Abstract
Objective
We characterized independent and joint impacts of maternal race/ethnicity and obesity on adverse birth outcomes, including preeclampsia, low birthweight (LBW), and macrosomia.
Methods
Retrospective cohort study of all 2007 California births using vital records and claims data. Maternal race/ethnicity and maternal BMI were the key exposures; we analyzed their independent and joint impact on outcomes using regression models.
Results
Racial/ethnic minority women of normal weight generally had higher risk as compared to white women of normal weight (e.g., African-American women, preeclampsia aOR, 1.60, 95% CI: 1.48 – 1.74, versus white women). However, elevated BMI did not usually confer additional risk (e.g., preeclampsia aOR comparing African-American women with morbid obesity to white women with morbid obesity; 1.17, 95% CI: 0.89 – 1.54). Obesity was a risk factor for LBW only among white women (morbid obesity aOR, 95% CI: 1.24, 1.04 – 1.49, versus white women of normal weight), and not among racial/ethnic minority women (e.g., African-American women, 0.95, 0.83 – 1.08).
Conclusions
These findings add nuance to our understanding of the interplay between maternal race/ethnicity, BMI, and perinatal outcomes. While the BMI/adverse outcome gradient appears weaker in racial/ethnic minority women, this reflects the overall risk increase in racial/ethnic minority women of all body sizes.
Keywords: Birth outcomes, maternal obesity, racial disparities, perinatal complications
Introduction
It is firmly established that maternal overweight and obesity are risk factors for a host of adverse perinatal outcomes, including gestational diabetes, preeclampsia, cesarean delivery, and stillbirth.(1, 2, 3, 4, 5) There are also well-documented racial/ethnic disparities in perinatal outcomes, with African-American women generally having higher rates of adverse outcomes.(6) Hispanic women are at elevated risk of some adverse perinatal outcomes (e.g., gestational diabetes), but decreased risk for others (e.g., preterm birth).(6, 7) Asian-American women are at increased risk for some perinatal outcomes (e.g., severe perineal lacerations), but not all.(6, 8) Racial disparities are also observed in prevalence of obesity: African Americans, Hispanics, and Pacific Islanders have a higher prevalence compared to whites, and Asian-Americans have a lower prevalence.(9) Despite these well-documented disparities, there are relatively few studies examining the joint impact of maternal race/ethnicity and obesity on a variety of perinatal outcomes.(10, 11)
There is reason to believe that the impact of maternal obesity on perinatal outcomes may not be uniform across race/ethnicity groups. In the case of gestational diabetes mellitus (GDM), it has been shown that Hispanic and especially Asian-American women are at elevated risk within a lower body mass index (BMI) range than their white counterparts, although outcomes vary amongst Hispanic and Asian-American ethnicities.(12, 13) Conversely, African-American women exhibit lower prevalence of GDM than white women with comparable BMI. Understanding the separate and joint contributions of maternal obesity and race/ethnicity to other adverse birth outcomes would provide public health professionals and clinicians with crucial information, helping tailor preventive messages and obstetric care to the women at the highest risk for adverse outcomes.
We aimed to help fill this evidence gap by examining the independent and joint impacts of maternal race/ethnicity and obesity on several adverse birth outcomes, including GDM, preeclampsia, low birthweight (LBW), and macrosomia. We analyzed a large administrative database of California births, containing a sufficient sample of racially diverse women to enable several stratifications. Outcomes were compared across and within racial categories of white, African-American, and Hispanic women in keeping with prior studies,(10, 12) as well as Asian-American women. We hypothesized there would be effect modification such that maternal obesity would confer greater odds of adverse outcomes among some subgroups of women relative to others.
Methods
Data source
This was a retrospective cohort study of all births occurring in the state of California in 2007. We analyzed the All-California, Rapid-Cycle, Maternal/Infant Database, which contains linked vital statistics data and hospital discharge data for all California deliveries. The dataset contains birth certificate data linked to maternal hospital discharge data for the nine months prior to birth and maternal/infant hospital discharge data for the year after birth. Mother-baby pairs were linked using probabilistic linkage methods. Details of the dataset have been published elsewhere.(14)
Exposure variables
Maternal race/ethnicity and maternal BMI were the key exposure variables; we were interested in their independent and joint impact on adverse perinatal outcomes. Using data from the birth certificate, we classified maternal race/ethnicity into the following mutually-exclusive categories: non-Hispanic white (n=123,150), non-Hispanic black (n=18,729), Hispanic (n=203,861), and Asian-American/Pacific Islander (n=39,667). Women who did not fall into these 4 categories (including American Indian/Alaska Native, multiracial, and other) were not analyzed here, due to concerns for heterogeneity in this subpopulation. Maternal BMI was derived from self-reported pre-pregnancy weight and self-reported height, and was then categorized using the WHO BMI categories (15): normal weight women (BMI 18.5 – 24.9 kg/m2), women who are overweight (25.0 – 29.9), women with obesity (30.0 – 39.9), and women with morbid obesity (BMI ≥ 40.0). Underweight women (BMI < 18.5 kg/m2) were excluded from analysis, as our focus was gradations of overweight/obesity, as compared to normal weight. Although there is evidence that further stratification of morbid obesity yields important health insights (e.g., super-obesity (16)), we analyzed only class I obesity and morbid obesity to preserve power to analyze rare outcomes.
Maternal height and weight data were derived from self-reported numbers on the birth certificate. Maternal weight data on birth certificates have noted shortcomings,(17) so we performed data cleaning prior to analysis. We excluded records that were missing for maternal weight (N=56,926) and maternal height (N=12,478); our final analytic sample comprised 385,407 women.
