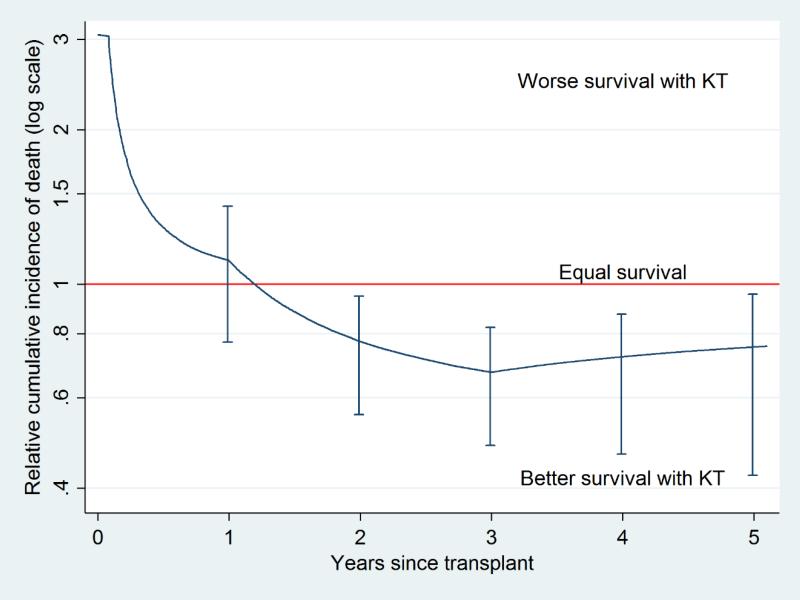
The recipients of the SCD/high-KDPI kidneys showed a decreased long-term mortality compared to those who remained on waitlist until receiving kidneys with lower KDPI. The relative cumulative incidence of death among the recipients of SCD/high-KDPI kidneys was 0.771.111.42 at 1 year after transplant, 0.560.770.94 at 2 years, and 0.480.670.82 at 3 years (Figure 3). The “break-even point” was at 1.20 years after transplant, implying that the recipients started to be at a lower risk of mortality since 1.20 years after transplant.
Figure 3. Survival benefit of transplanting SCD kidneys with KDPI>85.
The cumulative incidence of death among the SCD/high-KDPI recipients compared to those who remained on waitlist was higher immediately after transplant. However, the relative cumulative incidence decreased over time, reaching a “break-even point” at 1.20 years after transplant.
ECD, expanded criteria donor; KDPI, Kidney Donor Profile Index; SCD, standard criteria donor.
DISCUSSION
In this national study of the impact of providing KDPI with kidney offers, we did not observe any change in discard rates in the entire population or in any of the KDPI strata. However, there was an increase in the discard rate of discordant SCD kidneys, i.e. those with KDPI>85. The increased discard rate observed in this discordant subgroup shows that a “labeling effect” may actually be present. We had hypothesized that the impact of providing a new label, KDPI, might be greater in cases where the old and new labels deliver different implications; our finding of a significant change in discard rate solely observed in the SCD/high-KDPI subgroup supports this hypothesis.
While KDPI could either encourage the use of marginal kidneys by providing a better risk assessment or discourage it by labeling such organs with high KDPI scores, the expectations on how KDPI would influence the discard practice has been mainly pessimistic. Lee and Abramowicz discussed the possible reluctance to transplant high-KDPI kidneys (7), and Gandolfini and colleagues suggested that some transplant centers might reject kidneys with KDPI values over a certain threshold, eventually increasing discard rates (8). Furthermore, the new Kidney Allocation System requires clinicians to set maximum acceptable KDPI thresholds for each candidate, as opposed to the old system where candidates simply opted in or out for ECD offers. Some viable high-KDPI kidneys may therefore be rejected automatically, without ever being offered to local candidates. Even before the introduction of KDPI, there have been barriers to using marginal kidneys such as the risk of regulatory citation or liability issues. Providing KDPI may have heightened these barriers. However, our findings regarding the overall discard rates suggest that the effect of providing KDPI was neither good nor bad, but only minimal and only limited to one discordant subgroup. This is further supported not only by our findings that the discard rates did not change in any KDPI strata, but also by the similarity in the patterns of the association between KDPI and discard rate across both the ECD and KDPI eras (Figure 2).
