Abstract
Major depressive disorder is the most common neuropsychiatric complication in human immunodeficiency virus (HIV) infections and is associated with worse clinical outcomes. We determined if detectable cerebrospinal fluid (CSF) HIV ribonucleic acid (RNA) at threshold ≥50 copies/ml is associated with increased risk of depression. The CNS HIV Anti-Retroviral Therapy Effects Research (CHARTER) cohort is a six-center US-based prospective cohort with bi-annual follow-up 674 participants. We fit linear mixed models (N=233) and discrete-time survival models (N=154; 832 observations), to evaluate trajectories of Beck Depression Inventory (BDI) II scores, and the incidence of new-onset moderate-to-severe depressive symptoms (BDI≥17) among participants, on combination antiretroviral therapy (cART), who were free of depression at study entry, and received a minimum of three CSF examinations over 2,496 person-months follow-up. Detectable CSF HIV RNA (threshold ≥50 copies/ml) at any visit was associated with a 4.7-fold increase in new-onset depression at subsequent visits adjusted for plasma HIV RNA and treatment adherence; hazard ratio (HR)=4.76, (95% CI: 1.58–14.3); P=0.006. Depression (BDI) scores were 2.53 points higher (95% CI: 0.47–4.60; P=0.02) over 6 months if CSF HIV RNA was detectable at a prior study visit in fully adjusted models including age, sex, race, education, plasma HIV RNA, duration and adherence of cART, and lifetime depression diagnosis by DSM-IV criteria. Persistent CSF but not plasma HIV RNA, is associated with an increased risk for new-onset depression. Further research evaluating the role of immune activation and inflammatory markers may improve our understanding of this association.
Keywords: Viral load, Cerebrospinal fluid, Psychiatry, Depression
INTRODUCTION
Major Depressive Disorder (MDD) is the most common neuropsychiatric disorder associated with HIV (Zanjani et al., 2007). In the United States (US), the lifetime prevalence of MDD in HIV remains high, estimated at between 22–45% even with combination antiretroviral therapy (cART) use (Rabkin, 2008). Globally, MDD is a leading cause of disability adjusted life years (DALYs) (Murray et al., 2013). Compared to the general US population with an estimated 9% prevalence of depression (Centers for Disease Control and Prevention (CDC), 2010), persons with HIV bear some of the highest burden of depression-associated disability. MDD is associated with negative outcomes such as low productivity and medication non-compliance, and with comorbidities such as cardiovascular disease, stroke, diabetes, substance use and suicidality (Sanchez-Gistau et al., 2012; Hees et al., 2013; Pacek et al., 2012; Grenard et al., 2011). MDD in HIV is associated with decreased adherence to cART, poor virologic outcomes, faster disease progression, increased hospitalization rates and higher mortality (Kacanek et al., 2010; Cook et al., 2004).
There is increasing evidence implicating the activation of inflammatory pathways in MDD through innate and adaptive immune responses (Miller et al., 2009). Elevated levels of plasma and CSF pro-inflammatory cytokines such as IL-6, TNF-α and IL-1b are associated with MDD (Raison et al., 2009). Hepatitis C treatment with interferon has been shown to be associated with CNS inflammatory response and depression. Elevated pro-inflammatory cytokines are associated with development of chronic diseases like diabetes and cardiovascular diseases (Miller et al., 2002; Wellen and Hotamisligil, 2005), further suggesting a shared pathogenesis between MDD and chronic disease. Evidence suggests that a new diagnosis of HIV can lead to onset of depression (Jin et al., 2006), while the progression of HIV further increases the risk of MDD (Lyketsos et al., 1996). Furthermore, synergistic mechanisms between major MDD and HIV may be related to stress and immune dysfunction (Evans et al., 2002; Cruess et al., 2005), with HIV making MDD worse, and MDD in turn leading to progression of HIV.
HIV replication creates an inflammatory environment through activation of the innate and adaptive immune systems (Boasso et al., 2008; Catalfamo et al., 2008). Chronic exposure to inflammatory mediators such as type I IFN is associated with dysregulation of T-cell homeostasis mediated by homeostatic cytokines such as IL-7, and is linked to decreased HIV survival (Boasso et al., 2008; Herbeuval et al., 2005). Because HIV RNA levels are the main drivers of CD8 T-cell proliferation (Catalfamo et al., 2008), coupled with increasing evidence on the role of inflammation in the pathogenesis of MDD, we hypothesized a priori that detectable levels of HIV RNA in the CSF would be associated with increased risk of depression. The study objectives were to determine if detectable CSF HIV RNA is associated with increased incidence of new-onset moderate-to-severe depressive symptoms, and to evaluate the association between detectable CSF HIV RNA and trajectories of depression (BDI) scores. We utilized data from the CNS HIV Anti-Retroviral Therapy Effects Research (CHARTER) cohort, which uniquely affords bi-annual CSF examinations during follow-up.
METHODS
Study design and Participants
CHARTER is a six-center, US-based prospective, observational cohort study started in 2004 and designed to comprehensively assess a demographically representative US population of individuals who are HIV-seropositive in clinical care. CHARTER aims to evaluate the changing presentation of HIV neurological complications in the context of cART (Heaton et al., 2010). There were no general exclusion criteria except for the inability to consent to participation in study assessments. Study sites include Johns Hopkins University, Baltimore, MD; Icahn School of Medicine at Mount. Sinai, New York, NY; University of California, San Diego, CA; University of Texas, Galveston, TX; University of Washington, Seattle, WA; and Washington University, St. Louis, MO. The study was approved by the Institutional Review Board (IRB) or Western IRB for each study site and each participant provided written informed consent.
At study entry, 1,561 CHARTER participants, of whom 1,053 were currently on cART, underwent extensive evaluation including HIV and treatment history verified by medical records, physical examination, neuropsychiatric evaluation, neuropsychological testing lumbar puncture, and venipuncture (Heaton et al., 2010). Thereafter 674 participants were selected if they agreed to undergo follow-up visits every 6 months as part of the longitudinal study component.
These analyses utilize data from the cohort of participants enrolled between 2004 and 2007, and followed through 2009 that were on cART and completed at least 3 study visits with CSF examination for HIV RNA (Heaton et al., 2010). To examine the effect of detectable CSF HIV RNA on trajectories of depressive symptoms assessed by the Beck Depression Inventory (BDI), we excluded 4 participants with missing MDD status and 31 participants with prevalent MDD based on Diagnostic Statistical Manual (DSM-IV) criteria assessed by the World Health Organization Composite International Diagnostic Interview (CIDI) (Kessler and Ustun, 2004) ; (N=223, with 832 total observations). Because we were also interested in calculating incidence of new-onset moderate-to-severe depressive symptoms (BDI ≥17), for those analyses we excluded 69 participants with BDI scores ≥17, N=154. Participants with prevalent MDD who were excluded, had significantly higher mean baseline BDI scores, 26.3 (95% CI: 22.0–30.6) relative to those without prevalent MDD, 12.2 (95% CI: 10.9–13.6). There were no other differences in other covariates between participants excluded and those retained in our analysis.
