Abstract
Objectives
One of the challenges facing hearing care providers when recommending hearing aids is the choice of device technology level. Major manufacturers market families of hearing aids that are described as spanning the range from basic technology to premium technology. Premium technology hearing aids include acoustical processing capabilities (features) that are not found in basic technology instruments. These premium features are intended to yield improved hearing in daily life compared to basic-feature devices. However, independent research that establishes the incremental effectiveness of premium-feature devices compared to basic-feature devices is lacking. This research was designed to explore reported differences in hearing abilities for adults using premium-feature and basic-feature hearing aids in their daily lives.
Design
This was a single-blinded, repeated, crossover trial in which the participants were blinded. All procedures were carefully controlled to limit researcher bias. Forty-five participants used carefully fitted bilateral hearing aids for one month and then provided data to describe the hearing improvements or deficiencies noted in daily life. Typical participants were 70 years old with mild to moderate adult-onset hearing loss bilaterally. Each participant used 4 pairs of hearing aids: premium- and basic-feature devices from brands marketed by each of two major manufacturers. Participants were blinded about the devices they used and about the research questions.
Results
All of the outcomes were designed to capture the participant’s point of view about the benefits of the hearing aids. Three types of data were collected: change in hearing-related quality of life, extent of agreement with six positively worded statements about everyday hearing with the hearing aids, and reported preferences between the premium- and basic-feature devices from each brand as well as across all four research hearing aids combined. None of these measures yielded a statistically significant difference in outcomes between premium- and basic-feature devices. Participants did not report better outcomes with premium processing with any measure.
Conclusions
It could reasonably be asserted that the patient’s perspective is the gold standard for hearing aid effectiveness. While the acoustical processing provided by premium features can potentially improve scores on tests conducted in contrived conditions in a laboratory, or on specific items in a questionnaire, this does not ensure that the processing will be of noteworthy benefit when the hearing aid is used in the real world challenges faced by the patient. If evidence suggests the patient cannot detect that premium features yield improvements over basic features in daily life, what is the responsibility of the provider in recommending hearing aid technology level? In the current research, there was no evidence to suggest that premium-feature devices yielded better outcomes than basic-feature devices from the patient’s point of view. All of the research hearing aids were substantially, but equally, helpful. Further research is needed on this topic with other hearing aids and other manufacturers. In the meantime, providers should insist on scientifically credible independent evidence to support effectiveness claims for any hearing help devices.
Keywords: hearing aid, technology, outcomes, quality of life
At least 30% of older adults in the USA have a hearing loss (Agrawal, Platz, & Niparko, 2008; Lin, Niparko, & Ferrucci, 2011). Many studies indicate that as hearing loss worsens, psychological, social, and emotional functioning tend to deteriorate, as well as communication and earning power (Arlinger, 2003; Kochkin, 2007; Nachtegaal et al., 2009). Unless medical treatment is indicated, hearing aids typically are the first treatment of choice for hearing loss. Although hearing loss is one of the most prevalent chronic health conditions endured by older people (Summer, O' Neill, & Shirey, 1999), less than one-quarter of older adults with hearing loss in the US use hearing aids (Chien & Lin, 2012). This societal problem, which will only worsen as our population ages, is recognized in the Healthy People 2020 national health initiative whose ten-year objectives include increasing the hearing aid adoption rate ("US Department of Health and Human Services. Office of Disease Prevention and Health Promotion. Healthy People 2020.")
Why do so few hearing-impaired older adults seek or use amplification? Although numerous contributing variables have been identified, one theme that consistently is observed is the belief that hearing aids do not provide sufficient value to justify their expense (Chien & Lin, 2012). It has been shown that patients perform a cost-benefit analysis to determine the value of hearing aids (Kochkin, 2003; Newman & Sandridge, 1998). Devices that provide more benefit for a given cost, or the same benefit for less cost are considered to provide greater value. This suggests that hearing aids are more likely to be accepted if they provide greater benefit per unit of cost to the patient. These and other similar data support the proposition that although cost is not the only variable involved in hearing aid adoption, perceived benefit per unit cost has a central role in patient decisions.
The issue of everyday life benefit per unit cost has received limited research attention for hearing aids. Hearing aid cost typically is determined by factors external to evidence-based benefit. Each hearing aid manufacturer commonly offers hearing aid “families” including 3-4 models that function at successively more sophisticated technology levels. As technology level rises, the hearing aid increases in cost. In 2015, modern hearing aids provide digital signal processing intended to improve outcomes in a variety of listening environments. Some common features are: multichannel compression which allows for independent adjustment of amplification in several frequency bands; directional microphones which can improve signal-to-noise ratio by amplifying sound from the front more than sound from other directions; and noise reduction algorithms which act to reduce gain for unwanted noises. Hearing aids at the basic (lower) level of technology typically include a version of each of these features. However, premium (higher) level hearing aids include more complex, automatic and adaptive versions of the features, as well as some features that do not occur at all in the basic paradigm. It might seem obvious that the more advanced features a hearing aid has the more benefit the wearer will receive in daily life. If this is true, the benefit per unit cost could remain approximately constant, or even increase, when premium-feature hearing aids are purchased. However, increased real life benefit from premium features has not been established by independent research.
It is conceivable that some patients’ difficulties might indicate a need for premium features whereas others might derive considerable benefit and greater cost-effectiveness from basic features. However, practitioners do not have any scientifically grounded guidelines to help them determine when to recommend basic-feature technology and when to recommend premium features (Giola et al., 2015). Because independent research is lacking, practitioners rely mostly on unverified manufacturer claims about feature benefits when they decide which hearing aid(s) to recommend to patients. Not surprisingly, patients often ask about the real world difference they could expect between premium-feature and basic-feature devices, because, as Kochkin and Rogin (2000) pointed out, “most consumers simply do not understand the connection between the hearing features that are marketed and the impact on their daily life”. As a result, practitioners tend to use analogies such as “it is like comparing DVDs with video tapes,” or “it is like having a 10 megapixel camera versus 4 megapixels” (personal communication and online sources). This is not an acceptable basis for recommending important healthcare devices. To meet patients’ needs, hearing aid providers should have access to scientifically based evidence about the real world improvements that can be delivered by the premium- and basic-feature instruments they recommend. This article is the first of several reporting results of research designed to address this evidence deficit. In this article we compare the effectiveness of premium-feature and basic-feature hearing aids as seen from the patient’s perspective in everyday life.
The research reported here focusses on the following questions:
What is the overall effectiveness of basic-feature and premium-feature hearing aids from the patient’s perspective?
What is the difference in overall effectiveness between basic-feature and premium-feature hearing aids from the patient’s perspective?
Do the answers to these questions differ across exemplars of basic-feature and premium-feature devices from two major hearing aid manufacturers?
Methods
Study procedures were reviewed and approved by the University of Memphis Institutional Review Board. Each subject gave written informed consent at the outset. Participants were compensated for their time. The work was performed at the University of Memphis Hearing Aid Research Laboratory.
The study was a participant-blinded, repeated, crossover trial. When the research was planned, the decision was made on logical grounds that a medium effect size due to hearing aid technology level would be the minimal interesting difference. This is consistent with a value of Cohen’s d = 0.5. As we have noted previously (Cox, Johnson, & Xu, 2014), a medium effect size would result in about 20% of individuals who wear premium-feature hearing aids performing above the distribution for persons wearing basic-feature devices. Also, a difference equal to a medium effect would create a probability of about .64 that an individual would perform better with premium-feature hearing aids than with basic-feature hearing aids (keep in mind that with a zero effect, this probability would be .5) (Grissom, 1994). Power computation used the software G*Power version 3.1.7, configured for: ANOVA: repeated measures, within factors; alpha=.05; and 4 measurements (Faul, Erdfelder, Lang, & Buchner, 2007). The research had >98% power to detect a medium effect. This was equivalent to power of 80% to detect a difference between basic-feature and premium-feature devices corresponding to an effect size of Cohen’s d=.36.
Participants
This research explored the effectiveness of hearing aids with basic features and premium features for older adults with mild to moderate hearing loss. Inclusion criteria were: bilateral, adult-onset, symmetrical, mild to moderate, non-fluctuating, sensorineural hearing loss; absence of observed middle- or outer-ear pathologies; absence of reported retrocochlear pathology; absence of any other observed or reported hearing problems needing medical attention; good or excellent self-rated physical and mental health; native English speaker; and observed adequate corrected vision and literacy to complete informed consent, questionnaires, and the test of speech understanding. In addition, to be accepted for the study, a participant needed to identify, in an initial interview, at least one specific situation in which he/she desired hearing help. Participants were recruited from a database of willing research participants maintained by our laboratory and through word-of-mouth referral. Of 15 older adults contacted by mail and telephone, 11 volunteered to participate. Thirty-seven other participants who independently contacted our laboratory and met the inclusion criteria participated. Forty-eight subjects began the study. One withdrew because of illness, one was discontinued due to non-compliance with the protocol, one was disqualified because he demonstrated a decline of cognitive abilities over the course of the study. Forty-five completed the research successfully. Participants were 30 men and 15 women, aged 61 to 81 years (mean = 70.3). Sixteen were employed full-time, 11 worked part-time, 18 were retired.
