Abstract
Background
The need for complete lymph node dissection (CLND) in patients with positive sentinel lymph node biopsy (SNB) is an important unanswered clinical question.
Study Design
Patients diagnosed with positive SNB at a melanoma referral center from 1991 to 2013 were studied. Outcomes of patients who underwent CLND were compared to those who did not undergo immediate CLND (observation group, OBS).
Results
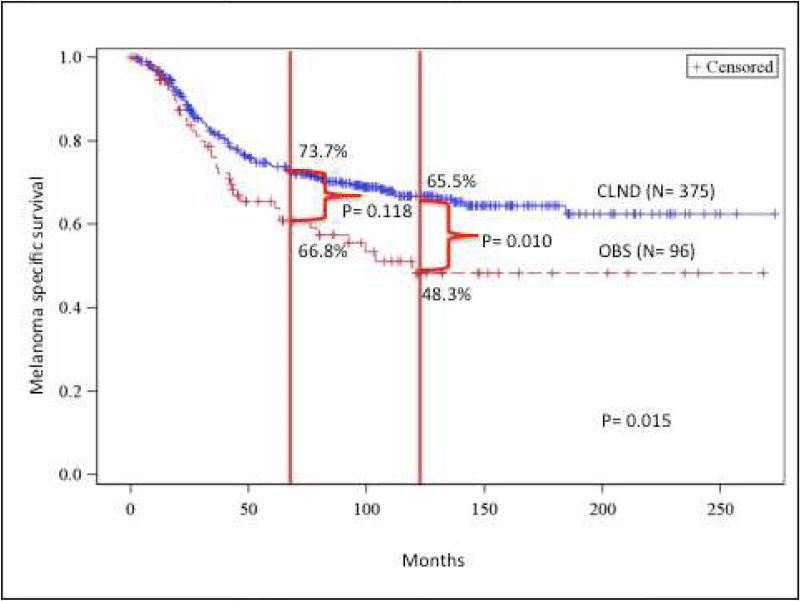
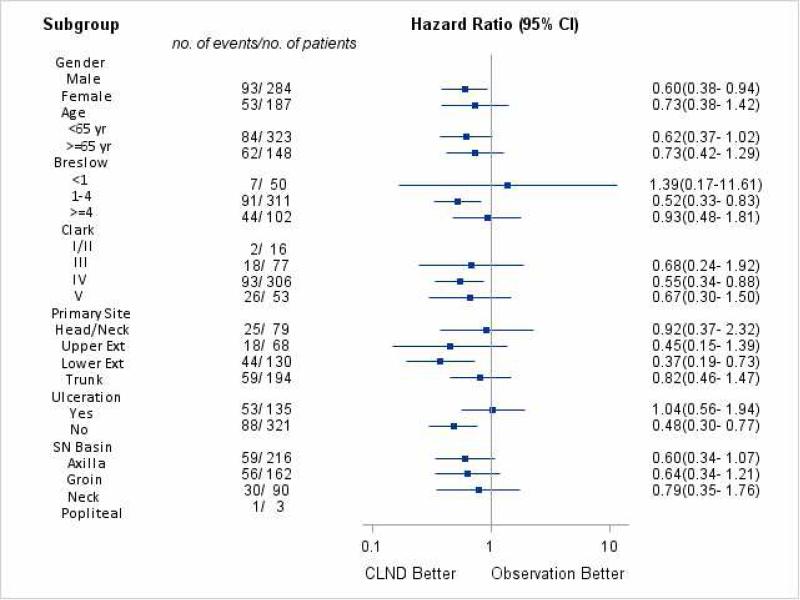
There were 471 patients who had positive SNB; 375 (79.6%) in the CLND group and 96 (20.4%) in the OBS group. The groups were similar except that the CLND group was younger and had more sentinel nodes removed. Five-year nodal recurrence free survival was significantly better in the CLND group compared to the OBS group (93.1% vs 84.4%, p= 0.005). However, the 5- (66.4% vs 55.2%) and 10- year (59.5% vs 45.0%) distant metastasis free survival was not significantly different (p= 0.061). The CLND group's melanoma specific survival (MSS) was superior to the OBS group; 5 year MSS was (73.7 vs 65.5%) and10 year MSS- (66.8 vs 48.3%, p=0.015). On multivariate analysis, CLND was associated with improved MSS (HR 0.60, 95% CI 0.40-0.89, p= 0.011) and lower nodal recurrence (HR 0.46, 95% CI 0.24-0.86, p=0.016). Increased Breslow thickness, older age, ulceration, and trunk melanoma were all associated with worse outcomes. On subgroup analysis, following factors were associated with better outcomes from CLND- male gender, non-ulcerated primary, intermediate thickness, Clark level IV or lower extremity tumors.
Conclusions
Treatment of positive SNB with CLND was associated with improved MSS and nodal recurrence rate. Follow up beyond 5 years was needed to see a significant difference in MSS.
Introduction
The prognostic value of sentinel lymph node biopsy (SNB) has been validated by retrospective studies as well as a large prospective randomized study, the Multicenter Selective Lymphadenectomy Trial (MSLT-1) (1-5). While SNB is now widely accepted as a useful staging tool in the management of cutaneous melanoma, there continues to be a debate regarding its impact on patient outcomes (6-8).
The current National Cancer Comprehensive (NCCN) guidelines recommend that patients with a positive sentinel lymph node undergo completion lymphadenectomy (CLND) or be enrolled into a clinical trial (9). However, a number of retrospective studies have shown that there may be limited survival or clinical benefits for those who are observed after a positive SNB compared to those who undergo immediate CLND after positive SNB (10-13).
The studies regarding CLND after positive SNB have been limited by small numbers and relatively short follow up. Furthermore, many of the series have a selection bias towards observing older patients and patients with positive SN from the groin (14). While individual studies have been limited, there is data to suggest that in a large patient population with enough power, survival benefit may be demonstrated (15). Therefore, we conducted a review of large prospectively maintained melanoma database at a melanoma referral center. We compared the outcomes of patients undergoing immediate CLND following positive SNB those were observed closely.
Methods
This study received an IRB-exemption status after independent regulatory review. The prospectively maintained melanoma database was used to study patients who underwent successful SNB for cutaneous melanoma at the John Wayne Cancer Institute (JWCI) from January of 1991 to June of 2013. Patients enrolled in the Multicenter Selective Lymphadenectomy Trial II (MSLT-II) were excluded from this study. Factors including age, gender, primary tumor site, SNB site, tumor characteristics, results of the SNB, information regarding CLND, recurrence and melanoma specific survival (MSS) data were analyzed.
