Abstract
In the fourth decade of the HIV epidemic, the relationship between host immunity and HIV central nervous system (CNS) disease remains incompletely understood. Using a simian immunodeficiency virus (SIV)/macaque model, we examined CNS outcomes in pigtailed macaques expressing the MHC class I allele Mane-A1*084:01 which confers resistance to SIV-induced CNS disease and induces the prototypic viral escape mutation Gag K165R. Insertion of gag K165R into the neurovirulent clone SIV/17E-Fr reduced viral replication in vitro compared to SIV/17E-Fr. We also found lower CSF, but not plasma, viral loads in macaques inoculated with SIV/17E-Fr K165R versus those inoculated with wildtype. Although escape mutation K165R was genotypically stable in plasma, it rapidly reverted to wildtype Gag KP9 in both CSF and in microglia cultures. We induced robust Gag KP9-specific CTL tetramer responses by vaccinating Mane-A*084:01-positive pigtailed macaques with a Gag KP9 virus-like particle (VLP) vaccine. Upon SIV/17E-Fr challenge, vaccinated animals had lower SIV RNA in CSF compared to unvaccinated controls, but showed no difference in plasma viral loads. These data clearly demonstrate that viral fitness in the CNS is distinct from the periphery and underscores the necessity of understanding the consequences of viral escape in CNS disease with the advent of new therapeutic vaccination strategies.
Keywords: SIV, escape, CNS, MHC class I, viral compartmentalization
Introduction
Shortly after human immunodeficiency virus-1 (HIV-1) was identified as the cause of acquired immune deficiency syndrome (AIDS), health care providers recognized that individuals with AIDS frequently demonstrated varying degrees of HIV-associated neurocognitive disorders (HAND) that ranged from HIV-associated dementia (HAD) to asymptomatic neurocognitive impairment (ANI) (Antinori et al, 2007). With widespread availability of effective antiretroviral therapy, the challenges of clinical management of HIV-infected individuals have shifted from treating life-threatening opportunistic infections to managing a chronic disease in an increasingly aging HIV-infected population. Consequently, although the incidence of HAD has decreased with the advent of effective ART, the cumulative prevalence of HAND has remained unchanged because of a relative increase in the mild to moderate clinical manifestations (Heaton et al, 2010). This trend is especially disturbing because individuals with HAND overall tend to have worse clinical outcome with higher rates of mortality and lower adherence to treatment (Mothobi and Brew, 2012).
Cytotoxic lymphocytes (CTLs) play an important role in the pathogenesis of HIV-associated neurologic disease. Perivascular CD8+ T cells have been demonstrated in the brains of patients with HIV-associated encephalitis (HIVE) (McCrossan et al, 2006; Petito et al, 2003; Petito et al, 2006; Tomlinson et al, 1999), even before the development of clinical neurologic signs (McCrossan et al, 2006). In addition, rhesus macaques (Macaca mulatta) depleted of CTLs through the use of anti-CD8 monoclonal antibodies and then infected with simian immunodeficiency virus (SIV) have an extremely high incidence of SIV-associated encephalitis (SIVE) (Marcondes et al, 2015; Schmitz et al, 1999).
Given the importance of CTLs in the pathogenesis of HIV CNS disease, it is surprising that few studies have directly examined the relationship between protective MHC class I allele expression and the development of lentiviral-associated neurologic disease. Using a well-characterized accelerated model of SIVE in pigtailed macaques (Macaca nemestrina) (Mankowski et al, 2002; Zink et al, 1997; Zink et al, 1999), our group has shown that the expression of the MHC class I allele Mane-A1*084:01 (formerly Mane-A*10) is significantly neuroprotective; animals that lack this allele are 2.5 times more likely to develop SIVE and have significantly higher CNS SIV replication (Mankowski et al, 2008), despite a lack of reduction in peripheral viral load (Beck et al, 2015; Gooneratne et al, 2014; Mankowski et al, 2008). Correspondingly, longitudinal CSF and plasma SIV RNA sequencing revealed the development of a canonical escape in SIV Gag KP9 (K165R) following the emergence of Mane-A1*084:01/Gag KP9 tetramer-specific CTLs, demonstrating that CTL-mediated immunologic pressure was driving the escape (Queen et al, 2011). Also, the SIV Gag K165R escape mutation was archived in latent proviral DNA and was present in resting CD4+ T cells, indicating that the replication competent SIV bearing the escape mutation persisted in latent reservoirs such as the brain (Queen et al, 2011).
In this study, we engineered the gag K165R point mutation into the neurovirulent molecular clone SIV/17E-Fr. Escape mutations in HIV and SIV are often associated with a loss of fitness. In the case of SIV Gag K165R escape, pronounced loss of fitness was demonstrated by rapid reversion to wildtype in animals that did not respond to KP9 (Mane-A1*084:01-negative animals) (Fernandez et al, 2005); however, the development of rapid and significant development of the compensatory mutation V145A reduced the fitness defect of K165R (Reece et al, 2013). The main goal of this study was to evaluate the potential fitness cost of SIV Gag K165R, an escape mutation that develops as the result of CTL-mediated immune pressure associated with the known neuroprotective MHC class I allele Mane-A1*084:01. We show that inoculation of Mane-A1*084:01-positive pigtailed macaques with SIV/17E-Fr K165R resulted in lowered CSF viral loads compared to macaques inoculated with wildtype SIV/17E-Fr. Reversion to wildtype SIV Gag KP9 in animals inoculated with SIV/17E-Fr K165R also only occurred in the CSF. Finally, we utilized a novel VLP-based vaccine platform to induce targeted CTL-mediated immune pressure on Gag KP9, and found that vaccinated animals challenged with wildtype SIV/17E-Fr had a lower viral load in the CSF when compared to unvaccinated SIV-infected controls. In contrast to CSF differences, plasma viral levels were similar in all of these studies, suggesting that Mane-A1*084:01-associated CTL control is especially crucial to control of viral replication in the central nervous system.