Because the risk of new-onset depression associated with detectable CSF HIV RNA is unknown, we hypothesized from clinical experience that persons with detectable CSF HIV RNA would have at least a 2-fold increased risk of depression over 12 months compared to persons without detectable CSF HIV RNA. A study of 20 persons with detectable CSF HIV RNA and 100 with undetectable CSF HIV RNA (1:5 ratio), over 24-month follow-up would have 83% power to detect a relative risk of 2.1, and reject the null hypothesis that the survival curves for persons with detectable CSF HIV RNA is equal to those without detectable CSF HIV RNA at Type 1 error (α) =0.05. Our study had sufficient statistical power to detect such a difference.
Measures
The principal outcome variable was new-onset moderate-to-severe depressive symptoms defined as BDI score ≥17, corresponding to a range of moderate-to-severe clinical depression (Aalto et al., 2012). We also examined trajectories of depression scores over time. The main exposure of interest was detectable CSF HIV RNA, threshold ≥50 copies/ml. CSF HIV RNA levels were measured at each 6-month follow-up visit and determined by commercial ultrasensitive reverse transcriptase-polymerase chain reaction (Amplicor, Roche Diagnostic Systems, Indianapolis, Indiana). Participants were included in these analyses if they completed a minimum of 3 CSF examinations. We defined persistence of CSF HIV RNA as a minimum of 2 CSF examinations positive for HIV RNA. Fixed covariates evaluated were patient demographics (age, sex, race, and years of education), self-reported duration of HIV seropositivity and nadir CD4+ T-cell, lifetime history of DSM-IV MDD and HCV seropositivity. We incorporated time-varying covariates assessed at 6 month intervals at each study visit, including cART regimen (protease inhibitor vs. non-nucleoside reverse transcriptase inhibitor based regimen) and duration of cART regimen, CNS penetration effectiveness (CPE) score of cART which is an estimate of the extent to which antiretroviral drugs affect the CNS (Letendre et al., 2010), current CD4+ T-cell count, plasma HIV RNA levels, cognitive impairment assessed by the Global Deficit Score (GDS) (Carey et al., 2004), a summary measure of the standardized comprehensive neurocognitive assessment; and medication adherence assessed by the AIDS Clinical Trials Group 4-Day Adherence Questionnaire (Chesney et al., 2000). All participants included in these analyses were on cART and changes in treatment regimen assessed at each study visit, was captured by changes in CPE scores.
Statistical analysis
Population characteristics were examined using summary statistics. We conducted a complete case analysis of the 832 out of 892 (93.3%) expected observations if all participants had completed each study visit. To examine the time-varying effect of detectable CSF HIV RNA, we fit discrete-time survival models to estimate hazard ratios between detectable CSF HIV RNA and new-onset moderate-to-severe depressive symptoms. Because protease inhibitor (PI)-based cART has been associated with decreased depression scores (Low-Beer et al., 2000), we examined interactions between lifetime MDD and cART regimen use. We also examined interactions between plasma HIV RNA and adherence to cART. Because relatively few endpoints were reached, we fit several discrete-time survival models, increasing the number of covariates adjusted in subsequent models.
To examine the trajectories of BDI scores over time, we fit linear mixed models with random intercepts and robust variance-covariance estimates of the model parameters (Laird and Ware, 1982). We examined the effect of detectable CSF HIV RNA on BDI scores throughout follow-up by fitting interactions between follow-up time and CSF HIV RNA, as well as other covariates. Markov transition matrix was used to assess the transition probability for detectable CSF HIV RNA between study visits We performed a Wald test to check the joint hypothesis that coefficients of the interactions were significant. The best-fitting functional form of each covariate were fit, performing collinearity checks and sensitivity analysis using BDI≥14 (mild depressive symptoms) as outcome. Author ERH performed the statistical analysis with Stata Statistical Software: Release 12. (College Station, TX: StataCorp LP).
RESULTS
The study population comprised 223 persons free of DSM-IV MDD at study entry. The mean age at entry was 44.8 years. Most were male (81.6%), with 44.8% Blacks, 39.5% White and 15.7% Hispanic or other race (Table 1). At study entry, 32 (14.4%) participants had detectable CSF HIV RNA at a threshold of ≥50 copies/ml, of which 28 (87.5%) had detectable plasma HIV RNA compared to 4 (12.5%) with undetectable plasma HIV RNA, p<0.001. Participants with detectable CSF HIV RNA were younger, had higher plasma HIV RNA, more likely to be on PI-based and lower CPE cART regimen, and <95% medication adherence. Participants with detectable CSF HIV RNA were also more likely to have experienced a previous lifetime depressive episode (P=0.05). The prevalence of a lifetime depressive episode among the study population was 46.2%. Persons with detectable CSF HIV RNA at baseline had detectable HIV RNA ≥50 copies/ml in 54.5% of CSF examinations during follow-up. Participants with undetectable CSF HIV RNA at baseline had undetectable HIV RNA (<50 copies/ml) in 92.3% of CSF examinations during follow-up. The Markov transition matrix probability that persons on cART with detectable CSF HIV RNA at prior visit, would present with undetectable CSF HIV RNA at the next subsequent visit was 45.8%.
Table 1.
Baseline Characteristics of CHARTER study participants free of DSM-IV major depressive disorder by presence of CSF HIV RNA (≥50 copies/ml) at study entry.