Surgical Technique
Details of our technique for SNB have been previously reported in detail (16-18). Briefly, the majority of the cases in this study were performed with the use of Vital blue dye and 99mTc-labeled sulfur colloid radiotracer as a dual agent utilizing the hand-held gamma probe. Vital blue dye (1cc of 1% Lymphazurine) was administered 5-15 minutes prior to incision while lymphoscintigraphy was performed 1-4 hours prior to the operation. Sentinel lymph nodes (SN) were defined as: lymph nodes that were blue or had evidence of blue channels entering the lymph node, radioactive lymph nodes that had counts ≥ 10% of the most radioactive nodes, or clinically suspicious lymph nodes. Histopathological analysis of the SN consisted of sectioning and staining with hematoxylin and eosin (H&E) as well as immunohistochemistry with antibodies including S100, HMB-45, and MART-1.
Patients with positive SNB were advised to undergo CLND. Patients who did not undergo immediate CLND and recurred in the nodal basin were advised to undergo CLND at the time of the regional recurrence in the absence of detectable distant metastases. Outcomes of those who underwent CLND for positive SNB (CLND group) were compared to those who did not undergo immediate CLND (observation group, OBS). Patients who were observed were seen every 3-6 months with clinical exam, and ultrasound to the nodal basin every 6 months. Patients who were initially observed but underwent CLND in a delayed fashion (salvage CLND group) were considered to be in the OBS group. We also compared the outcomes of those who underwent immediate CLND and were found to have additional tumor cells in non-sentinel lymph nodes to those who were initially observed and underwent CLND at a later time point (salvage CLND group).
Statistics
Univariate comparison of clinico-pathologic factors between the CLND and the OBS groups were performed using chi-square test, or Student's T-test as appropriate. Survival analyses were performed by constructing Kaplan-Meier survival curves and compared using log-rank tests. Factors associated with regional and distant metastasis, and melanoma specific survival (MSS) were identified by constructing Cox-multivariate models. Distant metastasis-free survival was defined as the time between initial surgical treatment and melanoma recurrence beyond the regional basin or death from melanoma. MSS was defined as the time from initial surgical treatment to death from melanoma. Patients who died of non-melanoma causes were censored at that point. All statistical analyses were performed on SAS version 9.3 (SAS Institute, Carey NC) and p values< 0.05 were considered significant.
Results
Patient demographics/Tumor characteristics
Our inclusion criteria yielded 471 patients with positive SNB with a mean and median follow up of 83.1 months and 76.0 months respectively. Of these patients, 375 (79.6%) underwent immediate CLND with median follow up of 81.4 months. Ninety-six (20.4%) patients with positive SNB were observed with median follow up of 45.4 months. Sixteen (16.7%) of these patients eventually underwent salvage CLND with median time to CLND of 15 months. Patients in the CLND group were younger than the OBS group, however other clinico-pathologic factors including gender and tumor characteristics between the two groups were similar (Table 1).
Table 1.
Patient Demographics and Tumor Characteristics
| Characteristics | OBS (n=96) | CLND (n=375) | p Value |
|---|---|---|---|
| Age, y, median (range) | 56 (7-95) | 53 (4-98) | |
| Age, y, mean ± SD | 57.6 ± 17.9 | 52.9 ± 18.4 | 0.026 |
| Age, y, n (%) | |||
| < 65 | 57 (59.4) | 276 (70.9) | 0.030 |
| ≥ 65 | 39 (40.6) | 109 (29.1) | |
| Sex, male, n (%) | 64 (66.7) | 220 (58.7) | 0.153 |
| Breslow thickness, mean mm ± SD | 3.1 ± 2.7 | 3.0 ± 3.6 | 0.604 |
| Breslow thickness, mm, n (%) | 0.606 | ||
| <1 | 11 (1.5) | 39 (10.4) | |
| 1-3.99 | 59 (61.5) | 252 (67.2) | |
| ≥ 4 | 25 (26.0) | 77 (20.5) | |
| Unknown | 1 (1.0) | 7 (1.9) | |
| Clark level, n (%) | 0.783 | ||
| I/II | 3 (3.1) | 13 (3.5) | |
| III | 17(17.7) | 60 (16.0) | |
| IV | 58 (60.4) | 248 (66.1) | |
| V | 14 (14.6) | 39 (10.4) | |
| Unknown | 4 (4.2) | 15 (4.0) | |
| Primary tumor site, n (%) | 0.394 | ||
| Head/neck | 19 (19.8) | 57 (15.2) | |
| Upper extremity | 11 (11.5) | 60 (16.0) | |
| Trunk | 44 (45.8) | 150 (40.0) | |
| Lower extremity | 22 (22.9) | 108 (28.8) | |
| Ulceration, n (%) | 0.133 | ||
| Present | 34 (35.4) | 101 (26.9) | |
| Absent | 61 (63.5) | 260 (69.3) | |
| Unknown | 1 (1.0) | 14 (3.7) |
OBS, observation; CLND, completion lymph node dissection.
Nodal basin/sentinel lymph node characteristics
The most common site of SNB was the axilla for both OBS (45.4%) and the CLND group (44.5%) with no significant differences between the other sites. The majority of the patients in the CLND group (85.9%) and the OBS group (89.6%) had SNB performed at only one nodal basin. On average, patients in the OBS group had higher number of sentinel lymph nodes harvested compared to patients in the CLND group (2.9 vs 2.4, p=0.029). If the patient had more than one nodal basin sampled for SNB, the number of sentinel lymph nodes harvested at the second site was higher in the OBS group compared to the CLND group but this did not reach statistical difference (4.7 vs 2.0, p= 0.104).
In majority of the cases (82.3% OBS vs 79.5% CLND, p= 0.537), only one tumor positive lymph node was identified via SNB. When CLND was performed immediately after positive SNB, additional tumor positive nodes were identified in 66 (17.4%) cases. The mean and the median number of additional tumor positive nodes identified were 2.1 and 1.0 respectively.
In the 16 cases of salvage CLND performed in the OBS group, 15 (93.4%) of the CLND identified additional tumor positive nodes. The mean and the median number of tumor positive nodes identified were significantly higher compared to the immediate CLND group at 5.1 and 3.0 respectively (p= 0.034). Details of the SNB and the characteristics of the nodal basins are found in Table 2.
Table 2.