Methods
Generation of the escape neurovirulent clone SIV/17E-Fr K165R
We constructed an infectious SIV molecular clone containing the K165R escape mutation by designing synthetic oligonucleotides with an A to G point mutation to modify the KP9 segment of Gag in the SIV/17E-Fr plasmid to K165R using the QuikChange Site-directed Mutagenesis kit (Stratagene, Agilent Technologies©). The KP9 sequence was excised from pUC19 SIV 17E-Fr and inserted into a smaller plasmid, pDSRed N1, for site-directed mutagenesis. After the sequence was confirmed by direct sequencing, the insert was excised with the restriction enzyme Sbf1 and re-ligated back into the original plasmid. Stocks for in vitro and in vivo experiments were generated by transfecting this plasmid into CEMx174 cells as previously described (Mankowski et al, 1997).
Primary cell isolation and culture
Primary pigtailed macaque (Macaca nemestrina) lymphocytes and macrophages were isolated from whole blood collected from adult macaques (healthy donors or animals in this study). PBMCs were isolated by discontinuous density gradient centrifugation with 25% Percoll and subsequently cultured for lymphocytes or monocyte derived macrophages as previously described (Babas et al, 2001; Flaherty et al, 1997). Microglia were isolated from macaque cerebral cortex as previously described (Babas et al, 2003). Fresh cortical tissue was minced and trypsinized with 0.25% trypsin, DNase (50 μg/mL), and gentamycin (50 μg/mL) in DMEM [4.5g/L glucose]), filtered into a clean 50mL conical tube using 183 μM sterile mesh, washed, resuspended in sterile PBS, and centrifuged with Percoll for 35 minutes at 15,000 rpm in an Oakridge tube. The microglia were then removed from beneath the myelin layer and filtered through a 40μm mesh. Cells were plated in DMEM with 10% FBS in 25-cm2 flasks overnight, washed, and cultured for 5 days in DMEM supplemented with 5% FBS, 5% giant-cell-growth-tumor conditioned media (OriGen, Gaithersburg, MD), 1mM sodium pyruvate, and 50 μg of gentamicin/mL.
In vitro viral replication kinetics assays
Primary macaque cells (lymphocytes, macrophages, and microglia) and CEMx174 cells were infected in duplicate with either SIV/17E-Fr alone (MOI=0.05), SIV/17E-Fr K165R alone (MOI=0.05), or a 50:50 mixture of wildtype SIV/17E-Fr (MOI=0.025) and SIV/17E-Fr K165R (MOI=0.025) in 6 well plates or 25-cm2 flasks. After 24 hours, the cells were washed three times with PBS to remove virus and then cultured in appropriate media (see above) for 15 days. Culture supernatants were subsequently collected at 48-hour intervals for p27 ELISA (Advanced Bioscience Laboratories, Rockville, MD) and viral RNA isolation.
Animal studies
A total of 8 male juvenile Mane-A1*084:01-positive pigtailed macaques (Macaca nemestrina) were used in this study. MHC class I expression by pigtailed macaques was evaluated by sequence specific PCR or Roche 454 Titanium pyrosequencing (Fernandez et al, 2011; Queen et al, 2011). Six macaques were intravenously inoculated with either SIV/17E-Fr or SIV/17E-Fr K165R (groups 1 and 2, Table 1) at a dose of AID50 10,000. As a proof-of-principle, the final two macaques (group 3, Table 1) were primed then boosted at approximately two-week intervals with 250 μg KP9-loaded VLP administered intradermally then intramuscularly (quadriceps muscle) until a Gag KP9-specific tetramer response was achieved, then challenged intravenously with SIV/17E-Fr (AID50 10,000). For all animals, blood and CSF samples were taken approximately every 14 days, and animals were perfused with sterile saline when euthanised at day 84 post inoculation. These studies were approved by the Johns Hopkins University Institutional Animal Care and Use Committee.
Table 1. Animal groups in this study.
Six pigtailed macaques were inoculated with either SIV/17E-Fr or SIV 17E-Fr K165R, and two macaques were vaccinated with SIV Gag KP9-conjugated VLP then challenged with SIV/17E-Fr.
| Group | Number of animals | Animal IDs | Inoculum
|
VLP vaccinated | |
|---|---|---|---|---|---|
| SIV/17E-Fr | SIV/17E-Fr K165R | ||||
| 1 | 3 | PT1, PT2, PT3 | + | − | − |
| 2 | 3 | PT4, PT5, PT6 | − | + | − |
| 3 | 2 | PT7, PT8 | + | − | + |
Quantitation and sequencing of SIV RNA
SIV RNA was measured in the plasma and CSF by quantitative reverse transcription PCR (qRT-PCR) using primers directed against SIV Gag as previously described (Queen et al, 2011). PCR was performed on cDNA generated from the plasma, CSF, and cultured cell supernatant with one of two pairs of SIV Gag KP9-specific primers, either (forward) 5-CACGCAGAAGAGAAAGTGAA-3 and (reverse) 5-GTTCCTCGAAT(AG)TC(GT)GATCC-3 or (forward) 5- AGCGGCAGAGGAGGAAATTAC-3 and (reverse) 5- GTCCTTGTTGTGGAGCTGGT using Platinum PCR supermix (Invitrogen) and specific cycle conditions (94°C for 2 min; 30 cycles of 94°C for 15 s, 56°C for 30 s, 72°C for 1 min; and a final extension of 72°C for 8 min). PCR product was cloned (TOPO TA) and colonies were selected for sequencing as described (Queen et al, 2011). Sequences were aligned and analyzed using Geneious 5.0.2 software.
Measuring SIV Gag KP9 specific CD3+/CD8+ T cells
Leukocytes in 0.1 mL whole blood were stained with allophycocyanin (APC)-conjugated Mane-A1*084:01/Gag KP9 tetramer at a 1:500 dilution and counterstained with anti-CD3-PE (BD, Cat #552127), anti-CD4-PerCP-Cy™5.5 (BD, Cat # 552838), and anti-CD8-FITC (BD, Cat #557085) conjugated antibodies as previously described (Queen et al, 2011). Flow cytometry was performed on a LSRFortessaTM flow cytometer and analysis was performed using FlowJo (Tree Star Inc., Ashland, OR). Tetramer-positive CTLs were normalized to uninfected control animals.