| CSF HIV RNA≥50 copies/ml | |||
|---|---|---|---|
| Total N=223 |
Detectable n=32 (14.4%) |
Undetectable n=191(85.6%) |
|
| Characteristics | |||
| Age, mean (SD) yrs | 44.8 (7.4) | 41.1 (8.3) | 45.5 (7.0)** |
| Sex | |||
| Male, n(%) | 182 (81.6) | 154 (80.6) | 28 (87.5) |
| Race | |||
| White | 88 (39.5 ) | 8 (25.0) | 80 (41.9) |
| Black | 100 (44.8) | 18 (56.3) | 82 (42.9) |
| Hispanic and Other | 35 (15.7) | 6 (18.8) | 29 (15.2) |
| Education, mean(SD) yrs | 12.8 (2.3) | 12.8(1.7) | 12.8 (2.3) |
| Log plasma RNA, mean (SD) | 2.3 (1.0) | 3.8 (1.1) | 2.1 (0.8)*** |
| Log CSF HIV RNA, mean (SD) |
- | 2.64 (0.76) | - |
| Current CD4, mean (SD) cells/mm3 |
477.6 (288.1) | 370.3 (270.3) | 495.6 (287.7) |
| CD4 nadir, mean (SD) | 145.2 (137.2) | 140.7 (145.2) | 146.0 (136.2) |
| cART Regimen, n(%) | |||
| PI | 124 (55.6) | 26 (81.3) | 98 (51.3) |
| NNRTI | 79 (35.4) | 4 (12.5) | 75 (39.3) |
| PI-NNRTI | 11 (4.9) | 1 (3.1) | 10 (5.2) |
| Other | 9 (4.0) | 1 (3.1) | 8 (4.2)* |
| CPE, mean(range) | 7.7 (4–14) | 7.2 (5–12) | 7.8 (4–14)* |
| Adherence, n (%) | |||
| ≥95% | 192 (86.1) | 22 (68.8) | 170 (89.0) |
| 85–94% | 17 (7.6) | 2 (6.2) | 15 (7.9) |
| <85% | 14 (6.3) | 8 (25.0) | 6 (3.1)*** |
| Current cART duration, mean(SD), months |
18.1 (21.1) | 13.6 (20.0) | 18.8 (21.2) |
| Duration of HIV infection, mean (SD), months |
139.6 (71.0) | 113.0 (66.1) | 144.0 (71.0)* |
| Lifetime DSM-IV depression, n(%) |
103 (46.2) | 20 (62.5) | 83 (43.5)* |
| Lifetime DSM-IV alcohol use disorder, n(%) |
62 (27.8) | 8 (25.0) | 54 (28.3) |
| Lifetime DSM-IV substance use disorder, n(%) |
73 (32.7) | 5 (15.6) | 68 (35.6)* |
| BDI score, mean (SD) | 12.2 (10.2) | 10.5 (9.0) | 12.5 (10.3) |
| Cognition, GDS, mean (SD) | 0.49 (0.47) | 0.37 (0.40) | 0.50 (0.48) |
| HCV positive, n(%) | 63 (28.6) | 5 (16.7) | 58 (30.5) |
| History of Opportunistic infection, n(%) |
38 (44.7) | 5 (45.5) | 33 (44.6) |
cART: Combination antiretroviral therapy; PI: Protease inhibitor; NNRTI: Non-nucleoside reverse transcriptase inhibitor; CPE: CNS penetration effectiveness score; Adherence assessed by the AIDS Clinical Trials Group 4-Day Adherence Questionnaire; Lifetime depression, substance and alcohol use disorder based on Diagnostic and Statistical Manual of Mental Disorders, IV using the Composite International Diagnostic Interview (CIDI); BDI: Beck Depression Inventory II; GDS: Global Deficit Score.
P < 0.05,
P < 0.01,
P < 0.001 for difference comparing participants with detectable vs. undetectable CSF HIV RNA.
Incidence and Risk of New-onset Moderate-to-Severe Depressive Symptoms
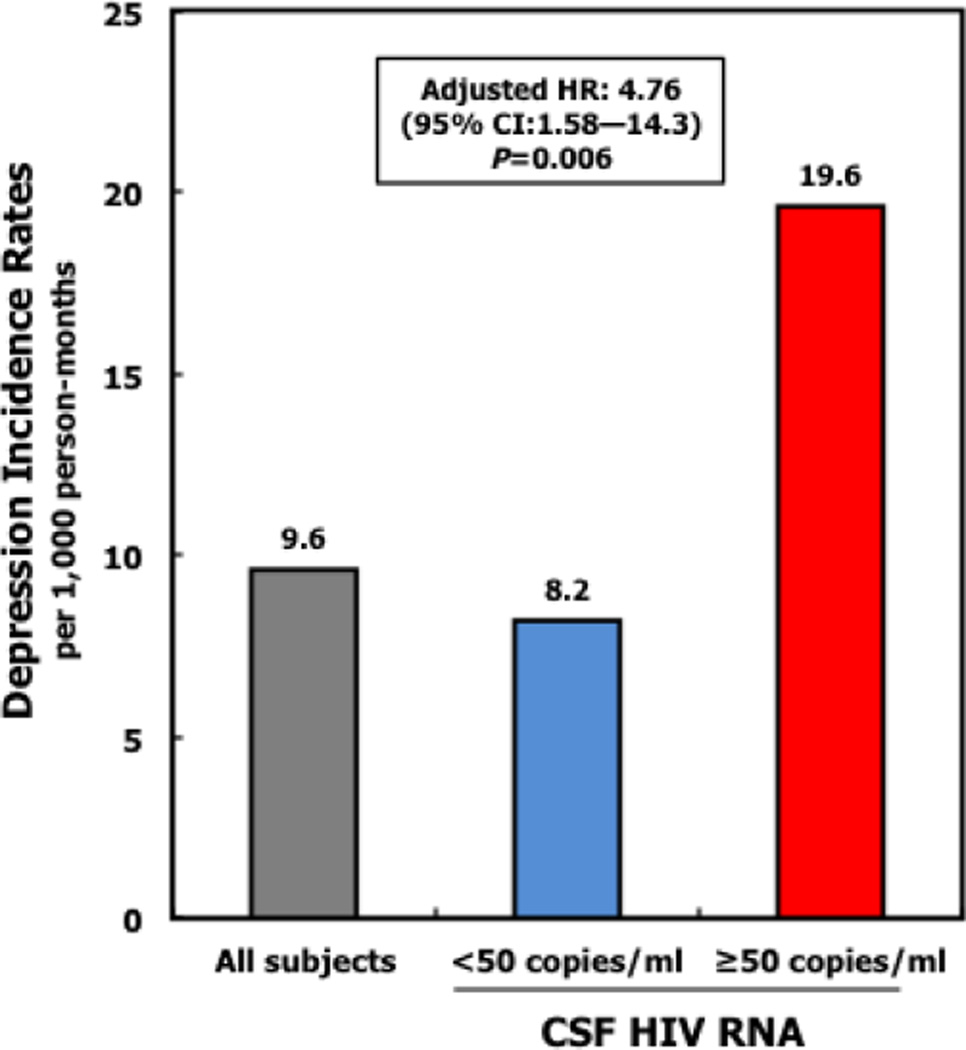
The overall incidence of new-onset moderate-to-severe depressive symptoms over 2,496 person-months of follow-up was 9.6 per 1,000 person-months (95% CI: 6.1 to 14.3) (Table 2). When CSF HIV RNA was detectable, the incidence rate of new-onset moderate– to-severe depressive symptoms was 19.6 per 1,000 person-months (95% CI: 8.8 to 43.6), compared to 8.2 per 1,000 person-months when CSF HIV RNA was undetectable (Figure 1).
Table 2.
