Abstract
Mitochondrial genomes compete for transmission from mother to progeny. We explored this competition by introducing a second genome into Drosophila melanogaster to follow transmission. Competitions between closely related genomes favored those functional in electron transport, resulting in a host-beneficial purifying selection1. Contrastingly, matchups between distant genomes often favored those with negligible, negative or lethal consequences, indicating selfish selection. Exhibiting powerful selfish selection, a genome carrying a detrimental mutation displaced a complementing genome leading to population death after several generations. In a different pairing, opposing selfish and purifying selection counterbalanced to give stable transmission of two genomes. Sequencing of recombinant mitochondrial genomes revealed that the non-coding region, containing origins of replication, governs selfish transmission. Uniparental inheritance prevents encounters between distantly related genomes. Nonetheless, within each maternal lineage, constant competition among sibling genomes selects for super-replicators. We suggest that this relentless competition drives positive selection promoting change in the sequences influencing transmission.
Natural selection culls populations of compromising mutations and favors traits that enhance organismal fitness. Nuclear genes have a relatively uniform exposure to natural selection as a result of regimented replication and segregation. In contrast, unconstrained competition among multiple mitochondrial genomes creates alternative ways to select for fit genomes, as well as opportunities for selfish genomes to increase in abundance.
In heteroplasmic lines of Drosophila, mitochondrial genomes harboring mutations detrimental to oxidative phosphorylation (OXPHOS) function suffered a transmission disadvantage, even when complemented by co-resident wild-type genomes. The resulting purifying selection is based on competition among genomes within the organism rather than survival of fit organisms1,2. However, a selfish advantage might also bias transmission and provide an evolutionary drive (i.e. selfish drive)3–5. For example, in S. cerevisiae, preferential replication promotes inheritance of hypersuppressive petite mitochondrial DNA (mtDNA)6–8. In multicellular organisms, defective mitochondrial genomes carried in animals can also benefit from selfish drive to enhance their propagation9–14.
To study head-to-head competition between mitochondrial genomes, we used cytoplasmic transplantation to bypass uniparental inheritance, which normally prevents encounters between unrelated genomes. In the resulting heteroplasmic lines, purifying selection dominated competition between closely related genomes1, but here we show that when distantly related genomes compete, a selfish selection drives success of genomes having negligible, or negative consequences to the fly. Stable heteroplasmy can occur when selfish selection benefits one genome and purifying selection benefits the other. Our findings indicate that competition among mitochondrial genomes influences their evolutionary trajectory.
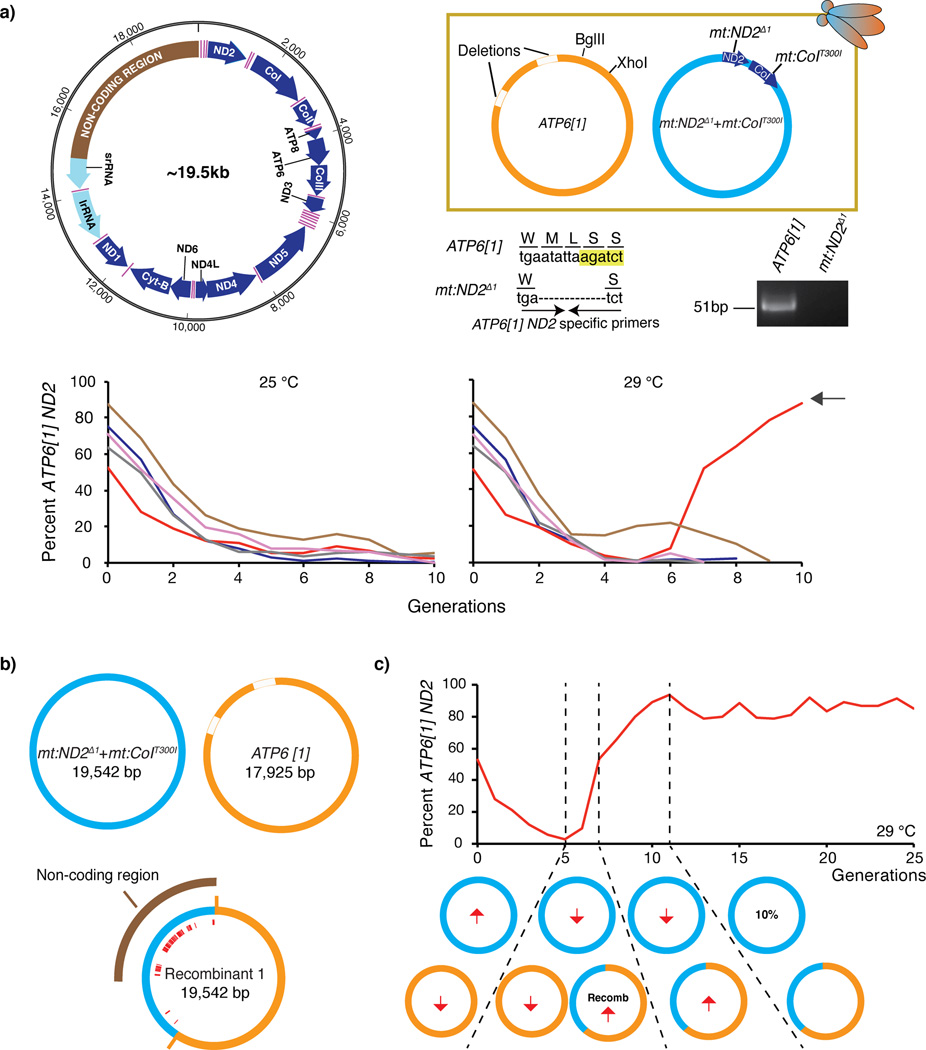
We impose selection for OXPHOS function by raising the temperature of a line carrying a marked genome with a temperature sensitive lethal mutation in the cytochrome c oxidase subunit I gene (mt:ND2del1 + mt:CoIT300I) (Figure 1a). When paired with a related wild-type genome in heteroplasmic strains, selection reduced the transmission of temperature sensitive genome due to an intra-organismal competition among mitochondria genomes1,2. In contrast, when paired with the ATP6[1] genome15,16, a diverged D. melanogaster mitochondrial genome with numerous sequence differences (Figure 1), the temperature sensitive genome completely displaced the ATP6[1] genome17. Unlike purifying selection, this displacement occurred at either 25 °C or 29 °C, hence was not substantially influenced by the ability of the genome to contribute to OXPHOS (Figure 1a). Even at the restrictive temperature, the population expanded as long as the ATP6[1] genome provided complementing mt:CoI function. However, after several generations, just as the ATP6[1] genome disappeared, the entire stock died. We conclude that the temperature sensitive genome has a transmission advantage despite carrying a detrimental allele that leads to lethality.
