Abstract
Background
We assessed Zimbabwe’s progress towards elimination of mother-to-child HIV transmission (eMTCT) under Option A.
Methods
We analyzed 2012 and 2014 cross-sectional serosurvey data from mother-infant pairs residing in the same 157 health facility catchment areas (CA) randomly sampled from 5 provinces. Eligible women were ≥16 years and mothers/caregivers of infants born 9–18 months prior. We aggregated individual-level questionnaire and HIV serostatus within CA or district to estimate MTCT and the number of HIV-infected infants; these data were mapped using facility GPS coordinates.
Findings
A weighted population of 8,800 and 10,404 mother-infant pairs was included from 2012 and 2014, respectively. In 2014, MTCT among HIV-exposed infants was 6.7% (95% confidence interval (CI): 5.2,8.6), not significantly different from 2012 (8.8%, 95%CI: 6.9,11.1, p=0.13). From 2012–2014, self-reported antiretroviral therapy or prophylaxis among HIV-infected women increased from 59% to 65% (p=0.05), as did self-reported infant antiretroviral prophylaxis (63% vs. 67%, p=0.08).
In 2014, 65 (41%), 55 (35%), and 37 (24%) CAs had the same, lower, and higher MTCT rate as in 2012, respectively. MTCT in 2014 varied by CA (median=0%, mean=4.9%, interquartile range (IQR)=0–10%) as did the estimated number of HIV-infected infants (median=0, mean=1.1, IQR=0–1.0). Also in 2014, 106 (68%) CAs had MTCT=0%. Geovisualization revealed clustering of CAs where both MTCT and the estimated number of HIV-infected infants were relatively high.
Interpretation
Although MTCT is declining in Zimbabwe, geospatial analysis indicates facility-level variability. CAs with high MTCT rates and a high burden of HIV-infected infants should be the highest priority for service intensification.
Keywords: Prevention of mother-to-child HIV transmission, geospatial, vertical HIV transmission, PMTCT cascade, HIV infection, Zimbabwe
INTRODUCTION
The Joint United Nations Programme on HIV/AIDS’ (UNAIDS) 2015 goal for “virtual elimination” of mother-to-child transmission (eMTCT) is within reach for many low- and middle-income countries.[1, 2] Countries that achieve both of the following impact targets for at least one year can apply to WHO for validation of having eliminated MTCT: <=50 new pediatric HIV infections per 100,000 live births, and either 1) a MTCT rate of <5% in breastfeeding populations, or 2) a MTCT rate of <2% in non-breastfeeding populations, based on infants’ final infection status which is typically determined at 18 months.[3] In addition, three coverage targets must have been be met for two years prior to certification of eMTCT: 1) ≥95% ANC attendance, 2) ≥95% HIV testing coverage of pregnant women, and 3) ≥90% antiretroviral treatment (ART) coverage of HIV-infected pregnant women.[3]
Although the eMTCT validation guidance emphasizes the need for ongoing, effective interventions and quality surveillance systems before and after eMTCT certification, the path towards elimination is not simple. Given limited resources, how should governments prioritize their short-term efforts to achieve eMTCT most efficiently? As part of an evaluation of Zimbabwe’s Accelerated National PMTCT Program, we had the opportunity to examine changes in MTCT rates and service uptake to inform prioritization of sub-regions for enhanced PMTCT service strengthening. Zimbabwe has been hailed for their scale up of PMTCT services, under Option A from August 2011 and under Option B+ starting in November 2013.[4, 5] For example, from 2007 to 2011, program monitoring data suggest that the percentage of HIV-positive pregnant women attending a health facility who received ARV prophylaxis increased from 22% to 82%,[6, 7] and recent estimates suggest that MTCT is now between 8%[8] and 13%.[9] Thus, Zimbabwe is an ideal setting to consider how to best allocate human and financial resources for the final push toward eMTCT.
Using data from two rounds of a community-based cross-sectional serosurvey, we examined Zimbabwe’s progress towards eMTCT by assessing changes in MTCT rates and uptake of key PMTCT services between 2012 and 2014. In addition, we conducted a geospatial analysis to examine variation in MTCT and the number of HIV-infected infants by facility catchment area over the same time period (2012–2014). Based on these results, we identified communities with the highest need for PMTCT service intensification from a simple algorithm based on MTCT and the number of pediatric infections in 2014. Lastly, we examined regional variability in MTCT and infant infections along with HIV prevalence among women and reported coverage of maternal ARVs/ART. These analyses were motivated by the President’s Emergency Plan for AIDS Relief’s (PEPFAR) goal to maximize resources by identifying and prioritizing geographic areas at the sub-national level with the greatest need for treatment and prevention.[10]
METHODS
Study Population
The accelerated PMTCT program was initiated in 2011 by the Zimbabwe Ministry of Health and Child Care (MoHCC) and was based on Option A of the 2010 WHO PMTCT guidelines.[11] Zimbabwe began rollout of Option B+ in late 2013.[12] The impact evaluation consisted of serial cross-sectional serosurveys in 2012 and 2014 to determine the population-level impact of the accelerated program on MTCT and HIV-free child survival.[8, 13–15] In brief, the surveys targeted mothers and caregivers of infants who were born 9 to 18 months prior in order to capture MTCT during pregnancy, delivery and in the first 9–18 months of breastfeeding. Women were eligible if they were ≥16 years old and biological mothers or caregivers of eligible infants (alive or deceased). Biological mothers represented 96% of the sample in both rounds. A verbal autopsy was performed to determine timing and cause of the mother’s death in cases where the mother had died and the caregiver was interviewed.