Incidence rates and Cumulative Incidence for new-onset moderate-to-severe depressive symptoms based on the Beck Depression Inventory (BDIa score ≥17) by detectable CSF HIV RNA (≥50 copies/ml) and using discrete-time survival models (N=154).
| New-onset moderate-to-severe depressive symptoms | |||||
|---|---|---|---|---|---|
| No. of Subjects at Study Entry (N) |
Time at Risk (Person-months) |
No. of Events |
Incidence rate (IR) per 1,000 person-months |
||
| IR | 95% CI | ||||
| Entire study cohort | 154 | 2,496 | 24 | 9.6 | (6.4, 14.3) |
| CSF HIV RNA | |||||
| Undetectable | 124 | 2,190 | 18 | 8.2 | (5.2, 13.0) |
| Detectable | 30 | 306 | 6 | 19.6 | (8.8, 43.6) |
BDI: Beck depression inventory II - depression severity scores: 0–9, minimal; 10–16, mild; 17–29, moderate; 30–63, severe; CSF HIV RNA detectable at ≥50 copies/ml
Markov transition matrix probability that persons on cART with detectable CSF HIV RNA at prior visit, would present with undetectable CSF HIV RNA at the next subsequent visit is 45.8%.
Fig 1.
Caption Incidence rates (IR) for new-onset moderate-to-severe depressive symptoms assessed using the Beck Depression Inventory (BDIa score ≥17) by detectable CSF HIV RNA (≥50 copies/ml) using discrete-time survival models.
Annotation Incidence rates expressed per 1,000 person months. Adjusted HR=4.76, 95% CI: 1.58–14.3; P=0.006, adjusted for plasma HIV RNA and treatment adherence.
CSF HIV RNA detectable at a threshold of ≥50 copies/ml. aDepression assessed by Beck depression inventory II (BDI); depression severity scores: 0–9, minimal; 10–16, mild; 17–29, moderate; 30–63, severe.
The time-varying effect of detectable CSF HIV RNA was associated with more than 4-fold increased risk of new-onset moderate-to-severe depressive symptoms adjusted for plasma HIV RNA and adherence to cART (adjusted HR: 4.76, 95% CI: 1. 58 to 14.3); P=0.006 (Table 3). When further adjusted for lifetime DSM-IV MDD, lifetime DSM-IV alcohol and substance use disorder, cART regimen, adherence, duration of cART and sex, age and race, we observed similar associations between detectable CSF HIV RNA and increased risk of moderate-to-severe depressive symptoms (Table 3). There was no interaction between plasma HIV RNA and adherence.
Table 3.
Hazard ratios for the association between detectable CSF HIV RNA (≥50 copies/ml) and new-onset moderate-to-severe depressive symptoms based on the Beck Depression Inventory (BDIa score ≥17) using discrete-time survival models displaying covariates adjusted for in various models (N=154).
| Model | Variables Adjusted in Model | HIV RNA Compartment |
Hazard Ratio |
95% CI | P-value |
|---|---|---|---|---|---|
| 1 | Unadjusted | CSF† | 2.39 | (0.98, 5.82) | 0.06 |
| Plasma‡ | 1.14 | (0.78, 1.67) | 0.51 | ||
| 2 | Log plasmab HIV RNA/CSF† HIV RNA and Adherence to CART |
CSF | 4.76 | (1.58, 14.3) | 0.006 |
| Plasma | 0.81 | (0.61, 1.08) | 0.15 | ||
| 3 | Fully Adjusted Modelb | CSF | 6.34 | (1.74, 23.3) | 0.005 |
| Plasma | 0.82 | (0.61, 1.23) | 0.23 | ||
BDI: Beck depression inventory II - depression severity scores: 0–9, minimal; 10–16, mild; 17–29, moderate; 30–63, severe;
Detectable CSF HIV RNA, ≥50 copies/ml;
Log plasma HIV RNA, per 10 RNA copies/ml, categorized plasma RNA: <50, 50–199, 200–9,999, ≥10,000 copies/ml.
Adjusted for Log plasma HIV RNA, cART regimen, CNS Penetration Effectiveness score (CPE), adherence to cART, duration of cART, Lifetime Diagnostic and Statistical Manual of Mental Disorders IV (DSM-IV) major depressive disorder, lifetime DSM-IV alcohol and substance use disorder, sex, age and race.
Trajectories of Beck Depression Inventory (BDI) II scores
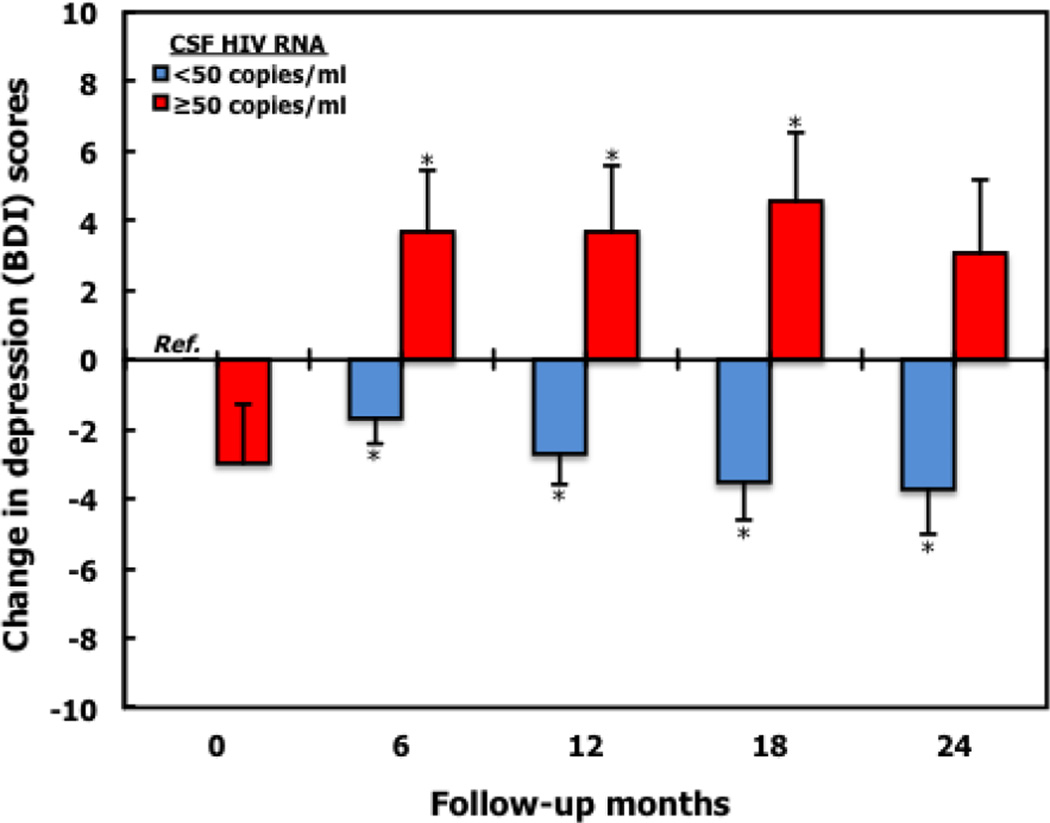
At study entry, mean BDI score for persons with detectable CSF HIV RNA was lower although not significantly different, compared to when CSF HIV RNA was undetectable, 10.5 (SD 9.0) vs. 12.5 (SD 10.3), with adjusted mean difference of −2.99 (95% CI: −6.29 to 0.31), p=0.08 (Table 4A). However throughout follow-up, BDI scores for persons with detectable CSF HIV RNA increased, whereas BDI scores decreased when CSF HIV RNA was undetectable in adjusted linear mixed models. (Figure 2, Table 4A). Unlike the findings for CSF HIV RNA, plasma HIV RNA (continuous and categorized: <50, 50–199, 200–9,999, ≥ 10,000 copies/ml), was not associated with an increase in BDI scores over time.