Nineteen participants had worn hearing aids before. Previous hearing aid use varied from 3 to >10 years. Admitting both new and experienced hearing aid users allowed us to sample typical individuals across the range of mild to moderate hearing loss. Most older adults with mild hearing loss do not currently wear hearing aids (although many are interested in whether amplification would help them); and many with moderate hearing loss do wear hearing aids. The presence/absence of pre-study experience with hearing aids was not expected to be a confounding variable because the lengthy field trial undertaken with each research hearing aid made all subjects experienced users by the time outcomes were measured. Figure 1 depicts the mean audiograms of all participants for left and right ears. Mean mono-syllabic word recognition scores in quiet were 81.6% (SD=14.2%).
Figure 1.
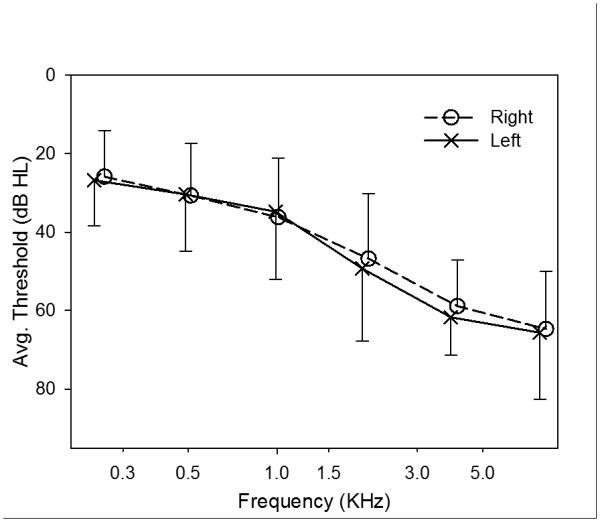
Mean audiometric thresholds of all participants for left (X) and right (O) ears. Error bars show one standard deviation.
Quality of life outcomes for 25 of these participants were presented in Cox et al. (2014). The present article expands on those data with the additional power provided by 20 more participants as well as presenting previously unreported outcomes reflecting the patient’s perspective.
Hearing Aids
Four pairs of commercially available hearing aids were evaluated. They exemplified basic-feature and premium-feature technology from one device family from each of two major manufacturers, under two well-known brands, released in 2011 and still in wide use at this writing. They were mini behind-the-ear thin-tube devices similar to the most popular style currently marketed. Each pair was linked with wireless communication. Basic and premium devices from the same brand were identical in appearance, but there were substantial differences in advertised features and functions between the two technology levels. A detailed list of advertised features in each model of hearing aid is given in Tables 1A and 1B. In most cases, the acoustic functioning of features for premium and basic technology devices was measured and verified to act as advertised. However, a few advertised functions, such as multi-channel adaptive directional microphones, could not be fully tested outside a specialized laboratory, and these were not verified.
Table 1A.
Feature capabilities activated for brand A research hearing aids, when fitting basic-feature and premium-feature devices. Settings are given for each of the three programs: ED=Everyday, LL=Look-and-Listen, SF=Speech-Finder.
| Feature | Premium A | Basic A | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| ED | LL | SF | ED | LL | SF | |
| Directional microphone |
Automatic multi- channel adaptive |
Dedicated multi- channel adaptive |
Dedicated multi- channel adaptive & front-null capable |
Automatic single- channel adaptive |
Dedicated single- channel adaptive |
Dedicated fixed omni- directional |
| Pinna simulation |
Yes | No | No | No | No | No |
| Noise management |
Automatic adjustment. More steps |
Automatic adjustment. More steps |
Automatic adjustment. More steps |
Automatic adjustment. Fewer steps |
Automatic adjustment. Fewer steps |
Automatic adjustment. Fewer steps |
| Wind noise reduction |
Yes | Yes | Yes | Yes | Yes | Yes |
| Impulse noise reduction |
Yes | Yes | Yes | Yes | Yes | Yes |
| Reverberation reduction |
(…………………………………Not available for this brand…………………………………) | |||||
| Compression channels |
16 | 16 | 16 | 8 | 8 | 8 |
| Proprietary high-frequency processing |
Yes | Yes | Yes | No | No | No |
| Binaural wireless link |
Yes | Yes | Yes | Yes | Yes | Yes |
| Binaural data streaming |
No | No | Yes | No | No | No |
| Environmental adaptation |
More categories |
More categories |
More categories |
Fewer categories |
Fewer categories |
Fewer categories |
| Self-learning | More levels & environ- ments |
More levels & environ- ments |
More levels & environ- ments |
Fewer levels & environ- ments |
Fewer levels & environ- ments |
Fewer levels & environ- ments |
| Feedback management |
Yes | Yes | Yes | Yes | Yes | Yes |
Table 1B.
Feature capabilities activated for brand B research hearing aids, when fitting basic-feature and premium-feature devices. Settings are given for each of the three programs: ED=Everyday, LL=Look-and-Listen, SF=Speech-Finder.
| Feature | Premium B | Basic B | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| ED | LL | SF | ED | LL | SF | |
| Directional microphone |
Automatic multi- channel adaptive |
Dedicated multi- channel fixed |
Dedicated multi- channel adaptive & front-null capable |
Automatic single- channel adaptive |
Dedicated single- channel fixed |
Dedicated fixed omni- directional |
| Pinna simulation |
Yes | No | No | No | No | No |
| Noise management |
Automatic adjustment. More steps |
Automatic adjustment. More steps |
Automatic adjustment. More steps |
Automatic adjustment. Fewer steps |
Automatic adjustment. Fewer steps |
Automatic adjustment. Fewer steps |
| Wind noise reduction |
Yes | Yes | Yes | No | No | No |
| Impulse noise reduction |
Yes | Yes | Yes | No | No | No |
| Reverberation reduction |
Yes | Yes | Yes | No | No | No |
| Compression channels |
20 | 20 | 20 | 6 | 6 | 6 |
| Proprietary high-frequency processing |
Yes | Yes | Yes | Yes | Yes | Yes |
| Binaural wireless link |
Yes | Yes | Yes | Yes | Yes | Yes |
| Binaural data streaming |
No | Yes | Yes | No | No | No |
| Environmental adaptation |
More categories |
More categories |
More categories |
Fewer categories |
Fewer categories |
Fewer categories |
| Self-learning | Yes | Yes | Yes | No | No | No |
| Feedback management |
Yes | Yes | Yes | Yes | Yes | Yes |
Hearing Aid Fittings
The hearing aids were newly released at the outset of the research. Each manufacturer provided a trainer who met with the research team and presented an in-depth tutorial about the design and goals of the instruments. In addition, the trainers described any useful modifications to the fitting software that had been learned from experience.
The method for coupling the device to the ear canal was individually chosen based on the participant’s hearing thresholds and the approach that yielded the best results for that person. Seventeen individuals were fitted using open domes. All of these had low-frequency hearing thresholds better than 35 dB HL. Twenty-five individuals were fitted using custom earmolds with 3mm parallel vents. Three participants were fitted with unvented custom earmolds.
Each bilateral hearing aid fitting was accomplished using a comprehensive best-practice five-step approach. First, hearing aids were programmed for the participant’s hearing thresholds using the manufacturer’s proprietary algorithm. In this step, all feature settings (e.g., microphone directionality mode, strength of noise management conditions, environment analysis, etc) were set according to the recommendation of the manufacturer for this hearing loss. These settings were not changed. Second, real-ear amplified levels for continuous speech were matched to the National Acoustics Laboratory Non-Linear prescription goals (Dillon 2006; Keidser and Dillon, 2006). This was accomplished with the Speechmap function of the Audioscan Verifit fitting system. Third, the fitting was fine-tuned using rule-based subjective assessments of bilateral loudness balance, loudness of average speech, loudness comfort, and quality of own voice. To achieve a fitting that was acceptable to the wearer, this step often resulted in some adjustment away from strict adherence to prescription targets. Fourth, follow-up telephone interviews and further fine tuning were completed as needed within the first week of use. Finally, remote controls and hearing aid learning capabilities, were available throughout the month of wearing time to further optimize hearing aid performance in daily life. Each pair of hearing aids was set to engage the maximum datalogging/datalearning available in that model. The most sophisticated learning capabilities allowed premium hearing aids to make volume adjustments automatically rather than manually.
