Abstract
Objectives
Empiric treatment with milrinone, a phosphodiesterase 3 inhibitor (PDE3i), has become increasingly common in patients with single ventricle heart disease of right ventricular morphology (SRV); our objective was to characterize the myocardial response to PDE3i in the pediatric population with SRV.
Study design
Cyclic adenosine monophosphate (cAMP) levels, phosphodiesterase (PDE) activity, and phospholamban phosphorylation (pPLN) were determined in explanted human ventricular myocardium from nonfailing pediatric donors (n=10) and pediatric patients transplanted secondary to SRV. SRV subjects were further classified by PDE3i treatment (n=13 with PDE3i and n=12 without PDE3i).
Results
In comparison with nonfailing RV myocardium, cAMP levels are lower in patients with SRV treated with PDE3i (p=0.021). Chronic PDE3i does not alter total PDE or PDE3 activity in SRV myocardium. When compared with nonfailing RV myocardium, SRV myocardium (both with and without PDE3i) demonstrates equivalent pPLN at the protein kinase A phosphorylation site.
Conclusions
As evidenced by preserved pPLN, the molecular adaptation associated with SRV differs significantly from that demonstrated in pediatric heart failure due to dilated cardiomyopathy. These alterations support a pathophysiologically distinct mechanism of heart failure in pediatric patients with SRV, which has direct implications regarding the presumed response to PDE3i treatment in this population.
Keywords: Cyclic adenosine monophosphate, phospholamban, phosphodiesterase, pediatric heart failure
Single ventricle congenital heart disease is a subset of heart defects characterized by a univentricular circulation with persistent hypoxia, which is universally fatal without intervention1. Despite advances in surgical technique and perioperative care, the outcome for single ventricle heart disease remains poor2–5, with only one-third of children surviving, free from heart transplant, to age 14 years6.
Progressive heart failure is both a common cause of death and indication for heart transplantation in infants and children with single ventricle heart disease4, 7. The dominant ventricle in single ventricle heart disease can be of right ventricular (RV), left ventricular (LV), or indeterminate morphology, and it is suggested that those with single ventricle heart disease of RV morphology (SRV) have worse outcomes8, 9. The RV in particular is at risk for progressive failure secondary to the non-physiologic high afterload in SRV, as well as intrinsic embryological and architectural differences between the RV and LV10, 11. As a whole, the RV is not well-suited to pump against sustained pressure overload, and the mechanisms that help the RV adapt to high pressures ultimately lead to maladaptive remodeling, with RV dilation and eventual failure. However, the molecular mechanisms underlying RV failure in SRV are poorly understood, limiting the ability to identify effective therapies. Treatments for LV failure are aimed at preventing or reversing pathologic remodeling; these therapies have been applied to RV failure under the hypothesis that the same pathophysiological events occur in LV and RV failure. However, given the significant differences between the LV and RV, perhaps it is not surprising that the extrapolation of proven adult LV systolic heart failure medications to the pediatric SRV population have demonstrated little benefit. Specifically, angiotensin-converting enzyme inhibitors (ACEi) and beta-blockers (BB) have been ineffective in preventing heart failure or even trended toward worsening heart failure in the setting of SRV respectively12, 13.
Management of SRV has included the empirical use of specific inhibitors targeting phosphodiesterase (PDE) enzymes. PDEs regulate the amplitude, duration, and compartmentalization of intracellular cyclic nucleotide signaling by hydrolyzing cyclic adenosine monophosphate (cAMP) and/or cyclic guanosine monophosphate (cGMP). Pharmacologic inhibition of select PDE enzymes has become an increasingly common therapy for SRV and is often used in pediatric heart failure as a bridge to transplant14; specifically, inhibition of PDE3 (PDE3i) with milrinone is thought to augment contractility through increasing intracellular cAMP. Classically, milrinone-mediated improvements in contractility are attributed to the downstream effects of increased cAMP through phosphorylation of proteins that affect excitation/contraction coupling, including the sarcoplasmic reticulum calcium ATPase 2 (SERCA)-regulatory protein phospholamban (PLN). By de-inhibiting SERCA, increased protein kinase A (PKA)-mediated phosphorylation of PLN (pPLN) accelerates calcium re-uptake into the sarcoplasmic reticulum and increases sarcoplasmic reticulum calcium content, contributing to both lusitropic and inotropic effects, respectively15. We have previously demonstrated that PKA-mediated pPLN is increased in children with dilated cardiomyopathy (DCM) chronically treated with PDE3i, and may contribute to increased contractility resulting in the sustained clinical benefit of PDE3i treatment in that population16. However, it is unlikely that the molecular perturbations in SRV are akin to those in pediatric DCM; thus, given the widespread clinical use our objective was to characterize the molecular response to PDE3i in this unique population.