Table 4.
Association between detectable CSF HIV RNA (≥50 copies/ml) and Beck Depression Inventory (BDIa) scores over time; (A) longitudinal change in BDI scores, using adjusted linear mixed models with random intercept in persons without depression (DSM IV criteria) at baseline, and (B) Cross-sectional effect of detectable CSF HIV RNA over time (N=223).
| (A) | ||||||
|---|---|---|---|---|---|---|
| Undetectable CSF HIV RNA | Detectable CSF HIV RNA | |||||
| Time, months | Coefficientb | (95% CI) | p value | Coefficientb | (95% CI | p value |
| Baseline | Ref | - | - | −2.99 | (−6.29, 0.31) | 0.08 |
| 6 | −1.67 | (−3.11, −0.23) | 0.02 | 3.72 | (0.32, 7.13) | 0.03 |
| 12 | −2.72 | (−4.41, −1.03) | 0.002 | 3.72 | (−0.03, 7.46) | 0.05 |
| 18 | −3.54 | (−5.65, −1.42) | 0.001 | 4.56 | (0.71, 8.41) | 0.02 |
| 24 | −3.74 | (−6.28, −1.20) | 0.004 | 3.02 | (−1.19, 7.22) | 0.16 |
| (B) | ||||||
|---|---|---|---|---|---|---|
| Unadjusted model estimates | Adjusted† model estimates | |||||
| Covariate | Coefficientb | 95% CI | p-value | Coefficientb | 95% CI | P-value |
| CSF HIV RNA | ||||||
| Undetectable | Ref | Ref | ||||
| Detectable | 0.51 | (−1.16, 2.19) | 0.55 | 2.52 | (0.44, 4.60) | 0.02 |
| Log Plasma HIV RNA (per 10 RNA copies/ml) |
0.53 | (−0.29, 1.35) | 0.21 | −0.12 | (−0.94, 0.70) | 0.78 |
BDI: Beck depression inventory II; depression severity scores: 0–9, minimal; 10–16, mild; 17–29, moderate; 30–63, severe.
Coefficient represents the longitudinal change in BDI scores from baseline with reference to persons without detectable CSF HIV RNA.
Multivariate linear mixed models adjusted for log plasma HIV RNA, race, age, sex, education, nadir and current CD4 counts, CNS Penetration Effectiveness score (CPE), lifetime DSM-IV depression and depression at prior visit, lifetime DSM-IV alcohol and substance use disorder, years infected with HIV, cognitive impairment (global deficit score- GDS), and prior diagnosis of HCV infection.
Fig 2.
Caption Longitudinal effect of detectable CSF HIV RNA (≥50 copies/ml) on Beck Depression Inventory (BDIa) scores using adjustedb linear mixed models with random intercept in persons without depression (DSM IV criteria) at baseline.
Annotation Change in depression (BDI) scores represents the longitudinal change in BDI scores from baseline with reference to persons without detectable CSF HIV RNA. *P<0.05
aDepression assessed by Beck depression inventory II (BDI); depression severity scores: 0–9, minimal; 10–16, mild; 17–29, moderate; 30–63, severe.
bMultivariate linear mixed model adjusted for log plasma HIV RNA, race, age, sex, education, nadir and current CD4 counts, CNS Penetration Effectiveness score (CPE), lifetime depression diagnosis, depression at prior study visit, years infected with HIV, cognitive impairment (global deficit score-GDS), and prior diagnosis of HCV infection.
Detectable CSF HIV RNA measured at 6-month intervals was associated with increasing BDI scores over time (Table 4B). In linear mixed models, the presence of CSF HIV RNA at a prior study visit was associated with an increase in BDI of 2.5 points (95% CI: 0.47–4.60; P=0.02) during the subsequent 6 months after adjusting for age, sex, race, education, log plasma HIV RNA, nadir and current CD4 count, CPE2, current duration and adherence of cART, duration of HIV infection, lifetime DSM-IV MDD, DSM-IV substance and alcohol use disorder, depression diagnosis at prior study visit, cognitive impairment and prior diagnosis of HCV infection.
DISCUSSION
We found that persistent CSF HIV RNA (threshold of 50 copies/ml) is associated with over 4-fold increase in new-onset moderate-to-severe depressive symptoms. Furthermore, persistent CSF HIV RNA is associated with an increase in BDI score, 2.5 points over 6 months. CSF rather than plasma HIV RNA is associated with new-onset moderate depression and worsening depression scores. These findings lend support to our a priori hypothesis that the presence of CSF HIV RNA is associated with increased depression in HIV.
At baseline our study population had 12.2% prevalence of DSM-IV MDD which when compared to HIV depression prevalence estimates of 22–45% (Rabkin, 2008), is rather low, but is similar to that found in the MultiCenter AIDS Cohort Study (MACS) using the Center for Epidemiologic Studies Depression (CES-D) Scale (Lyketsos et al., 1996). This suggests that our study population was not at additional or higher risk of new-onset depression than the general HIV population. In this regard, our reported estimates of the association between detectable CSF HIV RNA and new-onset moderate-to-severe depressive symptoms may be conservative.
The evaluation of trajectories of BDI scores over time allows ascertainment of changes in mood along a continuum. The presence of HIV RNA in CSF is associated with an increasing BDI score over time. Of equal importance is our finding that undetectable CSF HIV RNA is associated with decreasing BDI scores over time (Figure 2). Persistent over time, detectable CSF HIV RNA may be a cause of increasingly severe depression. Over the study period, there was 54.5% transitional probability that persons with detectable CSF HIV RNA would present at a subsequent study visit with detectable CSF HIV RNA. Therefore the likelihood that detectable CSF HIV RNA could have been misclassified due to false positive CSF HIV is reduced, more so because all patients were on cART.
We speculate that one mechanism for increased depression in HIV is HIV-induced CNS inflammation. This theory, though not yet evaluated in humans, may however be supported by evidence from animal studies in which intraventricular administration of HIV-1 Tat and HIV-1 gp120 has been associated with increased depressive-like behavior (Lawson et al., 2011; Barak et al., 2002).