Each hearing aid was fitted with three user-selectable programs. The programs were as follows:
One selectable program was labeled the Everyday program (ED) and it was configured with all features implemented as recommended by the manufacturer to optimize that hearing aid for that individual’s hearing loss. The Everyday program automatically adjusted the hearing aids’ features in different environments. It is theoretically expected that these automatic adjustments will be more advantageous for the listener when premium features are used compared to basic features. The Everyday program was the default program which was activated when the hearing aid was turned on.
Another selectable program was labeled the Look-&-Listen program (LL). This program was configured like the Everyday program with the exception that it engaged the strongest non-adaptive (when possible) front-facing microphone directionality.
Another selectable program was labeled the Speech-Finder program (SF) and it was configured like the Everyday program with the exception that it included the most effective technology in each hearing aid for detecting speech that is produced from the sides and behind as well as the front.
The feature settings for each program are shown in Tables 1A and 1B. Subjects were trained about the purposes of the different programs. Training materials comprised printed statements and demonstrative photos to show listening environments where each program might be most appropriate. This was followed by a discussion of schematic diagrams to explore optimal positioning and program use when in typical listening situations (e.g., restaurant, audience member). The training was exactly the same for all hearing aids and was given to each participant only when he/she received the first pair of hearing aids. Participants were told that the research hearing aids might perform differently in various situations and they should explore the three programs in each hearing aid to find the best setting in their daily lives. Remote controls were supplied and these were used by subjects to explore and select programs in daily life listening and to change sound volume as preferred in different environments. Practice in use of the remote controls was provided.
Procedures
Participants were told that the research was about “how people benefit from different types of modern hearing aids”. They were otherwise blinded about the study. Participants used each pair of hearing aids for one month in their daily lives, and they agreed in advance to use the instruments for at least four hours per day. They knew from the outset that the hearing aids were on loan to them and would be returned after one month of use. Participants did not pay for the hearing aids and the potential cost of hearing aids was not discussed. Batteries were provided. Counterbalancing controlled the order in which the two brands were presented and, within brands, the order of using basic and premium features. When the basic and premium hearing aids by the first brand had been evaluated, there was a one-month washout period before the basic and premium hearing aids from the second brand were used. This was a precautionary measure since there is no information about whether a washout period is essential for this type of research. During the wash-out period, previous users were allowed to wear their personal hearing aids.
Because the researchers were not blinded about the hearing aid conditions, we were aware of the potential for subtle unintended influences to be conveyed to the participants. For this reason, the research was carefully planned to standardize all interactions with each participant, including verbal and visual interactions for all aspects of the protocol. Written materials were prepared and practiced before any participants were enrolled. The 6-item questionnaire and the QoL measure (described below) were provided to the participant in a binder when the hearing aids were fitted, accompanied by written instructions. These were completed at the end of the 1-month field trial prior to meeting with the researcher. In addition, each participant was debriefed after his/her protocol was complete (a process of 5-6 months). The debriefing included the question “Was there anything about us, or this research, that might have influenced your opinions about the hearing aids?” Forty-four individuals answered “no” to this question. The 45th reported that she thought she detected some subtle facial expressions from the researcher on one occasion that she interpreted as meaningful.
Outcomes
When research focuses on the functioning of hearing aid technological features, it is typical for outcomes to be measured under strictly controlled laboratory conditions. These conditions might attempt to simulate daily life listening or they might be contrived to highlight the feature being examined. This type of research is a valuable and necessary step towards establishing the potential worth of the feature for the hearing-impaired listener. Nevertheless, it is well understood that sound processing that improves test scores under laboratory conditions might, or might not, be useful in daily life. In the long run, it is the performance in daily living in the circumstances of the particular listener that determines the usefulness of a hearing aid fitting. Only the subjective observations of the hearing-impaired listener can furnish this type of outcome data. It is reasonable to assert that the patient’s perspective is the gold standard for determining whether one type of hearing aid is better than another for that patient in the particular circumstances of his/her world (O'Boyle, 1997; Fischer et al. 1999; Asadi-Lari et al. 2004). Accordingly, in this article, we report outcomes measured from the patient’s perspective with minimal structuring by the researchers.
Three types of patient-point-of-view data were collected: changes in quality of life (QoL) related to hearing; a six-item questionnaire encompassing topics that are considered critical for satisfaction with amplification and often targeted in the engineering design of new premium features; and in-depth qualitative interviews to further explore participants’ personal experiences and preferences with the hearing aids.
Quality of life measurement
There is no single widely accepted definition of QoL because it is a complicated construct and its specific elements vary among individuals and across the lifespan (Carr et al. 1996). Nevertheless, it is generally agreed that health-related QoL includes an individual’s emotional, social and physical well-being. Ability to hear affects communication and information exchange, which are critical to maintaining quality of life. Further, there is likely to be substantial heterogeneity across persons regarding the specific details of emotional, social and physical well-being that are impacted by hearing in a given individual. For example, one individual’s quality of life might be strongly affected by his ability to hear well enough to participate easily in a weekly poker game. Another person might be very concerned with improving her ability to interact with children in a preschool program where she volunteers. It is easy to recognize that for both of these persons a definition of QoL changes involves improved communication but the specific life situations and listening demands where this need arises are quite different. These kinds of considerations caused us to choose a global QoL outcome measure rather than one which asks about specific communication situations. With a global type of approach, each subject was able to respond based on his/her own particular perspective in daily life.
Our approach to measuring hearing related QoL was informed by work indicating that a direct question about the amount of change produces data that are at least as sensitive and valid as comparison of measures of QoL state at two points in time (Fischer et al. 1999). In addition, we were cognizant of a body of literature that supports the conclusion that a change of 0.5 on a 7-point outcome scale, or one half of a standard deviation on other scales, corresponds to a minimal important change from the patient’s perspective (eg., Norman et al. 2003). These considerations were explored and combined by Juniper et al (Juniper et al. 1994), who quantified QoL change using a 15-point response scale. The Juniper et al. (1994) research supports the conclusion that a score of +2 (a little better) on this response scale is a minimally important positive change and would be consistent with a management recommendation in favor of choosing the hearing aid in daily life. This questionnaire was slightly adapted for the current research (shown in Appendix A, Supplemental Digital Content I). After a one-month field trial with bilaterally fitted devices, subjects marked the response scale to report change in quality of life related to hearing with that device, compared to using no hearing aids. This questionnaire was completed by the participant following each field trial before meeting with the researcher. It preceded administration of standardized questionnaires and laboratory tests that will be reported in other articles.
Six-item questionnaire
The questionnaire was modeled on the one devised by Bentler et al. (2008). Each item was a statement and the participant marked his/her extent of agreement/disagreement with the statement on a scale from 1 (do not agree at all) to 10 (completely agree). The statements were worded so that a higher score indicated a better outcome (see Appendix B, Supplemental Digital Content II). The aspects of hearing aid performance addressed by the statements are ones that often are targeted in the engineering design of new premium features: speech clarity, noise acceptability, amount of daily use, listening effort, sound comfort, and localization. For each bilateral hearing aid fitting, the participant recorded extent of agreement with each statement every day for the last five days of the field trial.
Interviews
Because the daily life impacts of hearing problems differ across individuals, a variety of factors might be relevant to the outcome of a given intervention for a given person. To capture these differences, investigators collected in-depth interview data after each field trial phase of the study. The interviews were structured to explore each participant’s experiences with the hearing aid model during the field trial and to illuminate any potential effects of hearing aid features that might not have been revealed using standard laboratory and self-report outcome measures. After both basic and premium hearing aids for one brand were trialed, the interview additionally explored participants’ preferences between the two hearing aid feature levels and the factors that influenced those preferences. After all four hearing aid models were trialed, the interview explored the participant’s overall preferences across the four devices. All interviews were conducted by one of two researchers in a private area. Interviews were audio-recorded and transcribed.
Results
Much of the analysis is descriptive. Where statistics were called for, parametric methods were used. It has been argued in some sources that when responses to questionnaire items are ordinal in nature, derived scores cannot accurately be treated using parametric statistical methods. However, many statistician-scientists do not subscribe to this view (e.g., Velleman and Wilkinson 1993; Nunnally and Bernstein 1994; Labovitz 1967). Prior to all analyses, data were examined for distribution and outliers. When outliers were discovered, transformation was considered and, if applied, the recommendations of Tabachnick and Fidell (2001) were followed. The QoL data included several outliers (all the negative scores) but these were not transformed because this would compromise data interpretation given the opposite meaning of negative vs positive QoL scores. Three of the 270 data points from the diary responses were outliers and these were transformed. Some of the distributions deviated from normality; however, it has been shown that analysis of variance is quite robust to violations of normality for groups of moderate size (Donaldson 1968; Glass et al. 1972).Values of p≤.05 are reported as significant. The analyses were driven by a priori hypotheses that correspond to the three research questions, namely:
Outcomes would be better when listeners wore hearing aids than when listeners did not wear hearing aids (a necessary precursor to 2 and 3).