Sampling Strategy
The two-stage sampling strategy has been previous described.[8] Briefly, in 2012 the research team randomly selected 157 catchment areas1 of 699 health facilities in five of ten provinces offering PMTCT services, proportionate to the number of facilities per district. The five provinces (Harare, Mashonaland West, Mashonaland Central, Manicaland, and Matabeleland South) were purposefully selected to include Zimbabwe’s capital, rural communities with higher and lower HIV prevalence, and both Shona and Ndebele ethnic groups. In each catchment area, all eligible infants were identified (using community health workers, immunization registers, and peer referral) and a pre-determined proportion was randomly sampled, depending on the size of the catchment area. In 2014, we followed the same sampling procedures in the same catchment areas.
Data Collection
At each healthcare facility serving a sampled catchment area, the field research team collected information about the facility and its global positioning system (GPS) coordinates. The team also conducted a household survey with identical data collection procedures in 2012 and 2014.[8] Mothers/caregivers providing written informed consent completed an anonymous interviewer-administered survey that collected information about maternal and household demographics, health services accessed by the mother and her infant during pregnancy and after delivery, and health behaviors germane to MTCT (e.g., breastfeeding). Pregnant women were asked to report about care received at any health facility, not only the one closest to her home. Patient-held maternal and infant health cards were examined when available; for example, 23% and 15% of women in 2012 and 2014 had receipt of ART/ARVs verified on the patient-held health card, respectively. The survey response rate was 98.9% in 2012 and 99.4% in 2014. The anonymous nature of the survey prevented the collection of GPS coordinates from women’s homes.
Assessment of HIV Status
Living mothers and infants provided blood spot samples for HIV testing, which were air-dried onto filter papers and stored at room temperature until transported biweekly to the laboratory. Maternal samples were tested for HIV-1 antibody using AniLabsytems EIA kit (AniLabsystems Ltd, OyToilette 3, FIN-01720, Vantaa, Finland) with positive specimens confirmed using Enzygnost Anti-HIV 1/2 Plus ELISA (Dade Behring, Marburg, Germany) and discrepant results resolved by Western Blot. Samples of infants born to HIV-positive mothers or to mothers with unavailable samples were tested for HIV with DNA polymerase chain reaction (Roche Amplicor HIV-1 DNA Test, version 1.5). The response rate for maternal blood samples was 97.4% in 2012 and 95.3% in 2014. Among eligible infants who were randomly selected to be surveyed, the response rate for blood samples was 96.5% in 2012 and 93.5% in 2014.
HIV test results were linked to questionnaire data through a unique code. Because the survey was anonymous, HIV status during pregnancy was not verified with medical records. In 2012, participants could retrieve their anonymous HIV test results at the local facility up to 3 months following the survey using a card with barcode numbers. In 2014, participants who wanted to receive their HIV test results had the option to provide identifying information, which allowed receipt of HIV test results at the local facility.
Data Analysis
MTCT
MTCT was defined as proportion of infants who were HIV-infected at 9–18 months of age among those born to HIV-infected mothers (MTCT is often referred to as a “rate” by convention).[8] The numerator (number of infants HIV-infected or deceased related to HIV/AIDS) was assessed based on i) laboratory-confirmed HIV test results based on specimens collected during the survey (98.1% and 99.5% of the final sample of infants in 2012 and 2014, respectively, which is different than the response rates indicated above due to the different denominators), ii) verbal autopsy data (for deceased infants, 1.3% and 0.2% of the 2012 and 2014 samples, respectively), and iii) information recorded on infant health cards (0.6% and 0.3% of the 2012 and 2014 samples, respectively), which reflected laboratory confirmed HIV-test results conducted at health facilities.