Figure 1.
Selection based on selfish drive in a heteroplasmic line containing the ATP6[1] genome and the temperature sensitive double-mutant: mt:ND2del1 + mt:CoIT300I. (a) Decline of the ATP6[1] genome when co-existing with mt:ND2del1 + mt:CoIT300I. A schematic (upper left) of D. melanogaster mitochondrial genome with protein coding genes (blue), rDNA loci (light cyan), tRNAs (pink) and the non-coding region (brown). Key features distinguishing the ATP6[1] and temperature sensitive genomes are indicated (upper right panel). A PCR primer set that selectively amplifies the intact ND2 locus of the ATP6[1] genome is indicated (BglII site, yellow highlight). The relative abundance of the ATP6[1] genome as assessed by qPCR for five lines maintained at 25 °C and 29 °C for multiple generations. After the ATP6[1] abundance fell to a low level (illustrated), the flies at 29 °C started to die (not shown), but in one line a few survivors expanded and showed an increasing abundance in a genome with the ATP6[1] ND2 region (red line, black arrow). (b) The map of the recombinant genome sequenced by PacBio SMRT technology. Red lines indicate the distribution of SNPs characteristic of mt:ND2del1 + mt:CoIT300I genome that are present in the recombinant. The ATP6[1] genome also lacks ~1.6 kb of the non-coding region17. (c) The transmission of the recombinant genome was favored when paired with the temperature sensitive genome. The directional arrows indicate how the abundance of a particular genotype was increasing or decreasing at any given generation.
Based on our recent demonstration of recombination among mitochondrial genomes17, we genetically mapped the sequence distinctions making the temperature sensitive genome the stronger competitor. Exchange events transferring these sequences should produce an enhanced ATP6[1] genome that would persist to give surviving flies. When five heteroplasmic lines were followed at 29 °C, one line gave surviving progeny carrying a recombinant genome (Figure 1a). This recombinant has the majority of the ATP6[1] coding sequence including the functional mt:CoI allele, but carried the entire non-coding segment and a small segment flanking coding sequence from the temperature sensitive genome (Figure 1b)17. Later, we isolated another recombinant with the entire coding sequence derived from the ATP6[1] genome but with the non-coding sequence from the temperature sensitive genome (Supplementary Figure 1a). At first, the surviving heteroplasmic flies had a low relative abundance of the recombinant genome and a preponderance of the temperature sensitive genome. Over subsequent generations, the relative abundance of recombinant genomes increased, showing their ability to successfully compete with the co-existing temperature sensitive genome (Figure 1c, Supplementary Figure 1b & c). These changes in the relative ratios of the mitochondrial genomes (as well as those described below) occurred in the absence of change in the total copy number of mitochondrial genomes. These data show that acquisition of the non-coding region from the temperature sensitive genome is sufficient to endow the recombinant with an improved ability to compete against the temperature sensitive genome. We conclude that, at least in this pairing of genomes, the difference in selfish drive maps to the non-coding region of the mitochondrial genome.
The non-coding region of the mitochondrial genome, also known as the control region or the regulatory region, contains the origins of replication, and is the most variable sequence for many metazoan species18–21. The D. melanogaster version is AT-rich (> 90%) and large (~4.6 kb), and is mainly (> 90%) composed of five tandem type I repeats and four tandem type II22 (Supplementary Figure 2a). Within D. melanogaster, this region exhibits frequent nucleotide and length polymorphisms23–25 (e.g. Supplementary Figure 2a & b), and the divergence is more extensive in other Drosophila species26,27 (e.g. Supplementary Figure 2c). For instance, D. yakuba mtDNA (NC_001322) has a shorter (~1 kb) and diverged non-coding region. We thus introduced mitochondrial genomes from other Drosophila species into D. melanogaster and examined their ability to compete.