Outcomes would be better for premium-feature hearing aids compared to basic-feature hearing aids for both brands combined.
Outcomes would be better for premium-feature hearing aids compared to basic-feature hearing aids for each brand separately.
Final hearing aid fittings
Figure 2 depicts the mean real ear aided responses for final hearing aid fittings after all fine tuning adjustments. Data are given for the left ear (the right ear results are essentially identical). The Figure demonstrates the extent to which the achieved amplification differed from the NAL-NL2 prescription targets. It is noteworthy that the mean fine-tuned amplification for average speech input matched prescription targets quite well below 4kHz, whereas for soft inputs the fine-tuned fittings were considerably lower than targets at most frequencies. In addition, as typically found, all mean maximum output levels were substantially lower than theoretical targets.
Figure 2.
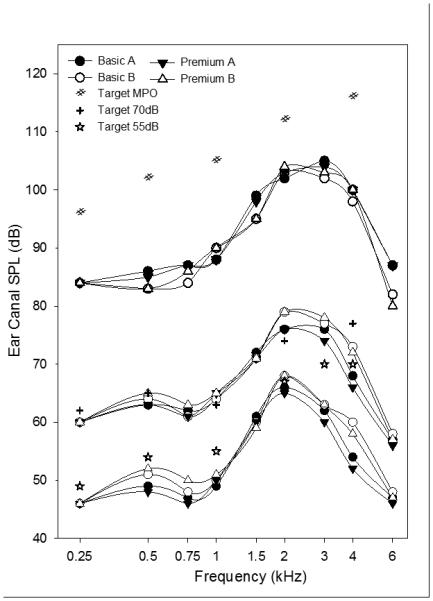
Mean real ear aided responses in the left ear for final hearing aid fittings after all fine tuning adjustments. The lower, middle, and upper sets of curves depict results for soft speech input (55 dB SPL), average speech input (70 dB SPL), and swept tone bursts at 85 dB SPL (to determine narrow-band maximum output), respectively. Each set shows results for each of the four research hearing aids (premium and basic models for each of two brands). Brand A is shown using filled symbols and brand B is shown using open symbols. In addition, mean target values for the NAL-NL2 prescription are shown for each set of curves.
Use of hearing aids in daily life
Use of the three programs
The data-logging function of the research hearing aids was analyzed to determine the details of hearing aid use during the one-month field trials. On average, the hearing aid models were used between 9.1 and 9.3 hours per day. There were no significant differences among the four models of hearing aids on this variable.
Patterns of use of the three programs were examined. Program 1 was always the default Everyday (ED) setting. The default ED program was used more than either of the other programs. Mean use of ED for the 4 hearing aid models ranged from 75% to 82% and the differences among them were not statistically significant.
The original intention had been to assign the Look-and-Listen (LL) setting to program 2 and the Speech-Finder (SF) setting to program 3. All subjects were informed of this during the fitting process, and additionally they were encouraged to explore all three programs before choosing the one that seemed to work best in a given listening environment. During the analysis of the data, it was discovered that for one hearing aid model (Premium B) the settings for programs 2 and 3 were accidentally reversed for 16 of the 45 subjects so that the SF settings were in program 2 and the LL settings were in program 3. This might have affected self-report outcomes for this hearing aid if the subjects used programs based solely on an expectation of LL in program 2 and SF in program 3, but would not be likely to affect outcomes if subjects were exploring all three programs before deciding which to use.
The post field trial interviews for the premium B hearing aid for all 45 participants were subjected to content analysis to determine whether the reversed locations of LL and SF might have affected the use of these programs for the 16 subjects who experienced it. Most participants reported that they used all three programs at some point during the field trial, and chose which program to use in a listening environment primarily by experimenting with the programs. At the same time, many participants could not detect substantial differences among the three programs. All comments about program usage (how the programs were selected, satisfaction with program performance, situations when programs were used, observations about quality and benefit from programs, etc.) were coded and categorized into a hierarchical structure. Then the 45 individuals were partitioned into reversed and non-reversed groups. The previously determined hierarchical structure was examined for each group. On the whole, similar proportions of each group’s participants contributed to each of the high-order categories. These data suggest that reversing the location of the LL and SF settings did not influence the way participants used the programs. Mean use of the LL setting ranged from 11% to 20% and there was a significant main effect for hearing aid model (F[3, 120] =4.74, p=.004). Further analysis revealed that this result was due to a difference in use of the LL setting between the brand B basic and premium models (p=.016). The LL setting was used more in the basic model than in the premium model. Mean use of the SF setting ranged from 4.6% to 9.4% for the 4 hearing aid models and the differences among them were not significant. However, further analysis revealed a difference in use of the SF setting between the brand B basic and premium models (p=.019). The SF setting was used more in the premium model than in the basic model. These results for use of the LL and SF settings, taken together with participant remarks in the post-trial interviews, suggest that because there were no noticeable differences between LL and SF, participants tended to use program 2 (regardless of whether it contained LL or SF) if they found program 1 to be unsatisfactory.
In summary, participants used all three programs at some point and explored their effectiveness. The automatic program (program 1) was used at least three-fourths of the time, on average. When the listener wanted to change the program he/she most often selected the next program (program 2), and program 3 was relatively rarely used.
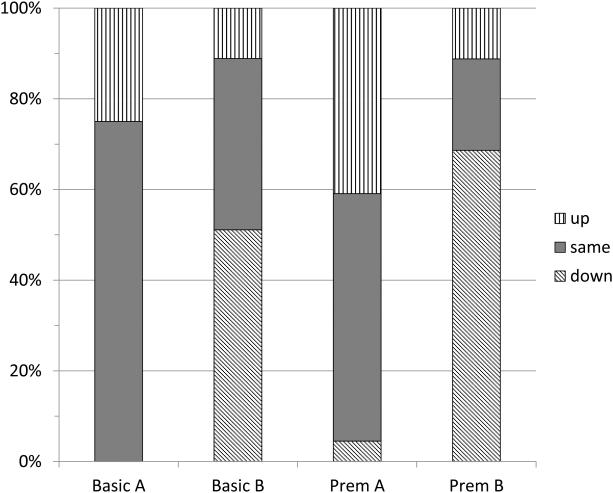
Volume control changes
Data-logging information also was used to estimate the extent to which participants adjusted the volume on each device during the field trial. Precise values could not be obtained from the displays provided by the programming software. However, it was possible to assess whether the volume control had been generally increased, decreased, or unchanged overall for each device. The results for the automatic program are summarized in Figure 3. The Figure shows that for both Brand A hearing aids, the volume tended to be unchanged or turned up. In contrast, for both Brand B devices, the volume tended to be unchanged or turned down. It is of interest to compare these tendencies with the pattern shown in Figure 2 for the mean ear canal levels when the hearing aids were first fitted. It is clear that the two Brand B devices produced levels several dB higher than the two Brand A devices. The volume changes illustrated in Figure 3 indicate that participants tended to counteract these differences by adjusting the volume setting during everyday use.
Figure 3.
Percent of subjects who increased or decreased their volume control setting during the field trial. Data are given for each hearing aid model when it was used in the automatic (Everyday) program.
Quality of life changes resulting from hearing aid use
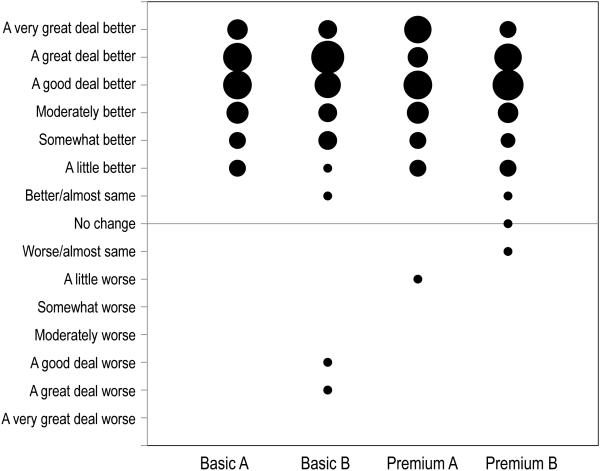
At the conclusion of a field trial with a particular pair of hearing aids, each participant used a 15-point response scale to report change in quality of life related to hearing with that device, compared to using no hearing aids. The quality of life changes reported are summarized in Figure 4 for each of the four research hearing aid models. The Figure represents 180 separate QoL judgments (4 hearing aid models × 45 participants). In this bubble chart, the size of each bubble is proportional to the number of participants who gave that response. Most individuals reported that QoL was at least “moderately better” (a score of +4) with the hearing aid compared to not using any amplification. Juniper et al. (1994), using this response scale, scored responses of “no change” and “almost same” (−1, 0, and +1) as indicating that QoL was not affected by the treatment. Following their example to evaluate these data, it can be seen that 4 individuals reported no effect on QoL related to hearing when they used one of the hearing aid models. Three additional participants reported that a hearing aid (brand B basic, or brand A premium) made their QoL worse. The remaining 173 responses reported improved QoL when using the hearing aid.