Outcome variables
We were interested in the impact of maternal race/ethnicity and BMI on a range of maternal and infant outcomes. The specific maternal outcomes we analyzed were: gestational diabetes mellitus (GDM), and preeclampsia. Both were defined by International Classification of Diseases, 9th Revision- Clinical Modification (ICD-9) codes, as recorded in the maternal hospital discharge data (GDM: 775.0, 648.80, 648.81, 648.82, 648.83, 648.84; preeclampsia: 401.0, 642.40, 642.41, 642.42, 642.43, 642.44, 642.50, 642.51, 642.52, 642.53, 642.54, 642.70, 642.71, 642.72, 642.73, 642.74). These outcome definitions contain diagnoses of both mild and severe preeclampsia, and the variety of gestational diabetes and abnormal glucose tolerance in the mother that are captured in claims data. We analyzed primary cesarean delivery, using mode of delivery as recorded on the birth certificate. Women with prior cesarean (defined from the birth certificate and ICD-9 codes) were excluded from the denominator for this outcome. Infant outcomes were preterm birth (gestational age <37 completed weeks), macrosomia (birthweight >4,000 grams [g]), and low birthweight (birthweight <2,500g). Birthweight and gestational age were recorded on the birth certificate. The clinical/obstetric estimate of gestational age was analyzed, as it is a more valid measure of pregnancy dating that last menstrual period dating.(18)
Analysis
We used descriptive statistics to characterize the demographic profile of our sample of California births. In addition to maternal race/ethnicity and BMI category, we described the parity, educational background, insurance status, and prenatal care initiation of our sample.
We analyzed unadjusted associations between BMI and perinatal outcomes, stratified by race/ethnicity, by graphing the prevalence of each outcome across increasing categories of BMI. For each outcome, we graphed white women, black women, Hispanic women, and Asian-American women using a unique line.
We employed several multivariable logistic regression modeling approaches to assess the independent and joint impact of maternal race/ethnicity and maternal BMI on perinatal outcomes. To analyze the independent effect of race/ethnicity and of maternal BMI on perinatal outcomes, we first fit a basic model (model 1) on our analytical sample, with indicator variables for maternal race/ethnicity (referent category: white) and maternal BMI category (referent: normal weight). We controlled for potential confounding variables including advanced maternal age (>35 years), maternal educational attainment (≥12 years versus <12), parity (nulliparous versus multiparous), maternal insurance status (private versus public/none), and prenatal care initiation (first trimester versus later/none).
Although it is common to control for gestational age in such analyses, recent methodologic advances in epidemiology have demonstrated that when gestational age is on the causal pathway between exposure and outcome (as it is in this research question), controlling for gestational age (1) blocks part of the causal effect of interest between exposure and outcome, and (2) introduces additional bias of an unpredictable nature when there is unmeasured confounding between gestational age and outcomes (as there is likely to be here)(19, 20). Therefore, we chose not to control for gestational age in regression models.
To dig deeper into the joint effects of race/ethnicity and BMI and test for interaction effects, we then fit stratified models. In model 2, we fit 2-race models comparing the risk of adverse outcomes among racial/ethnic minority women compared to white women, stratified by BMI category. For each outcome, a model was fit comparing normal weight African-American women to normal weight white women, African-American women who are overweight to white women who are overweight, and so on. This process was repeated with BMI-stratified Hispanic and Asian-American women, in all cases comparing outcomes to the referent category (white women of the same BMI status). This enabled us to isolate the impact of maternal race/ethnicity on outcomes, assessing whether this effect varied by BMI status (i.e., effect modification).
Our third and final modeling approach examined the converse association, analyzing the impact of maternal BMI category on perinatal outcomes, within racial/ethnic groups. For each outcome, we fit a model among white women, with indicator variables for women with obesity, and then for women with morbid obesity BMI category (referent category: normal weight and overweight combined). We repeated this process with African-American women, then Hispanic women, then Asian-American women. This enabled us to assess whether the impact of maternal obesity on perinatal outcomes differed by racial/ethnic group. We controlled for the same confounders in models 2 and 3 as in model 1.
We obtained human subjects approval from the Institutional Review Board at Oregon Health & Science University and the California Maternity Quality Care Collaborative. Data management and analysis was conducted using Stata (version 12, StataCorp; College Station, TX) and R (version 3.2.0, R Foundation for Statistical Computing; Vienna, Austria).
Results
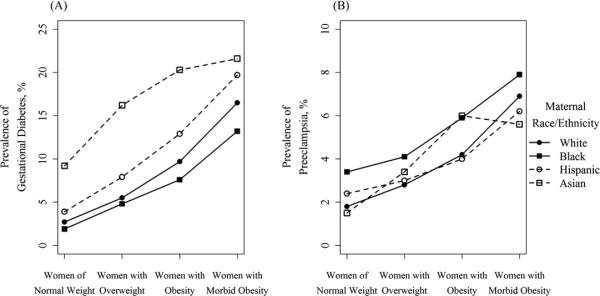
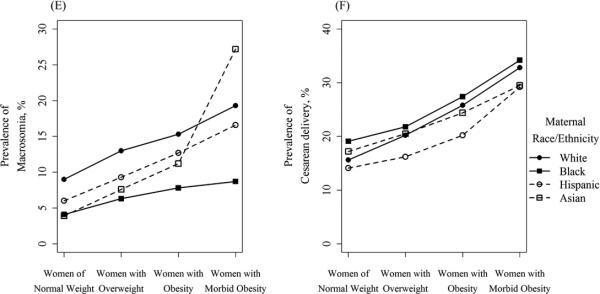
Our analytical sample comprised 385,407 women who delivered in California in 2007. There was a majority of Hispanic women (52.9%), with substantial proportions of white women (32.0%), Asian-American women (10.3%), and 4.9% African-American women (Table 1). Among all women, 53.5% were classified as normal weight by the WHO definitions, with higher proportions of white and Asian-American women being normal-weight (58.7% and 76.9%, respectively), as compared to Hispanic and African-American women (46.6% and 45.0%, respectively). 26.7% of the analytical sample were women with overweight BMI status, and women with obesity comprised 17.1% of the sample, with prevalence of obesity highest in African-American women (22.3%) and Hispanic women (20.3%), as compared to white women (14.9%) and Asian-American women (5.6%). Morbid obesity was observed in a small minority of women (2.6%), with a notably higher rate of morbid obesity in African-American women (5.7%). Unadjusted prevalence of all outcomes, by race/ethnicity and BMI category, are presented in Figure 1.
Table 1.