Nodal Basin and Sentinel Lymph Node Characteristics
| Characteristics | OBS (n=96) | CLND (n=375) | p Value |
|---|---|---|---|
| Sentinel lymph nodal basin, n (%) | 0.803 | ||
| Axilla | 49 (45.4) | 170 (44.5) | |
| Groin | 34 (31.5) | 136 (35.6) | |
| Neck | 24 (22.2) | 74 (19.4) | |
| Popliteal | 1 (0.9) | 2 (0.5) | |
| No. of basins (%) | 0.340 | ||
| 1 | 86 (89.6) | 322 (85.9) | |
| ≥2 | 10 (10.4) | 53 (14.1) | |
| Median LN sampled (quartile) | |||
| SNB site 1 | 2 (1-12) n= 96 | 2 (1-15) n= 375 | |
| SNB site 2 | 3 (1-16) n= 10 | 1 (1-10) n= 53 | |
| SNB site 3 | 1 (1-2) n= 4 | ||
| Mean no. of SN | |||
| SNB Site 1 | 2.9 ± 2.1 | 2.4 ± 1.8 | 0.029 |
| SNB Site 2 | 4.7± 4.6 | 2.0 ± 1.7 | 0.104 |
| Single positive SN/SNB site 1, n (%) | 79 (82.3) | 298 (79.5) | 0.537 |
| Patients with additional nodes on CLND, n/N (%) | 15/16 (93.4) | 66/373 (17.4) | <0.001 |
| Median no. additional positive nodes (range) | 3 (1-20) | 1 (1-13) | |
| Mean no. additional positive nodes ± SD | 5.1 ± 4.8 (n=15) | 2.1 ± 2.3 (n=66) | 0.034 |
OBS, observation; CLND, completion lymph node dissection; LN, lymph node; SN, sentinel lymph node; SNB, sentinel lymph node biopsy.
Recurrence patterns
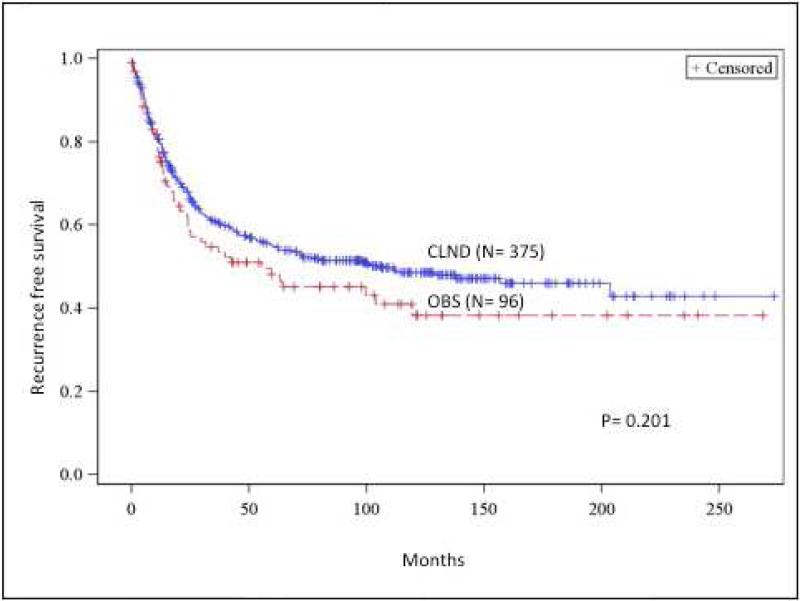
The 5- and 10-year disease free survival in the CLND group was higher compared to the OBS group (54.9% and 48.6%, vs 48.1% and 38.2%, p=0.201), however this difference was not statistically significant (Figure 1a).
Fig 1.
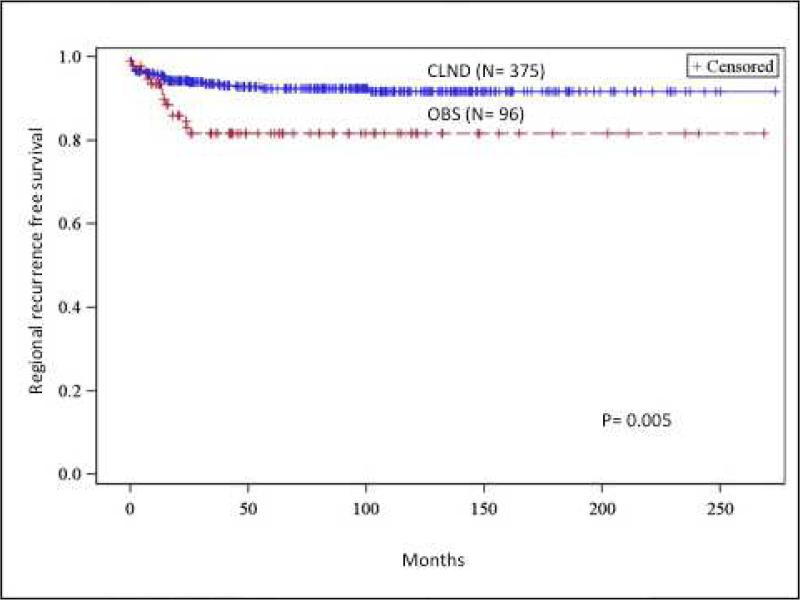
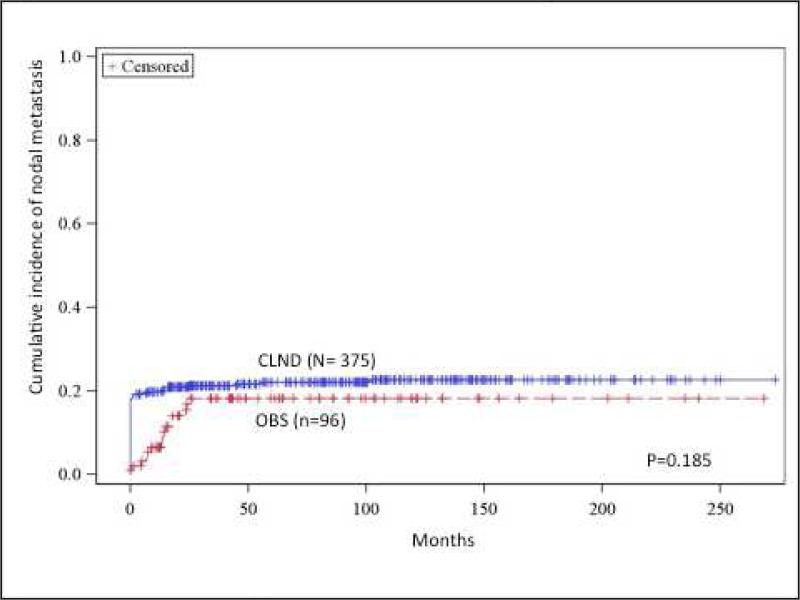
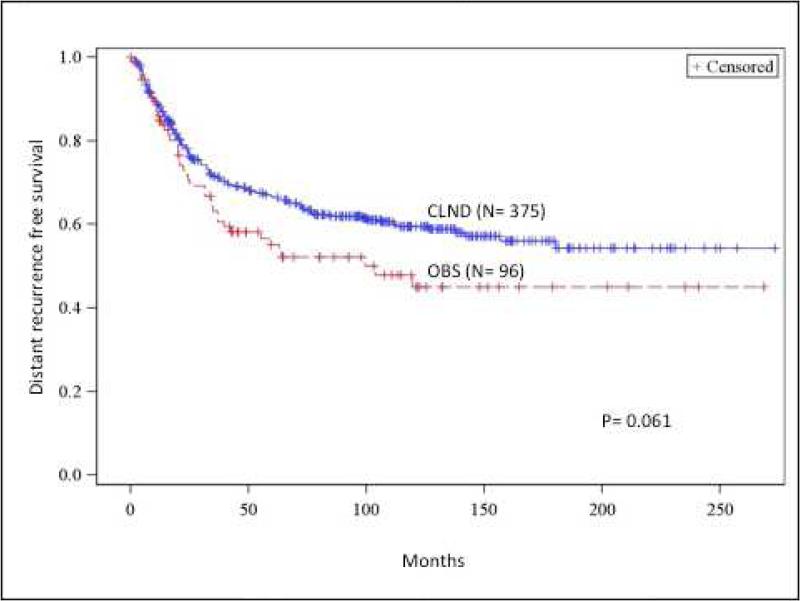
(A) Recurrence-free survival comparison between completion lymph node dissection (CLND) and observation (OBS). (B) Regional recurrence-free survival comparison between CLND and OBS. (C) Cumulative incidence of nodal metastasis comparison between CLND and OBS. (D) Distance recurrence-free survival comparison between CLND and OBS. OBS, Observation
There was significantly less nodal recurrence in the CLND group compared to the OBS group. In the CLND group, nodal basin as the first site of nodal recurrence was 6.4% compared to 12.5% for the OBS group (p=0.045). Five and 10-year nodal recurrence free survival for the CLND group was 93.1% and 92.8% respectively. This was significantly better compared to the OBS group, which had 5- and 10- year nodal recurrence free survival of 84.4% (p=0.005) (Figure 1b).
For those who developed nodal recurrence, the time to recurrence was similar between the two groups. The mean time to nodal recurrence was 15 months (median 6 months) for the CLND group and 13 months (median 14 months) for the OBS group. On multivariate analysis, CLND was associated with improved nodal recurrence (HR 0.46, 95% CI 0.24-0.86, p= 0.016) while age ≥ 65 years was significantly associated with a higher rate of nodal recurrence (HR 2.17, 95% CI 1.17-4.02, p= 0.013).
The cumulative incidence of non-sentinel nodal metastasis in the entire cohort was 21.7%. While there was better nodal control in the CLND group, the cumulative incidence of non-sentinel nodal metastasis (defined as a sum of patients who harbored tumor positive non-sentinel node at the time of the initial CLND or were found to have non-sentinel tumor positive nodes at a later time) were similar between the CLND and the OBS group (22.7% vs 18.3%, p=0.185) (Figure 1c).