Because depression is associated with increased risky behavior such as multiple lifetime sexual partners, sex when intoxicated by drugs or alcohol, sex for money or drugs and decreased medication adherence, all of which increase the risk of HIV transmission (Hutton et al., 2004; Kacanek et al., 2010; High et al., 2012), it is important that clinicians and providers be aware of its association with detectable CSF HIV RNA. Unfortunately, mental health services for persons infected with HIV are still grossly unavailable in high-income countries, and much more so in mid-to-low income countries (High et al., 2012; Chander et al., 2006). Presently there are no recommended guidelines for testing CSF in management of HIV or for that matter depression in HIV. The European AIDS Clinical Society guidelines recommends CSF analysis for resistance patterns when neurocognitive impairment is detected (European AIDS Clinical Society, 2012), but US guidelines do not address CSF monitoring. In a recent Consensus Report, the Mind Exchange Program recommends CSF testing for HIV RNA in persons with suspected or demonstrated neurocognitive impairment (Mind Exchange Working Group, 2013).
The association between depression and persistent CSF HIV RNA may be bidirectional. In this study we did not evaluate whether incident depression was associated with future loss of CSF virologic suppression, an unlikely finding given our approach. The present findings may suggest a clinical role both for monitoring depressive symptoms through valid and reliable assessment tools, and for CSF viral load testing. Its value might include indicators for changes to cART regimen (including treatment intensification), discussions about adherence to medication, counseling aimed at reducing high risk HIV behavior, and evaluation of antidepressant therapy. Depression in HIV can be effectively treated with psychotropic medication, psychological interventions, and by incorporating cognitive-behavioral components (Sherr et al., 2011). In persons with refractory HIV-associated depression, knowledge about presence of CSF HIV RNA might become useful to guide treatment choices for both antidepressant and cART regimen.
Some limitations to our analyses need to be considered. First, although our prospective study is one of the largest available to assess CSF over time, because of the relatively low prevalence of participants at baseline with detectable CSF HIV RNA without MDD, we were unable to assess a dose response association. Also, the measures of association we report for new-onset moderate-to-severe depressive symptoms have wide 95% CIs. However our study had sufficient statistical power to detect a difference in risk of depression by detectable CSF HIV RNA status. It is conceivable that higher levels of CSF HIV RNA may result in further increases in the incidence and severity of depressive symptoms. Second, we detected CSF HIV RNA at a threshold of 50 copies/ml. New ultrasensitive HIV RNA assays enable lower thresholds of HIV RNA detection and might allow refinement of our findings. Majority of the participants included in these analyses were male, which may make our findings a conservative estimate when applied to females, who may report more depressive symptoms compared to males (Piccinelli and Wilkinson, 2000). Although we accounted for the effect of lifetime substance and alcohol use disorder in these analyses, we did not evaluate the effect of current substance and alcohol use disorder because of the low diagnosis of current DSM-IV substance and alcohol use disorder, 0.9% and 0.8% respectively.
Strengths of our study include the availability of data from HIV participants with at least three CSF examinations during follow-up. This allowed us to harness the strength of longitudinal data analysis and robust statistical techniques, adequately accounting for correlations between repeated measures thereby providing robust estimates (Laird and Ware, 1982). We utilized CSF HIV testing performed at each follow-up visit and incorporated its time-varying nature. We did not account directly for psychotropic medications received during follow-up. However by adjusting for lifetime MDD and depression at a prior study visit, we indirectly accounted for antidepressant therapy effects any participants may have received. Although persons with a prior history of depression have an increased risk for new depression, we further restricted our analysis to persons free of MDD at baseline making it unlikely that our findings are a result of lifetime MDD.
We assessed depressive symptoms by the BDI, a validated tool with high content and construct validity (Aalto et al., 2012). By assessing outcomes at BDI score of ≥17, we captured moderate-to-severe depressive symptoms while excluding mild symptoms. Our approach results in less potential misclassification of depression and helps to ensure that our outcome is more likely to represent clinically relevant depressive disorder rather than distress symptoms associated with HIV. Furthermore the use of the BDI allows depressive symptoms to be assessed on a continuum; enabling ascertainment of BDI changes over time, and assessment of extreme BDI scores.
In conclusion, persistent CSF HIV RNA is associated with increased risk of new-onset moderate-to-severe depressive symptoms. Assessment of depressive symptoms using screening tools, and techniques to determine which patients may benefit most from CSF testing may be beneficial. Because detectable plasma HIV RNA was not associated with depression even though patients with detectable CSF HIV RNA were more likely to have detectable plasma HIV RNA, persons with HIV presenting with persistent or worsening depression may benefit from CSF testing for HIV RNA, which may help guide HIV and depression treatment. We speculate that depression may be a surrogate of ongoing CNS inflammation and injury.
Acknowledgments
The CNS HIV Anti-Retroviral Therapy Effects Research (CHARTER) group is affiliated with the Johns Hopkins University, Mount Sinai School of Medicine, University of California, San Diego, University of Texas, Galveston, University of Washington, Seattle, Washington University, St. Louis and is headquartered at the University of California, San Diego and includes: Igor Grant, M.D. (UCSD, Director); Ronald J. Ellis, M.D., Ph.D. (UCSD, Co-Director); Scott L. Letendre, M.D. (UCSD, Co-Director); Ian Abramson, Ph.D. (UCSD, Co-Investigator); Muhammad Al-Lozi, M.D. (Washington University, Co-Investigator); J. Hampton Atkinson, M.D.(UCSD, Co-Investigator); Edmund Capparelli, Pharm.D. (UCSD, Co-Investigator); David Clifford, M.D. (Washington University, Site PI), Ann Collier, M.D. (University of Washington, Site Co-PI), Christine Fennema-Notestine, Ph.D. (UCSD, Core PI), Anthony C. Gamst, Ph.D. (UCSD, Core PI), Benjamin Gelman, M.D., Ph.D. (University of Texas, Site PI), Robert K. Heaton, Ph.D. (UCSD), Thomas D. Marcotte, Ph.D. (UCSD, Core PI), Christina Marra, M.D. (University of Washington, Site Co-PI), J. Allen McCutchan, M.D. (UCSD, Site PI), Justin McArthur, M.D. (Johns Hopkins, Site PI), Susan Morgello, M.D. (Mount Sinai, Site Co-PI), David M. Simpson, M.D. (Mount Sinai, Site Co-PI), Davey M. Smith, M.D. (UCSD, Core PI), Michael J. Taylor, Ph.D. (UCSD, Core Co-Investigator), Rebecca Theilmann, Ph.D. (UCSD, Imaging Physicist), Florin Vaida, Ph.D. (UCSD, Co-Investigator), Steven Paul Woods, Psy.D.(UCSD, Co-Investigator); Study Coordinators: Terry Alexander, R.N. (UCSD, Neuromedical Coordinator), Clint Cushman (UCSD, Data Manager), Matthew Dawson (UCSD, Neurobehavioral Coordinator), Donald Franklin, Jr. (UCSD, Center Manager), Eleanor Head, R.N., B.S.N. (University of Texas, Site Coordinator), Trudy Jones, M.N., A.R.N.P. (University of Washington, Site Coordinator), Jennifer Marquie-Beck, M.P.H (UCSD, Recruitment Coordinator), Letty Mintz, N.P. (Mount Sinai, Site Coordinator), Vincent Rogalski, C.C.R.P (Johns Hopkins, Site Coordinator), Mengesha Teshome, M.D. (Washington University, Site Coordinator), Will Toperoff, B.S., N.D. (UCSD, Site Coordinator).