Demographic characteristics of California women delivering in 2007 (N [%])
| Total N= 385,407 | White N= 123,150 | Black N= 18,729 | Hispanic N= 203,861 | Asian-American N= 39,667 | |
|---|---|---|---|---|---|
| Race/ethnicity | |||||
| White | 123,150 (32.0) | ||||
| Black | 18,729 (4.9) | ||||
| Hispanic | 203,861 (52.9) | ||||
| Asian-American | 39,667 (10.3) | ||||
| BMI category | |||||
| Normal weight | 206,303 (53.5) | 72,285 (58.7) | 8,419 (45.0) | 95,085 (46.6) | 30,514 (76.9) |
| Overweight | 102,930 (26.7) | 29,324 (23.8) | 5,072 (27.1) | 61,777 (30.3) | 6,757 (17.0) |
| BMI 30-40 | 65,993 (17.1) | 18,313 (14.9) | 4,173 (22.3) | 41,273 (20.3) | 2,234 (5.6) |
| BMI > 40 | 10,181 (2.6) | 3,228 (2.6) | 1,065 (5.7) | 5,726 (2.8) | 162 (0.4) |
| Nulliparous | 147,591 (38.3) | 52,450 (42.6) | 7,453 (39.8) | 69,951 (34.3) | 17,737 (44.7) |
| Education (≥12 yrs) | 166,420 (44.6) | 79,275 (65.3) | 8,314 (45.2) | 48,465 (24.9) | 30,366 (77.9) |
| Age (≥35 yrs) | 65,756 (17.1) | 27,103 (22.0) | 2,337 (12.5) | 25,539 (12.5) | 10,777 (27.2) |
| Non-private insurance | 182,773 (48.2) | 31,575 (26.7) | 11,015 (60.0) | 132,058 (64.9) | 8,125 (20.8) |
| Prenatal care 1st tri. | 314,831 (82.8) | 104,952 (86.4) | 14,439 (78.4) | 161,408 (80.3) | 34,032 (86.9) |
| Prior cesarean | 59,274 (15.4) | 16,815 (13.7) | 3,010 (16.1) | 34,063 (16.7) | 5,386 (13.6) |
| Chronic hypertension | 4,313 (1.1) | 1,394 (1.1) | 533 (2.9) | 1,896 (0.9) | 490 (1.2) |
| Preexisting diabetes | 2,656 (0.7) | 641 (0.5) | 151 (0.8) | 1,604 (0.8) | 260 (0.7) |
Figure 1.
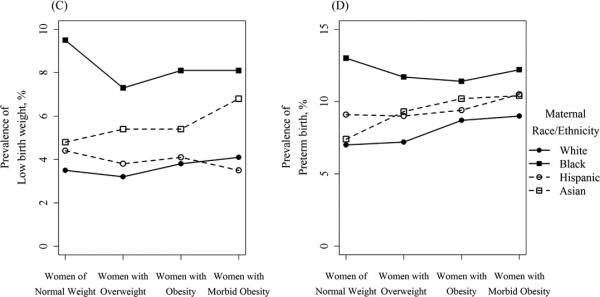
Unadjusted prevalence of adverse birth outcomes by BMI category, stratified by maternal race/ethnicity
Model 1 assessed the independent effects of maternal race/ethnicity and BMI category on perinatal outcomes. Black race was a risk factor for all perinatal outcomes compared to white women (e.g., preeclampsia adjusted odds ratio [aOR], 95% confidence interval [CI]: 1.60, 1.48 – 1.74), except for GDM and macrosomia, for which black race was protective (macrosomia aOR, 95% CI: 0.46, 0.43 – 0.50; Table 2). Black race was most strongly associated with low birthweight (aOR, 95% CI: 2.53, 2.38 – 2.70). Hispanic ethnicity was a risk factor for all outcomes, as compared to white women (e.g., GDM aOR, 95% CI: 1.53, 1.47 – 1.58), except for macrosomia, for which it was protective. Asian-American ethnicity was most strongly associated with GDM (aOR, 95% CI: 3.08, 2.95 – 3.22), and was protective against macrosomia and preeclampsia (preeclampsia aOR, 95% CI: 0.91, 0.84 – 0.98). Compared to other racial/ethnic groups, non-Hispanic white women were at increased risk for macrosomia.
Table 2.
Multivariable adjusted* independent impact of maternal race/ethnicity and maternal BMI category on perinatal outcomes (model 1)
| Maternal race/ethnicity | Maternal BMI category | |||||||
|---|---|---|---|---|---|---|---|---|
| White | Black | Hispanic | Asian-American | Women of normal weight | Women with overweight | Women with obesity | Women with morbid obesity | |
| GDM | Ref. | 0.86 (0.8 - 0.93) | 1.53 (1.47 - 1.58) | 3.08 (2.95 - 3.22) | Ref. | 2.04 (1.97 - 2.11) | 3.56 (3.43 - 3.68) | 6.27 (5.91 - 6.64) |
| Preeclampsia | Ref. | 1.60 (1.48 - 1.74) | 1.18 (1.12 - 1.24) | 0.91 (0.84 - 0.98) | Ref. | 1.61 (1.53 - 1.69) | 2.38 (2.26 - 2.51) | 3.80 (3.48 - 4.15) |
| Primary CD | Ref. | 1.34 (1.28 - 1.40) | 1.04 (1.01 - 1.06) | 1.06 (1.02 - 1.10) | Ref. | 1.46 (1.42 - 1.49) | 2.14 (2.08 - 2.20) | 3.40 (3.21 - 3.60) |
| PTB | Ref. | 1.65 (1.56 - 1.74) | 1.15 (1.12 - 1.19) | 1.12 (1.07 - 1.17) | Ref. | 0.99 (0.96 - 1.02) | 1.07 (1.04 - 1.11) | 1.18 (1.10 - 1.27) |
| Macrosomia | Ref. | 0.46 (0.43 - 0.50) | 0.76 (0.74 - 0.78) | 0.47 (0.45 - 0.49) | Ref. | 1.56 (1.52 - 1.61) | 2.09 (2.03 - 2.15) | 2.76 (2.60 - 2.92) |
| LBW | Ref. | 2.53 (2.38 - 2.70) | 1.17 (1.12 - 1.22) | 1.46 (1.38 - 1.55) | Ref. | 0.92 (0.88 - 0.96) | 1.03 (0.98 - 1.07) | 1.01 (0.91 - 1.11) |
Results in bold indicate statistical significance (P<0.05)
Results are adjusted odds ratio (95% confidence interval). Multivariable logistic regression models controlled for advanced maternal age, maternal education (≥12 years versus <12), parity (nulliparous versus parous), public insurance status, and prenatal care initiation in 1st trimester.