Methods
All subjects gave informed consent and donated their hearts to the Institutional Review Board-approved Pediatric Cardiac Transplant Tissue Bank at the University of Colorado Denver. Non-failing tissues were from pediatric (<18 years of age) organ donors with normal heart function, whose hearts could not be placed for technical reasons (size or blood type mismatch). Hearts from patients transplanted with single ventricle heart disease of RV morphology were included in this study (single ventricle disease of LV or indeterminate morphology were excluded). 2 groups of SRV patients were included: (1) patients with SRV listed for transplant secondary to surgical palliation failure and (2) patients with SRV listed for primary transplant that met predefined criteria. Surgical palliation failure included those patients suffering from RV failure, protein losing enteropathy and/or plastic bronchitis. Patients with SRV listed for primary transplant were included only if they were > 4 months of age at the time of transplant and had signs and symptoms of heart failure, evidence of ventricular dilation, hypertrophy or decreased cardiac function. Patients that were transplanted primarily for SRV lacking these defined clinical characteristics of heart failure were excluded. Patients with SRV were then categorized based on PDE3i treatment at the time of transplant and all patients with SRV were compared with nonfailing RV controls. At the time of cardiac transplantation (SRV group) or donation (nonfailing group), the heart tissue was rapidly dissected in the operating room, flash frozen and stored at −80°C until further use.
cAMP levels were measured by ELISA in the core facility at Children’s Hospital Colorado, Aurora, CO using the R&D Parameter immunoassay kit (R&D Systems, Minneapolis, MN) according to manufacturer’s recommendations.
Approximately 150 mg of myocardium was homogenized and separated into nuclear, cytosolic and sarcoplasmic reticulum-enriched microsomal fractions by differential sedimentation, as previously described16.
cAMP-hydrolytic activity was quantified at 30°C by the two-step snake-venom method with [3H]cAMP (1 μmol/L) as substrate (previously described16).
Western blots were performed as described previously16. Serine 16 (Ser16) PLN phosphorylation (pPLN) (A010-12 - Badrilla) and total PLN (05-205 - Millipore) were quantified on separate blots and normalized to GAPDH (Santa Cruz Biotechnology). Blots were quantified using ImageJ version 1.46r.
Statistical analyses
Statistical analyses were performed using GraphPad Prism version 6.0c. Statistical significance was set a priori at p<0.05. Normality of data was confirmed and when appropriate, comparison of three normally distributed groups was conducted using one-way ANOVA; if the overall comparison reached significance, Tukey post hoc tests were performed. Non-normally distributed data were analyzed using non-parametric testing (Mann-Whitney or Kruskal-Wallis with Dunn’s multiple comparisons test). Comparisons of nonfailing LV and RV were conducted using unpaired t-test (cAMP), paired t-tests (PDE activity in RV and LV from the same patient) or Wilcoxon matched-pairs signed rank test (pPLN). Linear regression was performed to evaluate for association between milrinone treatment, duration of heart failure and dose of milrione and cAMP levels, PDE activity and PLN expression.
Results
Subject characteristics and medications are listed in Table (available at www.jpeds.com). Median age at tissue collection for pediatric nonfailing subjects was 8.0 years with an interquartile range (IQR) of 8.9 years; for SRV subjects, 0.67 years with an IQR of 5.5 years; and, for SRV+ milrinone (SRV+M) subjects, 2.9 years with an IQR of 4.1 years. The nonfailing group was significantly older than the SRV group (p=0.005), however the ages of the SRV and SRV+M groups were similar. Mean duration of milrinone therapy was 139 days, with a median of 100 days (range 19–355 days). Duration of heart failure was determined for all patients with SRV transplanted after palliation failure. There was no significant difference in duration of heart failure between the SRV and the SRV+M groups. The mean duration of heart failure for the SRV group was 1.56 years with a median of 0.84 years (range 0.15–5.73 years). The mean duration of heart failure for the SRV+M group was 0.76 years with a median of 0.7 years (range 0.05–2.35 years). The nonfailing subjects were 62% male, SRV subjects were 83% male, and SRV+M subjects were 62% male.
Table 1.
Subject Characteristics
| Patient Characteristics | Medications | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ID | Group | Sex | Age at Tissue Collection (years) |
Anatomic Diagnosis |
Last Surgical Palliation |