PMTCT Service Utilization
In each survey, we computed the proportion of women or HIV-exposed infants completing each step in the PMTCT cascade using the total weighted number of women or infants as the denominator.[15] Uptake of the cascade of services targeted to all pregnant women was based on self-report and included: ANC (any and the WHO-recommended ≥4 visits[16]), HIV testing during ANC or labor and delivery or prior knowledge of one’s HIV-positive status, receipt of HIV test results or prior knowledge of one’s HIV-positive status, institutional delivery, postnatal visit attendance (6–8 weeks after birth), and current use of a modern contraceptive method.[17] Uptake of services targeted to HIV-infected women and their HIV-exposed infants was based on self-report and included: reported maternal ART and/or ARV prophylaxis (grouped), infant ARV prophylaxis, ≥1 immunization visit, receipt of cotrimoxazole, exclusive breastfeeding for ≥6 months,[18] HIV testing, receipt of HIV test results, and treatment (if infected).
This portion of the analysis was restricted to biological mothers and their eligible infants (representing 96% of the survey samples). When receipt of the service or engagement in the behavior was unknown or missing, women or infants were classified as not having completed the cascade step; however, this applied to no more than 1% of any service or behavior. We compared service utilization between years with an F test adjusted for the study design and alpha=0.05. These analyses were conducted with STATA 14 (College Station, Texas) and were weighted to account for the sampling fraction and survey non-response.
Geographical Analysis
Overall, 156 of 157 catchment areas had valid GPS coordinates. In each survey year we assigned mother-infant pairs the GPS coordinates of the nearest maternal and child health facility. We aggregated individual-level data within each catchment area to estimate: 1) the catchment-level MTCT rate, 2) the estimated number of HIV-infected infants per catchment area, which was the product of the unweighted number of HIV-infected infants and the inverse of the catchment area’s sampling fraction, 3) HIV prevalence among women in the survey, aggregated to the district level and 4) maternal coverage of ART/ARV prophylaxis. These indicators were selected because they provide information about service uptake and loss to follow-up (MTCT and maternal coverage of ART/ARV prophylaxis), the burden of pediatric infections (number of HIV-infected infants), and the potential for MTCT (maternal HIV prevalence and ART/ARV coverage). These data were displayed on 2012 and 2014 maps created with ArcGIS (Esri, Redlands, California). On the 2014 maps, we indicated high priority areas for intensification of PMTCT service provision, based on the algorithm described below.
Determination of Priority Areas for PMTCT Service Intensification
We prioritized catchment areas based on their 2014 MTCT rates and the estimated number of HIV-infected infants. Priority groups were intended to differentiate a reasonable subset of catchment areas where enhanced intervention was urgent (high priority) or necessary (moderate priority) compared to those areas where the current level of service provision should be maintained (low priority). High priority areas were those where we estimated ≥3 HIV-infected infants 9–18 months of age and MTCT ≥10% (the 75th percentile of the MTCT distribution); moderate priority areas were those where we estimated 1–2 HIV-infected infants 9–18 months of age or 0%<MTCT<10%; and low priority groups were those where we estimated no HIV-infected infants 9–18 months of age and MTCT=0%. We examined whether maternal ART/ARV prophylaxis varied by priority area using an F test adjusted for the study design and alpha=0.05. We hypothesized that catchment areas in the highest priority groups would have the lowest coverage levels of maternal ART/ARV prophylaxis.
Human Subjects Protection
The Medical Research Council of Zimbabwe and the ethical review boards at the University of California, Berkeley and University College London approved the impact evaluation.
RESULTS
Participant Characteristics
Overall, a weighted population of 8,800 and 10,404 mother-infant pairs were included in the 2012 and 2014 analysis, respectively, based on 8,662 and 10,225 interviews with mothers and caregivers of infants who were or would have been between 9 and 18 months of age. The median age of women was 26 years (range: 16–68), 93.2% were married or had a regular sexual partner, and they had a median of 2 lifetime births (range: 0–15), with no important differences by survey year. In 2012 and 2014, 1,075 (12.4%) and 1,324 (13.4%) women were HIV-infected (p=0.11).
MTCT Estimates
In 2014, MTCT was 6.7% (95% confidence interval (CI): 5.2, 8.6) but this did not differ significantly from 2012 (8.8%, 95% CI: 6.9, 11.1, p=0.13). In 2 of the 5 provinces in 2014 (Manicaland and Matabeleland South), MTCT was <5% (the eMTCT target for breastfeeding populations) although the upper bound of the 95% confidence interval was >5% (Table 1).
TABLE 1.