GDM, gestational diabetes mellitus; CD, Cesarean delivery; PTB, preterm birth; LBW, low birth weight
Maternal overweight and obesity were risk factors for all adverse outcomes as compared to women of normal weight, with risk increasing in a dose-response fashion with increasing BMI, with the exception of low birthweight, which was generally not associated with maternal BMI category.
When comparing adverse outcomes between racial minority women and white women, elevated BMI status did not generally confer additional risk beyond the risk associated with maternal race/ethnicity. For example, normal-weight Hispanic women had elevated odds of GDM compared to normal-weight white women (aOR, 95% CI: 1.63, 1.53 – 1.75), as did normal-weight Asian-American women (aOR, 95% CI: 3.48, 3.28 – 3.70, Table 3). Both of these racial/ethnic disparities attenuated as BMI increased. Among Hispanic women with morbid obesity, odds of GDM were 30% increased relative to their white counterparts with morbid obesity (aOR, 95% CI: 1.30, 1.15 – 1.47), and among Asian-American women with obesity, the odds of GDM were increased 2-fold compared to white women with obesity (aOR, 95% CI: 2.26, 2.01 – 2.54). Similarly attenuated racial disparities were observed for preeclampsia and primary cesarean in Black and Hispanic women, as BMI category increased. Normal-weight Asian-American women had decreased odds of preeclampsia compared to normal-weight white women (aOR, 95% CI: 0.79, 0.71 – 0.88), an association that reversed among Asian-American women with obesity (aOR, 95% CI: 1.43, 1.18 – 1.74).
Table 3.
Adjusted odds ratios (95% CIs)* comparing adverse maternal outcomes among racial minority women to white women (referent group), stratified by BMI status (model 2)
| Black | Hispanic | Asian-American | ||
|---|---|---|---|---|
| GDM | Women of normal weight | 0.77 (0.65 - 0.92) | 1.63 (1.53 - 1.75) | 3.48 (3.28 - 3.70) |
| Women with overweight | 0.91 (0.78 - 1.05) | 1.51 (1.42 - 1.62) | 3.12 (2.87 - 3.40) | |
| Women with obesity | 0.84 (0.74 - 0.96) | 1.41 (1.32 - 1.50) | 2.26 (2.01 - 2.54) | |
| Women with morbid obesity | 0.72 (0.59 - 0.89) | 1.30 (1.15 - 1.47) | 1.32 (0.87 - 1.99) | |
| Preeclampsia | Women of normal weight | 1.79 (1.55 - 2.06) | 1.33 (1.23 - 1.44) | 0.79 (0.71 - 0.88) |
| Women with overweight | 1.53 (1.30 - 1.80) | 1.20 (1.09 - 1.31) | 1.18 (1.01 - 1.38) | |
| Women with obesity | 1.45 (1.24 - 1.70) | 1.03 (0.93 - 1.13) | 1.43 (1.18 - 1.74) | |
| Women with morbid obesity | 1.17 (0.89 - 1.54) | 0.91 (0.76 - 1.10) | 0.79 (0.38 - 1.64) | |
| Primary CD | Women of normal weight | 1.44 (1.35 - 1.55) | 1.10 (1.06 - 1.14) | 1.09 (1.05 - 1.14) |
| Women with overweight | 1.22 (1.11 - 1.33) | 1.00 (0.95 - 1.04) | 1.06 (0.98 - 1.15) | |
| Women with obesity | 1.24 (1.13 - 1.36) | 0.94 (0.89 - 0.99) | 0.93 (0.82 - 1.05) | |
| Women with morbid obesity | 1.18 (0.97 - 1.43) | 1.05 (0.93 - 1.20) | 0.91 (0.58 - 1.43) |
Results in bold indicate statistical significance (P<0.05)
models controlled for advanced maternal age, maternal education (≥12 years versus <12), parity (nulliparous versus parous), public insurance status, and prenatal care initiation in 1st trimester.
GDM, gestational diabetes mellitus; CD, Cesarean delivery
Not all outcomes displayed this pattern of attenuating racial disparities with increased BMI. Odds of macrosomia were decreased in all racial/ethnic groups as compared to white women, an associated that was largely constant across BMI categories (Table 4). Black and Asian-American women remained at increased risk for low birthweight in all weight categories, compared to white women (aORs ranging between 2.03 and 2.50 for black women, and 1.45 and 1.82 for Asian-American women; all but one significant at the P<0.05 level).
Table 4.
Adjusted odds ratios (95% CIs)* comparing adverse neonatal outcomes among racial minority women to white women (referent group), stratified by BMI status (model 2)
| Black | Hispanic | Asian-American | ||
|---|---|---|---|---|
| PTB | Women of normal weight | 1.71 (1.58 - 1.85) | 1.16 (1.11 - 1.22) | 1.10 (1.04 - 1.16) |
| Women with overweight | 1.62 (1.46 - 1.80) | 1.21 (1.14 - 1.29) | 1.37 (1.24 - 1.51) | |
| Women with obesity | 1.31 (1.16 - 1.47) | 1.06 (0.99 - 1.14) | 1.23 (1.05 - 1.44) | |
| Women with morbid obesity | 1.37 (1.09 - 1.74) | 1.10 (0.93 - 1.30) | 1.29 (0.75 - 2.21) | |
| Macrosomia | Women of normal weight | 0.50 (0.44 - 0.56) | 0.74 (0.71 - 0.77) | 0.41 (0.38 - 0.43) |
| Women with overweight | 0.49 (0.44 - 0.56) | 0.73 (0.70 - 0.77) | 0.52 (0.47 - 0.57) | |
| Women with obesity | 0.50 (0.44 - 0.57) | 0.82 (0.78 - 0.87) | 0.66 (0.57 - 0.76) | |
| Women with morbid obesity | 0.39 (0.31 - 0.50) | 0.84 (0.75 - 0.95) | 1.43 (0.98 - 2.09) | |
| LBW | Women of normal weight | 2.50 (2.28 - 2.74) | 1.16 (1.09 - 1.24) | 1.45 (1.35 - 1.55) |
| Women with overweight | 2.25 (1.96 - 2.57) | 1.23 (1.12 - 1.34) | 1.82 (1.59 - 2.07) | |
| Women with obesity | 2.25 (1.94 - 2.59) | 1.16 (1.05 - 1.28) | 1.49 (1.21 - 1.83) | |
| Women with morbid obesity | 2.03 (1.52 - 2.73) | 0.89 (0.70 - 1.14) | 1.72 (0.88 - 3.36) |
Results in bold indicate statistical significance (P<0.05)
models controlled for advanced maternal age, maternal education (≥12 years versus <12), parity (nulliparous versus parous), public insurance status, and prenatal care initiation in 1st trimester.
