Abstract
Objective
It is well known that Uncoupling protein 1 (UCP1) in brown adipose tissue plays an important role in diet induced thermogenesis. In this study we investigated whether SLN a regulator of SERCA in muscle is also an important player of diet-induced thermogenesis and if loss of SLN could be compensated by increased UCP1 expression and vice versa.
Methods
Age and sex matched UCP1−/− (UKO), SLN−/− (SKO) and double knockout for both UCP1 and SLN (DKO) mice maintained in C57Bl/6J background were challenged to high fat diet for 12 weeks and then analyzed for weight gain, alterations in serum metabolites and changes in thermogenic protein expression.
Results
We found that loss of either SLN or UCP1 alone was sufficient to cause diet-induced obesity. There was no compensatory up regulation of UCP1 in SLN−/− mice or vice versa. Paradoxically loss of both mechanisms failed to exacerbate the obesity phenotype.
Conclusions
Our data suggests that both SLN and UCP1 based adaptive thermogenic mechanisms are essential for achieving maximal diet-induced thermogenesis. When both mechanisms are absent, less efficient thermogenic mechanisms are activated to counter energy imbalance.
Keywords: Diet-induced obesity, High fat diet, metabolic rate, thermogenic mechanisms
INTRODUCTION
Studies have shown that rodents can increase energy expenditure in response to overfeeding to prevent excessive weight gain, a phenomenon termed diet-induced thermogenesis (DIT) (1, 2, 3, 4). The role of Brown adipose tissue (BAT) and UCP1 (4) has been extensively studied as a major mechanism for DIT in rodents (5, 6, 7). The role of BAT in humans has gained renewed interest as a target to treat obesity (4, 5). Although skeletal muscle has been proposed to play an important role in DIT, the mechanisms are less well explored (8). Skeletal muscle comprises approximately 40% of mammalian body mass and accounts for ~30% of resting energy expenditure (during activity its contribution can increase to 90%) (9).
In skeletal muscle, Sarcolipin (SLN), a regulator of the Sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) pump plays a role in cold- and diet-induced thermogenesis (10, 11). More recently we investigated the importance of muscle and BAT for cold adaptation utilizing SLN−/− (SKO) and UCP1−/− (UKO) mouse models. We found that loss of SLN was compensated by increased expression of UCP1 and vice versa during cold adaptation. These findings prompted us to further explore the importance of skeletal muscle and SLN in DIT in comparison to BAT. We performed these studies at thermoneutrality (29.0±1.0 °C) to eliminate cold stress and its associated effects on energy expenditure.
MATERIALS AND METHODS
Generation of UKO, SKO and UCP1−/−.SLN−/− (DKO) mice have been described previously (6, 12, 13). Complete experimental procedure is described in the supplemental materials.
RESULTS
The HFD studies were performed at 29.0±1.0 °C, which is considered as thermoneutral temperature for mice, to eliminate cold stress and its effect on metabolism. The SKO and UKO gained significantly more weight than WT upon HFD-feeding, (Figure 1A) interestingly the rate of weight gain was similar. Paradoxically, the DKO mice gained less weight than the single knockouts (Figure 1A and B). Food intake was not significantly different among genotypes (Figure 1C), thus the differences in weight gain could not be attributed to differences in caloric intake. Metabolic efficiency (ratio of food intake and weight gain) is an important measure of metabolic control. We found that the metabolic efficiency was higher in SKO, UKO and DKO mice compared to WT mice (Figure 1D). Interestingly, the metabolic efficiency of the DKO was lower than single KO animals. Measurements of fat pad weights after HFD-feeding showed a large portion of the weight gained could be attributed to increased fat deposition in adipose tissue depots. The white adipose tissue (WAT) and BAT weights were higher in SKO and UKO mice as compared to WT and DKO mice (Figure 1E–H). Interestingly, the greatest differences in WAT weights occurred in the subcutaneous fat depot, but not in the epidydymal depot (Figure 1G and H). No compensatory beiging in the WAT depots was observed in any genotype. The weights of muscles, heart and blood metabolites levels were not different among genotypes (Supplementary Table 1 and Figure 1I–K).
Figure 1. Results of high fat diet feeding on body/organ weight and serum metabolites.