Our hypothesis on the effect of providing KDPI on discards relies on the assumption that the clinicians had sufficient knowledge on KDPI and implemented it in their decision making. One possible alternative explanation as to why providing KDPI barely affected the overall discard rates could be that the KDPI scores were simply ignored by those offering the kidneys. Introducing a change in clinical practice has been long known to be challenging for various reasons including physician resistance or incomplete understanding (17,18), and KDPI might not have been an exception. This explanation is also supported by a survey (19) on liver transplant surgeons’ attitudes toward the Liver Donor Risk Index (LDRI), which is analogous to KDRI for liver donors: 73% of liver transplant surgeons surveyed believed that LDRI does not adequately describe donor-associated risk and 83% rarely or never discussed LDRI with their patients. Additionally, given the association between KDPI and discard, the factors constituting KDPI could have already been affecting discard practice, and KDPI may have not provided much additional information.
In contrast, we found that the discard rates of the discordant kidneys, namely SCD/high-KDPI kidneys, did increase significantly after the provision of KDPI. This increase may be the result of a labeling effect, because a significant change in discard rate was only observed in this subgroup where the new label, KDPI, contradicts the old label, SCD/ECD. The increase is unlikely caused by individual donor factors, such as age or race, because it was statistically significant after adjusting for potential confounders.
Our findings would imply that providing KDPI has led to either appropriate discards of nonviable kidneys or inappropriate discards of viable kidneys. Our survival benefit analysis lends support to the latter, as we have demonstrated that these SCD/high-KDPI kidneys also confer long-term survival benefit. Moreover, the discard rate of the kidneys from older donors is much higher in the United States than in Europe (20), suggesting that some kidneys discarded in the US might be considered viable elsewhere.
An inherent limitation of our study is that we cannot confirm the causality between the provision of KDPI and the increase in discard rate among SCD/high-KDPI kidneys. Due to the observational nature of our study, we cannot rule out the possibility that the increase was driven by other causes such as secular trends, or continued changes over time. However, the absence of any change in discard, uniformly observed within all KDPI quintiles and the concordant subgroups, lessens the likelihood that secular trends had caused the observed changes only in the SCD/high-KDPI subgroup. Another limitation of our study is that registry data may not convey every detail of the clinical information provided with the kidney offers (i.e. unmeasured or residual confounding). This risk is minimized by including all relevant information available on SRTR in our analyses, and by the fact that any bias would have to come from a change in an unmeasured confounder that happened at the same time as the introduction of KDPI, a scenario that is quite unlikely.
Given the severe organ shortage, one would hope that a novel clinical assessment tool would drive the current practice toward a more effective utilization of donated kidneys. However, our findings suggest that the implementation of KDPI had only a minimal impact on the overall discard of deceased donor kidneys. It is at least relieving that the implementation did not cause a notable increase in overall discard rates, but it also seems possible that the lack of change is because this novel metric has not yet been widely adopted. Moreover, our findings suggest that the KDPI implementation might have introduced a labeling effect which increased the discard rate of SCD/high-KDPI kidneys that would have been otherwise dubbed SCD kidneys and improved patient survival. Further study on the attitudes of those making the kidney offers, as well as the patients on the waiting list receiving these offers, would broaden our understanding of how KDPI and other clinical information are affecting our discard practice.
ACKNOWLEDGMENTS
Dr. Segev is supported by grant number K24DK101828 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). The analyses described here are the responsibility of the authors alone and do not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products or organizations imply endorsement by the U.S. Government.
Abbreviations
- aOR
adjusted Odds Ratio
- AUC
Area under the Receiver Operating Characteristic Curve
- DCD
Donation after Cardiac Death
- ECD
Expanded Criteria Donor
- GEE
Generalized Estimating Equation
- HRSA
Health Resources and Services Administration
- IQR
Interquartile Range
- KDPI
Kidney Donor Profile Index
- KDRI
Kidney Donor Risk Index
- LDRI
Liver Donor Risk Index
- OPTN
Organ Procurement and Transplant Network
- ROC
Receiver Operating Characteristic
- SCD
Standard Criteria Donor
- SRTR
Scientific Registry of Transplant Recipients
Footnotes
DISCLAIMER
The data reported here have been supplied by the Minneapolis Medical Research Foundation (MMRF) as the contractor for the Scientific Registry of Transplant Recipients (SRTR). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the SRTR or the U.S. Government.
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
REFERENCES
- 1.Rao PS, Schaubel DE, Guidinger MK, Andreoni KA, Wolfe RA, Merion RM, et al. A comprehensive risk quantification score for deceased donor kidneys: the Kidney Donor Risk Index. Transplantation. 2009;88(2):231–236. doi: 10.1097/TP.0b013e3181ac620b. [DOI] [PubMed] [Google Scholar]
- 2.The Organ Procurement and Transplantation Network [July 24, 2014];A guide to calculating and interpreting the Kidney Donor Profile Index (KDPI) http://optn.transplant.hrsa.gov/ContentDocuments/Guide_to_Calculating_Interpreting_KDPI.pdf.