PTB, preterm birth; LBW, low birth weight
These results were confirmed by model 3, which analyzed the impact of maternal BMI among racial/ethnic groups. Obesity (as compared to normal weight status) conferred a greater increase in odds of GDM and preeclampsia among white women, as compared to racial minority women (Table 5). For example, white women with morbid obesity were at 5-fold increased risk of GDM as compared to their white counterparts with no obesity (aOR, 95% CI: 5.72, 5.16 – 6.33), whereas the increase was 4-fold for Hispanic women (4.17, 3.88 – 4.48) and 2.5-fold among Asian-American women (2.48, 1.66 – 3.70). Obesity and morbid obesity were risk factors for LBW only among white women compared to white women of normal weight (morbid obesity aOR, 95% CI: 1.24, 1.04 – 1.49); obesity was not associated with LBW among racial/ethnic minority women (e.g., aOR comparing Hispanic women with obesity to Hispanic women of normal weight, 95% CI: 1.02, 0.97 – 1.08). Obesity increased the odds of PTB among white women, Hispanic women, and Asian-American women, but not black women. Obesity increased the odds of macrosomia and primary cesarean delivery among women of all races/ethnicities. This association was fairly uniform, except that Asian-American women with morbid obesity had far increased odds of macrosomia as compared to their Asian-American counterparts with no obesity (aOR, 95% CI: 7.14, 4.93 – 10.36).
Table 5.
Adjusted odds ratios (95% CIs)* comparing adverse perinatal outcomes among women with obesity & women with morbid obesity, compared to women with no obesity (normal & overweight), stratified by maternal race/ethnicity
| White | Black | Hispanic | Asian-American | ||
|---|---|---|---|---|---|
| GDM | Women with obesity | 3.07 (2.88 - 3.26) | 2.65 (2.27 - 3.10) | 2.42 (2.33 - 2.51) | 2.30 (2.06 - 2.58) |
| Women with morbid obesity | 5.72 (5.16 - 6.33) | 4.52 (3.66 - 5.59) | 4.17 (3.88 - 4.48) | 2.48 (1.66 - 3.70) | |
| Preeclampsia | Women with obesity | 2.31 (2.12 - 2.52) | 1.80 (1.53 - 2.12) | 1.75 (1.65 - 1.86) | 3.85 (3.16 - 4.69) |
| Women with morbid obesity | 3.97 (3.43 - 4.61) | 2.48 (1.94 - 3.18) | 2.82 (2.51 - 3.17) | 3.67 (1.78 - 7.56) | |
| Primary CD | Women with obesity | 2.05 (1.96 - 2.15) | 1.76 (1.60 - 1.94) | 1.76 (1.70 - 1.82) | 1.93 (1.71 - 2.19) |
| Women with morbid obesity | 3.04 (2.76 - 3.35) | 2.36 (2.00 - 2.80) | 2.95 (2.73 - 3.18) | 2.92 (1.86 - 4.58) | |
| PTB | Women with obesity | 1.19 (1.12 - 1.26) | 0.89 (0.80 - 1.00) | 1.03 (0.99 - 1.07) | 1.30 (1.12 - 1.51) |
| Women with morbid obesity | 1.22 (1.07 - 1.39) | 0.96 (0.79 - 1.18) | 1.18 (1.08 - 1.29) | 1.36 (0.81 - 2.30) | |
| Macrosomia | Women with obesity | 1.64 (1.57 - 1.72) | 1.61 (1.40 - 1.86) | 1.77 (1.71 - 1.84) | 2.57 (2.22 - 2.97) |
| Women with morbid obesity | 2.16 (1.97 - 2.37) | 1.74 (1.37 - 2.20) | 2.40 (2.23 - 2.58) | 7.14 (4.93 - 10.36) | |
| LBW | Women with obesity | 1.13 (1.03 - 1.23) | 0.95 (0.83 - 1.08) | 1.02 (0.97 - 1.08) | 1.15 (0.95 - 1.40) |
| Women with morbid obesity | 1.24 (1.04 - 1.49) | 0.97 (0.77 - 1.22) | 0.91 (0.78 - 1.05) | 1.43 (0.75 - 2.74) |
Results in bold indicate statistical significance (P<0.05)
models controlled for advanced maternal age, maternal education (≥12 years versus <12), parity (nulliparous versus parous), public insurance status, and prenatal care initiation in 1st trimester.
GDM, gestational diabetes mellitus; CD, Cesarean delivery; PTB, preterm birth; LBW, low birth weight
Discussion
Consistent with prior literature,(3, 6, 14, 21, 22) we found racial/ethnic disparities in a variety of adverse outcomes and increased rates of adverse outcomes among women who are overweight and women with obesity. However, the joint impacts of maternal race/ethnicity and obesity were not uniform, varying among racial groups and also by the specific outcome being analyzed. For maternal outcomes and mode of delivery, there was evidence of racial disparities among normal-weight women, which attenuated or disappeared with increasing BMI category. This was the case with Asian-American women and GDM, African-American and Hispanic women and preeclampsia, and all racial/ethnic minority women and primary cesarean delivery. This suggests that while racial/ethnic disparities exist in these outcomes, white women who are overweight and white women with obesity have a steeper gradient of risk increase with increasing BMI, effectively leveling off racial/ethnic disparities at high BMIs. This trend was confirmed in the race/ethnicity-stratified models, which found that white women had generally higher odds ratios associated with obesity (e.g., for GDM, preeclampsia, and primary cesarean delivery), as compared to their racial/ethnic minority counterparts.