The 5- and 10- year distant metastasis free survival was 66.4% and 59.5% for the CLND group. This was not statistically different from the OBS group, which had 5- and 10- year distant metastasis free survival of 55.2% and 45.0 % (p= 0.061) (Figure 1d). The mean time to distant metastasis was 32 months (median 20 months) for the CLND group and 29 months for the OBS group (median 21 months).
On multivariate analysis, male gender (HR 1.53, 95% CI 1.1-2.1, p= 0.014), and age ≥ 65 years (HR 2.02, 95% CI 1.46-2.81, p< 0.001) were associated the occurrence of distant metastasis. Having a single positive sentinel lymph node identified during SNB was associated with less likelihood of distant metastasis (HR 0.56, 95% CI 0.39-0.80, p = 0.002). However, CLND was not associated with distant metastasis (HR 0.85, 95% CI 0.58-1.26, p= 0.422).
Survival analysis
The CLND group had superior MSS compared to the OBS group. The 5- and 10-year MSS for the CLND group was 73.7%, and 66.8% compared to the OBS group at 65.5%, and 48.3% respectively (p= 0.015) (Figure 2a). On multivariate analysis, CLND was associated with improved MSS (HR 0.60, 95% CI 0.40-0.89, p = 0.011). Increased Breslow thickness, age ≥ 65 years, ulcerated primaries, and truncal primaries were all associated with worse MSS (Table 3). On subgroup analysis, the following factors were associated with better outcomes related to CLND: male gender, intermediate Breslow thickness (≥1 and < 4mm), Clark level IV, lower extremity and non-ulcerated tumors (Figure 2b).
Fig 2.
(A) Melanoma specific free survival comparison between completion lymph node dissection (CLND) and observation. (B) Subgroup analysis of melanoma specific survival (MSS). OBS, observation.
Table 3.
Cox Proportional Hazard Ratio of Factors Associated with Melanoma-Specific Survival
| Clinical factors | Hazard ratio | 95% CI | p Value |
|---|---|---|---|
| Lymph node dissection | |||
| OBS | Referent | ||
| CLND | 0.60 | 0.40-0.89 | 0.011 |
| Breslow thickness | |||
| <1 mm | Referent | ||
| 1-3.99 mm | 2.74 | 1.23-6.07 | 0.013 |
| ≥ 4 mm | 3.05 | 1.35-6.86 | 0.007 |
| Ulceration | |||
| No | Referent | ||
| Yes | 1.6 | 1.14-2.35 | 0.007 |
| Primary tumor site | |||
| Trunk | Referent | ||
| Upper extremity | 0.54 | 0.31-0.94 | 0.028 |
| Lower extremity | 0.62 | 0.41-0.93 | 0.021 |
| Head and neck | 0.63 | 0.38-1.02 | 0.062 |
OBS, observation; CLND, completion lymph node dissection;
Comparison of salvage CLND vs immediate CLND with non-sentinel positive nodes
To analyze the impact of delayed treatment on additional positive non-sentinel lymph nodes, we compared the outcomes of 16 patients from the OBS group who underwent salvage CLND with clinical recurrence during observation to those who underwent immediate SNB and were found to have additional metastases on CLND in non-sentinel nodes (n=66). This analysis functions on the assumption that additional disease was present in the lymph nodes of the salvage group at time of sentinel lymph node biopsy.