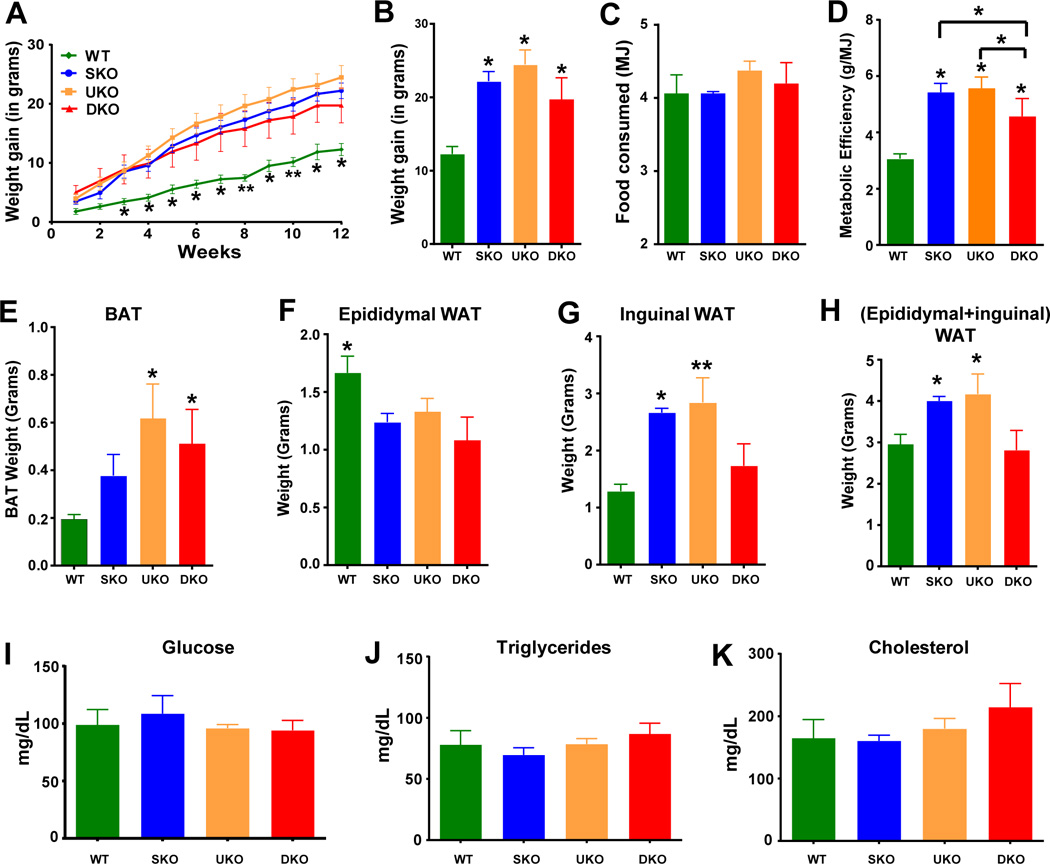
A. Weight gained at each week during high-fat diet (HFD) feeding at 29.0±1.0°C. B. Cumulative weight gain on 12th week of HFD feeding. C. Total food intake in MJ during the 12-week high-fat diet feeding protocol. D. Metabolic efficiency (total weight gain/total food intake) following the high-fat diet. * = p<0.05, ** = p<0.001 compared to WT. Wet tissue weights of brown adipose tissue (BAT; E), epidydymal WAT (F), inguinal WAT (G) and sum of epidydymal and inguinal WAT (H), after 12-weeks HFD feeding. Statistically significant differences are compared to WT. * = p<0.05, ** = p<0.01, compared to WT. I. Fasted (16-hour fast) blood glucose levels after HFD-feeding. Serum cholesterol (J) and triglycerides (K) after a 4-hour fast after 12-weeks HFD feeding.
We next determined if differences in average daily energy expenditure could explain the differences in weight gain in these genotypes. Therefore we measured oxygen consumption before and after 12 weeks of HFD-feeding and data are presented in two ways: 1) normalized to body weight and 2) without normalization. There were no differences in oxygen consumption among the genotypes before HFD feeding (Figure 2A and B). As shown in Figure 2A, all genotypes displayed increased oxygen consumption after 12 weeks of HFD-feeding. The increase was significantly greater in the SKO and UKO, as compared to WT. All genotypes displayed a decreased respiratory exchange ratio (RER), after HFD-feeding (Figure 2B), indicating a shift in substrate utilization from carbohydrates to fats. Loss of SLN caused a significant reduction in energy expenditure as found for UKO (Figure 2C).
Figure 2. Effect of HFD-feeding on metabolic rates and protein expression of SLN and UCP1.