The picture was considerably simpler for neonatal outcomes. The racial/ethnic disparities observed (e.g., increases in PTB for black women, increases in LBW and decreases in macrosomia for all racial minority women), were relatively constant across BMI categories. In race-stratified analyses, maternal obesity was a risk factor for macrosomia in all racial/ethnic groups. However, maternal obesity was a risk factor for PTB and LBW most consistently and strongly in white women. Again, this highlights the fact that normal-weight racial/ethnic minority women were at increased risk of these outcomes, compared to white women. In fact, distributions of birthweight (and perhaps to a lesser extent gestational age) are known to vary by race/ethnicity. It is still unclear whether the lower birthweights and shorter gestations observed in African-American women, for example, are universally pathological (i.e., they may in part represent physiological differences between racial/ethnic groups).(23, 24) Further research is required to fully elucidate these complex associations.
Other factors in addition to the baseline racial/ethnic disparities may contribute to our findings of different BMI/outcome gradients by race/ethnicity. Biometric features of obesity vary between races. On average, Asian-Americans have higher relative amounts of visceral adipose tissue (VAT) than whites of the same BMI; African-Americans on average have lower amounts of VAT and higher levels of subcutaneous adipose tissue (SAT).(25, 26, 27) Because VAT is more strongly predictive of metabolic syndrome (including diabetes) than SAT, this suggests that BMI-defined obesity should have differing prognostic abilities among women of different racial/ethnic groups, particularly for outcomes related to metabolic syndrome (e.g., GDM, preeclampsia, and macrosomia).
Although we used standard racial/ethnic categories used in the United States, there is considerable variation within these racial groups. The category of Asian-American/Pacific Islander, in particular, comprises several distinct ethnicities with varying rates of obesity and adverse perinatal outcomes.(28, 29, 30, 31) It is possible that stratifying Asian-American women by BMI category disaggregated different ethnicities of women (e.g., Samoan or other Pacific Islander women who have higher prevalence of obesity, versus Japanese-American women who have lower prevalence), which complicates BMI comparisons within this group. Also, while we analyzed maternal race/ethnicity here, more research is needed on the contribution of paternal race/ethnicity, which has been the subject of fewer studies to date.(32)
The limitations of our study must be taken into account when interpreting our results. In our study, maternal BMI was based on mothers’ self-reported height and prepregnancy weight. In addition to the unreliability that is generally observed in self-reported weight,(33) several studies have documented that misreporting varies by racial/ethnic group.(34, 35, 36) Studies on the validity of birth certificate-derived self-reported weight and height are relatively limited in number, and have demonstrated varying levels of agreement between BMI as recorded on birth certificates and BMI as recorded on medical records, across BMI categories and racial groups at term (e.g., between 51% and 99%, with a majority above 75% agreement)(17). As the birth certificate becomes more commonly used to study maternal BMI, future research should continue to characterize the validity of such data (particularly in Asian-American and Hispanic women, given that prior research has focused on white and African-American women).
In contrast with BMI, maternal race/ethnicity as recorded on the birth certificate has been found to have high reliability.(37) However, adverse perinatal outcomes have variable reliability and validity in vital statistics data.(38, 39) Vital records and claims data are administrative data, not collected for research purposes. Encouragingly, linked claims data and vital records have higher accuracy than either source on its own, and all of the outcomes analyzed here are captured with a either a moderate degree of accuracy (e.g., preeclampsia and GDM, sensitivities ranging from 62% - 96%), or a very high degree of accuracy (i.e., specificity universally above 95% and for cesarean delivery, preterm birth, and low birthweight, sensitivities universally above 80% and often close to 100%).(38) Although California is a large and racially diverse state with one eighth of all US births, our findings cannot be generalized to the entire US population.
Our study strengths include a large and diverse sample containing adequate power to analyze uncommon outcomes, stratifying by both race and BMI category. We used multiple modeling approaches to characterize in detail the individual and joint impact of maternal race/ethnicity and BMI. We analyzed a variety of maternal and infant outcomes, in contrast with prior research which has focused mostly on GDM.(12, 40) It is important to note that these outcomes are not independent of one another (e.g., GDM and preeclampsia predict cesarean delivery; preeclampsia strongly predicts preterm birth; preterm birth and also timing of cesarean delivery decrease the chance of macrosomia). Our analysis included multiple outcomes with the aim of allowing readers to assess this complex interplay, weighing various outcomes at once. We also stratified BMI category to examine 2 classes of maternal obesity, which enabled a more detailed examination of the race-specific BMI/outcome gradient. BMI/outcome gradients have been observed in many perinatal outcomes (e.g., cesarean delivery, preeclampsia, gestational diabetes),(2, 14) as we observed here, most universally in white women.
These findings add nuance to our understanding of the interplay between maternal race/ethnicity and BMI in predicting perinatal outcomes. While the BMI/adverse outcome gradient appears to be weaker in racial/ethnic minority women, this seems to reflect the overall risk increase in racial/ethnic minority women of all body sizes. These persistent racial/ethnic disparities have been documented extensively, and clinicians and public health officials should continue to work to close them.
Conclusion
The obesity epidemic continues to take a high toll on US health, including the health of pregnant women and their children. The racial disparities in obesity, and in adverse outcomes of pregnancy, demand to be addressed. By furthering our understanding of the complex interplay between race/ethnicity and BMI in predicting perinatal outcomes, we aim to provide clinicians and public health officials with a stronger evidence base to design individual and population-level strategies to improve maternal-infant health, and reduce racial/ethnic disparities.
Study Importance Questions.
What is already known about this subject?
For some perinatal outcomes, there are interactions between maternal obesity and race/ethnicity.
At all BMI levels, Asian-American and Hispanic women are at higher risk of gestational diabetes.
