Abstract
In our cross-sectional sample of 7289 serologic tests for Lyme disease, we identified 167 instances of a positive IgM immunoblot but a negative IgG immunoblot test result. Considering that only 71% (95% CI 64%-78%) of patients had Lyme disease, a positive IgM immunoblot alone should be interpreted with caution to avoid over-diagnosis of Lyme disease.
Current diagnostic testing for Lyme disease lacks sensitivity during early infection. Localized disease, which manifests with the distinctive erythema migrans (EM) skin lesion, can be diagnosed clinically in endemic areas. All later manifestations, however, require serologic testing to distinguish Lyme disease from other diseases with similar presentations. The current diagnostic standard for Lyme disease is performance of an enzyme immunoassay (EIA) followed by IgM and IgG immunoblots in cases of a positive or equivocal EIA.1 IgM reactivity to at least 2 of 3 tested antigens is a biomarker of early infection and is a component of the currently recommended 2-tiered serologic testing algorithm. In a recent adult study, almost one-third of positive IgM immunoblots alone were obtained from adults who were unlikely to have Lyme disease.2 However, the clinical significance of a positive Lyme disease IgM immunoblot result alone has not been rigorously evaluated in children. To this end, we examined the clinical presentations of children from a highly endemic region with a positive IgM immunoblot alone.
Methods
We performed a retrospective cross-sectional study at Boston Children's Hospital located in a Lyme disease-endemic area (Boston, Massachusetts). The institutional review board approved the study protocol with waiver of informed consent. We identified patients serologically tested for Lyme disease through an electronic query of the institutional data warehouse. We included individuals 21 years of age and younger who had a Lyme EIA obtained between January 1, 2007, and June 30, 2014. We included multiple Lyme disease tests from the same patient over the study period.
All serologic Lyme disease tests from the study institution were performed at a single commercial reference laboratory (ARUP National Laboratories, Salt Lake, Utah). This laboratory used a Borrelia burgdorferi quantitative whole cell sonicate EIA, followed by reflex IgM and IgG immunoblot (MarDx; Trinity Biotech, Tray, Ireland) testing for those with a positive or equivocal EIA (≥1.0) in accordance with recommended testing standards. We limited our primary analysis to children who had a positive EIA followed by a positive Lyme IgM immunoblot but a negative IgG immunoblot. For all eligible children, we abstracted the following data from the medical record: demographics (age, sex), clinical presentation, and duration of symptoms.
We considered the following clinical syndromes to be compatible with Lyme disease: early localized (EM), early disseminated (multiple EM, meningitis, radiculoneuropathy, or carditis), and late (arthritis). In our study population, children with signs compatible with early or early disseminated Lyme disease with duration of ≤60 days had Lyme disease. Those with nonspecific clinical findings, late manifestations of Lyme disease, or duration of signs >60 days did not have Lyme disease.2 Conventionally, duration of symptoms >1 month is recommended as a cutoff beyond which the IgM should be disregarded.1 We extended this interval to 60 days given the difficulty of identifying precisely the duration of symptoms from the medical record. As children with late Lyme disease should have a robust IgG response, a positive IgM alone was not deemed diagnostic of Lyme arthritis.
Our primary analysis was the proportion of children with a positive IgM immunoblot who had Lyme disease. We utilized SPSS ver. 23.0 (SPSS Inc, Chicago, Illinois) for all data analysis.
Results
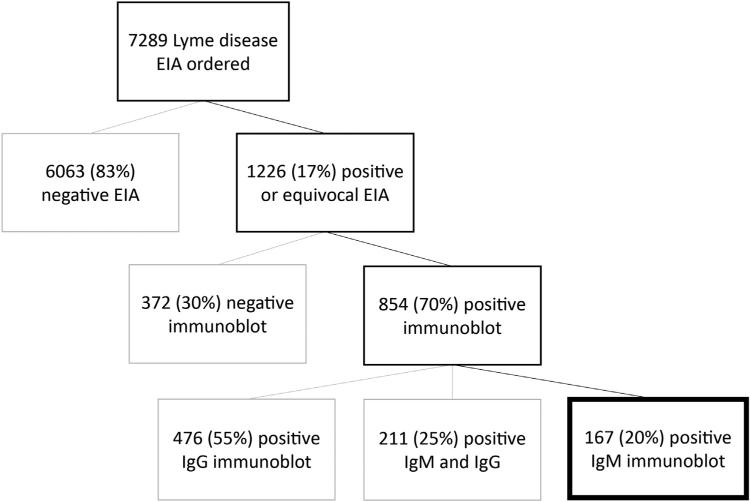
Over the 7-year study period, 7289 Lyme disease tests were obtained from 7043 unique patients. Reflex immunoblots were performed for the 1216 (17%) specimens with a positive or equivocal EIA result. Of the tests with confirmatory immunoblots performed, we identified 167 cases with positive IgM and negative IgG immunoblot result (2.2% of Lyme tests) from 167 unique children (Figure; available at www.jpeds.com). The median patient age was 10.9 years (IQR 7.5 years to 14.6 years), and 106 (64%) were male.