Median number of HIV-infected infants per catchment area, stratified by province, and estimated MTCT rate per province, Zimbabwe, 2012 and 2014
| Province | No. CAs sampled | No. of HIV-infected infants per CA Median (Range)a
|
Estimated MTCT rate % (95% CI)b
|
||
|---|---|---|---|---|---|
| 2012 | 2014 | 2012 | 2014 | ||
| Harare | 8 | 0 (0, 12) | 8 (0, 24) | 6.1% (2.2, 15.5) | 9.5% (6.4, 14.0) |
| Manicaland | 57 | 0 (0, 8) | 0 (0, 6) | 9.1% (6.1, 13.5) | 4.6% (2.6, 8.2) |
| Mashonaland Central | 30 | 0 (0, 8) | 0 (0, 6) | 7.6% (4.1, 13.4) | 6.0% (3.6, 9.7) |
| Mashonaland West | 37 | 1 (0, 10) | 0 (0, 6) | 10.7% (6.8, 16.5) | 7.8% (4.7, 12.5) |
| Matabeleland South | 24 | 0.5 (0, 11) | 0 (0, 4) | 9.4% (5.7, 15.0) | 4.1% (1.6, 10.0) |
|
| |||||
| Total | 156 | 0 (0,12) | 0 (0,24) | 8.8% (6.9, 11.1) | 6.7% (5.2, 8.6) |
CA: catchment area; CI: confidence interval; MTCT: mother-to-child HIV transmission
The estimated number of HIV-infected infants per catchment area was calculated as the product of the unweighted number of HIV-infected infants in the survey and the inverse of the catchment area’s sampling fraction.
The estimated proportion of infants per catchment area who were HIV-infected at 9–18 months of age among those born to HIV-infected mothers, weighted to account for the sampling fraction and survey non-response.
PMTCT Service Utilization and Engagement in Prevention Behaviors
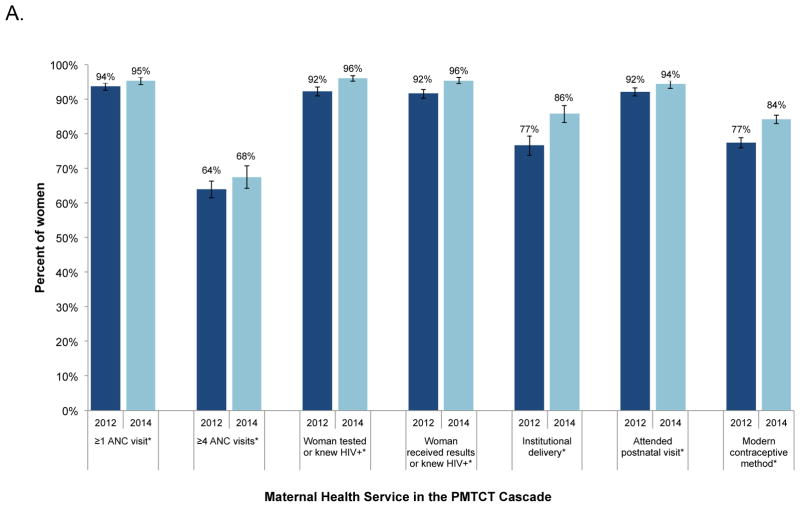
Service utilization increased among all pregnant and postpartum women from 2012–2014, including ≥4 ANC visits (64% to 68%), receiving HIV test results (92% to 96%), institutional delivery (77% to 84%), and current use of a modern contraceptive method (77% to 84%, Figure 1, Panel A). Among women who were HIV-infected, the proportion reporting ART or ARV prophylaxis increased from 59% to 65% (p=0.05, Figure 1, Panel B) as did the proportion of HIV-infected women reporting ARV prophylaxis for their HIV-exposed infant (63% to 67%, p=0.08). Coverage of cotrimoxazole increased 15 percentage points and receipt of early infant diagnosis results increased 11 percentage points.
FIGURE 1.
The PMTCT cascade in Zimbabwe, 2012–2014. The percentages for each step are the weighted proportion of the total number of all women with a recent birth (Panel A) or HIV-infected women and their HIV-exposed infants (Panel B) in the survey receiving each service. Asterisks (*) indicate statistically significant differences at the alpha=0.05 level. Analysis restricted to biological mothers and their eligible infants.
Geospatial Analysis (2012–2014)
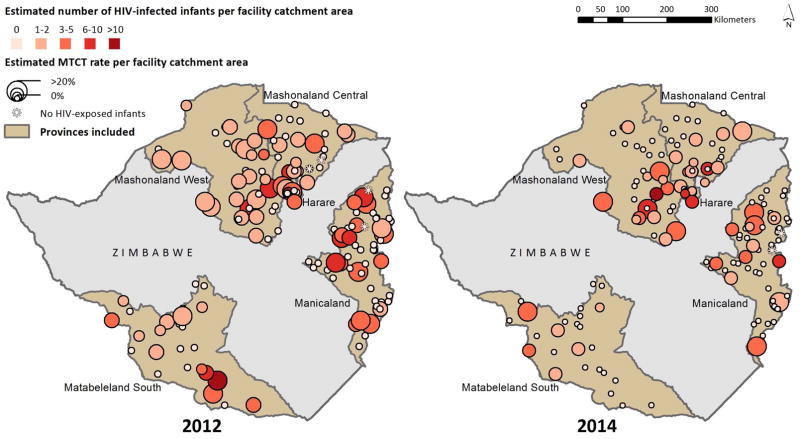
In 2014, MTCT varied by catchment area (median: 0%, mean: 4.9%, interquartile range (IQR): 0–10%). The estimated number of HIV-infected infants 9–18 months of age also varied by catchment area (median: 0, mean: 1.1, IQR: 0–1.0). In 2014, 68% of CAs had no MTCT. Overall, visual inspection of the maps depicted a reduction in both the catchment-level MTCT rate and the number of infected infants between 2012 and 2014 (Figure 2). This change was particularly evident in Matabeleland South and part of Mashonaland West. Nevertheless, there were a few sites where the MTCT rate and/or the number of HIV-infected infants increased. In 2014, “hotspots” containing catchment areas with both high MTCT rates and high numbers of HIV-infected infants were clustered in Harare, eastern Mashonaland West, and western Manicaland. Overall, 65 (41%) catchment areas had the same MTCT rate as in 2012, 55 (35%) had a lower MTCT rate, and 37 (24%) had a higher MTCT rate.