It is unknown if the association between obesity and other perinatal outcomes differs by race/ethnicity.
What does this study add?
There are interaction effects for maternal obesity and race/ethnicity on perinatal outcomes, and the joint impacts of maternal race/ethnicity and obesity are not uniform.
For maternal outcomes (e.g., preeclampsia and primary cesarean delivery), there were racial disparities among women of normal weight, which attenuated or disappeared with increasing BMI category.
For neonatal outcomes, the racial/ethnic disparities observed (e.g., increases in LBW for all racial/ethnic minority women), were relatively constant across BMI categories.
Acknowledgments
JMS is supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (grant number K99 HD079658-02).
Footnotes
Disclosure:
We declare that we have no real or perceived competing interests.
References
- 1.Catalano PM, Ehrenberg HM. The short- and long-term implications of maternal obesity on the mother and her offspring. BJOG : an international journal of obstetrics and gynaecology. 2006;113:1126–1133. doi: 10.1111/j.1471-0528.2006.00989.x. [DOI] [PubMed] [Google Scholar]
- 2.Chu SY, Kim SY, Schmid CH, Dietz PM, Callaghan WM, Lau J, et al. Maternal obesity and risk of cesarean delivery: a meta-analysis. Obes Rev. 2007;8:385–394. doi: 10.1111/j.1467-789X.2007.00397.x. [DOI] [PubMed] [Google Scholar]
- 3.Bodnar LM, Catov JM, Klebanoff MA, Ness RB, Roberts JM. Prepregnancy body mass index and the occurrence of severe hypertensive disorders of pregnancy. Epidemiology. 2007;18:234–239. doi: 10.1097/01.ede.0000254119.99660.e7. [DOI] [PubMed] [Google Scholar]
- 4.Chu SY, Callaghan WM, Kim SY, Schmid CH, Lau J, England LJ, et al. Maternal obesity and risk of gestational diabetes mellitus. Diabetes Care. 2007;30:2070–2076. doi: 10.2337/dc06-2559a. [DOI] [PubMed] [Google Scholar]
- 5.Yao R, Ananth CV, Park BY, Pereira L, Plante LA. Obesity and the risk of stillbirth: a population-based cohort study. American journal of obstetrics and gynecology. 2014;210457:e451–459. doi: 10.1016/j.ajog.2014.01.044. [DOI] [PubMed] [Google Scholar]
- 6.Bryant AS, Worjoloh A, Caughey AB, Washington AE. Racial/ethnic disparities in obstetric outcomes and care: prevalence and determinants. American journal of obstetrics and gynecology. 2010;202:335–343. doi: 10.1016/j.ajog.2009.10.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown HL, Chireau MV, Jallah Y, Howard D. The “Hispanic paradox”: an investigation of racial disparity in pregnancy outcomes at a tertiary care medical center. American journal of obstetrics and gynecology. 2007;197197:e191–197. doi: 10.1016/j.ajog.2007.04.036. discussion 197 e197-199. [DOI] [PubMed] [Google Scholar]
- 8.Grobman WA, Bailit JL, Rice MM, Wapner RJ, Reddy UM, Varner MW, et al. Racial and ethnic disparities in maternal morbidity and obstetric care. Obstetrics and gynecology. 2015;125:1460–1467. doi: 10.1097/AOG.0000000000000735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011-2012. Jama. 2014;311:806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marshall NE, Guild C, Cheng YW, Caughey AB, Halloran DR. Racial disparities in pregnancy outcomes in obese women. J Matern Fetal Neonatal Med. 2014;27:122–126. doi: 10.3109/14767058.2013.806478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramos GA, Caughey AB. The interrelationship between ethnicity and obesity on obstetric outcomes. American journal of obstetrics and gynecology. 2005;193:1089–1093. doi: 10.1016/j.ajog.2005.06.040. [DOI] [PubMed] [Google Scholar]
- 12.Hedderson M, Ehrlich S, Sridhar S, Darbinian J, Moore S, Ferrara A. Racial/ethnic disparities in the prevalence of gestational diabetes mellitus by BMI. Diabetes Care. 2012;35:1492–1498. doi: 10.2337/dc11-2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Makgoba M, Savvidou MD, Steer PJ. An analysis of the interrelationship between maternal age, body mass index and racial origin in the development of gestational diabetes mellitus. BJOG : an international journal of obstetrics and gynaecology. 2012;119:276–282. doi: 10.1111/j.1471-0528.2011.03156.x. [DOI] [PubMed] [Google Scholar]
- 14.Chung JH, Melsop KA, Gilbert WM, Caughey AB, Walker CK, Main EK. Increasing pre-pregnancy body mass index is predictive of a progressive escalation in adverse pregnancy outcomes. J Matern Fetal Neonatal Med. 2012;25:1635–1639. doi: 10.3109/14767058.2011.648970. [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization (WHO) Obesity: preventing and managing the global epidemic. Report of a WHO Consultation. WHO Technical Report Series. 2000;894 [PubMed] [Google Scholar]
- 16.Marshall NE, Guild C, Cheng YW, Caughey AB, Halloran DR. Maternal superobesity and perinatal outcomes. American journal of obstetrics and gynecology. 2012;206417:e411–416. doi: 10.1016/j.ajog.2012.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bodnar LM, Abrams B, Bertolet M, Gernand AD, Parisi SM, Himes KP, et al. Validity of birth certificate-derived maternal weight data. Paediatric and perinatal epidemiology. 2014;28:203–212. doi: 10.1111/ppe.12120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ananth CV. Menstrual versus clinical estimate of gestational age dating in the United States: temporal trends and variability in indices of perinatal outcomes. Paediatric and perinatal epidemiology. 2007;21(Suppl 2):22–30. doi: 10.1111/j.1365-3016.2007.00858.x. [DOI] [PubMed] [Google Scholar]