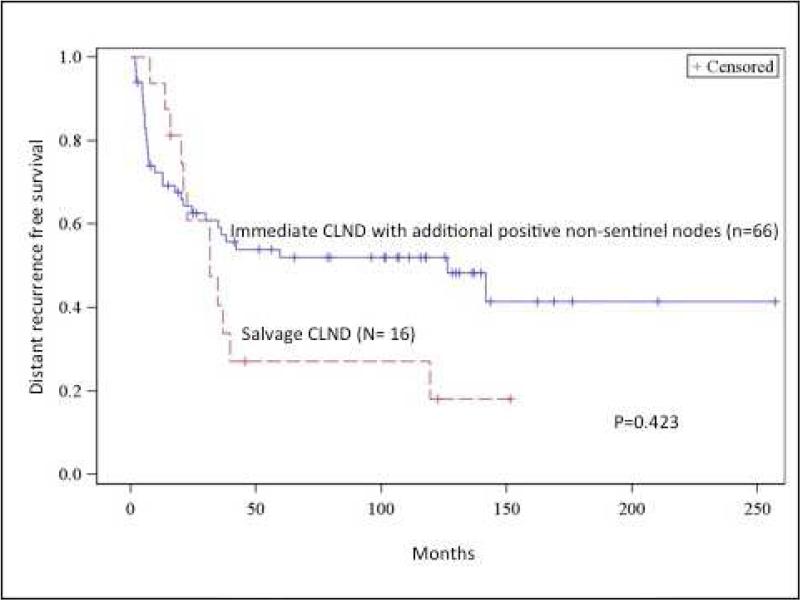
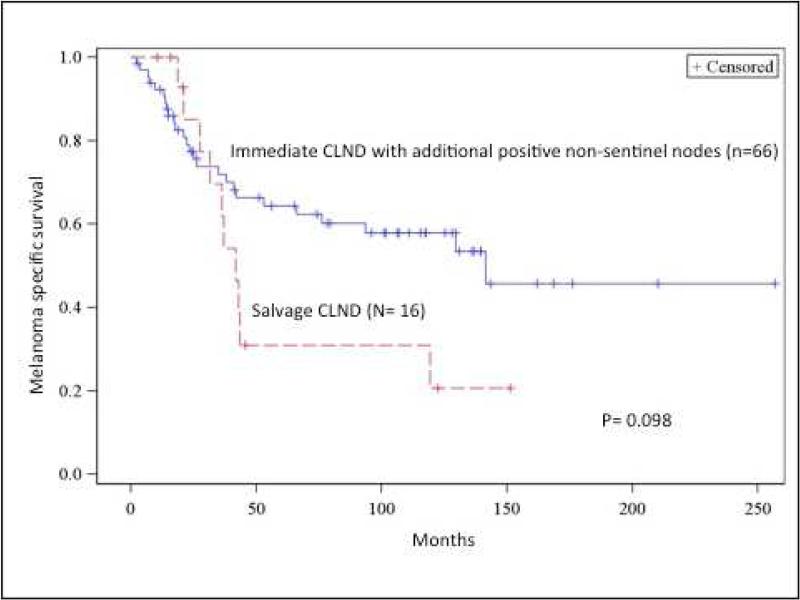
The 5- and 10- year distant metastasis free survival in the group who had additional non-sentinel positives nodes on CLND (N2-N3 patients) were 59.2% and 55.0% compared to 34.1% for the salvage CLND group, however this was not statistically significant (p= 0.423) (Figure 3a). While MSS favored the immediate CLND group with occult non-sentinel nodes with tumors compared to the salvage group, this did not reach statistical significance either. At 5 and 10 years, MSS for the immediate CLND group with non-sentinel tumor positive nodes were 68.2% and 63.6% compared to 43.8%, and 37.5% for the salvage group (p= 0.098) (Figure 3b).
Fig 3.
(A) Distant recurrence-free survival comparison between salvage completion lymph node dissection (CLND) vs immediate CLND patients with additional tumor-positive non-sentinel nodes. (B) Melanoma specific survival comparison between salvage CLND vs immediate CLND patients with additional tumor-positive non-sentinel nodes. OBS, observation.
Discussion
While CLND after positive SNB remains standard treatment in melanoma, there has been increasing debate about whether all patients with positive SNB should undergo CLND (12, 15, 19-21). In theory, CLND would only potentially benefit patients with additional tumor cells in the same nodal basin as the biopsied sentinel nodes. In our series, 17% of the patients with positive SNB had additional nodal metastases identified on CLND. This number is similar to other retrospective series in the literature, which ranges between 10-25% (10, 22-24). Thus the majority of the patients who undergo CLND after positive SNB do not have additional tumor containing nodes and may not benefit from CLND.
Unfortunately there are no reliable techniques to identify and selectively offer CLND to patients who will have additional positive nodes based on imaging or clinico-pathologic features. While ultrasound has been used in clinical trials and is likely to be a useful adjunct in follow up, it is highly operator dependent with reported sensitivities of 20-65% and it also requires metastases to reach a certain size (usually >5 mm) to be detectable (25, 26). Newer technology such as PET-CT is expensive and also requires a minimum tumor size (usually ≥1cm) for detection. Furthermore, its role in the management of patients with nodal disease in melanoma is not well defined in either the diagnostic or surveillance settings (27-29).
Many investigators have studied patient and tumor factors as well as the characteristics of sentinel lymph node tumor burden to identify patients who may have occult tumor positive nodes (30-32). Murali et al. developed a scoring scheme that includes gender, regression in the primary tumor, proportion of harvested nodes containing metastatic melanoma, peri-nodal lymphatic invasion, and maximum size of largest tumor deposit to risk stratify patients (33). This study was subsequently validated by two other retrospective series (34, 35). While the study was accurate in identifying patients with very high or low risk of having additional tumor positive nodes, the majority of the patients in the study were assigned intermediate to high-risk scores with risks for additional lymph nodes positivity between 5 to 40% thereby limiting the scoring scheme's clinical utility.
Our finding that patients who undergo immediate CLND have superior regional control compared to those who undergo OBS at the time of the positive SNB, corroborate earlier series (10, 36, 37). In contrast to those series, however, our study detected a significant MSS difference favoring those who underwent immediate CLND compared to all those who were observed. This difference could be due to an increased length of the follow up combined with a large number of patients in the current study and relatively well-balanced patient populations between the CLND and the OBS group.