Indication for Transplant |
PDE3i | PDE5i | Non PDEi inotropes* |
Digoxin | ACEi | Beta- Blocker |
Diuretic | Anti- arrhythmic |
PDE3i (duration in days/ dose#) |
| Non-Failing (n=13) | |||||||||||||||
| 1 | NF | F | 1.3 | Normal | None | NA | N | N | YD,E | N | N | N | N | N | None |
| 2 | NF | M | 1.4 | Normal | None | NA | N | N | N | N | N | N | N | N | None |
| 3 | NF | F | 3.0 | Normal | None | NA | N | N | YD,E,V | N | N | N | N | N | None |
| 4 | NF | M | 7.0 | Normal | None | NA | N | N | N | N | N | N | N | N | None |
| 5 | NF | M | 7.0 | Normal | None | NA | N | N | YD | N | N | N | N | N | None |
| 6 | NF | F | 8.0 | Normal | None | NA | N | N | N | N | N | N | N | N | None |
| 7 | NF | M | 9.0 | Normal | None | NA | N | N | YD,V | N | N | Y | N | N | None |
| 8 | NF | M | 12 | Normal | None | NA | N | N | N | N | N | N | N | N | None |
| 9 | NF | M | 13 | Normal | None | NA | N | N | YD, N | N | N | Y | N | N | None |
| 10 | NF | M | 14 | Normal | None | NA | N | NA | NA | NA | NA | NA | NA | NA | NA |
| 11 | NF | F | 11.7 | Normal | None | NA | N | N | YD,N,E | N | N | N | N | N | None |
| 12 | NF | F | 8.6 | Normal | None | NA | N | N | YD | N | N | N | Y | N | None |
| 13 | NF | M | 2.9 | Normal | None | NA | N | N | YE,V | N | N | N | N | N | None |
| SRV (n=12) | |||||||||||||||
| 14 | SRV | M | 0.33 | HLHS | None | Primary transplant, >4 months, RVH | N | N | N | N | N | N | N | N | None |
| 15 | SRV | M | 0.34 | HLHS | None | Primary transplant, >4 months, RVH | N | N | N | N | N | N | N | N | None |
| 16 | SRV | M | 0.4 | HLHS | None | Primary transplant, >4 months, RVH | N | N | N | N | N | N | N | N | None |
| 17 | SRV | M | 0.45 | HLHS | PAB | Primary transplant, >4 months, RVH and RV dilation | N | N | N | N | N | N | N | N | None |
| 18 | SRV | M | 0.56 | HLHS | PAB | Primary transplant, >4 months, RV dilation | N | N | N | N | N | N | N | N | None |
| 19 | SRV | M | 0.63 | HLHS | PAB | Primary transplant, >4 months, RVH and RV dilation | N | N | N | N | N | N | N | N | None |
| 20 | SRV | F | 0.7 | HLHS | PDA Stent, PAB | Pulmonary hypertension, RVH | N | N | N | Y | N | N | Y | N | None |
| 21 | SRV | F | 1.0 | Unbalanced AVSD, RV dominant | Norwood/Sano | RV failure | N | N | N | Y | Y | N | Y | N | None |
| 22 | SRV | M | 1.3 | DORV, hypoplastic LV | Coarctation repair, PAB, Pacemaker | RV failure | N | N | N | N | Y | N | Y | N | None |
| 23 | SRV | M | 7.5 | Unbalanced AVSD, RV dominant | Glenn, Kawashima | RV failure | N | NA | N | NA | NA | NA | NA | NA | None |
| 24 | SRV | M | 8.0 | TGA, PA, hypoplastic LV | Fontan | PLE | N | N | N | Y | Y | N | Y | N | None |
| 25 | SRV | M | 12 | HLHS | Glenn | RV failure | N | N | N | Y | Y | N | N | N | None |
| SRV with Milrinone (n=13) | |||||||||||||||
| 26 | SRV+M | M | 0.4 | HLHS | PDA Stent, PAB | Pulmonary hypertension, RVH, RV dilation | Y | N | YD | N | N | N | Y | N | 31/ 0.75 |
| 27 | SRV+M | M | 0.4 | HLHS | Norwood/BTS | RV failure | Y | N | N | N | Y | N | Y | N | 100/ 0.5 |
| 28 | SRV+M | F | 0.8 | HLHS | Norwood/BTS | RV failure | Y | N | N | N | N | N | Y | N | 97/ 0.75 |
| 29 | SRV+M | F | 0.9 | HLHS | Norwood/Sano | RV failure | Y | N | N | Y | Y | N | N | N | 235/ 0.5 |
| 30 | SRV+M | M | 2.3 | HLHS | Glenn | RV failure | Y | N | N | Y | Y | N | Y | N | 180/ 0.5 |
| 31 | SRV+M | F | 2.8 | HLHS | Glenn | RV failure | Y | N | N | Y | Y | N | Y | N | 94/ 0.5 |
| 32 | SRV+M | M | 2.9 | HLHS | Glenn | RV failure | Y | Y | N | Y | Y | N | Y | N | 279/ 1.0 |
| 33 | SRV+M | M | 3.8 | Single RV | Fontan | RV failure | Y | N | N | N | NA | N | NA | NA | 137/ 0.5 |
| 34 | SRV+M | F | 3.9 | HLHS | Fontan | RV failure | Y | Y | N | Y | N | N | Y | Y | 19/ 0.75 |
| 35 | SRV+M | M | 4.8 | HLHS | Fontan | Plastic bronchitis | Y | Y | N | N | Y | N | Y | N | 355/ 0.5 |
| 36 | SRV+M | M | 5.0 | Single RV | Fontan | RV failure | Y | N | N | Y | Y | N | Y | N | 194/ 0.75 |
| 37 | SRV+M | F | 6.1 | HLHS | Fontan | PLE | Y | Y | N | N | N | N | Y | N | 30/ 0.5 |
| 38 | SRV+M | M | 10 | DORV, mitral atresia | Fontan | PLE | Y | Y | N | N | Y | N | Y | N | 60/ 0.5 |
ID = identification, NF = non-failing, SRV = single right ventricle, SRV+M = single right ventricle treated with milrinone, M = Male, F = Female, ACEi = angiotensin-converting enzyme inhibitor, PDEi = phosphodiesterase inhibitor, RV = right ventricle, LV = left ventricle, HLHS = hypoplastic left heart syndrome, AS = aortic stenosis, AVSD = atrioventricular septal defect, DORV = double outlet right ventricle, TGA = transposition of the great arteries, PA = pulmonary atresia, PDA = patent ductus arteriosus, PAB = pulmonary artery banding, BTS = Blalock-Taussig Shunt, RVH = right ventricular hypertrophy, PLE = protein losing enteropathy, NA = not available or not-applicable.