- 19.VanderWeele TJ, Hernandez-Diaz S. Is there a direct effect of pre-eclampsia on cerebral palsy not through preterm birth? Paediatric and perinatal epidemiology. 2011;25:111–115. doi: 10.1111/j.1365-3016.2010.01175.x. [DOI] [PubMed] [Google Scholar]
- 20.Cole SR, Hernan MA. Fallibility in estimating direct effects. International journal of epidemiology. 2002;31:163–165. doi: 10.1093/ije/31.1.163. [DOI] [PubMed] [Google Scholar]
- 21.Bryant AS, Washington S, Kuppermann M, Cheng YW, Caughey AB. Quality and equality in obstetric care: racial and ethnic differences in caesarean section delivery rates. Paediatric and perinatal epidemiology. 2009;23:454–462. doi: 10.1111/j.1365-3016.2009.01059.x. [DOI] [PubMed] [Google Scholar]
- 22.Castro LC, Avina RL. Maternal obesity and pregnancy outcomes. Curr Opin Obstet Gynecol. 2002;14:601–606. doi: 10.1097/00001703-200212000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Steer PJ. Prematurity or immaturity? BJOG : an international journal of obstetrics and gynaecology. 2006;113(Suppl 3):136–138. doi: 10.1111/j.1471-0528.2006.01136.x. [DOI] [PubMed] [Google Scholar]
- 24.Sparks PJ. Do biological, sociodemographic, and behavioral characteristics explain racial/ethnic disparities in preterm births? Social science & medicine. 2009;68:1667–1675. doi: 10.1016/j.socscimed.2009.02.026. [DOI] [PubMed] [Google Scholar]
- 25.Carroll JF, Chiapa AL, Rodriquez M, Phelps DR, Cardarelli KM, Vishwanatha JK, et al. Visceral fat, waist circumference, and BMI: impact of race/ethnicity. Obesity (Silver Spring) 2008;16:600–607. doi: 10.1038/oby.2007.92. [DOI] [PubMed] [Google Scholar]
- 26.Park YW, Allison DB, Heymsfield SB, Gallagher D. Larger amounts of visceral adipose tissue in Asian Americans. Obes Res. 2001;9:381–387. doi: 10.1038/oby.2001.49. [DOI] [PubMed] [Google Scholar]
- 27.Araneta MR, Barrett-Connor E. Ethnic differences in visceral adipose tissue and type 2 diabetes: Filipino, African-American, and white women. Obes Res. 2005;13:1458–1465. doi: 10.1038/oby.2005.176. [DOI] [PubMed] [Google Scholar]
- 28.Deurenberg P, Deurenberg-Yap M, Guricci S. Asians are different from Caucasians and from each other in their body mass index/body fat per cent relationship. Obes Rev. 2002;3:141–146. doi: 10.1046/j.1467-789x.2002.00065.x. [DOI] [PubMed] [Google Scholar]
- 29.Hedderson MM, Darbinian JA, Ferrara A. Disparities in the risk of gestational diabetes by race-ethnicity and country of birth. Paediatric and perinatal epidemiology. 2010;24:441–448. doi: 10.1111/j.1365-3016.2010.01140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rao AK, Cheng YW, Caughey AB. Perinatal complications among different Asian- American subgroups. American journal of obstetrics and gynecology. 2006;194:e39–41. doi: 10.1016/j.ajog.2006.01.027. [DOI] [PubMed] [Google Scholar]
- 31.Wong LF, Caughey AB, Nakagawa S, Kaimal AJ, Tran SH, Cheng YW. Perinatal outcomes among different Asian-American subgroups. American journal of obstetrics and gynecology. 2008;199382:e381–386. doi: 10.1016/j.ajog.2008.06.073. [DOI] [PubMed] [Google Scholar]
- 32.Caughey AB, Cheng YW, Stotland NE, Washington AE, Escobar GJ. Maternal and paternal race/ethnicity are both associated with gestational diabetes. American journal of obstetrics and gynecology. 2010;202616:e611–615. doi: 10.1016/j.ajog.2010.01.082. [DOI] [PubMed] [Google Scholar]
- 33.Connor Gorber S, Tremblay M, Moher D, Gorber B. A comparison of direct vs. self- report measures for assessing height, weight and body mass index: a systematic review. Obes Rev. 2007;8:307–326. doi: 10.1111/j.1467-789X.2007.00347.x. [DOI] [PubMed] [Google Scholar]
- 34.Brunner Huber LR. Validity of self-reported height and weight in women of reproductive age. Matern Child Health J. 2007;11:137–144. doi: 10.1007/s10995-006-0157-0. [DOI] [PubMed] [Google Scholar]
- 35.Gillum RF, Sempos CT. Ethnic variation in validity of classification of overweight and obesity using self-reported weight and height in American women and men: the Third National Health and Nutrition Examination Survey. Nutr J. 2005;4:27. doi: 10.1186/1475-2891-4-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wen M, Kowaleski-Jones L. Sex and ethnic differences in validity of self-reported adult height, weight and body mass index. Ethn Dis. 2012;22:72–78. [PMC free article] [PubMed] [Google Scholar]
- 37.Baumeister L, Marchi K, Pearl M, Williams R, Braveman P. The validity of information on “race” and “Hispanic ethnicity” in California birth certificate data. Health Serv Res. 2000;35:869–883. [PMC free article] [PubMed] [Google Scholar]
- 38.Lain SJ, Hadfield RM, Raynes-Greenow CH, Ford JB, Mealing NM, Algert CS, et al. Quality of data in perinatal population health databases: a systematic review. Medical care. 2012;50:e7–20. doi: 10.1097/MLR.0b013e31821d2b1d. [DOI] [PubMed] [Google Scholar]
- 39.Goff SL, Pekow PS, Markenson G, Knee A, Chasan-Taber L, Lindenauer PK. Validity of using ICD-9-CM codes to identify selected categories of obstetric complications, procedures and co-morbidities. Paediatric and perinatal epidemiology. 2012;26:421–429. doi: 10.1111/j.1365-3016.2012.01303.x. [DOI] [PubMed] [Google Scholar]
- 40.Sridhar SB, Ferrara A, Ehrlich SF, Brown SD, Hedderson MM. Risk of large-for gestational-age newborns in women with gestational diabetes by race and ethnicity and body mass index categories. Obstetrics and gynecology. 2013;121:1255–1262. doi: 10.1097/AOG.0b013e318291b15c. [DOI] [PMC free article] [PubMed] [Google Scholar]