Abstract
Objective
Diabetes mellitus is common in the nursing home (NH) population, yet little is known is known about prescribing of glucose-lowering medications in the NH setting. We describe trends in initiation of glucose-lowering medications in a national cohort of NH residents.
Design and Setting
Retrospective cohort study using Part A and D claims for a random 20% of Medicare enrollees linked to NH Minimum Data Set (MDS) and Online Survey, Certification, And Reporting (OSCAR) databases in 7,158 U.S. NHs.
Participants
11,531 long-stay (continuous residence of ≥90 days) NH residents ≥65 years old with diabetes who received a glucose-lowering medication between 2008 and 2010 after four months of non-use.
Measurements
Medicare Part D drug dispensings of glucose-lowering treatments; resident and facility characteristics preceding medication initiation.
Results
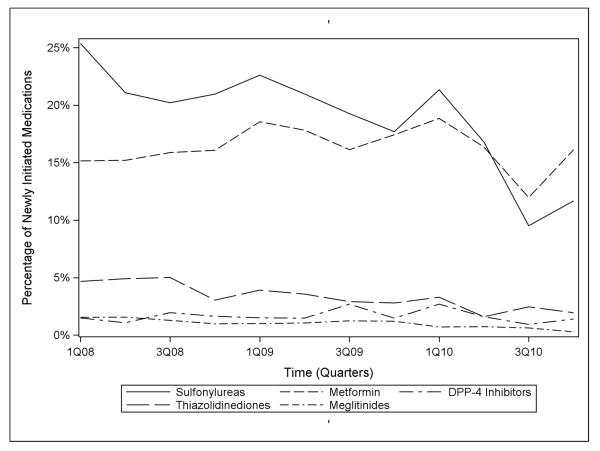
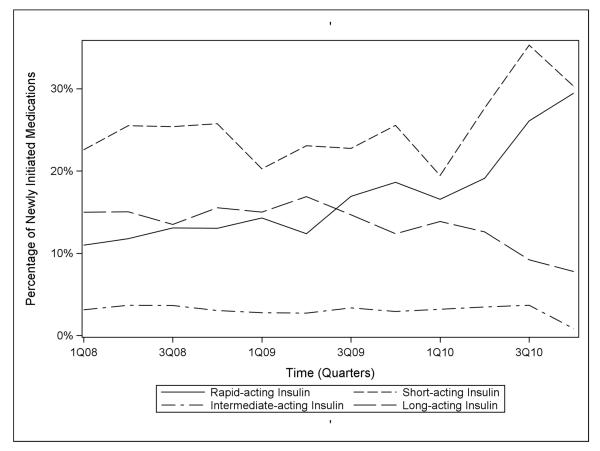
We observed decreasing sulfonylurea initiation from 25.4% of initiations in 2008 to 11.7% in 2010, an average decrease of 1% per quarter (95% CLs: −1.5, −0.5). Thiazolidinedione initiation decreased from 4.7% to 1.9%, an average decrease of 0.3% per quarter (95% CLs: −0.4, −0.2), and meglitinide initiation from 1.5% to 0.3%. No appreciable linear trends were observed for metformin (range 12-18.8%) and dipeptidyl peptidase-4 (DPP-4) inhibitors (range 0.9-2.7%). In contrast, insulin use increased from 51.7% to 68.3% during the same time period, driven by a marked increase in initiation of rapid-acting insulin (11% to 29.4%; average increase of 1.4% per quarter, 95% CLs: 0.9, 1.9) and a modest increase in short-acting insulin (22.6% to 30.3%; an average increase of 0.6% per quarter, 95% CLs: −0.1, 1.3).
Conclusions
Between 2008 and 2010 there were substantial decreases in the use of oral glucose-lowering agents and corresponding increases in the use of insulin among long-term residents of U.S. nursing homes.
Keywords: Nursing home, diabetes, long-term care, pharmacotherapy, treatment choice variation, glucose-lowering, quality of care
Introduction
One and one-half million older United States (U.S) adults live in a nursing home (NH) and 25% of all adults spend the final stages of their lives in these settings.1 Available estimates suggest that 25 to 35% of elderly NH residents have diabetes mellitus.2, 3 Information on how these NH residents are treated pharmacologically is limited despite the necessity for monitoring the quality of prescribing in all health care settings.4-7 Quantifying patterns of treatment initiation is an essential part of assessing quality of pharmaceutical care and informs future efforts to provide guidance for selecting treatments for diabetes.8-10 The objective of this study was to describe national trends in the use of different glucose-lowering medications in the management of diabetes in NH residents from 2008 to 2010.
Methods
Data and Study Population
The Institutional Review Board of Brown University reviewed and approved this study. The study cohort consisted of a random 20% national sample of Medicare Fee-For-Service beneficiaries. We linked data for this sample from Parts A and D claims with the Minimum Data Set (MDS) for the years 2007 to 2010 and obtained information about beneficiaries’ NHs from the Online Survey, Certification, And Reporting (OSCAR) data. The Medicare claims data provided information on patient demographics, Medicare eligibility, hospitalizations, and dispensings of prescription drugs for each patient. Medicare Part D provided information on drug name, dosage, route of administration, quantity dispensed, and days supplied. Part D is ubiquitous in NHs with approximately 81% of residents enrolled in 2006.11 The MDS is a federally-mandated health assessment tool that captures information on cognitive, physical, and psychosocial functioning; active clinical diagnoses and health conditions; and treatments and services. NH staff assess each resident at least annually for all MDS measures, at three-month intervals for many measures, and at any time that a significant change in resident status occurs.12 We used the MDS 2.0, which has been found generally reliable and valid for measuring domains when used by trained staff.13 The OSCAR database consists of facility-level data elements collected at NHs for the purpose of certification for Medicare and Medicaid programs.14 The OSCAR database includes NH facility characteristics, staffing level information, survey deficiencies, and aggregate resident characteristics.