Generation of the Gag KP9-specific virus-like particle (VLP) vaccine
The virus-like particle (VLP)-based vaccine was composed of bovine papillomavirus L1 capsid protein that was genetically engineered to contain 8 glutamic acids and a cysteine in a surface exposed loop of the protein with Gag KP9 covalently linked to the particle (Pejawar-Gaddy et al, 2010). The negatively charged glutamic acids served as docking sites for protein/peptide antigens with an NH2 tag of 8 positively charged arginines and flanking cysteines. The SIV Gag KP9 antigen (CR8CAAYKKFGAEVVP) was covalently linked to the particle by an oxidation-reduction reaction as previously described (Pejawar-Gaddy et al, 2010).
VLP vaccination
Blood and CSF were collected from two Mane-A1*084:01-positive pigtailed macaques (group 4) three times at one-week intervals before vaccination (prebleed samples). Following the final prebleed sample collection, both macaques initially received intradermal SIV Gag KP9-VLP (100 μg in 0.1 μL buffered saline). On subsequent days 42, 56, 84, and 98, both animals were boosted with KP9-VLP by both intradermal (100 μg) and intramuscular (250 μg in 0.25 μL buffered saline) routes. Blood and CSF were collected on days 14, 28, 42, 56, 62, 70, 84, and 98 post VLP prime at day 0. Because SIV Gag KP9 specific CTL responses were observed by tetramer FACS analysis by this point, VLP boosts were discontinued. Animals were then challenged with SIV/17E-Fr intravenously 7 days after the final VLP boost.
Results
Kinetics of viral replication in vitro
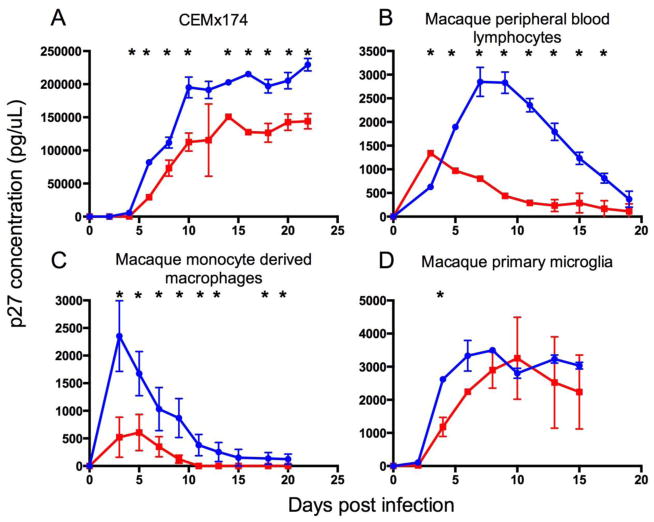
Replication of wildtype SIV/17E-Fr and escape SIV/17E-Fr K165R were directly compared in vitro in the lymphocyte cell line CEMx174, macaque peripheral blood lymphocytes, macrophages, and microglia (Figure 1). The goals of these in vitro studies were to 1) confirm that the escape mutation-bearing virus construct SIV/17E-Fr K165R was replication competent in vitro, and 2) compare the replication kinetics of SIV/17E-Fr K165R versus the wildtype clone SIV/17E-Fr at the cellular level. Previous studies using SHIV strains have shown rapid reversion of the Gag K165R mutation to wildtype KP9 when inoculated into animals that do not mount an immunologic response against the KP9 epitope, suggesting that the K165R mutation incurs a dramatic fitness cost (Fernandez et al, 2005).
Fig. 1. Replication kinetics of SIV/17E-Fr K165R vs wildtype SIV/17E-Fr.
In vitro, replication of SIV/17E-Fr K165R (red line) was lower than wildtype SIV/17E-Fr (blue line) in the CEMx174 B/T cell hybrid line (A) and in cultured primary lymphocytes (B), macrophages (C), and microglia (D). Asterisks denote significant differences between the groups (t-test, P<0.05), error bars represent standard deviation, and data points represent replicate means.
By infecting the HIV/SIV permissive hybrid B/T cell line CEMx174, we showed that the molecular clone SIV/17E-Fr K165R was able to replicate successfully, but produced less virus compared to wildtype SIV/17E-Fr (Figure 1a). When CEMx174 cells were infected with 50:50 wildtype SIV/17E-Fr and mutant SIV/17E-Fr K165R, viral growth kinetics were intermediate, as would be expected (data not shown). Interestingly, terminal SIV Gag sequencing showed 65% wildtype Gag KP9 (52/80 clones) and only 35% escape mutant Gag K165R (28/80 clones), suggesting that wildtype virus outcompeted SIV/17E-Fr K165R. Reversion to wildtype Gag KP9 in cells infected with escape mutant SIV/17E-Fr K165R occurred at a very low rate (0.5%, or 1/81 clones, terminally). Since CEMx174 cells are a transformed cell line, we extended the viral fitness assays to primary macaque cells isolated from blood (lymphocytes and macrophages). As the backbone of SIV/17E-Fr is a macrophage-tropic SIV clone (Babas et al, 2001), the Gag escape mutant was expected to have similar macrophage tropism. As expected, SIV/17E-Fr K165R was also replication competent in primary lymphocytes and macrophages, but similar to the findings in CEMx174 cells, had lower growth curves compared to the parental virus containing wildtype Gag KP9 (Figure 1b,c).
As a neurovirulent clone, wildtype SIV/17E-Fr also replicates readily in microglia (Babas et al, 2003). As an in vitro surrogate measure of viral replication in the brain, we isolated and infected macaque microglia. Although the viral growth curve of SIV/17E-Fr K165R was more similar to wildtype SIV/17E-Fr in infected primary microglia than in primary lymphocytes or macrophages, escape virus still demonstrated lower peak virus and a lower growth curve (Figure 1d), although this difference was only significant at d4 post infection. Reduced in vitro viral replication regardless of cell type is consistent with the premise that the Gag K165R mutation incurs a general fitness cost.