FIGURE 2.
Estimated number of HIV-infected infants and MTCT rate in 156 selected health facility catchment areas in five provinces in Zimbabwe, 2012 (Panel A) and 2014 (Panel B). Data from pregnant women and their infants were linked to health facility catchment area; their inclusion in a particular area does not necessarily imply that the mother-infant pair received care at that facility, or received care at all.
Priority Geographic Areas in 2014 and Maternal ART/ARV Coverage
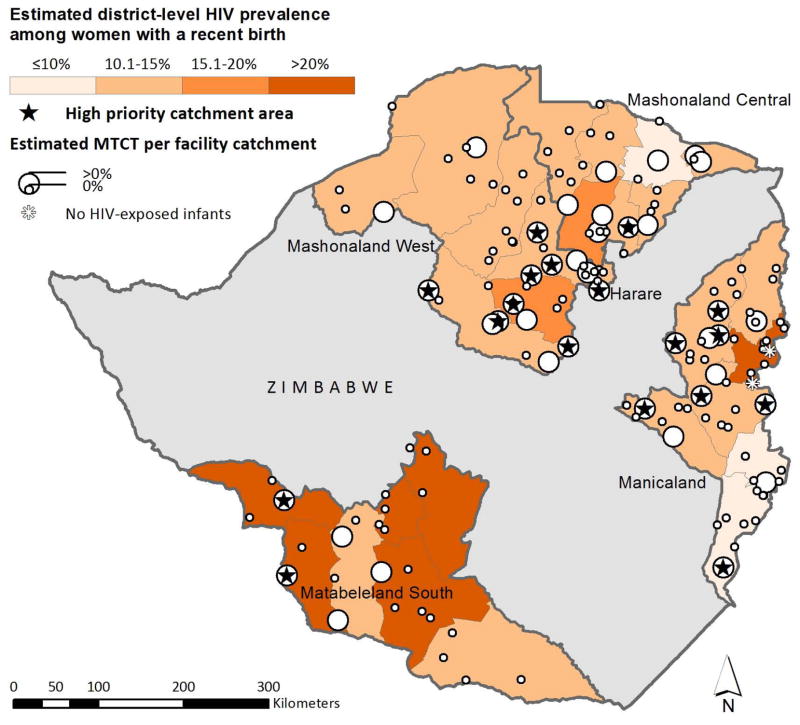
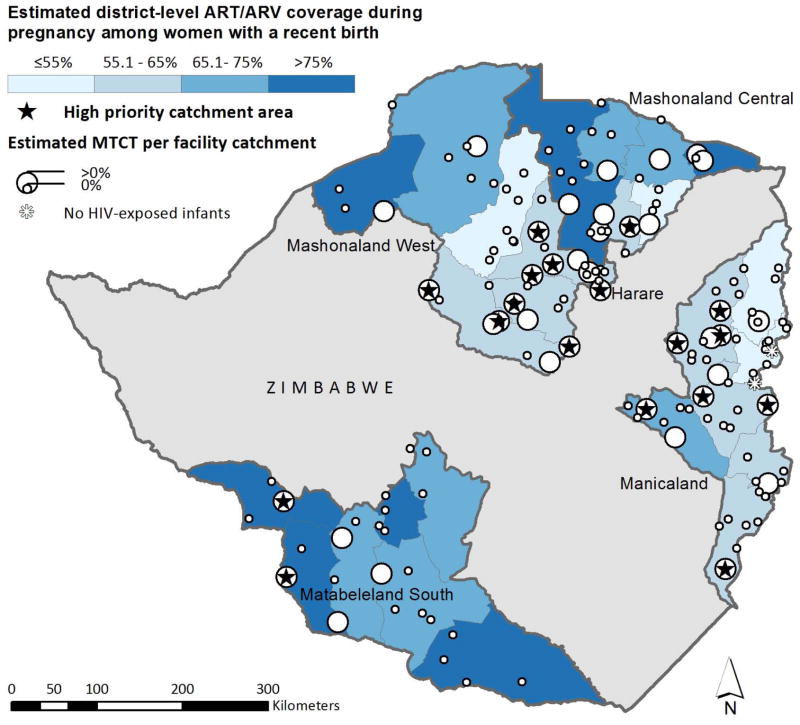
Overall, 18 (11%), 33 (21%), and 106 (68%) catchment areas were classified into priority levels ‘high’, ‘moderate’, and ‘low’, respectively (Table 2). High priority catchment areas were generally located within districts with greater than 15% HIV prevalence (Figure 3a) and/or below 65% maternal ARV coverage (Figure 3b). Self-reported coverage of maternal ART/ARV prophylaxis during pregnancy was non-significantly associated with catchment area priority level; HIV-infected pregnant women living in the highest priority areas self-reported lower coverage of ART/ARVs (57%) compared to women in the lowest priority areas (68%, p=0.17). The maps also revealed some sites with no MTCT and no infant infections despite being located in areas of high HIV prevalence and/or low maternal ART/ARV coverage.