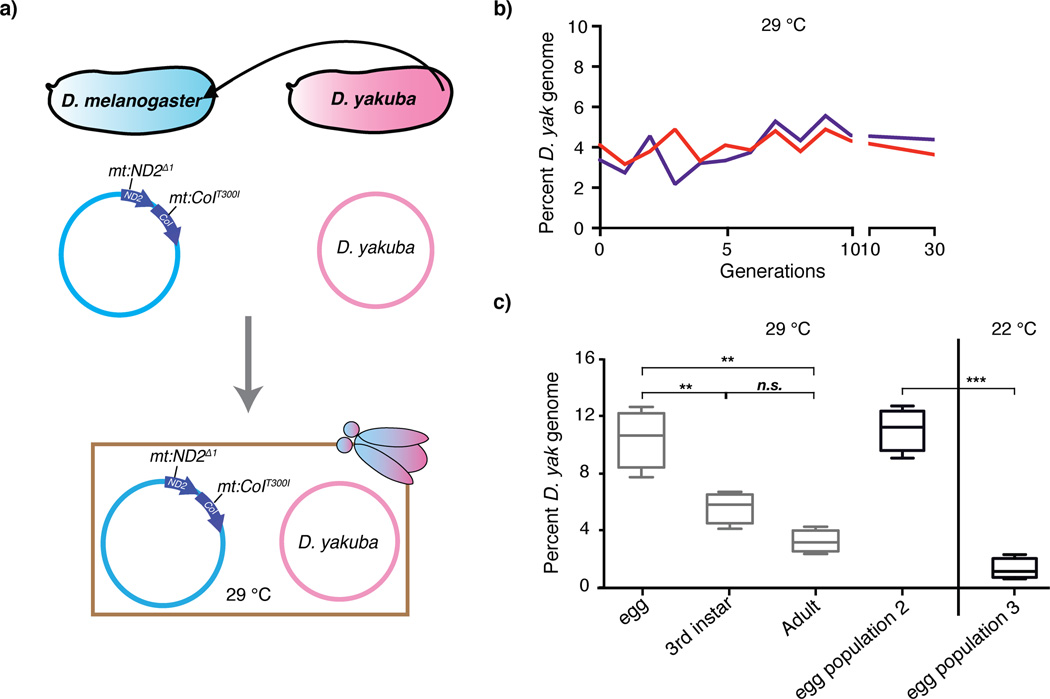
We first introduced cytoplasm of D. yakuba (diverged ~10 MY) embryos into D. melanogaster embryos carrying the temperature sensitive genome (Figure 2a). However, no lines retaining the D. yakuba mitochondrial genome were recovered. On the other hand, at the restrictive temperature, where selection favored retention of the D. yakuba genome, two of 50 injected females gave viable progeny heteroplasmic for the D. yakuba genome and a persisting temperature sensitive genome. The D. yakuba genome was carried at a low but stable level (~4%) for many generations (> 100) under constant temperature selection (Figure 2b).
Figure 2.
Stable transmission of the D. yakuba mtDNA in the D. melanogaster nuclear background. (a) A heteroplasmic line was established by transferring cytoplasm of D. yakuba embryos into embryos carrying the mt:ND2del1 + mt:CoIT300I genome. (b) The proportion of D. yakuba mtDNA was maintained at ~4% for over 30 generations in two independent heteroplasmic lines at 29 °C. (c) The abundance of the D. yakuba mtDNA oscillated during development: high in newly deposited eggs, declined during development and rose in oogenesis to reach a high level in eggs again at 29 °C. The first three entries come from analysis of different stages of the lifecycle across one generation. As expected for a stably propagated stock, an analysis of eggs collected at a different time (egg population 2) gave the same relative abundance for the S. yakuba genome. However, when mothers were shifted to 22 °C at the end of 3rd instar larval stage so that oogenesis occurred at the permissive temperature, the eggs laid had a reduced abundance of the D. yakuba genome (egg population 3). Results are means ± SD (n = 4 for each data point). Unpaired Student’s t-test was performed to compare the difference in the abundance of D. yakuba mtDNA between newly deposited eggs, 3rd instar larvae and adult flies (** = p < 0.01, *** = p < 0.001).
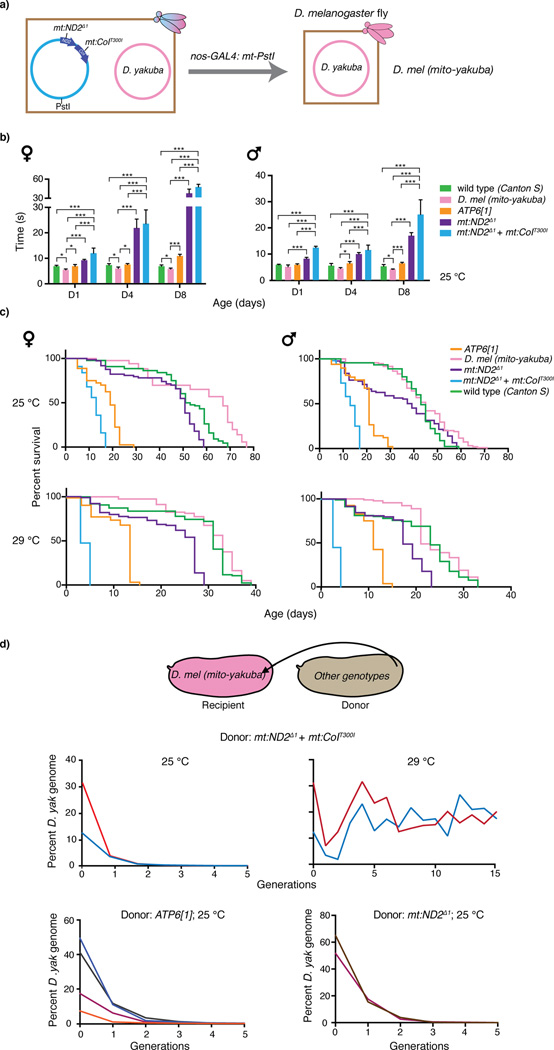
Expression of mitochondrially-targeted PstI selectively eliminated D. melanogaster mtDNA (site at mt7496) giving lines containing only the D. yakuba genome (Figure 3a). These lines (named D. mel (mito-yakuba)) were viable and, in our measures, at least as robust as wild-type flies (Figure 3b & c). This was surprising because examples of nuclear-mitochondrial incompatibility28–31 had suggested that mismatches between the nuclear genes and the foreign mitochondrial genes would compromise OXPHOS and hence the D. mel (mito-yakuba) flies. Instead, the result shows that the only incompatibility is between the two mitochondrial genomes, apparently the result of unequal competition. Similarly, incompatibility between mitochondrial genomes had been noted to influence transfer of ape mtDNA into human tissue culture cells12.
Figure 3.