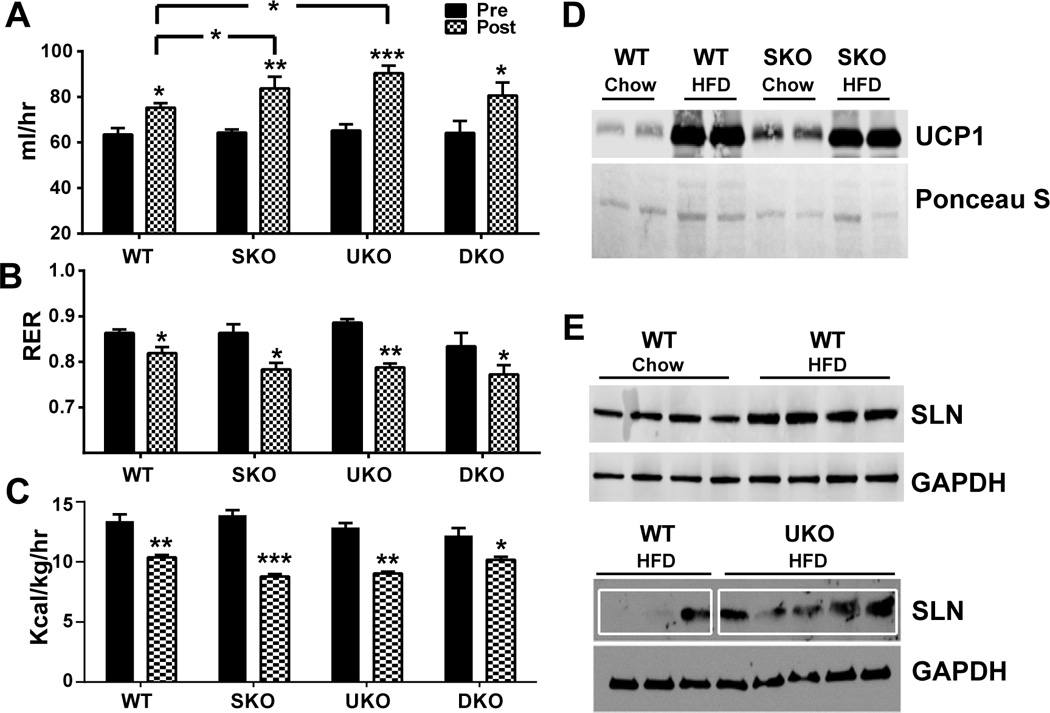
Average oxygen consumption over a 24-hour period before (Pre-HFD) and after (Post-HFD) 12-weeks of HFD-feeding. A. Oxygen consumption (ml/hr) per mouse. B. Respiratory exchange ratio before and after HFD feeding. C. Heat or energy expenditure (Kcal/kg/hr) before and after HFD feeding. Statistically significant differences are between the same genotype Pre-HFD and Post-HFD are indicated on the top of post-HFD column. The differences between the genotypes is indicated by lines. * = p<0.05, ** = p<0.01 and *** = p<0.001. D. Uncoupling protein 1 (UCP1) protein expression in interscapular brown adipose tissue of chow-fed WT, HFD-fed WT, and HFD-fed SKO mice. Ponceau S blot provided to show equal protein loading. E. Sarcolipin (SLN) protein expression in soleus muscles from Chow-fed WT, HFD-fed WT and HFD-fed UKO mice. GAPDH: glyceraldehyde 3-phosphate dehydrogenase.
At temperatures below thermoneutrality, UKO mice show reduced gain in obesity likely due to increased energy expenditure from cold-induced thermogenesis (5). It has also been shown that both SLN and UCP1 are upregulated in mice in response to HFD feeding (5, 10). We found that UCP1 protein was increased in response to the HFD in the BAT (Figure 2D), as previously reported. However, we did not find any compensatory upregulation of UCP1 protein levels in the BAT of SKO mice (Figure 2D). Interestingly, SLN expression was upregulated in WT animals upon HFD-feeding (Figure 2E), as reported previously (10). However, the SLN upregulation was less dramatic when the mice were housed at 29.0±1.0 °C all the times compared to the previous HFD studiesperformed at 22°C (mild cold stress) using mice reared at 22°C (10). Moreover, we did not find compensatory upregulation of SLN in UKO mice following HFD feeding (Figure 2E).