In our study, the follow-up for the overall cohort was 76 months, with 81 months for those who underwent observation and 45 months for the patients who were observed. In comparison, the largest previously published series (which had a trend toward improved MSS in the OBS group) had a median follow up of 20 months for those who underwent nodal observation compared to 83 months for those who underwent immediate CLND (10). If survival differences emerge after several years of follow up, which appears to be supported by our data, longer follow up may be required to observe a difference. OBS patients in the prior series were also an average of 10 years older than those in the CLND group, which might tend to skew MSS in their favor as they have competing causes for mortality. In addition, the two groups in the prior had markedly different distributions of primary tumor sites, therefore is not a balanced comparison. While there was a trend to improved distant-metastasis free survival among patients undergoing immediate CLND, the difference did not reach statistical significance. This may have been due to an increased rate of censoring for distant metastasis versus melanoma death, which limited the power of the former analysis.
While smaller individual studies have not been able to detect survival difference between CLND and OBS groups, these studies may have lacked power or sufficient length of follow up to detect a survival difference. In a meta-analysis of 2,633 patients from 6 studies by Pasquali et al, a significant survival benefit for those who underwent immediate CLND was demonstrated (15). Furthermore, the latent subgroup analysis of the MSLT-1 trial, showed that patients who develop regional disease during observation and undergo CLND at a later time period have worse outcomes than those who undergo immediate CLND after positive SNB, suggesting that there may be a benefit to removing tumor positive nodes at an earlier time point (3). However, this sub-group of patients was not pre specified and the trial was not designed to answer the outcomes of those who had positive SNB but were observed.
While MSS was superior in the immediate CLND group as a whole, we also performed subgroup analyses to identify the patients who might derive a greater benefit from CLND. In our study, male gender, non-ulcerated primary, intermediate thickness, Clark level IV, or lower extremity location were factors associated with better outcomes from CLND relative to OBS. Several of these factors are very similar to subgroups identified in earlier elective lymph node dissection randomized trials (38). This suggests that tumor biology determines the patients who are most likely to benefit from early nodal surgery, and conversely the timing of nodal surgery does not alter outcome for those with poor biologic features, such as ulcerated primary melanoma.
There are several limitations to this study. In our study, we did not examine the impact of adjuvant therapy on survival. However, only 15% of our study group underwent adjuvant therapy and the likelihood of receiving adjuvant therapy was not related to undergoing immediate CLND. It is therefore very unlikely that this effected outcomes in this analysis. Another factor that may be useful, to refine the selection of patients for CLND, is the volume of tumor burden in the SLN. There is provocative data emerging from the prospective Sunbelt Melanoma Trial suggesting improved outcomes among patients with sentinel node metastases detected by reverse transcriptase polymerase chain reaction (RT-PCR) if they were managed with immediate CLND (39). Unfortunately, standardized means of quantification were not established throughout our study period and are not available for this analysis. However, our practice is to offer CLND to all patients with isolated tumor cells in the sentinel lymph node and this practice did not change during the study period.
The therapeutic impact of CLND would likely be limited to those patients who had additional positive nodes on CLND and those who had salvage CLND. While the 5, and 10 year MSS for patients who had immediate CLND with additional non-sentinel nodes were superior to those who underwent salvage CLND (68.2, 63.6% vs 43.8%, 37.5) this did not reach statistical significance (p= 0.0977). However, due to the small number of patients in these sub groups (66 immediate with non-sentinel positive nodes vs 16 salvage CLND), we lacked the statistical power to adequately examine these subgroups. Improved methods for prospectively identifying these subgroups and examination of these non-sentinel node positive patients in MSLT-2 would be desirable.
Lastly, our study is retrospective in nature and reflects the referral bias and practice pattern of a single institution. Since this is not a randomized study, there may be a selection bias for those who underwent OBS compared to those who underwent CLND. The discordant results of our study and those of prior series suggest that the final answer to this important clinical question cannot be definitively determined from retrospective analysis. Only controlled, prospective study will provide such guidance. A recent interim analysis of randomized trial conducted by the German Dermatologic Cooperative Oncology Group, examining the utility of CLND in patients with sentinel lymph node metastases, did not show a survival benefit for those who underwent upfront CLND. However the limited sample size and follow up for that study suggests that the currently data may not be adequate to definitively answer the question (40). Fortunately, the MSLT-2 clinical trial is now fully accrued and should have adequate statistical power and long-term follow up to provide guidance in the future.
Conclusions
While the current ongoing MSLT-2 trial is expected to answer many of the questions regarding the utility of CLND, our study is one of the largest series in the literature specifically looking at the outcomes of patients who undergo CLND in patients with positive SNB. We confirmed the ability of CLND to decrease regional recurrence risk. In addition, ours is the first study to demonstrate improved MSS when treated with immediate CLND, relative to OBS. Pending definitive prospective evaluation, CLND should remain a standard for patients with SLN melanoma metastases.
Acknowledgment
The authors are grateful for the editorial assistance of Gwen Berry.
Support: Dr DY Lee is the Harold McAlister Charitable Foundation Fellow. This study was supported by grant R01 CA189163 from the National Cancer Institute and by funding from the Amyx Foundation, Inc, (Boise, ID), The Borstein Family Foundation (Los Angeles, CA), Dr. Miriam & Sheldon G Adelson Medical Research Foundation (Boston, MA), and the John Wayne Cancer Institute Auxiliary (Santa Monica, CA). The content is solely the responsibility of the authors and does not necessarily represent the official view of the National Cancer Institute or the National Institutes of Health.
Abbreviations and Acronyms
- CLND
Completion lymph node dissection
- HMB-45
Human melanoma black 45
- H&E
hematoxylin and eosin
- IRB
Institutional review board
- JWCI
John Wayne Cancer Institute
- MART-1
melanoma antigen recognized by T-cells 1 = Melan-A
- MSLT
Multicenter Selective Lymphadenectomy Trial
- MSS
Melanoma specific survival
- NCCN
National Comprehensive Cancer Network
- OBS
Observation
- SN
Sentinel lymph node
- SNB
Sentinel lymph node biopsy
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Information: Nothing to disclose.
Disclosures outside the scope of this work: Dr Faries has served as a consultant for Amgen Inc, Astellas Pharma Inc, Myriad Genetics, Inc, and Genentech, Inc.
Presented at the Western Surgical Association 123rd Scientific Session, Napa Valley, CA, November 2015.