The views expressed in this article are those of the authors and do not reflect the official policy or position of the United States Government.
Disclosures and Sources of Funding
Funding: The CNS HIV Anti-Retroviral Therapy Effects Research (CHARTER) is supported by awards N01 MH22005, HHSN271201000027C, and HHSN271201000030C from the National Institutes of Health. The current analyses were funded by R03 MH095640-02 from the National Institutes of Mental Health. The study sponsor had no role in the design, analyses, interpretation, or reporting of the study.
Footnotes
Conflict of Interest Statement
Rosa M. Crum declares no conflict of interest. Ronald J. Ellis declares no conflict of interest. Igor Grant declares no conflict of interest. Benjamin B. Gelman declares no conflict of interest. Edward R. Hammond declares no conflict of interest. Justin C. McArthur declares no conflict of interest. David B. Clifford has been a Consultant to Biogen Idec, M.illennium, Bristol Myers Squibb, Pfizer, Genzyme, Amgen, Quintiles, Sun Biopharma. Scott L. Letendre has research grants/grants pending from Abbvie, GlaxoSmithKline and Merck. Christina M. Marra has received payment for lectures from Universities and Societies, and royalties from LLW, UpToDate. Shruti H. Mehta has received payment for lectures at a University. Susan Morgello has lectured for Health Clear Strategies. David M. Simpson has been a Consultant for Astellas, Merz, Ipsen, Acorda Therapeutics, Depomed, Syntaxin, Viromed, Biogen, Allergan. Glenn J. Treisman has received payment for lectures from Universities.
References
- Aalto AM, Elovainio M, Kivimaki M, Uutela A, Pirkola S. The beck depression inventory and general health questionnaire as measures of depression in the general population: A validation study using the composite international diagnostic interview as the gold standard. Psychiatry Res (Ireland) 2012;197:163–171. doi: 10.1016/j.psychres.2011.09.008. [DOI] [PubMed] [Google Scholar]
- Barak O, Goshen I, Ben-Hur T, Weidenfeld J, Taylor AN, Yirmiya R. Involvement of brain cytokines in the neurobehavioral disturbances induced by HIV-1 glycoprotein120. Brain Res (Netherlands) 2002;933:98–108. doi: 10.1016/s0006-8993(02)02280-1. [DOI] [PubMed] [Google Scholar]
- Boasso A, Hardy AW, Landay AL, Martinson JL, Anderson SA, Dolan MJ, Clerici M, Shearer GM. PDL-1 upregulation on monocytes and T cells by HIV via type I interferon: Restricted expression of type I interferon receptor by CCR5-expressing leukocytes. Clin Immunol (United States) 2008;129:132–144. doi: 10.1016/j.clim.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey CL, Woods SP, Gonzalez R, Conover E, Marcotte TD, Grant I, Heaton RK HNRC Group. Predictive validity of global deficit scores in detecting neuropsychological impairment in HIV infection. J Clin Exp Neuropsychol (Netherlands) 2004;26:307–319. doi: 10.1080/13803390490510031. [DOI] [PubMed] [Google Scholar]
- Catalfamo M, Di Mascio M, Hu Z, Srinivasula S, Thaker V, Adelsberger J, Rupert A, Baseler M, Tagaya Y, Roby G, Rehm C, Follmann D, Lane HC. HIV infection-associated immune activation occurs by two distinct pathways that differentially affect CD4 and CD8 T cells. Proc Natl Acad Sci U S A (United States) 2008;105:19851–19856. doi: 10.1073/pnas.0810032105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control, Prevention (CDC) Current depression among adults---united states, 2006 and 2008. MMWR Morb Mortal Wkly Rep (United States) 2010;59:1229–1235. [PubMed] [Google Scholar]
- Chander G, Himelhoch S, Moore RD. Substance abuse and psychiatric disorders in HIV-positive patients: Epidemiology and impact on antiretroviral therapy. Drugs (New Zealand) 2006;66:769–789. doi: 10.2165/00003495-200666060-00004. [DOI] [PubMed] [Google Scholar]
- Chesney MA, Ickovics JR, Chambers DB, Gifford AL, Neidig J, Zwickl B, Wu AW. Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: The AACTG adherence instruments. patient care committee & adherence working group of the outcomes committee of the adult AIDS clinical trials group (AACTG) AIDS Care (ENGLAND) 2000;12:255–266. doi: 10.1080/09540120050042891. [DOI] [PubMed] [Google Scholar]
- Cook JA, Grey D, Burke J, Cohen MH, Gurtman AC, Richardson JL, Wilson TE, Young MA, Hessol NA. Depressive symptoms and AIDS-related mortality among a multisite cohort of HIV-positive women. Am J Public Health (United States) 2004;94:1133–1140. doi: 10.2105/ajph.94.7.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruess DG, Douglas SD, Petitto JM, Have TT, Gettes D, Dube B, Cary M, Evans DL. Association of resolution of major depression with increased natural killer cell activity among HIV-seropositive women. Am J Psychiatry (United States) 2005;162:2125–2130. doi: 10.1176/appi.ajp.162.11.2125. [DOI] [PubMed] [Google Scholar]
- European AIDS Clinical Society. Guidelines version 6.1. 2012 [Google Scholar]
- Evans DL, Ten Have TR, Douglas SD, Gettes DR, Morrison M, Chiappini MS, Brinker-Spence P, Job C, Mercer DE, Wang YL, Cruess D, Dube B, Dalen EA, Brown T, Bauer R, Petitto JM. Association of depression with viral load, CD8 T lymphocytes, and natural killer cells in women with HIV infection. Am J Psychiatry (United States) 2002;159:1752–1759. doi: 10.1176/appi.ajp.159.10.1752. [DOI] [PubMed] [Google Scholar]
- Grenard JL, Munjas BA, Adams JL, Suttorp M, Maglione M, McGlynn EA, Gellad WF. Depression and medication adherence in the treatment of chronic diseases in the united states: A meta-analysis. J Gen Intern Med (United States) 2011;26:1175–1182. doi: 10.1007/s11606-011-1704-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER study. Neurology (United States) 2010;75:2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hees HL, Koeter MW, Schene AH. Longitudinal relationship between depressive symptoms and work outcomes in clinically treated patients with long-term sickness absence related to major depressive disorder. J Affect Disord. 2013 doi: 10.1016/j.jad.2012.12.007. [DOI] [PubMed] [Google Scholar]