Non-PDEi Inotrope includes:
dopamine,
epinephrine,
norepinephrine,
vasopressin.
Dose of milrinone is in mcg/kg/min.
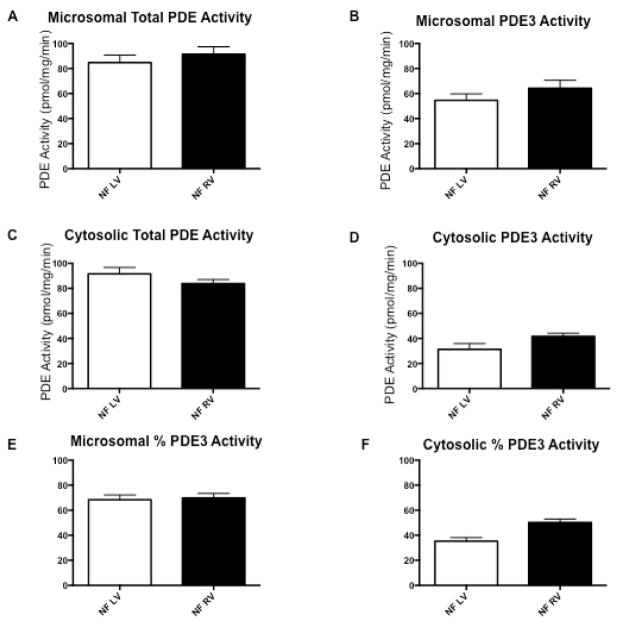
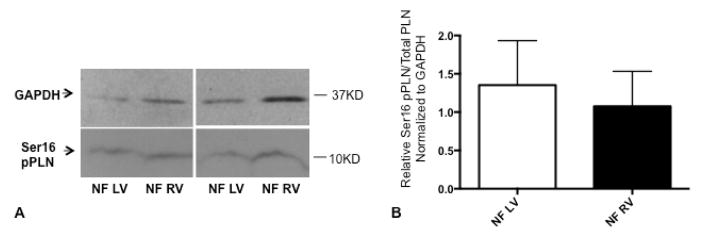
PDE activity and pPLN were quantified in the LV and RV of nonfailing pediatric controls to determine if there was an effect of ventricular morphology. In both microsomal and cytosolic fractions, total PDE activity, and PDE3 activity are similar in nonfailing LV and nonfailing RV (Figure 1, A–D; available at www.jpeds.com). The percent PDE3 activity in the microsomal fraction is higher than that of the cytosolic fraction in pediatric nonfailing LV (microsomal 68.5% versus cytosolic 35.3%, p<0.0001; Figure 1, E and F). Of note, PDE3 activity in the nonfailing RV microsomal fraction is also higher than in the nonfailing RV cytosolic fraction (microsomal 69.8% versus cytosolic 50.3%, p=0.002; Figures 1, E and F). In the microsomal fraction, the percent PDE3 activity is equivalent between nonfailing LV and nonfailing RV (p=0.802) and in the cytosolic fraction, the percent PDE3 is higher in the nonfailing RV than nonfailing LV (p=0.0017; Figure 1, E and F). Additionally, the ratio of pPLN at the PKA site to total PLN is similar between nonfailing LV and nonfailing RV (p=0.69, n=7 per group) (Figure 2; available at www.jpeds.com).
Figure 1.
Comparison of cAMP-hydrolyzing PDE activity profiles between nonfailing, pediatric left and right ventricular myocardium. (A) Total PDE activity in the microsomal fraction. (B) PDE3-specific activity in the microsomal fraction. (C) Total PDE activity in the cytosolic fraction. (D) PDE3-specific activity in the cytosolic fraction. (E) Fraction of PDE3 activity in the microsomal fraction. (F) Fraction of PDE3 activity in the cytosolic fraction. N=8 for each group. PDE, phosphodiesterase; NF, nonfailing; LV, left ventricle; RV, right ventricle.
Figure 2.
Phospholamban phosphorylation at the serine 16 residue in nonfailing, pediatric left and right ventricular myocardium; representative Western blot (A) and quantitation (B) are shown. GAPDH was used as a loading control. N=7 for each group. Ser16, serine 16 residue; pPLN, phospholamban phosphorylation; PLN, phospholamban; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; NF, nonfailing; LV, left ventricle; RV, right ventricle.
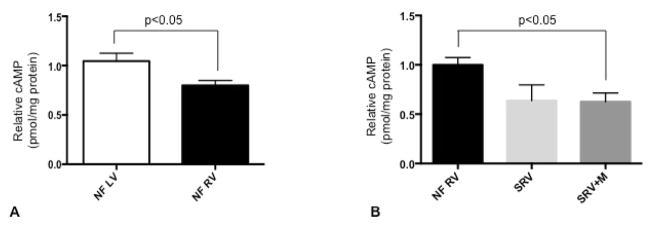
Comparison of cAMP levels between RV and LV in nonfailing pediatric patients suggests that cAMP levels are lower in RV myocardium compared with that in LV (p=0.03, n=13 LV and n=9 RV) (Figure 3, A; available at www.jpeds.com. Global myocardial cAMP levels are significantly lower in pediatric SRV despite milrinone treatment (p=0.021 between nonfailing RV and SRV+M; Figure 3, B). There is a trend toward decreased cAMP in SRV without milrinone treatment (p=0.095 between nonfailing RV and SRV) and no significant difference between SRV with and without milrinone (p=0.99 between SRV and SRV+M). There was no association between duration of SRV heart failure and cAMP levels.