DISCUSSION
Obesity often results from an imbalance between energy intake and expenditure. DIT can also be an important component in regulating whole-body energy expenditure and thus defects in DIT could play a role in obesity (14). In rodents, BAT has been shown to be an important player in DIT (5) but the relevance of BAT in adult humans has been debated. Emerging data also suggest that skeletal muscle is equally an important player in DIT. We recently showed that SLN-based thermogenesis in skeletal muscle is one such mechanism (10, 11). In this study, we investigated the relative importance of SLN and UCP1 in DIT; and if loss of UCP1 would be compensated by increased expression of SLN and vice versa. We further explored whether a loss of both SLN and UCP1 would severely compromise DIT.
A key finding was that SKO mice gained comparable weight to UKO littermates suggesting that loss of muscle based thermogenesis has similar consequences on body mass as loss of BAT-mediated DIT. This may indicate that in rodents SLN- and UCP1-based thermogenesis can contribute to DIT to a similar extent. Despite having intact BAT, SLN deficiency was sufficient to cause increased obesity, which suggests that muscle-based NST is a critical component of DIT or may be the only important component if BAT content is negligible. Considering the sizeable mass of skeletal muscle; even small perturbations in the energetic efficacy of muscle can have significant effects on whole body energy expenditure (15, 16). Although we earlier reported that loss of SLN was compensated by increased UCP1 expression and vice versa during gradual cold adaptation, such a compensatory response was not observed in HFD challenge at thermoneutrality.
An unexpected finding was that, the DKO mice were less obese compared to UKO and SKO mice. The milder obese phenotype of the DKO mice may be the result of 1) activation of other inefficient thermogenic pathways to minimize the effects of diet overload and/or 2) unknown developmental adaptations to compensate for loss of adaptive thermogenic mechanisms. Existence of various futile cycling processes has been proposed including phosphocreatine cycling in beige fat (17), triglyceride/fatty-acid cycling (18), futile protein turnover cycle (19) and phosphofructokinase (PFK)/fructose-1,6-bisphosphatase (FbPase) cycle (20). We earlier reported that the DKO mice were able to survive gradual cold acclimatization to 4°C, suggesting that when UCP1 and SLN based mechanisms are absent, less efficient thermogenic pathways requiring higher energy are activated for survival. Taken together these finding suggests that mice can adapt and survive without these two facultative thermogenic mechanisms. Future studies should be aimed at defining the involvement of other unknown mechanisms that might come to the rescue when these systems are absent. In conclusion, this study shows that muscle and BAT are important players in DIT.
Supplementary Material
What is already known about this subject?
UCP1 is recruited in diet-induced thermogenesis.
SLN is an important regulator of cold-induced thermogenesis.
What does this study add?
Muscle is an important site of DIT.SLN plays a key role in diet-induced thermogenesis in mice raised at thermoneutrality.
Acknowledgments
This work was supported by National Institutes of Health (NIH) Grants R01-HL-088555 and R01-DK098240-01 and by American Diabetes Association Basic Science Research Award (7-13-BS-131) to M.P and by the NIDDK-NIH Award (K01-DK102772) to N.C.B. We apologize for the omission of other pertinent references due to space constraints.
Footnotes
CONFLICT OF INTEREST
Authors declare no conflict of interest.