Cross species analysis of functional conservation and competitive strength of mitochondrial genomes. (a) The D. melanogaster genome was eliminated from a heteroplasmic line by expressing PstI that is targeted to mitochondria. (b) D. mel (mito-yakuba) flies climb faster than D. melanogaster flies carrying various native mitochondrial genomes. Time (means ± SD, n = 3) required for 50% of flies (25 °C) of the indicated age (D = days) and sex to climb to a prescribed height after being gently knocked down was recorded. Significance of differences is based on unpaired Student’s t-test (* = p < 0.05, ** = p < 0.01, *** = p < 0.001). (c) Lifespan of congenic flies with different mitochondrial genotypes at 25 °C and 29 °C. Survivorship was recorded every two days (n > 80, see Supplementary Table 1a). The D. yakuba mitochondrial genome supports robust survival that exceeds that supported by several native genomes (see Supplementary Table 1b & c for statistical analysis). (d) The D. yakuba mitochondrial genome was quickly outcompeted by various D. melanogaster genomes at 25 °C. Native mitochondrial genomes were introduced into the D. mel (mito-yakuba) line, and the relative abundance of the D. yakuba genome was followed over generations by qPCR (see Online Methods). The differently colored lines represent independently produced heteroplasmic lines. The D. yakuba mtDNA was only maintained when partnered with the temperature sensitive genome at 29 °C.
In the above heteroplasmic line, the D. yakuba genome benefits from purifying section at 29 °C because it provides a functional mt:CoI gene. Disappearance of D. yakuba mtDNA within two generations at the permissive temperature shows the importance of this advantage (data not shown). In contrast, the D. melanogaster temperature sensitive genome, whose OXPHOS function is compromised by mutation, is sustained by selfish drive. These two selections oppose and balance each other to maintain stable transmission of the two genomes. Since purifying selection operates during oogenesis to limit transmission of the temperature-sensitive genome1, this stage-limited selection against the temperature sensitive genome ought to give the D. yakuba genome a transient relative advantage. Indeed, at the restrictive temperature, newly deposited eggs have an especially high abundance of the D. yakuba genome. At the permissive temperature, eggs showed no increase, arguing that this increase depends on selection against the temperature sensitive electron transport defect (Figure 2c). The relative abundance of D. yakuba genome then declined during development, only to increase again during oogenesis (Figure 2c). Thus, selfish selection favoring the D. melanogaster genome influences competition at many stages of the life cycle, while the purifying selection favoring the D. yakuba genome is largely restricted to oogenesis1,2,32. This temporal distinction might underlie previously observed oscillations in the relative abundance of other heteroplasmic genomes33.
To further demonstrate that D. melanogaster mitochondrial genomes benefits from a stronger selfish drive, we introduced other D. melanogaster mitochondrial genotypes, many of which do not support organismal vigor as effectively as the D. yakuba genome (Figure 3b & c)16,32,34, into the D. mel (mito-yakuba) line. These tested D. melanogaster genomes outcompeted the D. yakuba genome at 25 °C (Figure 3d), an outcome detrimental to the fitness of the flies.
Notably, the competitive strengths of D. yakuba, ATP6[1] and the temperature sensitive genomes do not fall on a simple hierarchy: D. yakuba mtDNA is displaced by ATP6[1], suggesting that it is the weaker competitor, yet, the D. yakuba genome is sustained in conjunction with the temperature sensitive genome at the high temperature while the ATP6[1] genome is eliminated. Apparently, competitive strength is not determined by potency of a single factor.
When we re-introduced the temperature sensitive genome into the D. mel (mito-yakuba) line, heteroplasmy was again stabilized at the restrictive temperature, but with a higher ratio of the D. yakuba genome (Figure 3d). Since the sequence of the D. yakuba mitochondrial genome was unchanged, the ratio change is likely due to accumulation of nuclear modifiers, which have previously been suggested to influence competition among mitochondrial genomes35–37. The modification of this balance between selfish selection and purifying selection acting on competing genomes ought to be sensitive to minor perturbations of either selection. It will be interesting to explore how nuclear genes modify the competition between mitochondrial genomes.
The native genome is not always the winner in interspecies heteroplasmic lines. For instance, when we introduced D. mauritiana (diverged ~2mya) mtDNA (maI), it replaced the D. melanogaster genomes within a few generations (Supplementary Figure 3). A different D. mauritiana/D. melanogaster heteroplasmic line behaved similarly38. Previously, De Stordeur ranked mitochondrial haplotypes from D. simulans and D. mauritiana for their potency to displace each other39. While these findings suggest that mismatches in competitive strength are common among diverged genomes, the role of selfish drive in determining the outcome in these competitions has yet to be determined.
While the mechanism of selfish drive is unknown, localization of the trait to the non-coding region constrains possibilities. The non-coding sequence is unlikely to influence drive by complex actions, such as evasion of mitophagy or localizing mitochondria to the germline. In contrast, the localization of the two origins for asymmetric replication of the mtDNA (Supplementary Figure 2a) suggests a link between replication and competitive strength. While specific sequence features characterize the start sites of replication40, associated sequences show numerous SNPs and variations in the number of repeat sequences (Supplementary Figure 2a & c). A previous study of large laboratory fly populations detected instability in repeat number and tracked transient heteroplasmy in individual flies to show that longer variants of the non-coding regions tended to increase in abundance during transmission24. We suggest that this finding is related to our observations of selfish drive. Though little is known about the control of Drosophila mtDNA replication, the control region is likely to influence copy control, primer synthesis and initiation efficiency. Even a subtle gain in replication, amplified over many rounds of genome doubling, would result in a large competitive advantage. We thus hypothesize that selfish drive can be equated with replicative drive.