Figure 4.
Change in hearing-related quality of life when using each hearing aid in daily life. The size of each bubble is proportional to the number of subjects who gave that score.
By inspection, there is not a clear difference in the pattern of QoL changes reported across the 4 hearing aids. The QoL change responses were scored from −7 (A very great deal worse) to +7 (A very great deal better). The mean scores were 4.93 (brand A basic), 4.62 (brand B basic), 4.87 (brand A premium) and 4.56 (brand B premium). These results address research question 1, showing that both basic and premium hearing aids were typically substantially effective in improving quality of life related to hearing. General Linear Model (GLM) repeated measures analysis of variance (ANOVA) with a priori planned independent contrasts was used to analyze these data. Compared to the more typical omnibus F-test followed by post-hoc comparisons, this analysis approach has been shown to provide increased statistical power while controlling the experiment-wise error rate (Rosenthal and Rosnow 1985; Buckless and Ravenscroft 1990). The planned contrasts explored differences between basic and premium devices for both brands combined, and for each brand separately (research questions 2 and 3). None of the results were significant or approached significance.
In summary, 96% of the responses across the 4 hearing aid models indicated that the devices improved quality of life compared to wearing no amplification. However, the data did not provide any evidence that the premium devices produced more quality of life improvement than the basic devices.
Six-item questionnaire
This questionnaire was completed on five separate days at the end of each field trial. Each item statement used a scale from 1 to 10 with a higher score indicating a better outcome. Each participant’s score for each statement was determined by averaging the responses for the five days. The results are summarized in Figure 5. This figure provides scatter plots in which circles show the scores for basic and premium features for each participant, and squares depict mean scores, for each item. Brand A is depicted using filled symbols, brand B is shown using open symbols. A symbol above the diagonal indicates a better score for premium features whereas one below the diagonal reveals a better score for basic features. The most obvious characteristic of all panels is that most symbols, and the mean scores, are quite close to the diagonal, indicating similar scores for basic and premium features. All items tended to receive quite high scores. Item 3 (wearing the device when needed) consistently received the highest mean agreement score (approximately 9), and item 2 (noise was not bothersome) consistently received the lowest mean agreement score (approximately 7.7). The other four items all produced mean agreement scores close to or slightly above 8. Repeated measures ANOVA on these data supported the validity of these observations by revealing a significant main effect of item (F[3.2, 140.4] =19.3, p<.001). Post hoc analyses, with Sidak adjustment for multiple comparisons, revealed that agreement responses for item 3 were significantly higher than those for every other item (p<.001). Further, agreement with item 2 was significantly lower than agreement with items 1, 3, and 5 (p≤ .001). Although these results do not directly address the research questions, they do confirm that participants were capable of discriminating amongst the items as they scored each hearing aid.
Figure 5.
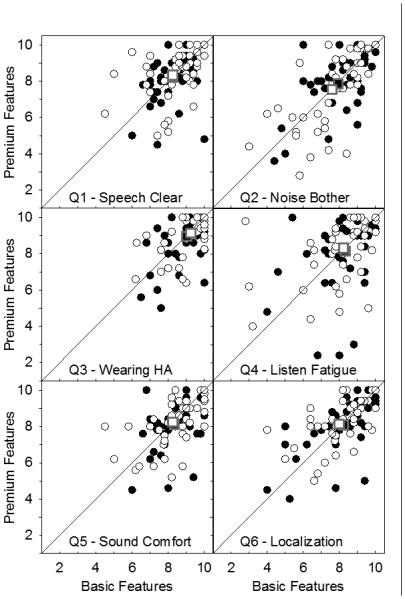
Each panel summarizes responses to one item of the 6-item questionnaire. Circles show scores for basic and premium features for each participant. Squares (sometimes overlapping) depict mean scores for basic and premium features for each item. Brand A is depicted using closed symbols. Brand B is shown using open symbols.
If premium feature technology resulted in fewer problems with the common challenges addressed by these items, it would be expected that scores for Premium A and Premium B would consistently be higher than those for Basic A and Basic B for some or all items. However, examination of the six panels reveals that there was not an obvious trend for more symbols to appear above the diagonal. Once again, analyses confirmed the observations. Planned contrasts exploring results for basic and premium devices for the two brands combined and for each brand separately failed to reveal any significant differences.
In summary, for each of the four hearing aid models, participants reported relatively high agreement with all six statements, although there were significant differences across items. This is indicative of substantial effectiveness for all hearing aid models. However, the data did not provide any evidence that the premium devices were superior to the basic devices in solving problems (with speech clarity, noise discomfort, etc.) commonly encountered in hearing aid use.
After real-world experience, did participants prefer premium devices?
An in-depth interview occurred at the end of each of four one-month field trials. Trials 1 and 2 comprised the basic and premium devices from one brand. Trials 3 and 4 comprised the basic and premium devices from the other brand. The two brands were separated by a one-month washout period. During interviews 2 and 4, the participant was asked “If you had to choose one pair of hearing aids [from these two pairs], which would you choose”? Note that, because the subjects were blinded about the nature of the differences between the devices, they did not know that they were choosing between basic or premium features.
An additional measure of overall preferences was performed at the conclusion of interview 4. At this time, the participant was shown a visual analog scale drawn on paper and labelled “Very Bad” on the left and “Very Good’ on the right with 10 equal intervals marked. The participant was provided with four stickers to indicate hearing aids 1, 2, 3, and 4. Instructions were: “This is a scale showing how the hearing aids were overall: from “very bad” on this end to “very good” on this end. Each of these four stickers represents a hearing aid. They are labeled according to the order you used them: 1st, 2nd, 3rd, 4th. You can put them anywhere along this line that you want. For example, they can all be really close together, or really far apart. It’s up to you. ” After the stickers were placed by the participant, the position of the sticker for each hearing aid was scored using the percent of the measuring line from the left edge to the sticker. A higher score was indicative of a better rating.
Preference data
Data produced by these two types of preference measures (models within brands and across all models in both brands) were analyzed to determine whether there was a tendency for participants to prefer the premium hearing aids over the basic hearing aids based on whatever criteria they felt were most salient. The pairwise preferences reported at interviews 2 and 4 are summarized in Table 2. Three people could not tell any difference at all between the two devices for one of the brands. Nine participants chose the premium feature devices for both brands. Twelve chose the basic feature devices for both brands. Twenty-one other participants chose one basic and one premium device for the two brands. Thus, 42 participants were able to express a preference in the interview between the basic and premium devices for both brands. Of these preferences, about half (46%) were described by participants as based on minimal or negligible differences between the two devices, whereas the rest were definite preferences. However, the definite preferences were equally divided between basic-feature and premium-feature devices.
Mean scores generated by the overall goodness ratings for the four models on the visual analog scale used in interview 4 were: Brand A basic =75.7, Brand B basic = 74.4, Brand A premium = 72.6, Brand B premium = 74.4. There were no significant differences among these means.
Effect of order of use
These preference data depended on participants’ ability to remember the relative effectiveness of the hearing aids they were judging. It seemed possible that participants might not remember all the hearing aids equally well or might be biased in their judgments towards, or against, devices experienced more recently. This possibility was explored for both types of measures with the following results: For the 90 paired-comparison preference judgments (45 participants × 2 brands), the hearing aid used second was chosen as preferred 52 times, whereas the hearing aid used first was chosen as preferred 35 times, and participants could not choose a preference 3 times. This suggests a trend in which the second pair of devices was chosen more often than the first, regardless of technology. These data were tested to determine if there was a significant difference between percent of participants preferring the first or second pair of devices. The effect did not quite meet the standard for significance (t(89)=1.86. p=.067). Nevertheless, this result is consistent with the trend seen towards a bias based on order of use.
The data obtained in the overall goodness ratings for the set of four field trials covering a period of five months, also were assessed for a possible order effect. When the devices were scored based on the order of use, rather than by brand and technology level, the mean scores for the 1st to the 4th pairs were 73.4, 78.6, 71.2, and 72.3, respectively. That is, despite the fact that both brand and technology level were counterbalanced variables, the second pair of devices trialed tended to be the highest rated when all four were rated together. The possibility that order of use had a significant effect on final overall goodness ratings was explored using an intraclass correlation (ICC). A two-way random model analysis generated a significant ICC of .475 (p = .003). This result implies that 47.5% of the variance in final overall goodness ratings of the devices could be accounted for by the order of use in the study.