REFERENCES
- 1.Tseng YH, Cypess AM, Kahn CR. Cellular bioenergetics as a target for obesity therapy. Nat Rev Drug Discov. 2010;9:465–482. doi: 10.1038/nrd3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tremblay A, Royer MM, Chaput JP, Doucet E. Adaptive thermogenesis can make a difference in the ability of obese individuals to lose body weight. Int J Obes (Lond) 2013;37:759–764. doi: 10.1038/ijo.2012.124. [DOI] [PubMed] [Google Scholar]
- 3.Bachman ES, Dhillon H, Zhang CY, Cinti S, Bianco AC, Kobilka BK, et al. betaAR signaling required for diet-induced thermogenesis and obesity resistance. Science. 2002;297:843–845. doi: 10.1126/science.1073160. [DOI] [PubMed] [Google Scholar]
- 4.Rothwell NJ, Stock MJ. A role for brown adipose tissue in diet-induced thermogenesis. Nature. 1979;281:31–35. doi: 10.1038/281031a0. [DOI] [PubMed] [Google Scholar]
- 5.Feldmann HM, Golozoubova V, Cannon B, Nedergaard J. UCP1 ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell Metab. 2009;9:203–209. doi: 10.1016/j.cmet.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 6.Enerback S, Jacobsson A, Simpson EM, Guerra C, Yamashita H, Harper ME, et al. Mice lacking mitochondrial uncoupling protein are cold-sensitive but not obese. Nature. 1997;387:90–94. doi: 10.1038/387090a0. [DOI] [PubMed] [Google Scholar]
- 7.Ricquier D, Bouillaud F. Mitochondrial uncoupling proteins: from mitochondria to the regulation of energy balance. J Physiol. 2000;529(Pt 1):3–10. doi: 10.1111/j.1469-7793.2000.00003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van den Berg SA, van Marken Lichtenbelt W, Willems van Dijk K, Schrauwen P. Skeletal muscle mitochondrial uncoupling, adaptive thermogenesis and energy expenditure. Curr Opin Clin Nutr Metab Care. 2011;14:243–249. doi: 10.1097/MCO.0b013e3283455d7a. [DOI] [PubMed] [Google Scholar]
- 9.Zurlo F, Larson K, Bogardus C, Ravussin E. Skeletal muscle metabolism is a major determinant of resting energy expenditure. J Clin Invest. 1990;86:1423–1427. doi: 10.1172/JCI114857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bal NC, Maurya SK, Sopariwala DH, Sahoo SK, Gupta SC, Shaikh SA, et al. Sarcolipin is a newly identified regulator of muscle-based thermogenesis in mammals. Nat Med. 2012;18:1575–1579. doi: 10.1038/nm.2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maurya SK, Bal NC, Sopariwala DH, Pant M, Rowland LA, Shaikh SA, et al. Sarcolipin Is a Key Determinant of the Basal Metabolic Rate, and Its Overexpression Enhances Energy Expenditure and Resistance against Diet-induced Obesity. J Biol Chem. 2015;290:10840–10849. doi: 10.1074/jbc.M115.636878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Babu GJ, Bhupathy P, Timofeyev V, Petrashevskaya NN, Reiser PJ, Chiamvimonvat N, et al. Ablation of sarcolipin enhances sarcoplasmic reticulum calcium transport and atrial contractility. Proc Natl Acad Sci U S A. 2007;104:17867–17872. doi: 10.1073/pnas.0707722104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rowland LA, Bal NC, Kozak LP, Periasamy M. Uncoupling Protein 1 and Sarcolipin Are Required to Maintain Optimal Thermogenesis, and Loss of Both Systems Compromises Survival of Mice under Cold Stress. J Biol Chem. 2015;290:12282–12289. doi: 10.1074/jbc.M115.637603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Silva JE. Thermogenic mechanisms and their hormonal regulation. Physiol Rev. 2006;86:435–464. doi: 10.1152/physrev.00009.2005. [DOI] [PubMed] [Google Scholar]
- 15.Gamu D, Bombardier E, Smith IC, Fajardo VA, Tupling AR. Sarcolipin provides a novel muscle-based mechanism for adaptive thermogenesis. Exerc Sport Sci Rev. 2014;42:136–142. doi: 10.1249/JES.0000000000000016. [DOI] [PubMed] [Google Scholar]
- 16.Maurya SK, Periasamy M. Sarcolipin is a novel regulator of muscle metabolism and obesity. Pharmacological research. 2015;102:270–275. doi: 10.1016/j.phrs.2015.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kazak L, Chouchani ET, Jedrychowski MP, Erickson BK, Shinoda K, Cohen P, et al. A creatine-driven substrate cycle enhances energy expenditure and thermogenesis in beige fat. Cell. 2015;163:643–655. doi: 10.1016/j.cell.2015.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tagliaferro AR, Dobbin S, Curi R, Leighton B, Meeker LD, Newsholme EA. Effects of diet and exercise on the in vivo rates of the triglyceride-fatty acid cycle in adipose tissue and muscle of the rat. International journal of obesity. 1990;14:957–971. [PubMed] [Google Scholar]
- 19.She P, Reid TM, Bronson SK, Vary TC, Hajnal A, Lynch CJ, et al. Disruption of BCATm in mice leads to increased energy expenditure associated with the activation of a futile protein turnover cycle. Cell Metab. 2007;6:181–194. doi: 10.1016/j.cmet.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Staples JF, Koen EL, Laverty TM. 'Futile cycle' enzymes in the flight muscles of North American bumblebees. J Exp Biol. 2004;207:749–754. doi: 10.1242/jeb.00825. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