Table 2.
Categorization of 157 catchment areas into priority levels for PMTCT service intensification, Zimbabwe, 2014.
| Priority level | Definition | No. (%) catchment areas |
|---|---|---|
| High | ≥ 3 HIV infected infants 9–18 months of age and MTCT ≥ 10%a | 18 (11%) |
| Moderate | 1–2 HIV-infected infants 9–18 months of age or 0 ≤ MTCT < 10% | 33 (21%) |
| Low | No (0) HIV-infected infants 9–18 months of age and MTCT = 0% | 106 (68%) |
The MTCT ≥ 10% cut point was the 75th percentile of the MTCT distribution.
FIGURE 3.
Estimated number of HIV-infected infants and MTCT rate in 156 selected health facility catchment areas in five provinces in Zimbabwe, 2014. High priority catchment areas (as determined with our algorithm) are labeled with a star. Panel A displays HIV prevalence in the background, Panel B displays maternal ART/ARV coverage in the background.
DISCUSSION
These data from Zimbabwean women sampled from the community suggest that MTCT in the five selected provinces continues to decrease, from 8.8% in 2012[8] to 6.7% in 2014. Thus, eMTCT may be within reach when countrywide data are examined. Furthermore, utilization of PMTCT-related services increased from 2012 to 2014, with improvements in institutional delivery, infant receipt of cotrimoxazole, and early infant diagnosis, some of the main gaps in the PMTCT cascade in 2012.[15] Other services, like ≥1 ANC visit, HIV testing during pregnancy or labor and delivery, and postnatal care, are approaching universal coverage. However, there remain services where further efforts are needed to increase engagement and retention as Zimbabwe approaches eMTCT. In particular, although the coverage of both maternal and infant ART/ARV prophylaxis improved over time, about a third of HIV-infected women reported that they and their infants did not receive ART/ARV prophylaxis in 2014.
Geospatial analysis and visualization provides a powerful tool for identifying regional variability of local MTCT rates and the number of pediatric infections and communicating these findings to programs and policymakers. Although from 2012–2014 we observed reduced MTCT and/or infant infections in some areas, there were both new and persistent “hotspots” in 2014. These catchment areas — clustered around Harare, eastern Mashonaland West, southern Mashonaland Central and Manicaland — were ranked as the highest priority for intensifying HIV prevention services to achieve and maintain eMTCT. Furthermore, high priority catchment areas also had lower levels of reported maternal ART/ARVs, indicating an opportunity for immediate intervention. However, it is important to note that these data are from five of the ten provinces in Zimbabwe, and a subset of catchment areas within these five provinces; other data sources are needed to understand the situation countrywide. Although imperfect, the application of a geospatial approach using routinely collected facility-based monitoring and evaluation data could complement community surveys like the one used in this analysis. Although monitoring and evaluation data are limited to women who seek healthcare services during pregnancy, they benefit from being verifiable with the medical record and may therefore reveal different patterns for individual health facilities.
Geovisualization indicates that there may be several areas in Zimbabwe, notably the comparatively rural sub-regions of Mashonaland West and Central, where contiguous catchment areas have lower MTCT rates and are located in districts with low levels of HIV-infected infants. Although some may argue that service provision could potentially be consolidated in these areas to maximize efficiency, the fact that some of these sites are located in areas with high maternal HIV prevalence suggests that consolidation or removal of PMTCT services in these areas would likely be highly detrimental to eMTCT efforts. Instead of consolidation, these sites might be considered as sources of “best practices” to guide other sites toward eMTCT amidst high levels of circulating HIV.