Each new mutation that enhances mtDNA transmission can displace genomes without this asset. The displacement raises the bar for the next mutation to produce a super-competitor that will again take over in an unending ‘arms race’41. Thus, as described in the ‘red queen’ hypothesis, we propose that a constant competition among co-existing mitochondrial genomes creates an intraorganismal positive selection for super-replicators during evolution. Uniparental inheritance confines each super-replicator to a lineage, fragmenting a species into multiple lineages within which local competition independently selects for winning mitochondrial genomes42. Positive selection accelerates sequence divergence, and the non-coding sequences of mtDNA diverge especially fast42–44. Divergence in the size of this region by alteration of repeat number is widespread45–50. This complicates alignment of these sequences between species making quantification of change problematic. Using sequences of twelve D. melanogaster wild isolates25, we show that divergence of the non-coding sequence exceeds neutral changes (Supplementary Figure 2b), a signature of positive selection. Thus, selfish selection, a positive selection, drives rapid change of noncoding sequences, while purifying selection, a negative or conserving selection, limits change of the coding sequences.
To conclude, our findings show that competition between distantly related mitochondrial genomes can be dominated by the selfish drive of a genome rather than its contribution to the fitness of an organism. As a caution, the incompatibilities observed suggest that the success of planned efforts to treat mitochondrial diseases using mitochondrial donors may well depend on the competitive strength of the donor’s mitochondrial genome.
Online Methods
Fly stocks
The D. melanogaster mutant alleles mt:ND2del1 and mt:CoIT300I were previously described1. These alleles were present either alone, or on a double mutant genome mt:ND2del1 + mt:CoIT300I. Flies homoplasmic for the ATP6[1] mitochondrial genome was kindly provided by Dr. Michael Palladino (University of Pittsburgh, U.S.). D. mauritiana and D. yakuba flies were obtained from Drosophila species stock center, San Diego. Flies with different mitochondrial genomes were backcrossed to Canton S for 10–30 generations to homogenize the nuclear background. Other strains used included UAS-mito-PstI and nos-Gal4. The stocks were cultured at 18–25 °C on standard fly medium.
Establishment of heteroplasmic lines
Poleplasm transplantation was used to generate heteroplasmic flies as previously described1. For the mt:ND2del1 + mt:CoIT300I / ATP6[1] line (‘+’ indicates alleles on the same genome and ‘/’ indicates the co-residence of the two indicated genomes), ATP6[1] flies were used as the recipient during poleplasm transplantation in order to obtain lineages with high initial abundance of the ATP6[1] genome. Numerous female progeny (G0) from injected embryos were individually crossed to mt:ND2del1 + mt:CoIT300I males for 2 days at 25 °C. After progeny collection, mothers were sacrificed for total DNA extraction and the proportion of ATP6[1] genome was estimated by qPCR as described below. The progeny (G1) of the mothers were either maintained at 25 °C, or shifted to 29 °C and maintained at 29 °C for multiple generations.
For the D. yakuba/mt:ND2del1 + mt:CoIT300I line, cytoplasm from D. yakuba embryos was transplanted into the mt:ND2del1 + mt:CoIT300I embryos and eclosed adults were kept at 29 °C to select for flies with the D. yakuba genome. By doing this, two independent lines were established and both stably transmitted D. yakuba mtDNA (~4%) from generation to generation at 29 °C. Subsequently, a mitochondrially-targeted restriction enzyme, mito-PstI, was expressed in the germline of the two heteroplasmic lines to eliminate the mt:ND2del1 + mt:CoIT300I genome, as only the D. melanogaster mtDNA contain a PstI site. Through this, several lines with only wild-type D. yakuba mtDNA were established. The D. mel (mito-yakuba) line was then used a recipient for subsequent cytoplasm transplantations.
For lines heteroplasmic for D. mauritiana and D. melanogaster mtDNA, cytoplasm from D. mauritiana embryos was transplanted into D. melanogaster embryos homoplasmic for wild-type mtDNA, mt:ND2del1 + mt:CoIT300I, or mt:ND2del1. Several G0 mothers were crossed to wild-type mtDNA, mt:ND2del1 + mt:CoIT300I, or mt:ND2del1 males respectively for 2 days at 25 °C to produce G1 females in order to establish independent lineages.
Phenotypic analysis of flies with different mitochondrial genotypes
Flies homoplasmic for mt:ND2del1 + mt:CoIT300I, ATP6[1], mt:ND2del1 and D. yakuba mitochondrial genome were backcrossed to Canton S males for at least 10 generations. To assay the lifespan, newly eclosed flies were separated by sex and over 80 flies of each sex were used to follow each population in each condition. To avoid crowding, these were housed at a density of 10 flies per vial or less throughout the analysis (Supplementary Table 1). The flies were transferred to fresh vials and survivorship was recorded every two days at both 25 °C and 29 °C. The climbing assay was performed as previously described1. Briefly, 20 flies (backcrossed for 30 generations) of various ages were transferred to a plastic cylinder (22 cm long, 1.5 cm diameter) with a mark 10 cm line from bottom. After 1 h for acclimation, the flies were knocked down to the bottom by gently tapping the tubes. The time required for 50% of the flies to climb to the marked 10 cm line was recorded. Three trials were conducted for each group, and three groups were used for each genotype. For all the above phenotypic studies, individual flies were picked randomly and the climbing assay was performed blind to avoid cognitive bias in scoring. For statistical analysis, Log-rank test and unpaired Student’s t-test was performed for the survivorship and climbing assay data, respectively, in order to compare flies homoplasmic with different mitochondrial genotypes.
DNA isolation
Total DNA was extracted from adults as described preiously1. Frequencies of mitochondrial genotypes were measured in individual founding females (G0) and their further generations via qPCR. When populations were analyzed, we extracted DNA from groups of 40 individuals.