Figure 3.
Relative cAMP levels (quantitated by ELISA). (A) Comparison between pediatric NF LV, nonfailing left ventricle (n=13) and NF RV, nonfailing right ventricle (n=9). (B) Comparison between NF RV (n=8); SRV, single right ventricle (n=5); and SRV+M, single right ventricle treated with milrinone (n=12). cAMP, cyclic adenosine monophosphate.
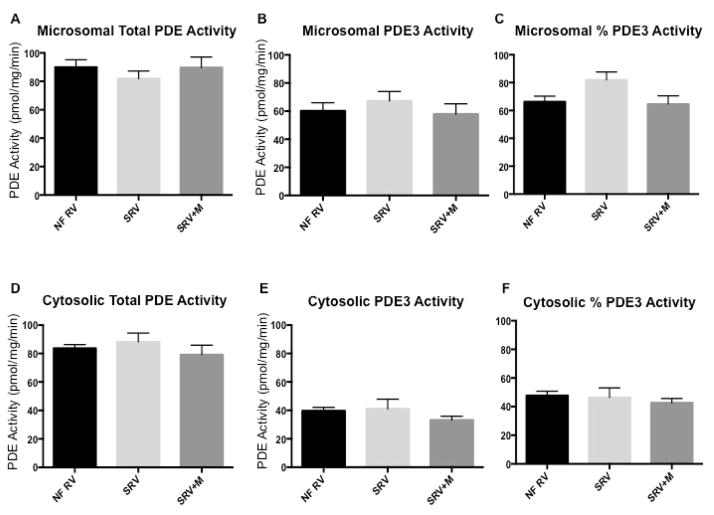
Total PDE activity in the microsomal fraction is similar in nonfailing RV and SRV, and unchanged in patients with SRV on chronic milrinone treatment (p=0.69). PDE3 activity in the microsomal fraction is also comparable among the three groups (p=0.68) (Figure 4, A and B; available at www.jpeds.com). Overall, percent PDE3 activity in the microsomal fraction is equivalent (p=0.11; Figure 4, C). Total PDE activity in the cytosolic fraction is not different between nonfailing RV, SRV, and SRV treated with milrinone (p=0.55; Figure 4, D). PDE3 activity in the cytosolic fraction is also similar among the three groups (p=0.25; Figure 4, E). Lastly, percent PDE3 activity in the cytosolic fraction is also similar (p=0.59; Figure 4, F). No association was found between duration of milrinone treatment and PDE activity (total and PDE3) in the SRV+M group. There was no association between duration of SRV heart failure and total PDE or PDE3 activity.
Figure 4.
cAMP-hydrolyzing PDE activity in nonfailing pediatric right ventricle, SRV, and SRV treated with milrinone. (A) Total PDE activity in the microsomal fraction. (B) PDE3-specific activity in the microsomal fraction. (C) Fraction of PDE3 activity in the microsomal fraction. (D) Total PDE activity in the cytosolic fraction. (E) PDE3-specific activity in the cytosolic fraction. (F) Fraction of PDE3 activity in the cytosolic fraction. PDE, phosphodiesterase; NF, nonfailing (n=9); SRV, single right ventricle (n=5); SRV+M, single right ventricle treated with milrinone (n=13).
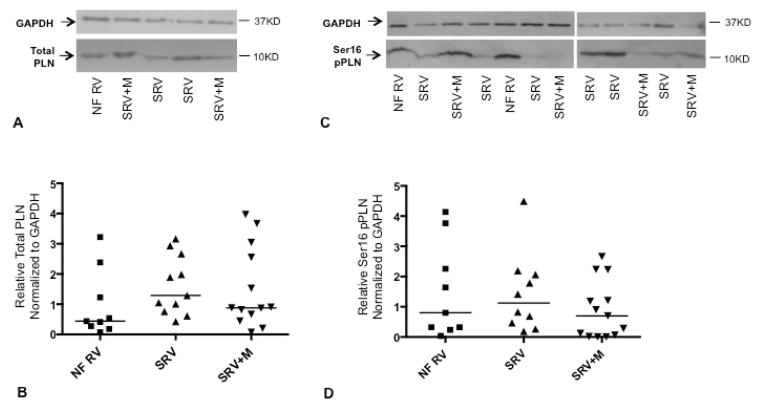
SRV myocardium has equivalent levels of total PLN when compared with nonfailing RV myocardium (p=0.44) (Figure 5, A and B; available at www.jpeds.com). Notably, milrinone treatment in patients with SRV does not significantly alter total PLN levels.
Figure 5.
Total phospholamban (A and B) and phospholamban phosphorylation at the serine 16 residue (C and D) in nonfailing pediatric right ventricle and SRV; representative Western blot and quantitation for each are shown. Horizontal lines in B and D represent population medians; scatter plot and median shown due to non-normally distributed data. GAPDH was used as a loading control. Ser16, serine 16 residue; pPLN, phospholamban phosphorylation; PLN, phospholamban; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; NF, nonfailing (n=9); SRV, single right ventricle (n=11); SRV+M, single right ventricle treated with milrinone (n=13).