References
- 1.Morton DL, Cochran AJ, Thompson JF, et al. Sentinel node biopsy for early-stage melanoma: accuracy and morbidity in MSLT-I, an international multicenter trial. Ann Surg. 2005 Sep;242(3):302–11. doi: 10.1097/01.sla.0000181092.50141.fa. discussion 11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morton DL CL, Wong J. Intraoperative lymphatic mapping and selective lymphadenectomy: Technical details of a new procedure for clinical stage I melanoma.. 42nd Annual Meeting of the Society of Surgical Oncology; Washington, DC. 1990 May 20-22; 1990. 1990. [Google Scholar]
- 3.Morton DL, Thompson JF, Cochran AJ, et al. Final trial report of sentinel-node biopsy versus nodal observation in melanoma. N Engl J Med. 2014 Feb 13;370(7):599–609. doi: 10.1056/NEJMoa1310460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nowecki ZI, Rutkowski P, Nasierowska-Guttmejer A, Ruka W. Sentinel lymph node biopsy in melanoma patients with clinically negative regional lymph nodes--one institution's experience. Melanoma Res. 2003 Feb;13(1):35–43. doi: 10.1097/00008390-200302000-00007. [DOI] [PubMed] [Google Scholar]
- 5.Rutkowski P, Nowecki ZI, Nasierowska-Guttmejer A, Ruka W. Lymph node status and survival in cutaneous malignant melanoma--sentinel lymph node biopsy impact. Eur J Surg Oncol. 2003 Sep;29(7):611–8. doi: 10.1016/s0748-7983(03)00118-5. [DOI] [PubMed] [Google Scholar]
- 6.Sladden M, Zagarella S, Popescu C, Bigby M. No survival benefit for patients with melanoma undergoing sentinel lymph node biopsy: critical appraisal of the Multicenter Selective Lymphadenectomy Trial-I final report. Br J Dermatol . 2015 Mar;172(3):566–71. doi: 10.1111/bjd.13675. [DOI] [PubMed] [Google Scholar]
- 7.Faries MB, Cochran AJ, Elashoff RM, Thompson JF. Multicenter Selective Lymphadenectomy Trial-I confirms the central role of sentinel node biopsy in contemporary melanoma management: response to 'No survival benefit for patients with melanoma undergoing sentinel lymph node biopsy: critical appraisal of the Multicenter Selective Lymphadenectomy Trial-I final report'. Br J Dermatol . 2015 Mar;172(3):571–3. doi: 10.1111/bjd.13676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ross MI, Gershenwald JE. How should we view the results of the Multicenter Selective Lymphadenectomy Trial-1 (MSLT-1)? Ann Surg Oncol. 2008 Mar;15(3):670–3. doi: 10.1245/s10434-008-9828-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coit DG, Andtbacka R, Anker CJ, et al. Melanoma, version 2.2013: featured updates to the NCCN guidelines. J Natl Compr Canc Netw. 2013 Apr 1;11(4):395–407. doi: 10.6004/jnccn.2013.0055. [DOI] [PubMed] [Google Scholar]
- 10.Bamboat ZM, Konstantinidis IT, Kuk D, et al. Observation after a positive sentinel lymph node biopsy in patients with melanoma. Ann Surg Oncol . 2014 Sep;21(9):3117–23. doi: 10.1245/s10434-014-3758-7. [DOI] [PubMed] [Google Scholar]
- 11.Kingham TP, Panageas KS, Ariyan CE, et al. Outcome of patients with a positive sentinel lymph node who do not undergo completion lymphadenectomy. Ann Surg Oncol. 2010 Feb;17(2):514–20. doi: 10.1245/s10434-009-0836-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wong SL, Morton DL, Thompson JF, et al. Melanoma patients with positive sentinel nodes who did not undergo completion lymphadenectomy: a multi-institutional study. Ann Surg Oncol. 2006 Jun;13(6):809–16. doi: 10.1245/ASO.2006.03.058. [DOI] [PubMed] [Google Scholar]
- 13.van der Ploeg AP, van Akkooi AC, Rutkowski P, et al. Prognosis in patients with sentinel node-positive melanoma without immediate completion lymph node dissection. Br J Surg. 2012 Oct;99(10):1396–405. doi: 10.1002/bjs.8878. [DOI] [PubMed] [Google Scholar]
- 14.Shah DR, Yang AD, Maverakis E, Martinez SR. Age-related disparities in use of completion lymphadenectomy for melanoma sentinel lymph node metastasis. J Surg Res. 2013 Nov;185(1):240–4. doi: 10.1016/j.jss.2013.05.090. [DOI] [PubMed] [Google Scholar]
- 15.Pasquali S, Mocellin S, Campana LG, et al. Early (sentinel lymph node biopsy-guided) versus delayed lymphadenectomy in melanoma patients with lymph node metastases : personal experience and literature meta-analysis. Cancer. 2010 Mar 1;116(5):1201–9. doi: 10.1002/cncr.24852. [DOI] [PubMed] [Google Scholar]
- 16.Bagaria SP, Faries MB, Morton DL. Sentinel node biopsy in melanoma: technical considerations of the procedure as performed at the John Wayne Cancer Institute. J Surg Oncol. 2010 Jun 15;101(8):669–76. doi: 10.1002/jso.21581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morton DL, Thompson JF, Essner R, et al. Validation of the accuracy of intraoperative lymphatic mapping and sentinel lymphadenectomy for early-stage melanoma: a multicenter trial. Multicenter Selective Lymphadenectomy Trial Group. Ann Surg. 1999 Oct;230(4):453–63. doi: 10.1097/00000658-199910000-00001. discussion 63-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zogakis TG, Essner R, Wang HJ, et al. Melanoma recurrence patterns after negative sentinel lymphadenectomy. Arch Surg. 2005 Sep;140(9):865–71. doi: 10.1001/archsurg.140.9.865. discussion 71-2. [DOI] [PubMed] [Google Scholar]
- 19.Nowecki ZI, Rutkowski P, Michej W. The survival benefit to patients with positive sentinel node melanoma after completion lymph node dissection may be limited to the subgroup with a primary lesion Breslow thickness greater than 1.0 and less than or equal to 4 mm (pT2-pT3). Ann Surg Oncol. 2008 Aug;15(8):2223–34. doi: 10.1245/s10434-008-9965-3. [DOI] [PubMed] [Google Scholar]