Sequencing the D. yakuba mtDNA
Three long-range PCR reactions using Expand Long Template PCR system (Roche) were performed using the total DNA from D. yakuba and D. mel (mito-yakuba) as template: mt186 – 7519, mt7229 – 14797 and mt12822 – 400 with the following program: 1 cycle of 93 °C for 3 min, 30 cycles of 93 °C 15 s, 50 °C 30 s, 60 °C 8 min, and 1 cycle of 60 °C for 10 min. Primers were designed to give full coverage of the D. yakuba mitochondrial genome (Supplementary Table 2) for sequencing by QuintaraBio (Albany, CA).
qPCR Parameters
qPCR assays were performed as described previously1. Briefly, the total mtDNA copy number in heteroplasmic flies was measured by qPCR of a 52 bp region (mt361 – 412) present in all mtDNA genotypes (primer mt361F and mt412R, Supplementary Table 2). Primers cognate to the mt:ND2 loci of specific genomes were designed to measure copy number of genomes with ATP6[1], or D. yakuba, or D. mauritiana genomes without amplifying a product from the D. melanogaster mt:ND2del1 allele: these were used for qPCR of a 51 bp from the mt:ND2 region of these genomes (See Supplementary Table 2 for primers). Standard curves were constructed using a series of 10-fold dilutions of purified PCR fragment containing both the common region and ATP6[1], or D. yakuba, or D. mauritiana mt:ND2 region. The efficiency of the 2 primer sets was normalized each time by comparing total mtDNA copy number estimated for the same wild-type DNA sample. qPCR was performed with the following reaction conditions: 95 °C for 10 min, 40 cycles of 95 °C 30 s and 48 °C 30 s. For each 20 µl qPCR reaction, 1% of a fly’s total DNA was used as template. The Ct values used ranged from 13 to 33 and each reaction was repeated 3 times or more. To distinguish the ATP6[1] genome from the D. yakuba mtDNA, two different sets of primers were designed for the qPCR assay (Supplementary Table 2): mt6237F and mt6314R as the common primers; and mt6652F and mt6811R as primers specific for recognizing D. yakuba mtDNA.
Monitoring abundance of the D. yakuba mitochondrial genome during development
Four females heteroplasmic for D. yakuba and mt:ND2del1 + mt:CoIT300I genomes were individually crossed (in separated vials) to mt:ND2del1 + mt:CoIT300I males for 2 days at 29 °C. The mothers of each vial were transferred to new vials to collect eggs for 16 h at 29 °C. Subsequently, the mothers and half of the collected eggs were sacrificed to measure the relative abundance of D. yakuba genome via qPCR described above. The rest of the eggs were allowed to developed into late 3rd instar larvae at 29 °C before they were sacrificed to measure the relative abundance of D. yakuba genome. To examine the abundance of D. yakuba mtDNA in newly laid eggs when oogenesis occurs at 22 °C, late 3rd instar larvae were shifted from 29 °C to 22 °C, and the eggs were collected at 22 °C and sacrificed to measure the abundance of D. yakuba genome.
Quantitative analyses of D. mauritiana mitochondrial genome based on restriction cleavage
For lines heteroplasmic for D. mauritiana wild-type mtDNA and D. melanogaster wild-type mtDNA, the sequence difference between the two genomes did not allow performance of qPCR. Thus, we quantified the relative amount of genomes distinguished by restrictions enzyme cleavage site by comparing the ratios of diagnostic restriction fragments. Total DNA was collected from 40 adult flies of various heteroplasmic lines from generation 1 to generation 4 (for generation 0, total DNA was collected from the mother after it was mated with males at 25 °C for two days to lay eggs). A mtDNA region (mt11517 – 12529) was amplified by PCR (30 cycles of 95 °C 30 s, 50 °C 30 s and 60 °C 60 s). The PCR products were then digested completely with XhoI under the conditions recommended by the supplier (NEB). The digested DNA was separated by gel electrophoresis, and the ratio of cut and uncut DNA was estimated by measuring the intensity of bands using ImageJ.
Southern analysis
Southern blotting was used to detect the recombinant genome and monitor the length variation in the non-coding region of the mitochondrial genomes. It was performed as described in Ma and O’Farrell17. In brief, digested DNA was separated on a 0.8% agarose gel by electrophoresis and transferred to Hybond N+ membrane by the capillary method. The blot was hybridized with PCR-generated probes (mt1577 – 2365 or mt21 – 400, see Supplementary Table 2) that were labeled with DIG-11-dUTP.
Supplementary Material
Acknowledgments
This research was supported by NIH (ES020725) funding to P.H.O’F. H.M. was supported by the long-term postdoc fellowship from Human Frontiers Science Program (LT000138/2010-L). We thank Prof. Michael J. Palladino at University of Pittsburgh for kindly providing us flies with the ATP6[1] mitochondrial genome.
Footnotes
Accession Codes
The complete sequence of a recombinant mitochondrial genome (Supplementary Figure 1a) between the two parental genomes (ATP6[1] and mt:ND2del1 + mt:CoIT300I) was deposited in Genbank and given the following accession number: KU764535.
Author Contributions
H.M. and P.H.O’F. designed research; H.M. performed research; H.M. and P.H. O’F. wrote the paper.
Competing financial interests
The authors declare no competing financial interests.