Kinetics of viral replication in vivo
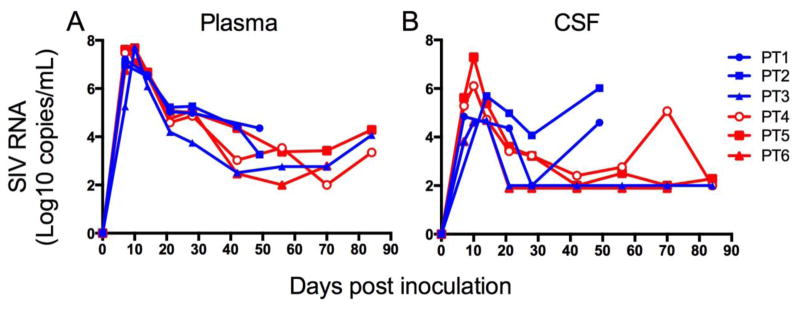
To determine whether fitness cost of the Gag K165R mutation demonstrated in vitro was recapitulated in vivo, we inoculated three Mane-A1*084:01-positive pigtailed macaques with SIV/17E-Fr K165R (table 1, group 2) and compared SIV replication with three Mane-A1*084:01-positive pigtailed macaques inoculated with wildtype SIV/17E-Fr (table 1, group 1). These studies were terminated at 84 days post inoculation (PI) for most animals but two of the three macaques in group 1 were only carried out to 50 days p.i.); longitudinal viral loads in the plasma and cerebrospinal fluid (CSF) were compared (Figure 2). Longitudinal plasma viral loads in animals inoculated with mutant SIV/17E-Fr K165R were nearly identical to those animals inoculated with wildtype SIV/17E-Fr (Figure 2a). In contrast, the CSF viral load of animals inoculated with SIV/17E-Fr/K165R was lower than two of three macaques inoculated with SIV/17E-Fr after acute infection at d49 p.i. (Figure 2b); These findings illustrate a potential CNS-specific fitness cost of the K165R SIV Gag escape mutation with reduced CNS replication without altering SIV replication in the periphery.
Fig. 2. Comparative longitudinal viral loads of SIV/17E-Fr versus SIV/17E-Fr/K165R in Mane-A1*08401 positive macaques.
Viral load in the plasma (A) was similar in Mane-A1*08401 positive pigtailed macaques infected with SIV/17E-Fr (blue lines) and SIV/17E-Fr K165R (red lines). CSF viral load in macaques infected with SIV/17E-Fr/K165R was lower than two of three macaques infected with SIV/17E-Fr by day 49 p.i.. Two animals (PT1 and PT2) were only carried out 50 days post inoculation.
Differences in CNS and peripheral viral genotype
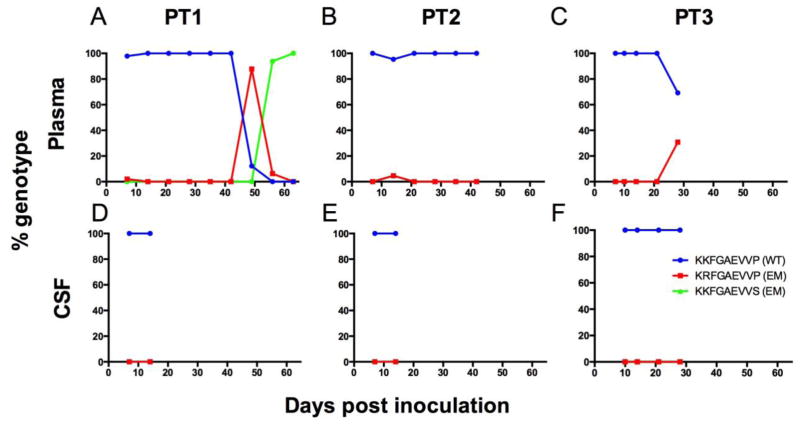
Consistent with previous findings from our group (Queen et al, 2011), we detected emergence of the escape mutation Gag K165R in plasma of all three Mane-A1*084:01 pigtailed macaques that were inoculated with wildtype SIV/17E-Fr (Figure 3a,b,c) although we failed to see escape mutations develop in the CSF of these animals (Figure 3d,e,f). We were unable to sequence SIV Gag in the CSF past day 14 in these animals because levels of viral RNA fell below our threshold of detection for cloning and sequencing in asymptomatic and chronic infection.
Fig. 3. Longitudinal SIV RNA genotyping in the plasma and CSF of three pigtailed macaques inoculated with wildtype SIV/17E-Fr.
In macaques inoculated with the cloned wildtype virus, SIV/17E-Fr, escape from CTL-mediated immune pressure developed in plasma of all three animals (A–C) but escape was not detected in the CSF (D–F). Peak plasma viral escape in the three animals was as follows: PT1 = 88%, PT2 = 5%, and PT3 = 31% escape. Viral RNA was isolated from CSF and plasma and viral genotype was established by cloning and sequencing. Blue line = KKFGAEVVP (wildtype KP9), red line = KRFGAEVVP (K165R escape), green line = KKFGAEVVS (P172S escape).