Importantly, our analyses linked MTCT in the community with the health facility catchment area. This does not necessarily mean that all pregnant women received care at that health facility, or received care at all (for example, in 2012 35% of women received ANC in a facility outside their place of residence, data not shown). Although HIV-infected pregnant women are encouraged to deliver in a health facility, those who deliver at home are advised to go to the nearest health facility to register the infant in order to obtain a child health card as well as post-partum ARVs. Thus, these data do not necessarily single out individual health facilities as having ‘failed’ in service provision; rather, the data indicate geographic areas where the supply and/or demand for PMTCT services should be intensified, which might require interventions targeted to the clinic or the community, or both. Future analyses with additional data sources could identify catchment-level factors associated with low and/or persistently high MTCT rates.
As Zimbabwe approaches eMTCT, new strategies may be required that are more targeted than previous one-size-fits-all approaches (which have been highly successful[19]). For example, one possible new approach is to dispatch response teams to visit MTCT hotspots in order to conduct rapid assessments and brainstorm and implement solutions. Supply-side factors might be remediated by additional staff or training, supply chain strengthening, service integration, stronger engagement of village health workers,[20] or performance-based financing.[21] Demand side factors might be addressed with, for example, transportation vouchers, incentives (e.g., for institutional delivery)[22], mother-to-mother groups, or community engagement.[23] Likewise, active case finding coupled SMS notification could minimize delays after early infant diagnosis.[24] In addition to these new strategies, the current strong foundation of PMTCT services must remain in place to sustain the accomplishments of the past decade.
This analysis has several important limitations.[14, 15] First, utilization of PMTCT services was self-reported. Although these reports were not verified against medical records, previous analyses demonstrate their association with MTCT, strengthening confidence in their validity.[15] Second, women and infants’ HIV status was measured 9–18 months postpartum; thus, some women may have been infected after their infant was born (biasing coverage estimates downward) and we may not have captured all possible infant infections (78% of HIV-exposed infants were still breastfeeding at the time of the survey, data not shown). Some women who migrated during pregnancy (e.g., to deliver in her parent’s community) may not have returned home by 9–18 months to be included in the survey, although customarily this would happen within a few weeks post delivery. In addition, only living infants were tested for HIV although we collected verbal autopsies for deceased infants to assess whether their deaths are attributable to HIV/AIDS. Lastly, the study was not designed to statistically distinguish MTCT rates and the number of HIV-infected infants between catchment areas. However, as Zimbabwe approaches eMTCT, decisions about human and financial resources will have to be made based on sparse data. Thus, these data simulate the real-world scenario of approaching the elimination target.
Zimbabwe has made impressive progress towards eMTCT. The analysis presented here suggests that periodic community-based surveys can complement to facility-based data by monitoring progress among all women, not only those who enter the health system. Furthermore, a geospatial approach to monitoring can identify weaker performance sites or regions where enhanced community-based efforts are warranted. Data from the ongoing population-based HIV impact assessments in Zimbabwe, Malawi, Zambia, and Uganda should be well-suited to this purpose.[25] Together, these approaches can guide efforts during the last mile toward eMTCT.
Acknowledgments
SOURCES OF SUPPORT: The evaluation of Zimbabwe’s Accelerated National PMTCT Program was supported by the Children’s Investment Fund Foundation (CIFF) and NIH/NICHD (Padian R01HD080492). Dr. McCoy is supported by Award Number K01MH094246 from the National Institute of Mental Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health or the National Institutes of Health.
Footnotes
Catchment areas are defined by the boundaries of the administrative ward(s), which is a subdivision of district. Typically, catchment areas of health facilities at the same level have comparable population sizes.
PREVIOUS PRESENTATIONS: The preliminary results from this study were presented in 2015 by Dr. McCoy and Dr. Cowan at the 8th IAS Conference on HIV Pathogenesis, Treatment and Prevention in Vancouver, Canada.
AUTHOR CONTRIBUTIONS
Conceived and designed the impact evaluation: NP FC RB SM. Analyzed the data presented in this manuscript: SM CF RB. Wrote the first draft of the manuscript: SM. Instrumental to the design of the study: AM AM. Provided substantial contributions to the manuscript: FC NP RB AM AM CF.