PLN phosphorylation at the serine 16 residue (PKA phosphorylation site) is equivalent between patients with SRV and nonfailing RV (p=0.43) (Figure 5, C and D). Furthermore, pPLN is not increased in subjects with SRV despite milrinone therapy and neither total PLN nor pPLN correlate with duration of heart failure.
Discussion
Milrinone therapy has been extrapolated to the SRV population, and its chronic use is intended to bridge children with SRV heart failure to oral heart failure therapy or to heart transplantation. However, the effects of chronic PDE3i in pediatric SRV disease (or even human RV myocardium in biventricular circulations) have not previously been well described. In the context of the unique pathophysiology of heart failure in children with SRV, we have demonstrated that the effects of chronic PDE3i therapy in patients with SRV are largely distinct from those described in pediatric DCM, and present several novel findings.
Despite significant differences in anatomy, myofiber architecture, embryology, and physiology between the LV and RV, total cAMP-hydrolyzing PDE activity and PDE3 activities are similar in the LV and RV of nonfailing hearts. Importantly, cAMP-dependent pPLN (Ser16) is also comparable between LV and RV myocardium despite lower baseline levels of cAMP in RV myocardium. Similar PDE activity and pPLN between LV and RV myocardium suggest that in children with a normal biventricular circulation and cardiac function, the mechanism of action and myocardial effects of PDE3i may be analogous in either ventricle. Furthermore, any differences in response to PDE3i in the SRV population are unlikely to be fully attributable to inherent differences between LV and RV myocardium.
Our results support the hypothesis that SRV disease differs from other biventricular forms of pediatric heart failure. Specifically, pPLN is not altered in SRV, and pPLN is decreased in the myocardium of children (and adults) with DCM. Maintenance of pPLN despite decreased global cAMP in SRV suggests that: (1) cAMP levels in the local, PLN-containing compartment are unaltered in SRV; (2) preservation of PKA-mediated pPLN is not sufficient to maintain cardiac contractility in SRV; and (3) unlike heart failure secondary to DCM, different molecular perturbations dominate to produce heart failure in the pediatric SRV population.
Decreased cAMP levels are a common and pathophysiologically important feature in both pediatric and adult heart failure due to DCM, and provide the putative rationale for the inotropic action of PDE3i. Prior analyses of cAMP levels in SRV disease have demonstrated similar levels of myocardial cAMP between nonfailing RV and SV17. The current study focuses on SRV with heart failure (excluding patients with “compensated” disease) and encompasses a larger number of subjects. Although cAMP levels in SRV are lower than those of nonfailing RV (Figure 3, B), this represents a global assessment of cAMP levels in the myocardium and may not reflect physiologically significant changes in cAMP secondary to the highly compartmentalized nature of this second messenger. Discrete pools of cAMP within the cell allow for small, local changes in cAMP concentration to have considerable impact on neighboring protein phosphorylation and activity. In other forms of heart failure, not only is global cAMP decreased, but downstream PKA-mediated pPLN is also decreased, implying that cAMP is reduced in the region of the PLN and SERCA compartment. PLN is a key regulator of SERCA activity, and specifically, an increase in the ratio of dephosphorylated PLN to SERCA (by overexpression of PLN, decreased PLN phosphorylation, or decreased SERCA expression) decreases the apparent calcium affinity of SERCA and contributes to contractile dysfunction18, 19. Thus, the maintenance of pPLN in SRV is in stark contrast to the decrease in pPLN seen in pediatric DCM and implies that the mechanism of contractile dysfunction in SRV does not appear to be mediated by PLN regulation of SERCA. The global decrease in cAMP in SRV likely has downstream effects in other compartments, including the nucleus via cAMP-responsive element binding protein (CREB), other domains in the sarcoplasmic reticulum organized around myocyte A- kinase anchoring protein (mAKAP) including ryanodine receptors, and mitochondria20 that may be a more dominant cause of contractile dysfunction in this population.
The lack of change in total PDE and PDE3 activity is similar to previous findings that PDE activity in the pediatric and adult DCM myocardium is not significantly altered by heart failure alone16, 21. The unchanged total PDE cAMP-hydrolyzing activity in both the microsomal and cytosolic compartments may suggest that the decrease in global cAMP levels is secondary to reduced cAMP production as opposed to increased hydrolysis. Additionally, the assessment of global cAMP may be mostly reflective of cAMP levels in the cytosolic compartment, where the majority of cAMP-hydrolyzing activity is performed by non-PDE3 isoforms. In adult left ventricular cardiomyocytes, PDE1 is the dominant PDE isoform in the cytosol and the PDE1 family is uniquely activated by calcium/calmodulin22. Further investigations of PDE activity in the presence of calcium/calmodulin, particularly in the cytosolic fraction, may reveal some differences in PDE1 activity between nonfailing RV and SRV that could contribute to a global decrease in cAMP levels.