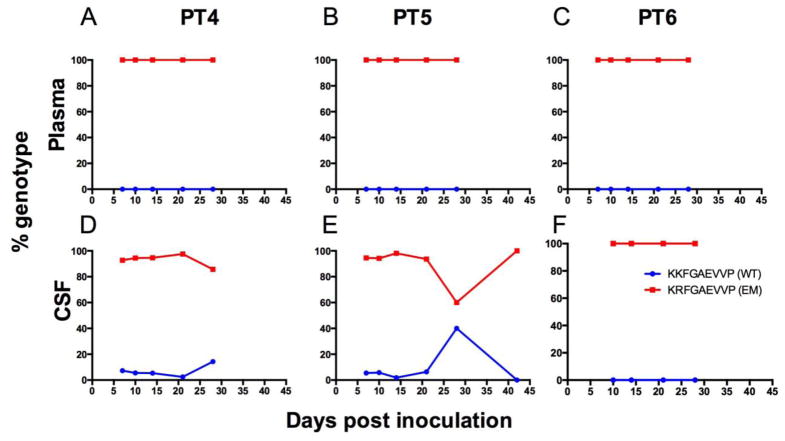
In addition, we sequenced SIV gag in both the peripheral (plasma) and CNS (CSF) compartments in animals inoculated with SIV/17E-Fr K165R (Figure 4) to track reversion from escape Gag K165R to wildtype Gag KP9 in vivo. Since we inoculated pigtailed macaques that express Mane-A1*084:01 that could mount immunologic pressure against reversion to Gag KP9, we did not expect to see significant reversion to wildtype. Previous studies that challenged Mane-A1*084:01-positive pigtailed macaques with a natural SIV isolate containing the Gag K165R mutation showed maintenance of the escape mutation (Fernandez et al, 2005). As expected, plasma genotyping of SIV gag in the three animals inoculated with SIV/17E-Fr K165R showed SIV Gag K165R stability up to day 28 post inoculation (Figure 4a,b,c); however, CSF genotyping showed reversion to wildtype Gag KP9 starting as early as 7 days post inoculation in two of three animals (Figure 3d,e,f). Replication-competent SIV obtained from microglia cultures prepared from one animal (PT5) showed a similar pattern of reversion, with 25% of virus genotyped having reverted to SIV Gag KP9 wildtype (data not shown).
Fig. 4. Longitudinal SIV RNA genotyping in the plasma and CSF of three pigtailed macaques inoculated with SIV/17E-Fr K165R.
In macaques inoculated with the cloned escape virus SIV/17E-Fr K165R, reversion to wildtype SIV Gag KP9 (blue) developed rapidly in two of three animals in the CSF (D–F) but not the plasma (A–C). Peak reversion to wildtype Gag KP9 in the CSF was as follows: PT4 = 14% and PT5 = 40%. Viral RNA was isolated from CSF and plasma and viral genotype was established by cloning and sequencing. Blue line = KKFGAEVVP (wildtype KP9), red line = KRFGAEVVP (K165R escape).
Virus-like particle vaccine platform targeting SIV Gag KP9
To induce KP9-specific CTL responses that specifically target the SIV Gag KP9 epitope and thereby accelerate K165R escape in animals inoculated with SIV/17E-Fr, we utilized a unique vaccine platform designed to induce a robust CTL response using a virus-like particle that solely presented the SIV Gag KP9 peptide. Two Mane-A1*084:01- positive pigtailed macaques were vaccinated five times approximately two weeks apart until a consistent Gag KP9-specific CTL response was observed. VLP vaccination induced a strong CTL response in both animals (0.62 and 0.98% tetramer positive CD3+/CD8+ lymphocytes), as measured by Gag KP9-tetramer response (Figure 5a). Intradermal vaccination had minimal ability to prime and boost an immune response, while the intramuscular route proved to be more immunogenic, at least in one animal (day −42 pre-inoculation, Figure 5a).
Fig. 5. Impact of Gag KP9 vaccination on SIV replication after SIV/17E-Fr challenge.
A) Two animals primed and boosted with Gag KP9 VLPs (PT7 = dashed red line, PT8 = dotted red line) mounted Gag KP9 tetramer responses pre-SIV challenge. B) When these same two vaccinated animals (red) were challenged with SIV/17E-Fr (at day 0), peak KP9 CTL responses developed rapidly at days 14 and 21 p.i. In contrast, the SIV Gag KP9-specific CTL response in an unvaccinated animal (PT3, blue) inoculated with SIV/17E-Fr peaked at day 56 p.i. Unvaccinated animals inoculated with SIV/17E-Fr K165R (grey) had no significant KP9 CTL responses (PT 4–6, n=3, median). C) Plasma viral loads in VLP vaccinated animals (red) challenged with SIV/17E-Fr were similar to the plasma viral load of unvaccinated animals (blue) inoculated with SIV/17E-Fr (PT1-3, n=3, median). However, the CSF viral loads (D) of VLP vaccinated animals (red) were lower than wildtype controls (blue line; PT1-3, n-3, median) after acute infection (> 1.12 logs lower at d42, > 0.76 logs lower at d56 and d84). CTL response was measured by calculating percentage of CD3+/CD8+/KP9-Mane-A1*08401 tetramer + lymphocytes. Plasma and CSF viral loads were measured by qRT-PCR.
After establishing that VLP vaccination increased Gag KP9-specific immunologic response, animals were challenged with a single intravenous inoculum of wildtype SIV/17E-Fr. Post-challenge, both animals responded with early and robust KP9 tetramer-specific anamnestic responses in acute infection (Figure 5b, red lines), considerably earlier than typically seen in unvaccinated animals at day 56 p.i. (Figure 5b, blue line). While VLP vaccination did not measurably alter plasma viral load, both animals had lower viral loads in the CSF compared to unvaccinated SIV/17E-Fr infected control animals.
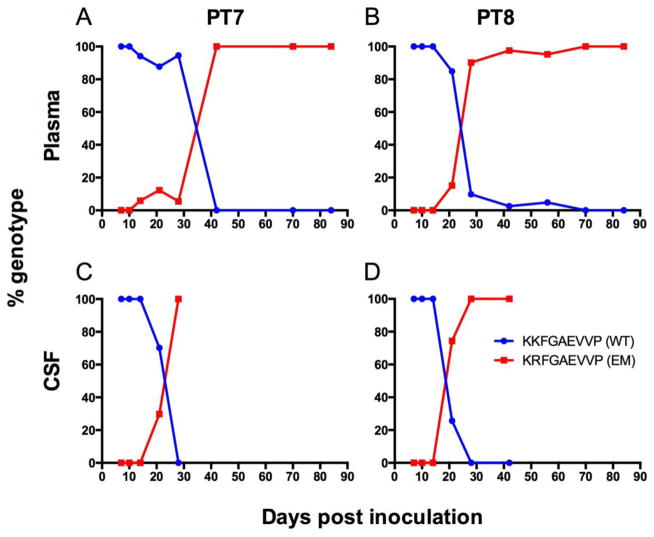
With induction of a robust SIV Gag KP9-specific CTL response with VLP Gag KP9-specific vaccination, we hypothesized that Gag KP9 escape would be more rapid and robust in vaccinated animals. Induction of such specific and rapid viral escape with single-epitope vaccine strategies is an often-used argument against single epitope vaccination. As expected, vaccinated animals developed the Gag K165R viral escape mutation rapidly in the plasma (Figure 6a,b) and developed more complete and sustained plasma viral escape mutations compared to the SIV/17E-Fr-inoculated, unvaccinated control animals (Figure 3a,b,c). Interestingly, the Gag K165R escape mutation developed more rapidly in the CSF of vaccinated animals compared to the periphery (Figure 6c,d), while CSF mutations did not develop in any of the SIV/17E-Fr- inoculated, unvaccinated animals (Figure 3d,e,f). The development of rapid escape in the CSF in vaccinated animals could imply that VLP Gag KP9-specific vaccination induced better CTL response in the CNS, although the reason is unclear.