References
- 1.Joint United Nations Programme on HIV/AIDS (UNAIDS) Global Plan Towards the Elimination of New HIV Infections Among Children By 2015 and Keeping their Mothers Alive, 2011–2015. Geneva: 2011. [Google Scholar]
- 2.Hayashi C. AIDS 2014. Melbourne: 2014. eMTCT Validation: Criteria, Process, Tools, Current Status. [Google Scholar]
- 3.World Health Organization. Global guidance on criteria and processes for validation: Elimination of mother-to-child transmission of HIV and syphillis. Geneva: 2014. [Google Scholar]
- 4.Lyons C. The BLOG. New York City: The Huffington Post; 2012. Zimbabwe’s Great Leap Toward Preventing HIV in Children. [Google Scholar]
- 5.UNAIDS. UNAIDS highlights Zimbabwe’s progress in responding to AIDS. Geneva: 2012. [Google Scholar]
- 6.Zimbabwe Ministry of Health and Child Welfare. Global AIDS Response Country Progress Report: Zimbabwe 2014. Harare: 2014. [Google Scholar]
- 7.World Health Organization. Global Update on HIV Treatment 2013: Results, Impact, and Opportunities. Geneva: WHO report in partnership with UNICEF and UNAIDS; 2013. [Google Scholar]
- 8.Buzdugan R, McCoy SI, Watadzaushe C, Kang Dufour MS, Petersen M, Dirawo J, et al. Evaluating the Impact of Zimbabwe’s Prevention of Mother-to-Child HIV Transmission Program: Population-Level Estimates of HIV-Free Infant Survival Pre-Option A. PLoS One. 2015;10:e0134571. doi: 10.1371/journal.pone.0134571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.UNAIDS. 2014 Progress report on the Global Plan towards the elimination of new HIV infections among children by 2015 and keeping their mothers alive. Geneva: 2014. [Google Scholar]
- 10.Office of the U.S. Global AIDS Coordinator. PEPFAR 3.0: Controlling the Epidemic: Delivering on the Promise of an AIDS-Free Generation. Washington, D.C: 2014. [Google Scholar]
- 11.World Health Organization. Antiretroviral Drugs for Treating Pregnant Women and Preventing HIV Infection in Infants: Recommendations for a public health approach. Geneva: 2010. [PubMed] [Google Scholar]
- 12.World Health Organization. Use of Antiretroviral Drugs for Treating Pregnant Women And Preventing HIV Infection in Infants. Geneva: 2012. Programmatic Update. [Google Scholar]
- 13.Buzdugan R, McCoy SI, Petersen M, Guay LA, Mushavi A, Mahomva A, et al. Feasibility of population-based cross-sectional surveys for estimating vertical HIV transmission: data from Zimbabwe. 7th IAS Conference on HIV Pathogenesis, Treatment and Prevention; Kuala Lumpur. 2013. [Google Scholar]
- 14.McCoy SI, Buzdugan R, Ralph LJ, Mushavi A, Mahomva A, Hakobyan A, et al. Unmet Need for Family Planning, Contraceptive Failure, and Unintended Pregnancy among HIV-Infected and HIV-Uninfected Women in Zimbabwe. PLoS One. 2014;9:e105320. doi: 10.1371/journal.pone.0105320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCoy SI, Buzdugan R, Padian NS, Musarandega R, Engelsmann B, Martz TE, et al. Implementation and Operational Research: Uptake of Services and Behaviors in the Prevention of Mother-to-Child HIV Transmission Cascade in Zimbabwe. J Acquir Immune Defic Syndr. 2015;69:e74–81. doi: 10.1097/QAI.0000000000000597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Health Organization. Antenatal Care Randomized Trial: Manual for the Implementation of the New Model. Geneva: 2002. [Google Scholar]
- 17.United Nations Department of Economic and Social Affairs. World Contraceptive Use 2011. United Nations: 2011. [Google Scholar]
- 18.World Health Organization. Guidelines on HIV and infant feeding: Principles and recommendations for infant feeding in the context of HIV and a summary of evidence. 2010. [PubMed] [Google Scholar]
- 19.UNAIDS. Report on the Global AIDS Epidemic. Geneva: 2013. [Google Scholar]
- 20.Chang LW, Kagaayi J, Nakigozi G, Ssempijja V, Packer AH, Serwadda D, et al. Effect of peer health workers on AIDS care in Rakai, Uganda: a cluster-randomized trial. PloS one. 2010;5:e10923. doi: 10.1371/journal.pone.0010923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Walque D, Gertler PJ, Bautista-Arredondo S, Kwan A, Vermeersch C, de Dieu Bizimana J, et al. Using provider performance incentives to increase HIV testing and counseling services in Rwanda. J Health Econ. 2015;40:1–9. doi: 10.1016/j.jhealeco.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 22.Gopalan SS, Durairaj V. Addressing maternal healthcare through demand side financial incentives: experience of Janani Suraksha Yojana program in India. BMC Health Serv Res. 2012;12:319. doi: 10.1186/1472-6963-12-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ackerman Gulaid L, Kiragu K. Lessons learnt from promising practices in community engagement for the elimination of new HIV infections in children by 2015 and keeping their mothers alive: summary of a desk review. J Int AIDS Soc. 2012;15(Suppl 2):17390. doi: 10.7448/IAS.15.4.17390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seidenberg P, Nicholson S, Schaefer M, Semrau K, Bweupe M, Masese N, et al. Early infant diagnosis of HIV infection in Zambia through mobile phone texting of blood test results. Bull World Health Organ. 2012;90:348–356. doi: 10.2471/BLT.11.100032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.ICAP. Population-based HIV Impact Assessments. New York, New York: Columbia University, Mailman School of Public Health; [Google Scholar]