Figure.
Lyme serology results for study patients.
Of these 167 children, 58 (35%) had EM, 71 (43%) had signs compatible with early-disseminated Lyme disease, 14 (8%) with late Lyme disease, and 24 (14%) had nonspecific clinical presentations. Of the 71 children with signs compatible with early-disseminated Lyme disease, 38 had radiculoneuropathy, 28 had meningitis, and 5 had carditis. The 10 children who had signs lasting more than 60 days did not have Lyme disease. Additionally, the 14 children with arthritis and the 24 with nonspecific signs of ≤60 days duration did not have Lyme disease. Only 3 children had a repeat immunoblot performed at the study institution, of which 1 result was negative, and 2 had a persistently positive IgM and negative IgG result. Overall, 119 of the 167 children with a positive IgM immunoblot alone had Lyme disease (71.2%; 95% CI 64.0%-77.6%).
Discussion
Among children with a positive IgM immunoblot alone, we have found that more than one-quarter were obtained from children who were unlikely to have Lyme disease. We considered a positive IgM immunoblot alone from a child who lacked clinical features specific for Lyme disease, had a long duration of illness, or had late manifestations as a false positive test result. Our study is consistent with a recent adult study in which approximately one-half of positive IgM immunoblots alone were false positive results.2 A false positive IgM immunoblot may lead to diagnostic uncertainty and the potential for over-diagnosis of Lyme disease.
The IgM immunoblot is a valuable test in early Lyme disease, given the relatively slow appearance of IgG antibodies.3 On the other hand, the potential for false positive IgM results impairs its diagnostic value. Investigators have reported a specificity of 96% for the IgM immunoblot compared with clinically relevant control specimens.3 With more than 3.4 million Lyme disease tests ordered annually in the US,4 this specificity would yield 136 000 false positive IgM immunoblots. Over-reading of weak IgM bands is responsible for some false positive results.2
Our findings underscore the importance of patient selection in Lyme disease testing, as the inferential value of a positive test depends greatly on the population being tested. The positive predictive value of the IgM immunoblot is low in patients who lack clinical features of Lyme disease.3 A prior model has suggested that Lyme disease testing should be restricted to patients whose pretest probability of Lyme disease is at least 20%,5 such as patients in endemic areas with classic Lyme disease-associated syndromes such as meningitis, 7th cranial nerve palsy, and arthritis.6-13 For less specific clinical presentations, the likelihood of a false positive test may exceed that of a true positive.14
Our study has several important limitations. First, our study was retrospective and we relied on accurate documentation for clinical signs and duration. However, we used objective abstraction criteria and had minimal missing data. Second, as all Lyme disease immunoblots were performed using a single commercial kit, our results may not be applicable to other assays. Third, we did not have many follow-up Lyme serologic tests in our cross-sectional study. Therefore, some of the children with potentially false positive IgM immunoblots may have developed positive IgG immunoblot results on follow-up testing. Fourth, we relied on the current “gold standard” for diagnosis of Lyme disease: EM lesion or a positive acute 2-tiered serology in the appropriate clinical scenario. As we did not obtain convalescent serology, we may have misclassified children with early Lyme disease. Although this gold standard is inherently flawed, more definitive tests such as Borrelia culture are seldom available and clinicians must rely on the best available diagnostic tests to make clinical decisions. Finally, our study was conducted at a pediatric referral center located in a Lyme disease endemic area, and our findings may not be generalizable to all clinical settings.
The Lyme disease IgM immunoblot is valuable in the diagnosis of early Lyme disease in children. However, a positive IgM and a negative IgG in a child with a long duration of symptoms, late manifestations, or nonspecific clinical presentation is likely a false positive result for Lyme disease. Clinicians should only obtain Lyme testing in children with a clinical constellation consistent with potential Lyme disease. A positive IgM immunoblot test result alone should be interpreted with caution to avoid Lyme disease over-diagnosis.
Acknowledgments
We would like to recognize Caroline Gordon and Catherine Gordon for their assistance with the data abstraction.
P.L. was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health (KL2 TR001115). L.N. was supported by the Harvard Catalyst Early Clinical Data Grant and Boston Children's Hospital (Research Faculty Council Pilot).