Fig. 6. Longitudinal SIV RNA genotyping in the plasma and CSF of two pigtailed macaques vaccinated with Gag KP9-VLP and challenged with SIV/17E-Fr.
In macaques primed and boosted with the Gag KP9-specific VLP vaccine, escape at Gag KP9 occurred earlier and at much greater frequency (A–D) compared to unvaccinated control animals (shown in Figure 3), consistent with early development of Gag KP9 CTL responses after KP9-VLP vaccination. Viral RNA was isolated from CSF and plasma and viral genotype was established by cloning and sequencing. Blue line = KKFGAEVVP (wildtype KP9), red line = KRFGAEVVP (K165R escape).
Discussion
This study demonstrated a CNS compartment-specific fitness cost to viral escape from MHC class I-mediated immunologic pressure. After inserting the canonical SIV Gag escape mutation K165R into the neurovirulent molecular clone SIV/17E-Fr, we inoculated Mane-A1*084:01-positive pigtailed macaques with the cloned escape mutant virus and demonstrated decreased viral load in CSF, but not plasma. Viral sequencing in these animals revealed transient reversion to wildtype Gag KP9 only in the CSF and not the plasma, corresponding with decreased CNS fitness of K165R. In a reciprocal experiment, we induced targeted immunologic pressure by vaccinating animals with a novel VLP-based construct that focused the CTL response on Gag KP9. Subsequent challenge with wildtype SIV/17E-Fr resulted in a decreased viral load in the CSF, but not plasma. Together, these findings provide compelling evidence that a CNS-specific loss in viral fitness results from a specific Gag escape mutation. Although the neuroprotective Mane-A1*084:01-associated phenotype could in part be explained by immunological selection for a CNS-specific viral variant with lower CNS fitness, some Mane-A1*084:01-positive animals with a high prevalence of the Gag K165R viral escape mutation are notable for progressing to develop encephalitis (Queen et al, 2011).
VLP Gag KP9 vaccination provided us with a novel way to induce targeted CTL-specific immunologic pressure. VLP particles are especially immunogenic because of their size (40–50 nm), repetitive surface docking sites that present foreign antigen, and their ability to self-adjuvanate (Chackerian et al, 2001; Grgacic and Anderson, 2006; Pejawar-Gaddy et al, 2010; Zhai et al, 2013). Memory phenotype FACS analysis of tetramer positive CTLs also showed that almost all of the KP9 specific memory CD8+ T cell response consisted of effector memory cells, with 42% of tetramer positive cells defined as EM1 transitional memory cells (CD95+/CD28+/CCR7−), 55% defined as EM2 fully differentiated effector memory (CD95+/CD28−/CCR7−), and only the remaining 3% as central memory (CD95+/CD28+/CCR7+). In rhesus macaques, vaccines that are able to induce robust effector memory T cell responses are believed to provide better protection against SIV infection than vaccines that produce predominantly central memory T cell responses (Hansen et al, 2011; Hansen et al, 2013; Hansen et al, 2009). As a single-epitope vaccine is not likely to effectively prevent SIV or HIV infection due to the development of escape and/or compensatory mutations, further studies vaccinating with multiple SIV Gag epitopes or larger segments of the SIV proteome on a VLP backbone are needed.
Evaluation of CD8+ T cell functional responses to Gag peptide pools by Smith et al (2005) first recognized Gag KP9 as an important immunodominant SIV Gag epitope in pigtailed macaques (Smith et al, 2005a). This is especially relevant because KP9 is a homolog of the HIV Gag epitope KF11, which is presented by the protective allele HLA-B57*01 (Fernandez et al, 2011). Consistently, SIV-infected Mane-A1*084:01-positive animals rapidly develop the canonical escape mutation Gag K165R (Smith et al, 2005a; Smith et al, 2005b). There is also rapid reversion to wildtype KP9 in the absence of Mane-A1*084:01, confirming the in vivo fitness cost to mutation (Loh et al, 2008). Although our lab found no difference in either plasma viral RNA or CD4+ cell counts between pigtailed macaques that expressed Mane-A1*084:01 and those that did not, CSF viral RNA was lower in Mane-A1*084:01-positive macaques (Mankowski et al, 2008). These data support the view that the efficacy of antigen-specific CTL control in the CNS clearly differs from the periphery (Doherty, 1995).
Surprisingly, few studies of HIV or SIV have specifically examined the association between MHC class I-mediated viral control and CNS outcome measurements (Mankowski et al, 2008; Queen et al, 2011). We have found compelling evidence for epitope-specific targeted immune pressure on viral fitness that is CNS-specific. In addition, we developed a vaccine strategy to target CTL-mediated immunologic pressure on this Gag epitope and found subsequent decreased SIV viral load in CNS. As the struggle to find an effective vaccine strategy for HIV-1 continues, it is extremely important to understand the interactions between host immunogenetics and organ specific HIV-induced disease as the field of HIV research moves beyond a focus on plasma-based end points. Our data provide the rationale for the development of a therapeutic vaccine-approach to control HIV-associated system-specific pathology, especially in the CNS.
Acknowledgments
Financial support. This work was supported by NIH grants R01 NS089482, R01 NS077869, P01 MH070306, P40 OD013117, and T32 OD011089.
Footnotes
Potential conflicts of interest. All authors report no potential conflicts of interest.