References
- 1.Ma H, Xu H, O'Farrell PH. Transmission of mitochondrial mutations and action of purifying selection in Drosophila melanogaster. Nat Genet. 2014;46:393–397. doi: 10.1038/ng.2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hill JH, Chen Z, Xu H. Selective propagation of functional mitochondrial DNA during oogenesis restricts the transmission of a deleterious mitochondrial variant. Nat Genet. 2014;46:389–392. doi: 10.1038/ng.2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hurst GD, Werren JH. The role of selfish genetic elements in eukaryotic evolution. Nat.. Rev. Genet. 2001;2:597–606. doi: 10.1038/35084545. [DOI] [PubMed] [Google Scholar]
- 4.Crow JF. Genes that violate Mendel's rules. Scientific American. 1979;240:134–147. doi: 10.1038/scientificamerican0279-134. [DOI] [PubMed] [Google Scholar]
- 5.Hickey DA. Selfish DNA: a sexually-transmitted nuclear parasite. Genetics. 1982;101:519–531. doi: 10.1093/genetics/101.3-4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.MacAlpine DM, Kolesar J, Okamoto K, Butow RA, Perlman PS. Replication and preferential inheritance of hypersuppressive petite mitochondrial DNA. EMBO J. 2001;20:1807–1817. doi: 10.1093/emboj/20.7.1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taylor DR, Zeyl C, Cooke E. Conflicting levels of selection in the accumulation of mitochondrial defects in Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 2002;99:3690–3694. doi: 10.1073/pnas.072660299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jasmin JN, Zeyl C. Rapid evolution of cheating mitochondrial genomes in small yeast populations. Evolution. 2014;68:269–275. doi: 10.1111/evo.12228. [DOI] [PubMed] [Google Scholar]
- 9.Samuels DC, et al. Recurrent tissue-specific mtDNA mutations are common in humans. PLoS Genet. 2013;9:e1003929. doi: 10.1371/journal.pgen.1003929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clark KA, et al. Selfish little circles: Transmission bias and evolution of large deletion-bearing mitochondrial DNA in Caenorhabditis briggsae nematodes. PLoS ONE. 2012;7 doi: 10.1371/journal.pone.0041433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Phillips WS, et al. Selfish Mitochondrial DNA Proliferates and Diversifies in Small, but not Large, Experimental Populations of Caenorhabditis briggsae. Genome Biol Evol. 2015;7:2023–2037. doi: 10.1093/gbe/evv116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moraes CT, Kenyon L, Hao H. Mechanisms of human mitochondrial DNA maintenance: the determining role of primary sequence and length over function. Mol. Biol. Cell. 1999;10:3345–3356. doi: 10.1091/mbc.10.10.3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Volz-Lingenhöhl A, Solignac M, Sperlich D. Stable heteroplasmy for a large-scale deletion in the coding region of Drosophila subobscura mitochondrial DNA. Proc Natl Acad Sci USA. 1992;89:11528–11532. doi: 10.1073/pnas.89.23.11528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsang WY, Lemire BD. Stable heteroplasmy but differential inheritance of a large mitochondrial DNA deletion in nematodes. Biochem. Cell Biol. 2002;80:645–654. doi: 10.1139/o02-135. [DOI] [PubMed] [Google Scholar]
- 15.Celotto AM, et al. Mitochondrial encephalomyopathy in Drosophila. J. Neurosci. 2006;26:810–820. doi: 10.1523/JNEUROSCI.4162-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Celotto AM, Chiu WK, Van Voorhies W, Palladino MJ. Modes of metabolic compensation during mitochondrial disease using the Drosophila model of ATP6 dysfunction. PLoS ONE. 2011;6:e25823. doi: 10.1371/journal.pone.0025823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma H, O'Farrell PH. Selections that isolate recombinant mitochondrial genomes in animals. Elife. 2015;4:e07247. doi: 10.7554/eLife.07247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stoneking M. Hypervariable Sites in the mtDNA Control Region Are Mutational Hotspots. The American Journal of Human Genetics. 2000;67:1029–1032. doi: 10.1086/303092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jamandre BW, Durand JD, Tzeng WN. High sequence variations in mitochondrial DNA control region among worldwide populations of flathead mullet (Mugil cephalus) International Journal of Zoology. 2014;2014:1–9. [Google Scholar]
- 20.Wilkinson GS, Mayer F, Kerth G, Petri B. Evolution of repeated sequence arrays in the D-loop region of bat mitochondrial DNA. Genetics. 1997;146:1035–1048. doi: 10.1093/genetics/146.3.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lunt DH, Whipple LE, Hyman BC. Mitochondrial DNA variable number tandem repeats (VNTRs): utility and problems in molecular ecology. Mol. Ecol. 1998;7:1441–1455. doi: 10.1046/j.1365-294x.1998.00495.x. [DOI] [PubMed] [Google Scholar]
- 22.Lewis DL, Farr CL, Farquhar AL, Kaguni LS. Sequence, organization, and evolution of the A+T region of Drosophila melanogaster mitochondrial DNA. Mol Biol Evol. 1994;11:523–538. doi: 10.1093/oxfordjournals.molbev.a040132. [DOI] [PubMed] [Google Scholar]
- 23.Chen S, et al. A cytoplasmic suppressor of a nuclear mutation affecting mitochondrial functions in Drosophila. Genetics. 2012;192:483–493. doi: 10.1534/genetics.112.143719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rand DM. Population genetics of the cytoplasm and the units of selection on mitochondrial DNA in Drosophila melanogaster. Genetica. 2011;139:685–697. doi: 10.1007/s10709-011-9576-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolff JN, Camus MF, Clancy DJ, Dowling DK. Complete mitochondrial genome sequences of thirteen globally sourced strains of fruit fly (Drosophila melanogaster) form a powerful model for mitochondrial research. Mitochondrial DNA. 2015:1–3. doi: 10.3109/19401736.2015.1106496. [DOI] [PubMed] [Google Scholar]