Did previous experience with amplification seem to affect the results?
As noted earlier, individuals both with and without a history of previous hearing aid experience were admitted into this study. This decision was made to allow us to sample the population of interest (typical persons with mild to moderate hearing loss). Although previous hearing aid experience was not a controlled variable, it is reasonable to reflect on whether there might have been differences in the results comparing basic with premium devices for the new and experienced user subgroups.
It is important to note that the new and experienced subgroups differed slightly in mean age (69 versus 72 years) and markedly in mean 4-frequency average hearing loss (37 dB versus 52 dB). Further, because previous experience was not controlled when hearing aid test orders were randomized, the order in which conditions were experienced is not balanced across this variable. Despite these limitations, the outcomes for new and experienced subgroups are reported here for their heuristic value.
The change in QoL data were examined separately for the 26 new users and the 19 experienced users. The average outcomes for the four research hearing aid models ranged from 3.6 to 4.5 for new users and from 5.0 to 5.8 for experienced users. This disparity is not surprising given the greater hearing loss of the experienced subgroup. However, neither subgroup revealed a statistically significant difference in QoL change between basic-feature and premium-feature hearing aids.
Each item in the 6-item questionnaire was analyzed to explore responses of new and experienced subgroups. The general trend in which all items received relatively high scores, with item 3 and item 2 eliciting the highest and lowest scores, respectively, was observed for both subgroups. The contrast test comparing basic-feature and premium-feature devices did not show a significant difference for any item in either subgroup.
-
Preference judgements provided during post-trial interviews 2 and 4 were examined separately for new and experienced subgroups. Of the 12 participants who chose basic features for both brands, 4 were new users and 8 were experienced. The difference between the percentage of new users who preferred basic features and the percentage of experienced users who preferred basic features barely exceeded the significance threshold of p≤.05, suggesting that experienced users tended to prefer the basic features more often, t(43)=1.99. p=.053 (2-tailed). Of the 9 participants who chose premium features for both brands, 7 were new users and 2 were experienced. There was no significant difference between the subgroups in preference for premium features.
Mean scores generated by the overall goodness ratings for the four models on the visual analog scale used in interview 4 were partitioned into the two subgroups. Overall goodness mean scores for three of the hearing aid models were similar for new and experienced users and similar to the values reported above for the whole group. However, experienced users gave a significantly higher mean score (84.6) than new users (66.9) for the brand B basic device, t(43)=2.694, p=.01(2-tailed).
Both types of preference data hint that the experienced users (who were also older and more hearing-impaired) might be more likely than new users to prefer basic-feature devices.
Order effects were examined for each subgroup in a manner parallel to the report for the full group, above. In the 90 paired comparisons, the hearing aid used second was chosen 28 times by new users and 24 times by experienced users. The device used first was chosen 22 times by new users and 13 times by experienced users. Of the 3 occasions when no preference could be expressed, 2 were new users and 1 was experienced. These results suggest that the trend towards choosing the second hearing aid was consistent across both subgroups.
To review the potential effect of order on the overall goodness ratings provided in interview 4, ICC coefficients were computed for both new and experienced subgroups. For both subgroups, the second hearing aid trialed (regardless of brand or feature level) secured the highest rating. The ICC coefficients were statistically significant for both subgroups (ICCnew = .427, p=.034; ICCexp = .518, p=.02). Thus, the finding that roughly half of the variance in overall goodness ratings could be accounted for by order of use in the study was observed for both new and experienced hearing aid users.
Discussion
This article presents some of the results of a comprehensive exploration of differences in effectiveness between premium-feature and basic-feature hearing aids. The complete set of research results includes both quantitative and qualitative methods and laboratory and daily life settings. The present article concentrates on the aspects of the research that explored basic-premium differences in daily life from the participant’s point of view with minimal structuring of responses by the researchers. In other words, we were interested in knowing the extent of differences in their own daily lives that participants would notice between the two types of hearing aids, when they had not been informed that these technological differences existed and had not been exposed to claims about what types of help could be expected from specific hearing aids. At the same time, participants were not ignorant about hearing aids: they were counselled fully about the ways in which hearing aids might potentially help, and about how to use the devices to achieve optimal benefit. They also were told that any given hearing aid might, or might not, deliver those benefits.
Did amplification improve hearing outcomes?
There are three types of data that address this question. First, each participant individually interpreted the phrase “quality of life related to hearing”, as it applied to the circumstances of his/her own life. As Figure 4 shows, 96% of participant’s judgements corresponded to a positive change in the quality of his/her own life related to hearing when any of the four research hearing aids was used. Second, in addition to considering quality of life, each participant reported extent of agreement with six positively-worded statements about their experience with the hearing aids. These general statements directed attention to broad areas, such as speech understanding, listening effort, etc. (topics that have been found to be of concern to many hearing aid users). As Figure 5 illustrates, agreement with all statements was high and, indeed, mean values all exceeded 7.5 on a 10-point scale. These data are consistent with a comparatively high personal opinion about the effectiveness of the hearing aids in daily life. Third, structured interviews with each participant after every field trial elicited their extemporaneous views about the value of the device to them in their lives. Although a formal qualitative analysis of the interviews is not provided here, participants almost always reported that the hearing aids were helpful in situations that were important to them. Typical statements were:
“I like being able… in a restaurant … talkin’ to the person at the table with me”
“… it helped with conversations that did have a lot of noise … I still had some problems sometime”
“… striking [that in large groups] there’s a multitude of distinctive voices… not just this diffuse sound”
“They work! I hear better, they’re easy to put in and out”
“…like church, and … hearing the priest … it seemed like I heard every word he said”
All three types of data are consistent in affirming that the research hearing aids did indeed improve hearing outcomes in participants’ daily lives. This type of result has been reported before (Larson et al. 2000; Kochkin 2011) and should be reassuring to health care providers who are inclined to doubt the effectiveness of modern hearing aids.
Were outcomes better for premium-feature hearing aids?
When hearing health care patients pay several thousand dollars extra to obtain premium-feature hearing aids, it is likely that essentially all of them expect to perform better with the purchased devices than they would with basic-feature devices. From this viewpoint, our a priori decision (see above for rationale) that a medium effect size would be a minimally interesting difference between premium and basic technology hearing aids is distinctly modest. Keep in mind also that the research was powered to have a very high probability of detecting a medium effect if one was present.
Ultimately, there was no basis for computing effect sizes due to the lack of any indication of overall differences between the premium-feature and the basic-feature hearing aids. A review of the reported results indicates that when the two basic devices were combined and the two premium devices were combined, there was no difference between the technology levels by any measure elicited from participants reporting on their daily life experiences: The difference between the premium-feature and basic-feature devices was 0.07 scale points of improvement in quality of life on a 15-point scale; There were no systematic differences between basic-feature and premium -feature hearing aids on any of the six items in the questionnaire, despite the fact that it was clear that participants could distinguish among the items (for example, all four hearing aids received lower scores on the item concerning problems with background noise); Lastly, in the interviews with participants, both measures of overall preference gave data consistent with a conclusion that, taken as a whole, participants did not prefer premium devices over basic devices following the field trials. Actually, these preference data pointed to a trend for selecting the second device used, regardless of technology. It is reasonable to speculate that this pattern of preferences occurred because the participant was more familiar with the brand characteristics when using the second pair and there was really no appreciable other difference between the two devices being compared. This is similar to a report by Naylor et al. that new hearing aid wearers tended to prefer the second device tried when two fittings were compared (2015).
Despite our failure to detect an overall trend for premium-feature devices to perform differently from basic-feature devices, both Figure 5 and Table 2 show that some participants gave notably better scores to premium-feature devices whereas others gave notably better scores to basic-feature devices. It would be of interest to determine whether these results are examples of measurement error or whether they represent individuals with specific characteristics that point to robust superior performance with one of the two feature levels. Perhaps manufacturers can devise exploratory tests that provide empirically supported guidelines for selection of specific sound processing strategies. For example, although this research explored outcomes for persons with typical, uncomplicated, age-related hearing loss, perhaps individuals with atypically irregular audiograms would perform better when fitted with hearing aids having more adjustable frequency bands. These types of issues can be explored to produce the evidence that is essential to back up fitting recommendations.
Another issue of potential interest arose when we compared new and experienced hearing aid users. Although the essentially equivalent outcomes for premium-feature and basic-feature devices were generally observed in both subgroups, there were two instances which hinted that experienced users might be more likely to prefer basic-feature hearing aids. Because the history of previous experience was confounded with several potentially important variables, this observation does not support any firm conclusion, but future research on this matter might provide useful insights.