References
- Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, Clifford DB, Cinque P, Epstein LG, Goodkin K, Gisslen M, Grant I, Heaton RK, Joseph J, Marder K, Marra CM, McArthur JC, Nunn M, Price RW, Pulliam L, Robertson KR, Sacktor N, Valcour V, Wojna VE. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–99. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babas T, Munoz D, Mankowski JL, Tarwater PM, Clements JE, Zink MC. Role of microglial cells in selective replication of simian immunodeficiency virus genotypes in the brain. J Virol. 2003;77:208–16. doi: 10.1128/JVI.77.1.208-216.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babas T, Vieler E, Hauer DA, Adams RJ, Tarwater PM, Fox K, Clements JE, Zink MC. Pathogenesis of SIV pneumonia: selective replication of viral genotypes in the lung. Virology. 2001;287:371–81. doi: 10.1006/viro.2001.1043. [DOI] [PubMed] [Google Scholar]
- Beck SE, Kelly KM, Queen SE, Adams RJ, Zink MC, Tarwater PM, Mankowski JL. Macaque species susceptibility to simian immunodeficiency virus: increased incidence of SIV central nervous system disease in pigtailed macaques versus rhesus macaques. J Neurovirol. 2015;21:148–58. doi: 10.1007/s13365-015-0313-7. [DOI] [PubMed] [Google Scholar]
- Chackerian B, Lowy DR, Schiller JT. Conjugation of a self-antigen to papillomavirus-like particles allows for efficient induction of protective autoantibodies. J Clin Invest. 2001;108:415–23. doi: 10.1172/JCI11849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty PC. Anatomical environment as a determinant in viral immunity. J Immunol. 1995;155:1023–7. [PubMed] [Google Scholar]
- Fernandez CS, Reece JC, Saepuloh U, De Rose R, Ishkandriati D, O’Connor DH, Wiseman RW, Kent SJ. Screening and confirmatory testing of MHC class I alleles in pig-tailed macaques. Immunogenetics. 2011;63:511–21. doi: 10.1007/s00251-011-0529-5. [DOI] [PubMed] [Google Scholar]
- Fernandez CS, Stratov I, De Rose R, Walsh K, Dale CJ, Smith MZ, Agy MB, Hu SL, Krebs K, Watkins DI, O’Connor DH, Davenport MP, Kent SJ. Rapid viral escape at an immunodominant simian-human immunodeficiency virus cytotoxic T-lymphocyte epitope exacts a dramatic fitness cost. J Virol. 2005;79:5721–31. doi: 10.1128/JVI.79.9.5721-5731.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaherty MT, Hauer DA, Mankowski JL, Zink MC, Clements JE. Molecular and biological characterization of a neurovirulent molecular clone of simian immunodeficiency virus. J Virol. 1997;71:5790–8. doi: 10.1128/jvi.71.8.5790-5798.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooneratne SL, Alinejad-Rokny H, Ebrahimi D, Bohn PS, Wiseman RW, O’Connor DH, Davenport MP, Kent SJ. Linking pig-tailed macaque major histocompatibility complex class I haplotypes and cytotoxic T lymphocyte escape mutations in simian immunodeficiency virus infection. J Virol. 2014;88:14310–25. doi: 10.1128/JVI.02428-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grgacic EV, Anderson DA. Virus-like particles: passport to immune recognition. Methods. 2006;40:60–5. doi: 10.1016/j.ymeth.2006.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen SG, Ford JC, Lewis MS, Ventura AB, Hughes CM, Coyne-Johnson L, Whizin N, Oswald K, Shoemaker R, Swanson T, Legasse AW, Chiuchiolo MJ, Parks CL, Axthelm MK, Nelson JA, Jarvis MA, Piatak M, Jr, Lifson JD, Picker LJ. Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature. 2011;473:523–7. doi: 10.1038/nature10003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen SG, Piatak M, Jr, Ventura AB, Hughes CM, Gilbride RM, Ford JC, Oswald K, Shoemaker R, Li Y, Lewis MS, Gilliam AN, Xu G, Whizin N, Burwitz BJ, Planer SL, Turner JM, Legasse AW, Axthelm MK, Nelson JA, Fruh K, Sacha JB, Estes JD, Keele BF, Edlefsen PT, Lifson JD, Picker LJ. Immune clearance of highly pathogenic SIV infection. Nature. 2013;502:100–4. doi: 10.1038/nature12519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen SG, Vieville C, Whizin N, Coyne-Johnson L, Siess DC, Drummond DD, Legasse AW, Axthelm MK, Oswald K, Trubey CM, Piatak M, Jr, Lifson JD, Nelson JA, Jarvis MA, Picker LJ. Effector memory T cell responses are associated with protection of rhesus monkeys from mucosal simian immunodeficiency virus challenge. Nat Med. 2009;15:293–9. doi: 10.1038/nm.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Clifford DB, Franklin DR, Jr, Woods SP, Ake C, Vaida F, Ellis RJ, Letendre SL, Marcotte TD, Atkinson JH, Rivera-Mindt M, Vigil OR, Taylor MJ, Collier AC, Marra CM, Gelman BB, McArthur JC, Morgello S, Simpson DM, McCutchan JA, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75:2087–96. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh L, Petravic J, Batten CJ, Davenport MP, Kent SJ. Vaccination and timing influence SIV immune escape viral dynamics in vivo. PLoS Pathog. 2008;4:e12. doi: 10.1371/journal.ppat.0040012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mankowski JL, Clements JE, Zink MC. Searching for clues: tracking the pathogenesis of human immunodeficiency virus central nervous system disease by use of an accelerated, consistent simian immunodeficiency virus macaque model. J Infect Dis. 2002;186(Suppl 2):S199–208. doi: 10.1086/344938. [DOI] [PubMed] [Google Scholar]