- 3.Kauffman HM, Bennett LE, McBride MA, Ellison MD. The expanded donor. Transplant Rev. 1997;11(4):165–190. [Google Scholar]
- 4.Akkina SK, Asrani SK, Peng Y, Stock P, Kim WR, Israni AK. Development of organ- specific donor risk indices. Liver Transplant. 2012;18(4):395–404. doi: 10.1002/lt.23398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gupta A, Chen G, Kaplan B. KDPI and donor selection. Am J Transplant. 2014;14(11):2444–2445. doi: 10.1111/ajt.12930. [DOI] [PubMed] [Google Scholar]
- 6.Remuzzi G, Cravedi P, Perna A, Dimitrov BD, Turturro M, Locatelli G, et al. Long-term outcome of renal transplantation from older donors. N Engl J Med. 2006;354(4):343–352. doi: 10.1056/NEJMoa052891. [DOI] [PubMed] [Google Scholar]
- 7.Lee APK, Abramowicz D. Is the Kidney Donor Risk Index a step forward in the assessment of deceased donor kidney quality? Nephrol Dial Transplant. 2014;30(8):1285–1290. doi: 10.1093/ndt/gfu304. [DOI] [PubMed] [Google Scholar]
- 8.Gandolfini I, Buzio C, Zanelli P, Palmisano A, Cremaschi E, Vaglio A, et al. The Kidney Donor Profile Index (KDPI) of marginal donors allocated by standardized pretransplant donor biopsy assessment: distribution and association with graft outcomes. Am J Transplant. 2014;14(11):2515–2525. doi: 10.1111/ajt.12928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. [July 24, 2014];The Organ Procurement and Transplantation Network. KDRI to KDPI mapping table. http://optn.transplant.hrsa.gov/ContentDocuments/KDRI_to_KDPI_Mapping_Table.pdf.
- 10.Zeger SL, Liang KY, Albert PS. Models for longitudinal data: a generalized estimating equation approach. Biometrics. 1988;44(4):1049–1060. [PubMed] [Google Scholar]
- 11.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837–845. [PubMed] [Google Scholar]
- 12.Tso PL. Access to renal transplantation for the elderly in the face of new allocation policy: A review of contemporary perspectives on “older” issues. Transplant Rev. 2014;28(1):6–14. doi: 10.1016/j.trre.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 13.Israni AK, Salkowski N, Gustafson S, Snyder JJ, Friedewald JJ, Formica RN, et al. New national allocation policy for deceased donor kidneys in the United States and possible effect on patient outcomes. J Am Soc Nephrol. 2014;25(8):1842–1848. doi: 10.1681/ASN.2013070784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The Organ Procurement and Transplantation Network [September 21, 2014];Kidney allocation system status update - August. 2014 http://optn.transplant.hrsa.gov/news/kidney-allocation-system-status-update-august-2014/.
- 15.Massie AB, Luo X, Chow EKH, Alejo JL, Desai NM, Segev DL. Survival benefit of primary deceased donor transplantation with high-KDPI kidneys. Am J Transplant. 2014;14(10):2310–2316. doi: 10.1111/ajt.12830. [DOI] [PubMed] [Google Scholar]
- 16.Louis TA, Zeger SL. Effective communication of standard errors and confidence intervals. Biostat. 2009;10(1):1–2. doi: 10.1093/biostatistics/kxn014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mittman BS, Tonesk X, Jacobson PD. Implementing clinical practice guidelines: social influence strategies and practitioner behavior change. Qual Rev Bull. 1992;18(12):413–422. doi: 10.1016/s0097-5990(16)30567-x. [DOI] [PubMed] [Google Scholar]
- 18.Grol R. Beliefs and evidence in changing clinical practice. BMJ. 1997;315(7105):418–421. doi: 10.1136/bmj.315.7105.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mataya L, Aronsohn A, Thistlethwaite JR, Ross LF. Decision making in liver transplantation-limited application of the liver donor risk index. Liver Transplant. 2014;20(7):831–837. doi: 10.1002/lt.23879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cecka JM, Cohen B, Rosendale J, Smith M. Could more effective use of kidneys recovered from older deceased donors result in more kidney transplants for older patients? Transplantation. 2006;81(7):966–970. doi: 10.1097/01.tp.0000216284.81604.d4. [DOI] [PubMed] [Google Scholar]