Glossary
- EIA
Enzyme immunoassay
- EM
Erythema migrans
Footnotes
The authors declare no conflicts of interest.
References
- 1.Centers for Disease Control and Prevention Recommendations for test performance and interpretation from the Second National Conference on Serologic Diagnosis of Lyme Disease. MMWR Morb Mortal Wkly Rep. 1995;44:590–1. [PubMed] [Google Scholar]
- 2.Seriburi V, Ndukwe N, Chang Z, Cox ME, Wormser GP. High frequency of false positive IgM immunoblots for Borrelia burgdorferi in clinical practice. Clin Microbiol Infect. 2012;18:1236–40. doi: 10.1111/j.1469-0691.2011.03749.x. [DOI] [PubMed] [Google Scholar]
- 3.Sivak SL, Aguero-Rosenfeld ME, Nowakowski J, Nadelman RB, Wormser GP. Accuracy of IgM immunoblotting to confirm the clinical diagnosis of early Lyme disease. Arch Intern Med. 1996;156:2105–9. [PubMed] [Google Scholar]
- 4.Hinckley AF, Connally NP, Meek JI, Johnson BJ, Kemperman MM, Feldman KA, et al. Lyme disease testing by large commercial laboratories in the United States. Clin Infect Dis. 2014;59:676–81. doi: 10.1093/cid/ciu397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tugwell P, Dennis DT, Weinstein A, Wells G, Shea B, Nichol G, et al. Laboratory evaluation in the diagnosis of Lyme disease. Ann Intern Med. 1997;127:1109–23. doi: 10.7326/0003-4819-127-12-199712150-00011. [DOI] [PubMed] [Google Scholar]
- 6.Cohn KA, Thompson AD, Shah SS, Hines EM, Lyons TW, Welsh EJ, et al. Validation of a clinical prediction rule to distinguish lyme meningitis from aseptic meningitis. Pediatrics. 2012;129:e46–53. doi: 10.1542/peds.2011-1215. [DOI] [PubMed] [Google Scholar]
- 7.Garro AC, Rutman M, Simonsen K, Jaeger JL, Chapin K, Lockhart G. Prospective validation of a clinical prediction model for Lyme meningitis in children. Pediatrics. 2009;123:e829–34. doi: 10.1542/peds.2008-2048. [DOI] [PubMed] [Google Scholar]
- 8.Avery RA, Frank G, Glutting JJ, Eppes SC. Prediction of Lyme meningitis in children from a Lyme disease-endemic region: a logistic-regression model using history, physical, and laboratory findings. Pediatrics. 2006;117:e1–7. doi: 10.1542/peds.2005-0955. [DOI] [PubMed] [Google Scholar]
- 9.Deanehan JK, Kimia AA, Tan Tanny SP, Milewski MD, Talusan PG, Smith BG, et al. Distinguishing Lyme from septic knee monoarthritis in lyme disease-endemic areas. Pediatrics. 2013;131:e695–701. doi: 10.1542/peds.2012-2531. [DOI] [PubMed] [Google Scholar]
- 10.Deanehan JK, Nigrovic PA, Milewski MD, Tan Tanny SP, Kimia AA, Smith BG, et al. Synovial fluid findings in children with knee mono-arthritis in lyme disease endemic areas. Pediatr Emerg Care. 2014;30:16–9. doi: 10.1097/PEC.0000000000000028. [DOI] [PubMed] [Google Scholar]
- 11.Halperin JJ, Golightly M. Lyme borreliosis in Bell's palsy. Long Island Neuroborreliosis Collaborative Study Group. Neurology. 1992;42:1268–70. doi: 10.1212/wnl.42.7.1268. [DOI] [PubMed] [Google Scholar]
- 12.Milewski MD, Cruz AI, Jr, Miller CP, Peterson AT, Smith BG. Lyme arthritis in children presenting with joint effusions. J Bone Joint Surg Am. 2011;93:252–60. doi: 10.2106/JBJS.I.01776. [DOI] [PubMed] [Google Scholar]
- 13.Thompson A, Mannix R, Bachur R. Acute pediatric monoarticular arthritis: distinguishing lyme arthritis from other etiologies. Pediatrics. 2009;123:959–65. doi: 10.1542/peds.2008-1511. [DOI] [PubMed] [Google Scholar]
- 14.Lantos PM, Branda JA, Boggan JC, Chudgar SM, Wilson EA, Ruffin F, et al. Poor positive predictive value of lyme disease serologic testing in an area of low disease incidence. Clin Infect Dis. 2015;61:1374–80. doi: 10.1093/cid/civ584. [DOI] [PMC free article] [PubMed] [Google Scholar]