Given the unique molecular perturbations leading to heart failure in patients with SRV, it is not unexpected that chronic milrinone treatment does not increase cAMP and pPLN in SRV. However, this is in stark contrast to the effects of chronic milrinone in the pediatric DCM population, where PDE3i is associated with a significant increase in cAMP and pPLN which likely contributes to its robust inotropic benefit16. Thus, the inotropic effect of milrinone in SRV is unlikely to be secondary to cAMP effects on the PLN/SERCA compartment. It is possible that clinical benefits seen with milrinone therapy in the SRV population may be secondary to off-target effects (possibly on PDEs other than PDE3) within the myocardium or non-myocardial effects on the pulmonary and systemic vasculature that are of principal importance in SRV.
Our results have several implications: (1) unique regulation in SRV myocardium results in preserved pPLN yet this does not prevent the development of clinical heart failure; (2) the global decrease in cAMP likely has downstream effects in other compartments; and (3) the direct myocardial effects from PDE3i are different in SRV than in pediatric heart failure due to DCM, which cannot be entirely attributable to differences in ventricular morphology.
There are several limitations to our study. First, all patients with SRV included in this study required heart transplantation; nevertheless, heart failure symptoms at the time of heart explant were varied and constituted a wide spectrum, as is common in this population. There are certainly clinical distinctions between those patients with SRV with systolic RV dysfunction and those that require transplant secondary to “Fontan failure” (ie, refractory protein-losing enteropathy) or plastic bronchitis. However, clinically, milrinone is empirically started in both types of SRV subjects; thus we sought to include both in our investigations. Sub-analyses of only patients with SRV with systolic RV dysfunction did not demonstrate any significant differences with regard to cAMP levels, PDE activity, or pPLN based on milrinone treatment (data not shown). Nevertheless, given the small number of patients, this type of sub-analysis is underpowered. Second, despite a non-statistically significant difference between the ages of the SRV and SRV+M groups, there may be age-related differences between the infant (<1 year) and pediatric (≥1 year) myocardium that we were underpowered to detect. In order to decrease the heterogeneity of the infant subjects, only infants that could be considered failing (greater than 4 months of age at the time of pulmonary artery banding or transplant, with evidence of RV hypertrophy and/or dilation) were included. Third, the volume loading status of patients at various stages of surgical palliation also provides another confounding factor. A greater number of patients in the SRV+M group, when compared with the SRV group, had already reached Fontan completion, with patients status post Fontan representing a decreased (or more “normal”) RV volume load. Although volume load cannot be discounted, the RV is thought to adapt more effectively to volume overload than to pressure overload, at least in the short-term. When classified by stage of surgical palliation, no significant differences in cAMP, pPLN, or PDE activity were present. Lastly, although our findings appear to support clinical observations, tissue bank-based studies are cross-sectional and cannot establish causality.
As evidenced by preserved PKA-mediated PLN phosphorylation, the molecular adaptation associated with SRV differs significantly from that demonstrated in pediatric heart failure due to DCM. These findings support a pathophysiologically distinct mechanism of heart failure in pediatric SRV patients, which has direct implications regarding the putative response to PDE3i treatment in this population.
Acknowledgments
Supported by the National Institutes of Health (NIH)/Eunice Kennedy Shriver National Institute of Child Health and Human Development (K12 HD068372 to [S.N.]), American Academy of Pediatrics Section on Cardiology and Cardiac Surgery (Research Fellowship Award [to S.N.]), NIH/National Center for Advancing Translational Sciences (UL1 TR000154 [to S.N. and S.M.]), NIH/ National Heart, Lung, and Blood Institute (R01 HL107715 [to B.S.], R01 HL126928 [to S.M.], R21 HL113846 [to S.M.]), Addison Scott Memorial Fund, Boedecker Foundation, and the Millisor Chair in Pediatric Heart Disease. C.S. is a scientific founder and shareholder at miRagen, Inc.
Abbreviations and Acronyms
- cAMP
cyclic adenosine monophosphate
- DCM
dilated cardiomyopathy
- LV
left ventricle
- PDE
phosphodiesterase
- PDE3i
phosphodiesterase 3 inhibition
- PKA
protein kinase A
- pPLN
phosphorylated phospholamban
- RV
right ventricle
- SRV
single ventricle heart disease of right ventricular morphology
Footnotes
The other authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Egbe A, Uppu S, Lee S, Ho D, Srivastava S. Changing Prevalence of Severe Congenital Heart Disease: A Population-Based Study. Pediatric cardiology. 2014 doi: 10.1007/s00246-014-0921-7. [DOI] [PubMed] [Google Scholar]