- Herbeuval JP, Hardy AW, Boasso A, Anderson SA, Dolan MJ, Dy M, Shearer GM. Regulation of TNF-related apoptosis-inducing ligand on primary CD4+ T cells by HIV-1: Role of type I IFN-producing plasmacytoid dendritic cells. Proc Natl Acad Sci U S A (United States) 2005;102:13974–13979. doi: 10.1073/pnas.0505251102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- High KP, et al. HIV and aging: State of knowledge and areas of critical need for research. A report to the NIH office of AIDS research by the HIV and aging working group. J Acquir Immune Defic Syndr (United States) 2012;60(Suppl 1):S1–S18. doi: 10.1097/QAI.0b013e31825a3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton HE, Lyketsos CG, Zenilman JM, Thompson RE, Erbelding EJ. Depression and HIV risk behaviors among patients in a sexually transmitted disease clinic. Am J Psychiatry (United States) 2004;161:912–914. doi: 10.1176/appi.ajp.161.5.912. [DOI] [PubMed] [Google Scholar]
- Jin H, Hampton Atkinson J, Yu X, Heaton RK, Shi C, Marcotte TP, Young C, Sadek J, Wu Z, Grant I HNRC China collaboration group. Depression and suicidality in HIV/AIDS in china. J Affect Disord (Netherlands) 2006;94:269–275. doi: 10.1016/j.jad.2006.04.013. [DOI] [PubMed] [Google Scholar]
- Kacanek D, Jacobson DL, Spiegelman D, Wanke C, Isaac R, Wilson IB. Incident depression symptoms are associated with poorer HAART adherence: A longitudinal analysis from the nutrition for healthy living study. J Acquir Immune Defic Syndr (United States) 2010;53:266–272. doi: 10.1097/QAI.0b013e3181b720e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Ustun TB. The world mental health (WMH) survey initiative version of the world health organization (WHO) composite international diagnostic interview (CIDI) Int J Methods Psychiatr Res (England) 2004;13:93–121. doi: 10.1002/mpr.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics (UNITED STATES) 1982;38:963–974. [PubMed] [Google Scholar]
- Lawson MA, Kelley KW, Dantzer R. Intracerebroventricular administration of HIV-1 tat induces brain cytokine and indoleamine 2,3-dioxygenase expression: A possible mechanism for AIDS comorbid depression. Brain Behav Immun (United States) 2011;25:1569–1575. doi: 10.1016/j.bbi.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letendre SL, Ellis RJ, Ances BM, McCutchan JA. Neurologic complications of HIV disease and their treatment. Top HIV Med (United States) 2010;18:45–55. [PMC free article] [PubMed] [Google Scholar]
- Low-Beer S, Chan K, Yip B, Wood E, Montaner JS, O'Shaughnessy MV, Hogg RS. Depressive symptoms decline among persons on HIV protease inhibitors. J Acquir Immune Defic Syndr (UNITED STATES) 2000;23:295–301. doi: 10.1097/00126334-200004010-00003. [DOI] [PubMed] [Google Scholar]
- Lyketsos CG, Hoover DR, Guccione M, Dew MA, Wesch JE, Bing EG, Treisman GJ. Changes in depressive symptoms as AIDS develops. the multicenter AIDS cohort study. Am J Psychiatry (UNITED STATES) 1996;153:1430–1437. doi: 10.1176/ajp.153.11.1430. [DOI] [PubMed] [Google Scholar]
- Miller AH, Maletic V, Raison CL. Inflammation and its discontents: The role of cytokines in the pathophysiology of major depression. Biol Psychiatry (United States) 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Stetler CA, Carney RM, Freedland KE, Banks WA. Clinical depression and inflammatory risk markers for coronary heart disease. Am J Cardiol (United States) 2002;90:1279–1283. doi: 10.1016/s0002-9149(02)02863-1. [DOI] [PubMed] [Google Scholar]
- Mind Exchange Working Group. Assessment, diagnosis, and treatment of HIV-associated neurocognitive disorder: A consensus report of the mind exchange program. Clin Infect Dis (United States) 2013;56:1004–1017. doi: 10.1093/cid/cis975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray CJ, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: A systematic analysis for the global burden of disease study 2010. Lancet (England) 2013;380:2197–2223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- Pacek LR, Martins SS, Crum RM. The bidirectional relationships between alcohol, cannabis, co-occurring alcohol and cannabis use disorders with major depressive disorder: Results from a national sample. J Affect Disord. 2012 doi: 10.1016/j.jad.2012.11.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccinelli M, Wilkinson G. Gender differences in depression. critical review. Br J Psychiatry (England) 2000;177:486–492. doi: 10.1192/bjp.177.6.486. [DOI] [PubMed] [Google Scholar]
- Rabkin JG. HIV and depression: 2008 review and update. Curr HIV/AIDS Rep (United States) 2008;5:163–171. doi: 10.1007/s11904-008-0025-1. [DOI] [PubMed] [Google Scholar]
- Raison CL, Borisov AS, Majer M, Drake DF, Pagnoni G, Woolwine BJ, Vogt GJ, Massung B, Miller AH. Activation of central nervous system inflammatory pathways by interferon-alpha: Relationship to monoamines and depression. Biol Psychiatry (United States) 2009;65:296–303. doi: 10.1016/j.biopsych.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Gistau V, Baeza I, Arango C, Gonzalez-Pinto A, de la Serna E, Parellada M, Graell M, Paya B, Llorente C, Castro-Fornieles J. Predictors of suicide attempt in early-onset, first-episode psychoses: A longitudinal 24-month follow-up study. J Clin Psychiatry. 2012 doi: 10.4088/JCP.12m07632. [DOI] [PubMed] [Google Scholar]
- Sherr L, Clucas C, Harding R, Sibley E, Catalan J. HIV and depression--a systematic review of interventions. Psychol Health Med (England) 2011;16:493–527. doi: 10.1080/13548506.2011.579990. [DOI] [PubMed] [Google Scholar]
- Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest (United States) 2005;115:1111–1119. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanjani F, Saboe K, Oslin D. Age difference in rates of mental health/substance abuse and behavioral care in HIV-positive adults. AIDS Patient Care STDS (United States) 2007;21:347–355. doi: 10.1089/apc.2006.0043. [DOI] [PubMed] [Google Scholar]