- 20.van Akkooi AC, Bouwhuis MG, de Wilt JH, et al. Multivariable analysis comparing outcome after sentinel node biopsy or therapeutic lymph node dissection in patients with melanoma. Br J Surg. 2007 Oct;94(10):1293–9. doi: 10.1002/bjs.5814. [DOI] [PubMed] [Google Scholar]
- 21.Pasquali S, Spillane AJ, de Wilt JH, et al. Surgeons' opinions on lymphadenectomy in melanoma patients with positive sentinel nodes: a worldwide web-based survey. Ann Surg Oncol. 2012 Dec;19(13):4322–9. doi: 10.1245/s10434-012-2483-3. [DOI] [PubMed] [Google Scholar]
- 22.McMasters KM, Wong SL, Edwards MJ, et al. Frequency of nonsentinel lymph node metastasis in melanoma. Ann Surg Oncol. 2002 Mar;9(2):137–41. doi: 10.1007/BF02557364. [DOI] [PubMed] [Google Scholar]
- 23.Sabel MS, Rice JD, Griffith KA, et al. Validation of statistical predictive models meant to select melanoma patients for sentinel lymph node biopsy. Ann Surg Oncol. 2012 Jan;19(1):287–93. doi: 10.1245/s10434-011-1979-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reeves ME, Delgado R, Busam KJ, et al. Prediction of nonsentinel lymph node status in melanoma. Ann Surg Oncol. 2003 Jan-Feb;10(1):27–31. doi: 10.1245/aso.2003.03.020. [DOI] [PubMed] [Google Scholar]
- 25.Voit C, Van Akkooi AC, Schafer-Hesterberg G, et al. Ultrasound morphology criteria predict metastatic disease of the sentinel nodes in patients with melanoma. J Clin Oncol. 2010 Feb 10;28(5):847–52. doi: 10.1200/JCO.2009.25.7428. [DOI] [PubMed] [Google Scholar]
- 26.Sanki A, Uren RF, Moncrieff M, et al. Targeted high-resolution ultrasound is not an effective substitute for sentinel lymph node biopsy in patients with primary cutaneous melanoma. J Clin Oncol. 2009 Nov 20;27(33):5614–9. doi: 10.1200/JCO.2008.21.4882. [DOI] [PubMed] [Google Scholar]
- 27.Singh B, Ezziddin S, Palmedo H, et al. Preoperative 18F-FDG-PET/CT imaging and sentinel node biopsy in the detection of regional lymph node metastases in malignant melanoma. Melanoma Res. 2008 Oct;18(5):346–52. doi: 10.1097/CMR.0b013e32830b363b. [DOI] [PubMed] [Google Scholar]
- 28.Bastiaannet E, Uyl-de Groot CA, Brouwers AH, et al. Cost-effectiveness of adding FDG-PET or CT to the diagnostic work-up of patients with stage III melanoma. Ann Surg. 2012 Apr;255(4):771–6. doi: 10.1097/SLA.0b013e31824a5742. [DOI] [PubMed] [Google Scholar]
- 29.Baker JJ, Meyers MO, Frank J, et al. Routine restaging PET/CT and detection of initial recurrence in sentinel lymph node positive stage III melanoma. Am J Surg. 2014 Apr;207(4):549–54. doi: 10.1016/j.amjsurg.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 30.Lee JH, Essner R, Torisu-Itakura H, et al. Factors predictive of tumor-positive nonsentinel lymph nodes after tumor-positive sentinel lymph node dissection for melanoma. J Clin Oncol. 2004 Sep 15;22(18):3677–84. doi: 10.1200/JCO.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 31.Sabel MS, Griffith K, Sondak VK, et al. Predictors of nonsentinel lymph node positivity in patients with a positive sentinel node for melanoma. J Am Coll Surg. 2005 Jul;201(1):37–47. doi: 10.1016/j.jamcollsurg.2005.03.029. [DOI] [PubMed] [Google Scholar]
- 32.Roka F, Mastan P, Binder M, et al. Prediction of non-sentinel node status and outcome in sentinel node-positive melanoma patients. Eur J Surg Oncol. 2008 Jan;34(1):82–8. doi: 10.1016/j.ejso.2007.01.027. [DOI] [PubMed] [Google Scholar]
- 33.Murali R, Desilva C, Thompson JF, Scolyer RA. Non-Sentinel Node Risk Score (N-SNORE): a scoring system for accurately stratifying risk of non-sentinel node positivity in patients with cutaneous melanoma with positive sentinel lymph nodes. J Clin Oncol. 2010 Oct 10;28(29):4441–9. doi: 10.1200/JCO.2010.30.9567. [DOI] [PubMed] [Google Scholar]
- 34.Wevers KP, Murali R, Bastiaannet E, et al. Assessment of a new scoring system for predicting non-sentinel node positivity in sentinel node-positive melanoma patients. Eur J Surg Oncol. 2013 Feb;39(2):179–84. doi: 10.1016/j.ejso.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 35.Feldmann R, Fink AM, Jurecka W, et al. Accuracy of the non-sentinel node risk score (N-SNORE) in patients with cutaneous melanoma and positive sentinel lymph nodes: a retrospective study. Eur J Surg Oncol. 2014 Jan;40(1):73–6. doi: 10.1016/j.ejso.2013.08.022. [DOI] [PubMed] [Google Scholar]
- 36.Morton DL, Thompson JF, Cochran AJ, et al. Sentinel-node biopsy or nodal observation in melanoma. N Engl J Med. 2006 Sep 28;355(13):1307–17. doi: 10.1056/NEJMoa060992. [DOI] [PubMed] [Google Scholar]
- 37.Chao C, Wong SL, Ross MI, et al. Patterns of early recurrence after sentinel lymph node biopsy for melanoma. Am J Surg. 2002 Dec;184(6):520–4. doi: 10.1016/s0002-9610(02)01102-9. discussion 5. [DOI] [PubMed] [Google Scholar]
- 38.Balch CM, Soong S, Ross MI, et al. Long-term results of a multi-institutional randomized trial comparing prognostic factors and surgical results for intermediate thickness melanomas (1.0 to 4.0 mm). Intergroup Melanoma Surgical Trial. Ann Surg Oncol. 2000 Mar;7(2):87–97. doi: 10.1007/s10434-000-0087-9. [DOI] [PubMed] [Google Scholar]
- 39.Kimbrough C, Martin R, McMasters K, et al. Molecular staging of sentinel lymph nodes identifies patients at increased risk of nodal recurrence. Oral presentation at the meeting of the Southern Surgical Association on December 7th, 2015. [Google Scholar]
- 40.Leiter U, Stadler R, Mauch C, et al. Survival of SLNB-positive melanoma patients with and without complete lymph node dissection: A multicenter, randomized DeCOG trial.. ASCO Annual Meeting; 2015. Abstract LBA 9002. Presented May 30,2015. [Google Scholar]