- 2.Ohye RG, Schonbeck JV, Eghtesady P, Laussen PC, Pizarro C, Shrader P, et al. Cause, timing, and location of death in the Single Ventricle Reconstruction Trial. The Journal of thoracic and cardiovascular surgery. 2012 doi: 10.1016/j.jtcvs.2012.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ohye RG, Sleeper LA, Mahony L, Newburger JW, Pearson GD, Lu M, et al. Comparison of shunt types in the Norwood procedure for single-ventricle lesions. The New England journal of medicine. 2010;362:1980–92. doi: 10.1056/NEJMoa0912461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Driscoll DJ, Offord KP, Feldt RH, Schaff HV, Puga FJ, Danielson GK. Five- to fifteen-year follow-up after Fontan operation. Circulation. 1992;85:469–96. doi: 10.1161/01.cir.85.2.469. [DOI] [PubMed] [Google Scholar]
- 5.Gentles TL, Mayer JE, Jr, Gauvreau K, Newburger JW, Lock JE, Kupferschmid JP, et al. Fontan operation in five hundred consecutive patients: factors influencing early and late outcome. J Thorac Cardiovasc Surg. 1997;114:376–91. doi: 10.1016/s0022-5223(97)70183-1. [DOI] [PubMed] [Google Scholar]
- 6.Menon SC, Keenan HT, Weng HY, Lambert LM, Burch PT, Edwards R, et al. Outcome and resource utilization of infants born with hypoplastic left heart syndrome in the Intermountain West. The American journal of cardiology. 2012;110:720–7. doi: 10.1016/j.amjcard.2012.04.050. [DOI] [PubMed] [Google Scholar]
- 7.Gutgesell HP, Massaro TA. Management of hypoplastic left heart syndrome in a consortium of university hospitals. The American journal of cardiology. 1995;76:809–11. doi: 10.1016/s0002-9149(99)80232-x. [DOI] [PubMed] [Google Scholar]
- 8.Lotto AA, Hosein R, Jones TJ, Barron DJ, Brawn WJ. Outcome of the Norwood procedure in the setting of transposition of the great arteries and functional single left ventricle. Eur J Cardiothorac Surg. 2009;35:149–55. doi: 10.1016/j.ejcts.2008.09.016. discussion 55. [DOI] [PubMed] [Google Scholar]
- 9.Daebritz SH, Nollert GD, Zurakowski D, Khalil PN, Lang P, del Nido PJ, et al. Results of Norwood stage I operation: comparison of hypoplastic left heart syndrome with other malformations. The Journal of thoracic and cardiovascular surgery. 2000;119:358–67. doi: 10.1016/S0022-5223(00)70192-9. [DOI] [PubMed] [Google Scholar]
- 10.Buckberg G, Hoffman JI. Right ventricular architecture responsible for mechanical performance: Unifying role of ventricular septum. The Journal of thoracic and cardiovascular surgery. 2014 doi: 10.1016/j.jtcvs.2014.05.044. [DOI] [PubMed] [Google Scholar]
- 11.Zaffran S, Kelly RG, Meilhac SM, Buckingham ME, Brown NA. Right ventricular myocardium derives from the anterior heart field. Circulation research. 2004;95:261–8. doi: 10.1161/01.RES.0000136815.73623.BE. [DOI] [PubMed] [Google Scholar]
- 12.Hsu DT, Zak V, Mahony L, Sleeper LA, Atz AM, Levine JC, et al. Enalapril in infants with single ventricle: results of a multicenter randomized trial. Circulation. 2010;122:333–40. doi: 10.1161/CIRCULATIONAHA.109.927988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shaddy RE, Boucek MM, Hsu DT, Boucek RJ, Canter CE, Mahony L, et al. Carvedilol for children and adolescents with heart failure: a randomized controlled trial. Jama. 2007;298:1171–9. doi: 10.1001/jama.298.10.1171. [DOI] [PubMed] [Google Scholar]
- 14.Price JF, Towbin JA, Dreyer WJ, Moffett BS, Kertesz NJ, Clunie SK, et al. Outpatient continuous parenteral inotropic therapy as bridge to transplantation in children with advanced heart failure. Journal of cardiac failure. 2006;12:139–43. doi: 10.1016/j.cardfail.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 15.Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 16.Nakano SJ, Miyamoto SD, Movsesian M, Nelson P, Stauffer BL, Sucharov CC. Age-related differences in phosphodiesterase activity and effects of chronic phosphodiesterase inhibition in idiopathic dilated cardiomyopathy. Circulation Heart failure. 2015;8:57–63. doi: 10.1161/CIRCHEARTFAILURE.114.001218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miyamoto SD, Stauffer BL, Polk J, Medway A, Friedrich M, Haubold K, et al. Gene expression and beta-adrenergic signaling are altered in hypoplastic left heart syndrome. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2014;33(8):785–93. doi: 10.1016/j.healun.2014.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pattison JS, Waggoner JR, James J, Martin L, Gulick J, Osinska H, et al. Phospholamban overexpression in transgenic rabbits. Transgenic research. 2008;17:157–70. doi: 10.1007/s11248-007-9139-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ablorh NA, Dong X, James ZM, Xiong Q, Zhang J, Thomas DD, et al. Synthetic phosphopeptides enable quantitation of the content and function of the four phosphorylation states of phospholamban in cardiac muscle. The Journal of biological chemistry. 2014;289:29397–405. doi: 10.1074/jbc.M114.556621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lefkimmiatis K, Zaccolo M. cAMP signaling in subcellular compartments. Pharmacology & therapeutics. 2014;143:295–304. doi: 10.1016/j.pharmthera.2014.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Movsesian MA, Smith CJ, Krall J, Bristow MR, Manganiello VC. Sarcoplasmic reticulum-associated cyclic adenosine 5′-monophosphate phosphodiesterase activity in normal and failing human hearts. The Journal of clinical investigation. 1991;88:15–9. doi: 10.1172/JCI115272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vandeput F, Wolda SL, Krall J, Hambleton R, Uher L, McCaw KN, et al. Cyclic nucleotide phosphodiesterase PDE1C1 in human cardiac myocytes. The Journal of biological chemistry. 2007;282:32749–57. doi: 10.1074/jbc.M703173200. [DOI] [PubMed] [Google Scholar]