- 26.Solignac M, Monnerot M, Mounolou JC. Concerted evolution of sequence repeats in Drosophila mitochondrial-DNA. J.. Mol. Evol. 1986;24:53–60. doi: 10.1007/BF02100996. [DOI] [PubMed] [Google Scholar]
- 27.Tsujino F, et al. Evolution of the A+T-rich region of mitochondrial DNA in the melanogaster species subgroup of Drosophila. J.. Mol. Evol. 2002;55:573–583. doi: 10.1007/s00239-002-2353-x. [DOI] [PubMed] [Google Scholar]
- 28.Schmitz-Linneweber C, et al. Pigment deficiency in nightshade/tobacco cybrids is caused by the failure to edit the plastid ATPase alpha-subunit mRNA. Plant Cell. 2005;17:1815–1828. doi: 10.1105/tpc.105.032474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meiklejohn CD, et al. An incompatibility between a mitochondrial tRNA and its nuclear-encoded tRNA synthetase compromises development and fitness in Drosophila. PLoS Genet. 2013;9:e1003238. doi: 10.1371/journal.pgen.1003238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee HY, et al. Incompatibility of nuclear and mitochondrial genomes causes hybrid sterility between two yeast species. Cell. 2008;135:1065–1073. doi: 10.1016/j.cell.2008.10.047. [DOI] [PubMed] [Google Scholar]
- 31.Špírek M, Poláková S, Jatzová K, Sulo P. Post-zygotic sterility and cytonuclear compatibility limits in S. cerevisiae xenomitochondrial cybrids. Front Genet. 2014;5:454. doi: 10.3389/fgene.2014.00454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen Z, et al. Genetic mosaic analysis of a deleterious mitochondrial DNA mutation in Drosophila reveals novel aspects of mitochondrial regulation and function. Mol. Biol. Cell. 2015;26:674–684. doi: 10.1091/mbc.E14-11-1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Petit N, et al. Developmental changes in heteroplasmy level and mitochondrial gene expression in a Drosophila subobscura mitochondrial deletion mutant. Curr. Genet. 1998;33:330–339. doi: 10.1007/s002940050344. [DOI] [PubMed] [Google Scholar]
- 34.Burman JL, et al. A Drosophila model of mitochondrial disease caused by a complex I mutation that uncouples proton pumping from electron transfer. Dis Model Mech. 2014;7:1165–1174. doi: 10.1242/dmm.015321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matsuura ET, Tanaka YT, Yamamoto N. Effects of the nuclear genome on selective transmission of mitochondrial DNA in Drosophila. Genes Genet Syst. 1997;72:119–123. doi: 10.1266/ggs.72.119. [DOI] [PubMed] [Google Scholar]
- 36.Doi A, Suzuki H, Matsuura ET. Genetic analysis of temperature-dependent transmission of mitochondrial DNA in Drosophila. Heredity. 1999;82:555–560. doi: 10.1038/sj.hdy.6885080. [DOI] [PubMed] [Google Scholar]
- 37.Dunbar DR, Moonie PA, Jacobs HT, Holt IJ. Different cellular backgrounds confer a marked advantage to either mutant or wild-type mitochondrial genomes. Proc Natl Acad Sci USA. 1995;92:6562–6566. doi: 10.1073/pnas.92.14.6562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.NIKI Y, Chigusa SI, Matsuura ET. Complete replacement of mitochondrial DNA in Drosophila. Nature. 1989;341:551–552. doi: 10.1038/341551a0. [DOI] [PubMed] [Google Scholar]
- 39.De Stordeur E. Nonrandom partition of mitochondria in heteroplasmic Drosophila. Heredity. 1997;79(Pt 6):615–623. doi: 10.1038/hdy.1997.207. [DOI] [PubMed] [Google Scholar]
- 40.Saito S, Tamura K, Aotsuka T. Replication origin of mitochondrial DNA in insects. Genetics. 2005;171:1695–1705. doi: 10.1534/genetics.105.046243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van VALEN L. A new evolutionary law. Evolutionary Theory. 1973;1:1–30. [Google Scholar]
- 42.van Oven M, Kayser M. Updated comprehensive phylogenetic tree of global human mitochondrial DNA variation. Human Mutation. 2009;30:E386–E394. doi: 10.1002/humu.20921. [DOI] [PubMed] [Google Scholar]
- 43.McMillan WO, Palumbi SR. Rapid rate of control-region evolution in Pacific butterfly fishes (Chaetodontidae) J.. Mol. Evol. 1997;45:473–484. doi: 10.1007/pl00006252. [DOI] [PubMed] [Google Scholar]
- 44.Stoneking M, Hedgecock D, Higuchi RG, Vigilant L, Erlich HA. Population variation of human mtDNA control region sequences detected by enzymatic amplification and sequence-specific oligonucleotide probes. Am.. J. Hum. Genet. 1991;48:370–382. [PMC free article] [PubMed] [Google Scholar]
- 45.Townsend JP, Rand DM. Mitochondrial genome size variation in New World and Old World populations of Drosophila melanogaster. Heredity. 2004;93:98–103. doi: 10.1038/sj.hdy.6800484. [DOI] [PubMed] [Google Scholar]
- 46.Casane D, Guéride M. Evolution of heteroplasmy at a mitochondrial tandem repeat locus in cultured rabbit cells. Curr. Genet. 2002;42:66–72. doi: 10.1007/s00294-002-0328-5. [DOI] [PubMed] [Google Scholar]
- 47.Hoelzel AR, Lopez JV, Dover GA, O'Brien SJ. Rapid evolution of a heteroplasmic repetitive sequence in the mitochondrial DNA control region of carnivores. J.. Mol. Evol. 1994;39:191–199. doi: 10.1007/BF00163807. [DOI] [PubMed] [Google Scholar]
- 48.Faber JE, Stepien CA. Tandemly repeated sequences in the mitochondrial DNA control region and phylogeography of the Pike-Perches Stizostedion. Molecular Phylogenetics and Evolution. 1998;10:310–322. doi: 10.1006/mpev.1998.0530. [DOI] [PubMed] [Google Scholar]
- 49.Lee WJ, Conroy J, Howell WH, Kocher TD. Structure and evolution of teleost mitochondrial control regions. J.. Mol. Evol. 1995;41:54–66. doi: 10.1007/BF00174041. [DOI] [PubMed] [Google Scholar]
- 50.Fumagalli L, Taberlet P, Favre L, Hausser J. Origin and evolution of homologous repeated sequences in the mitochondrial DNA control region of shrews. Mol Biol Evol. 1996;13:31–46. doi: 10.1093/oxfordjournals.molbev.a025568. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.