- Mankowski JL, Flaherty MT, Spelman JP, Hauer DA, Didier PJ, Amedee AM, Murphey-Corb M, Kirstein LM, Munoz A, Clements JE, Zink MC. Pathogenesis of simian immunodeficiency virus encephalitis: viral determinants of neurovirulence. J Virol. 1997;71:6055–60. doi: 10.1128/jvi.71.8.6055-6060.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mankowski JL, Queen SE, Fernandez CS, Tarwater PM, Karper JM, Adams RJ, Kent SJ. Natural host genetic resistance to lentiviral CNS disease: a neuroprotective MHC class I allele in SIV-infected macaques. PLoS One. 2008;3:e3603. doi: 10.1371/journal.pone.0003603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcondes MC, Morsey B, Emanuel K, Lamberty BG, Flynn CT, Fox HS. CD8+ T cells maintain suppression of simian immunodeficiency virus in the central nervous system. J Infect Dis. 2015;211:40–4. doi: 10.1093/infdis/jiu401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrossan M, Marsden M, Carnie FW, Minnis S, Hansoti B, Anthony IC, Brettle RP, Bell JE, Simmonds P. An immune control model for viral replication in the CNS during presymptomatic HIV infection. Brain. 2006;129:503–16. doi: 10.1093/brain/awh695. [DOI] [PubMed] [Google Scholar]
- Mothobi NZ, Brew BJ. Neurocognitive dysfunction in the highly active antiretroviral therapy era. Curr Opin Infect Dis. 2012;25:4–9. doi: 10.1097/QCO.0b013e32834ef586. [DOI] [PubMed] [Google Scholar]
- Pejawar-Gaddy S, Rajawat Y, Hilioti Z, Xue J, Gaddy DF, Finn OJ, Viscidi RP, Bossis I. Generation of a tumor vaccine candidate based on conjugation of a MUC1 peptide to polyionic papillomavirus virus-like particles. Cancer Immunol Immunother. 2010;59:1685–96. doi: 10.1007/s00262-010-0895-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petito CK, Adkins B, McCarthy M, Roberts B, Khamis I. CD4+ and CD8+ cells accumulate in the brains of acquired immunodeficiency syndrome patients with human immunodeficiency virus encephalitis. J Neurovirol. 2003;9:36–44. doi: 10.1080/13550280390173391. [DOI] [PubMed] [Google Scholar]
- Petito CK, Torres-Munoz JE, Zielger F, McCarthy M. Brain CD8+ and cytotoxic T lymphocytes are associated with, and may be specific for, human immunodeficiency virus type 1 encephalitis in patients with acquired immunodeficiency syndrome. J Neurovirol. 2006;12:272–83. doi: 10.1080/13550280600879204. [DOI] [PubMed] [Google Scholar]
- Queen SE, Mears BM, Kelly KM, Dorsey JL, Liao Z, Dinoso JB, Gama L, Adams RJ, Zink MC, Clements JE, Kent SJ, Mankowski JL. Replication-competent simian immunodeficiency virus (SIV) Gag escape mutations archived in latent reservoirs during antiretroviral treatment of SIV-infected macaques. J Virol. 2011;85:9167–75. doi: 10.1128/JVI.00366-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reece JC, Alcantara S, Gooneratne S, Jegaskanda S, Amaresena T, Fernandez CS, Laurie K, Hurt A, O’Connor SL, Harris M, Petravic J, Martyushev A, Grimm A, Davenport MP, Stambas J, De Rose R, Kent SJ. Trivalent live attenuated influenza-simian immunodeficiency virus vaccines: efficacy and evolution of cytotoxic T lymphocyte escape in macaques. J Virol. 2013;87:4146–60. doi: 10.1128/JVI.02645-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz JE, Kuroda MJ, Santra S, Sasseville VG, Simon MA, Lifton MA, Racz P, Tenner-Racz K, Dalesandro M, Scallon BJ, Ghrayeb J, Forman MA, Montefiori DC, Rieber EP, Letvin NL, Reimann KA. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science. 1999;283:857–60. doi: 10.1126/science.283.5403.857. [DOI] [PubMed] [Google Scholar]
- Smith MZ, Dale CJ, De Rose R, Stratov I, Fernandez CS, Brooks AG, Weinfurter J, Krebs K, Riek C, Watkins DI, O’Connor DH, Kent SJ. Analysis of pigtail macaque major histocompatibility complex class I molecules presenting immunodominant simian immunodeficiency virus epitopes. J Virol. 2005a;79:684–95. doi: 10.1128/JVI.79.2.684-695.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MZ, Fernandez CS, Chung A, Dale CJ, De Rose R, Lin J, Brooks AG, Krebs KC, Watkins DI, O’Connor DH, Davenport MP, Kent SJ. The pigtail macaque MHC class I allele Mane-A*10 presents an immundominant SIV Gag epitope: identification, tetramer development and implications of immune escape and reversion. J Med Primatol. 2005b;34:282–93. doi: 10.1111/j.1600-0684.2005.00126.x. [DOI] [PubMed] [Google Scholar]
- Tomlinson GS, Simmonds P, Busuttil A, Chiswick A, Bell JE. Upregulation of microglia in drug users with and without pre-symptomatic HIV infection. Neuropathol Appl Neurobiol. 1999;25:369–79. doi: 10.1046/j.1365-2990.1999.00197.x. [DOI] [PubMed] [Google Scholar]
- Zhai Y, Zhong Z, Zariffard M, Spear GT, Qiao L. Bovine papillomavirus-like particles presenting conserved epitopes from membrane-proximal external region of HIV-1 gp41 induced mucosal and systemic antibodies. Vaccine. 2013;31:5422–9. doi: 10.1016/j.vaccine.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zink MC, Amedee AM, Mankowski JL, Craig L, Didier P, Carter DL, Munoz A, Murphey-Corb M, Clements JE. Pathogenesis of SIV encephalitis. Selection and replication of neurovirulent SIV. Am J Pathol. 1997;151:793–803. [PMC free article] [PubMed] [Google Scholar]
- Zink MC, Suryanarayana K, Mankowski JL, Shen A, Piatak M, Jr, Spelman JP, Carter DL, Adams RJ, Lifson JD, Clements JE. High viral load in the cerebrospinal fluid and brain correlates with severity of simian immunodeficiency virus encephalitis. J Virol. 1999;73:10480–8. doi: 10.1128/jvi.73.12.10480-10488.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]