Were the differences between basic and premium technology devices consistent across brands?
Review of the data in Figure 4, Figure 5, and Table 2, as well as the statistical results comparing basic and premium hearing aids separately for brand A and brand B showed identical patterns for the two brands. From the typical participant’s perspective, the hearing aids used in this research were all quite helpful, but there were no differences on average between the two feature levels in terms of effectiveness in daily life.
Does this result suggest that technology advances have not improved hearing aids over time?
Not at all. It is important to keep in mind that this research examined outcomes for fittings of basic-feature and premium-feature devices marketed at a single specific point in time. Consequently, our results do not cast light on the extent to which technological improvements and developments over historical time have resulted in better real world outcomes when amplification is used. Studies that explore this question have examined outcomes from devices developed and marketed over a period of multiple years (eg., Bentler and Duve 2000; Kochkin 2010). As observed by Humes and Krull (2012) after an extensive review of the evidence, technological developments have, indeed, tended to produce small improvements in outcomes over time.
Further, our results do not reflect on whether future innovations in technology (e.g., Bluetooth capabilities, smartphone controls, bilateral beamformer) will additionally improve outcomes or, perhaps, bring about clinically significant differences in daily life between premium-feature and basic-feature devices for typical hearing-impaired individuals. However, given our results and the absence of evidence to the contrary, it would seem reasonable to believe that this result does apply to today’s hearing aids unless proven otherwise.
Was there something wrong with the research methods that produced this result?
Because these outcomes contradict many conventional beliefs and product claims, they might not be easy to accept. Several potential rebuttals could be put forward for these results, and some of those are addressed below.
It could be argued that the quantitative methods we used were not optimally designed to elicit the particular advantages of the premium features in the premium devices. It is true that investigations of the efficacy of newly developed technological features frequently are designed specifically to showcase feature functioning. Other features often are disabled for this type of study. This type of research can produce knowledge that has an important role in bringing new technologies to market. However, the patient reacts to the hearing aid as an integrated system rather than as a collection of independent technological features. This research considered the effectiveness of hearing aids as complete systems with all their features working together. We asked whether the devices with premium features were more effective in daily living than visually identical devices with basic features that were available for a substantially lower cost (although matters of cost were not known to the participant). Under these circumstances, using basic-feature and premium-feature devices produced essentially equal and indistinguishable improvements from the average participant’s point of view in their own real life situations.
Some hearing health providers might assert that hearing aid wearers simply can’t appreciate the advantages of premium features even though those features actually make the hearing aids more effective. Others may take the view that if hearing aid wearers, after careful, informed, and mindful comparisons, cannot detect a consistent difference between basic-feature and premium-feature devices, then probably there are no important differences for those individuals.
Another consideration that has implications for the validity of this research is the issue of whether there was optimal hearing aid fitting and counselling. If the devices were not optimally fit, any differences or similarities among them are less meaningful. For this reason, detailed accounts are provided (see methods) about how the fittings and post-fit instructions were conceived and documented. The goal was to standardize a protocol that embodied best practices for real ear performance and verification. In addition, every effort was made to utilize the hearing aids’ sound processing features in the most advantageous method as envisioned by the respective device manufacturers. Figure 2 illustrates that, on average, the real ear aided responses achieved were good approximations of prescribed goals for average speech and the overall patterns of real ear performance at different input levels were typical of those currently seen in clinical settings that follow best practice guidelines. Data logging confirmed significant daily use of the hearing aids and volume adjustments that were coherent with real ear performance (Figure 3). It should be reassuring to practitioners that wearers used the automatic program very frequently in daily life. They did experiment with the other programs, but patterns of use as well as the interviews suggested that mostly these did not seem to offer much advantage over the automatic setting. Overall, the hearing aid fittings were meticulously optimized for both basic and premium technology and this is a strength of this research. Nevertheless, we recognize that some practice surveys have suggested that many hearing aids are not fit using standard best practice methods. We cannot speculate about what the outcomes of this research might have been if sub-standard or unvalidated fitting methods had been used.
Because of the nature of null hypothesis statistical testing, a finding of “not significantly different” cannot automatically be assumed to mean that there are no important differences between the conditions compared. If the study is underpowered, meaningful differences might not be statistically significant. However, we do not believe that a lack of power is the reason for these findings. As noted earlier on theoretical grounds, a medium effect size appears to be a reasonable minimum practically important difference between premium-feature and basic-feature conditions. The power of the research provided a very high probability of a statistically significant result if such an effect was present.
This research focused on hearing aids representing brands from two of the six leading manufacturers of high-technology devices (Kirkwood, 2013). Practitioners and hearing aid wearers are likely to wonder whether the results can be generalized to other hearing aid brands. A review of the websites maintained for each of the leading brands indicates that the six manufacturers maintain a competitive similarity in claims for the features of the products they market. However, because the technological/engineering execution of feature functions are developed independently for the various brands, we cannot assume that they function equally. It also is noteworthy that this research was carried out using hearing aids that were newly brought to market in 2011. Because new products are unveiled by each manufacturer about every 18 months in the hearing aid industry, there probably have been two newer models of each hearing aid brand since this research was started. Might the difference in effectiveness of premium features and basic features be greater now than it was in 2011? Perhaps, but probably not. Although new products are developed regularly, changes in the technology that underlie each feature typically are small and incremental. Also, as new premium features are developed, formerly premium-feature technology migrates down to basic-feature devices. So, both technology levels tend to advance in tandem over time. These considerations drive home the point that additional independent research is urgently needed to explore real-world differences that might exist between premium-feature and basic-feature devices for different brands, as well as overall differences among brands, in terms of their effectiveness in daily life situations.
Conclusions
This article provides important evidence for patients and hearing health care providers about what might be expected when different levels of hearing-aid technology are used in the real world by persons with adult-onset mild to moderate sensorineural hearing loss. When the data from two major brands of hearing aids were examined from the point of view of typical hearing aid wearers in their daily lives, it appeared that the outlay of substantially higher dollar amounts to purchase premium feature engineering technology typically would not have resulted in meaningful incremental gain in overall effectiveness relative to basic-feature technology. Purchasers of hearing aids are appropriately concerned with the value delivered for dollars expended. This has been demonstrated by private payers (Kochkin 2003; Bridges et al. 2012;) but also should inform the decisions made by public health entities and other third party payers such as health insurers. Application of the results of this research should result in more cost-effective hearing aid recommendations and increased access to successful, lower cost, hearing health care for many hearing-impaired individuals. Although the results of this study are clear, it should be replicated by other independent researchers with other hearing aids and other brands. The relatively low uptake of hearing aids by persons who could benefit from them, and the long-standing intransigence of this fact, demands that those committed to hearing health care re-evaluate the paradigm for hearing rehabilitation. Although twenty-plus years of engineering improvements in amplification devices have undoubtedly resulted in better hardware, confidence in the potential for plug-and-play hearing aids that fix problems immediately has probably exceeded reality. Perhaps the time has come to de-emphasize technological sophistication and re-energize commitment to non-technological aspects of hearing care. This largely untapped field of study (Barker et al. 2014) has the potential to point the way to valuable incremental effectiveness in the treatment of chronic adult-onset mild to moderate hearing loss.
Supplementary Material
Table 2.
Cross tabulation showing reported preference for basic or premium model in each brand of hearing aid
| Brand A |
||||
|---|---|---|---|---|
| Basic | Premium | No Difference | ||
| Brand B | Basic | 12 | 9 | |
| Premium | 12 | 9 | 2 | |
| No Difference | ||||
Acknowledgements
This research was funded by a grant to the first author from the U. S. National Institute on Deafness and other Communication Disorders (R01DC011550). All authors contributed equally to this work.
Footnotes
Conflicts of Interest and Source of Funding: No conflicts of interest were declared by any author.
List of Supplemental Digital Content
Supplemental Digital Content 1. Appendix that provides the hearing-related quality of life change questionnaire used for this research. pdf
Supplemental Digital Content 2. Appendix that provides the 6-item questionnaire used for this research. pdf
References
- Agrawal Y, Platz EA, Niparko JK. Prevalence of hearing loss and differences by demographic characteristics among US adults: data from the National Health and Nutrition Examination Survey, 1999-2004. Arch Intern Med. 2008;168(14):1522–1530. doi: 10.1001/archinte.168.14.1522. [DOI] [PubMed] [Google Scholar]
- Arlinger S. Negative consequences of uncorrected hearing loss - a review. International Journal of Audiology. 2003;42(2):S17–S20. [PubMed] [Google Scholar]
- Asadi-Lari M, Tamburini M, Gray D. Patient's needs, satisfaction, and health related quality of life: Towards a comprehensive model. Health and Quality of Life Outcomes. 2004;2(32) doi: 10.1186/1477-7525-2-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker F, Mackensie E, Elloit L, Jones S, de Lusignan S. Interventions to improve hearing aid use in adult auditory rehabilitation. Cochrane database of Systematic Reviews. 2014;2014(7) doi: 10.1002/14651858.CD010342.pub2. doi: 10.1002/14651858.CD010342.pub2. [DOI] [PubMed] [Google Scholar]
- Bentler R, Duve M. Comparison of hearing aids over the 20th century. Ear Hear. 2000;21(6):625–639. doi: 10.1097/00003446-200012000-00009. [DOI] [PubMed] [Google Scholar]
- Bentler R, Wu YH, Kettel J, Hurtig R. Digital noise reduction: outcomes from laboratory and field studies. Int J Audiol. 2008;47(8):447–460. doi: 10.1080/14992020802033091. [DOI] [PubMed] [Google Scholar]
- Bridges JFP, Lataille AT, Buttorff C, White S, Niparko JK. Consumer preferences for hearing aid attributes: A comparison of rating and conjoint analysis methods. Trends Amplif. 2012;16(1):40–48. doi: 10.1177/1084713811434617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckless FA, Ravenscroft SP. Contrast coding: A refinement of ANOVA in behavioral analysis. The Accounting Review. 1990;65(4):933–945. [Google Scholar]
- Carr AJ, Thompson PW, Kirwan JR. Quality of life measures. British Journal of Rheumatology. 1996;35:275–281. doi: 10.1093/rheumatology/35.3.275. [DOI] [PubMed] [Google Scholar]
- Chien W, Lin F. Prevalence of hearing aid use among older adults in the United States. Arch Intern Med. 2012;172(3):292–293. doi: 10.1001/archinternmed.2011.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RM, Johnson JA, Xu J. Impact of advanced hearing aid technology on speech understanding for older listeners with mild to moderate, adult-onset, sensorineural hearing loss. Gerontology. 2014;60(6):557–568. doi: 10.1159/000362547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon H. What's new from NAL in hearing aid prescriptions? Hearing Journal. 2006;59(10):10–16. [Google Scholar]
- Donaldson TS. Robustness of the F-test to errors of both kinds and the correlation between the numerator and denominator of the F-ratio. Journal of the American Statistical Association. 1968;63(322):660–676. [Google Scholar]
- Faul F, Erdfelder E, Lang A-G, Buchner A. G*Power3: A flexible statistical power analysis for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- Fischer D, Stewart AL, Bloch DA, Lorig K, Laurent D, Holman H. Capturing the patient's view of change as a clinical outcome measure. JAMA. 1999;282(12):1157–1162. doi: 10.1001/jama.282.12.1157. [DOI] [PubMed] [Google Scholar]
- Giola C, Ben-Akiva M, Kirkegaard M, Jorgensen O, Jensen K, Schum D. Case factors affecting hearing aid recommendations by hearing care professionals. J Am Acad Audiol. 2015;26(3):229–246. doi: 10.3766/jaaa.26.3.4. [DOI] [PubMed] [Google Scholar]
- Glass G, Peckham P, Sanders J. Consequences of failure to meet assumptions underlying the fixed effects analyses of variance and covariance. Review of Educational Research. 1972;42(3):237–288. [Google Scholar]
- Grissom RJ. Probability of the superior outcome of one treatment over another. Journal of Applied Psychology. 1994;79(2):314–316. [Google Scholar]
- Humes L, Krull V. Hearing aids for adults. In: Wong L, Hickson L, editors. Evidence-Based Practice in Audiology. Plural Publishing; San Diego: 2012. pp. 61–91. [Google Scholar]
- Juniper EF, Guyatt GH, Willan A, Griffith LE. Determining a minimal important change in a disease-specific quality of life questionnaire. J Clin Epidemiol. 1994;47(1):81–87. doi: 10.1016/0895-4356(94)90036-1. [DOI] [PubMed] [Google Scholar]
- Keidser G, Dillon H. What's new in prescriptive fittings down under?. In: Palmer C, Seewald R, editors. Hearing Care for Adults: An International Conference; Stafa, Switzerland: Phonak AG; 2006. pp. 133–142. [Google Scholar]
- Kirkwood DH. Research firm analyzes market share, retail activity, and prospects of major hearing aid manufacturers. 2013 Retrieved June 17, 2015, from http://hearinghealthmatters.org/hearingnewswatch/2013/research-firm-analyzes-market-share-retail-stores-prospects-of-major-hearing-aid-makers/
- Kochkin S, Rogin C. Quantifying the obvious: the impact of hearing instruments on quality of life. Hearing Review. 2000;7(1):6–34. [Google Scholar]
- Kochkin S. MarkeTrak VI: On the issue of value: Hearing aid benefit, price, satisfaction, and repurchase rates. Hearing Review. 2003;10(2):12–26. (2) [Google Scholar]
- Kochkin S. The impact of untreated hearing loss on household income. 2005 Retrieved June 17, 2015, from http://www.hearingindustries.org/uploadedFiles/Content/impact_of_untreated_hearing_loss_on_income.pdf.
- Kochkin S. MarkeTrak VIII: Consumer satisfaction with hearing aids is slowly increasing. Hearing Journal. 2010;63(1):19–32. [Google Scholar]
- Kochkin S. MarkeTrak VIII:Patients report improved quality of life with hearing aid usage. Hearing Journal. 2011;64(6):25–32. [Google Scholar]
- Labovitz S. Some observations on measurement and statistics. Social Forces. 1967;46(2):151–160. [Google Scholar]
- Larson VD, Williams DW, Henderson WG, Luethke LE, Beck LB, Noffsinger D, Rappaport BZ. Efficacy of 3 commonly used hearing aid circuits: A crossover trial. JAMA. 2000;284(14):1806–1813. doi: 10.1001/jama.284.14.1806. doi: joc00017 [pii] [DOI] [PubMed] [Google Scholar]
- Lin F, Niparko J, Ferrucci L. Hearing loss prevalence in the United States. Arch Intern Med. 2011;171(20):1851–1852. doi: 10.1001/archinternmed.2011.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naylor G, Oberg M, Wanstrom G, Lunner T. Exploring the effects of the narrative embodied in the hearing aid fitting process on treatment outcomes. Ear Hear. 2015;36(5):517–526. doi: 10.1097/AUD.0000000000000157. [DOI] [PubMed] [Google Scholar]
- Nachtegaal J, Smit JH, Smits C, Bezemer PD, van Beek JH, Festen JM, Kramer SE. The association between hearing status and psychosocial health before the age of 70 years: results from an internet-based national survey on hearing. Ear Hear. 2009;30(3):302–312. doi: 10.1097/AUD.0b013e31819c6e01. doi: 10.1097/AUD.0b013e31819c6e01. [DOI] [PubMed] [Google Scholar]
- Newman CW, Sandridge SA. Benefit from, safisfaction with, and cost-effectiveness of three different hearing aid technologies. American Journal of Audiology. 1998;7(2):115–128. doi: 10.1044/1059-0889(1998/021). [DOI] [PubMed] [Google Scholar]
- Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Medical Care. 2003;41(5):582–592. doi: 10.1097/01.MLR.0000062554.74615.4C. [DOI] [PubMed] [Google Scholar]
- Nunnally JC, Bernstein IH. Psychometric Theory. Third McGraw-Hill, Inc.; New York: 1994. [Google Scholar]
- O'Boyle CA. Measuring the quality of later life. Philosophical Transactions of the Royal Society B: Biological Sciences. 1997;352(1363):1871–1879. doi: 10.1098/rstb.1997.0173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal R, Rosnow R. Contrast Analysis: Focused Comparisons in the Analysis of Variance. Cambridge University Press; Cambridge, England: 1985. [Google Scholar]
- Summer L, O' Neill G, Shirey L. Chronic conditions: A challenge for the 21st century (Vol. Number 1) National Academy on an Aging Society; Washington, DC: 1999. Retreived June 17, 2015, from http://www.agingsociety.org/agingsociety/pdf/chronic.pdf. [Google Scholar]
- Tabachnick BG, Fidell LS. Using multivariate statistics. Allyn & Bacon; Needham Heights: 2001. [Google Scholar]
- US Department of Health and Human Services. Office of Disease Prevention and Health Promotion Healthy People 2020. Retrieved June 11, 2015, from http://www.healthypeople.gov/2020/topics-objectives/topic/hearing-and-other-sensory-or-communication-disorders/objectives.
- Velleman PF, Wilkinson L. Nominal, ordinal, interval, and ratio typologies are misleading. Am Stat. 1993;47(1):65–72. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.