Our study cohort comprised all long-stay NH residents 65 years or older with diabetes who initiated a glucose-lowering treatment between January 1, 2008 and December 31, 2010 (Appendix Figure A1). We defined long-stay residents as those with 90 or more consecutive days in a NH. We defined the date of each resident’s first eligible prescription as the index date. We excluded residents who were prescribed any glucose-lowering medication in the four months preceding the index date (prevalent users of any glucose-lowering medication) and those who were prescribed a combination product (consisting of two or more glucose-lowering agents) on their index date. All patients were required to have maintained continuous Medicare Part D insurance eligibility for the six months preceding the index date. Residents were not required to be in the NH throughout the entirety of the four months preceding the index date, but were required to be in the NH at the time the glucose-lowering medication was initiated. Residents were identified as having diabetes if a diagnosis was present on any MDS assessment, which has good validity for identifying diabetes diagnoses in NHs.15
Glucose-lowering Medications
We began assessing medication initiation in 2008 and used 2007 data to identify previous dispensings of glucose-lowering medication. We considered a medication as newly initiated in a quarter if it was newly dispensed on one of the days included in that quarter. We categorized patients based on the class of medications on which they were initiated in the NH. Drug classes were mutually exclusive and based on mechanism of action. We only considered drugs if they were U.S. Food and Drug Administration (FDA) approved and on the U.S. market during the study period. The classes of non-insulin glucose-lowering medications considered were biguanides (i.e., metformin, the only available biguanide), sulfonylureas, thiazolidinediones, dipeptidyl peptidase-4 inhibitors (DPP-4 inhibitors; i.e., sitagliptin, the only one available in this cohort), meglitinides, alpha-glucosidase inhibitors, glucagon-like peptide-1 receptor agonists (GLP-1 agonists), and amylin mimetics. The types of insulin considered included rapid-acting (i.e., aspart, glulisine, lispro), short-acting (regular human recombinant), intermediate-acting (neutral protamine Hagedorn human recombinant, lente), and long-acting (i.e., glargine, detemir, ultralente). The insulins were then subcategorized by onset of effect as either bolus (rapid- and short-acting) or basal (intermediate- and long-acting). In a secondary analysis, we also assessed initiations of animal insulins, including isophane beef-pork, isophane pork pure, regular beef-pork, zinc beef-pork, and zinc pork purified. We also performed a secondary analysis where we included the previously excluded cohort of combination product initiators and examined them. A full list of the individual noninsulin glucose-lowering medications and combination products can be found in Appendix Table A2.
Resident and Facility Characteristics
To ascertain the presence of clinically-active conditions, comorbidities, and geriatric conditions, we used the MDS 2.0 data up to one year prior to the initiation of a glucose-lowering medication. We calculated the MDS Activities of Daily Living (MDS-ADL) score using seven ADLs (bed mobility, transfer, locomotion, dressing, eating, toilet use, and personal hygiene), each ranging from 0 (total independence) to 4 (total dependence).16 The sum of the seven individual items forms a 28-point scale, where higher scores indicate greater physical impairment.
We examined the following NH characteristics in the OSCAR data that was closest and up to fifteen months prior to each resident’s index date: structure and size (membership in a multi-facility corporation, for-profit status, inclusion in a hospital system, and number of beds), staffing (direct care hours/resident/day provided by nursing staff), quality indicators (percentage of residents physically restrained, percentage of residents on psychoactive medications, number of quality-of-life survey deficiencies), and proportion of residents paid for by Medicaid.
Statistical Analysis
Means with standard deviations, medians with interquartile range, and proportions were used to describe the characteristics of patients and NHs preceding initiation. We plotted the initiation of glucose-lowering medications in each of the 12 quarters of our three-year study period. We assessed linear trends in the use of each class of medications over time using simple linear regression models fit with ordinary least squares. These models provided an estimate (with 95% confidence limits [CLs]) of the difference in the proportion of each class of glucose-lowering medications by quarter during the study period. We checked model assumptions (linearity, normality, homoscedasticity, high-leverage observations) by plotting observed versus predicted values, residuals versus predicted values, and normal quantile plots.17 We completed all analyses using SAS version 9.4 (SAS Institute, Cary, NC).
Results
Characteristics of Study Cohort
There were 11,531 long-term care NH residents who initiated a glucose-lowering medication. The mean age of the cohort was 81.0 years and more than 38% were 85 years of age or older (Table 1). Approximately 40% of the cohort was male. Nearly one-third (30.1%) had a hospitalization in the year prior to initiating a glucose-lowering medication and most residents had multiple comorbidities, including hypertension (77.8%), congestive heart failure (29.0%), and ischemic heart disease (26.1%). More than three-fourths (76.6%) of the cohort required at least extensive assistance with activities of daily living prior to initiating a glucose-lowering medication.
Table 1.
Baseline* Characteristics of Study Long-term Care Residents (N=11,531) and Nursing Homes (N=7,158)
| Resident Characteristics | |
|---|---|
| Male sex—n (%) | 4,598 (39.9) |
| Age at time of medication initiation—mean (SD) years | 81.0 (8.5) |
| ≥65 to <75 | 2,837 (24.6) |
| ≥75 to <85 | 4,279 (37.1) |
| ≥85 to <95 | 3,939 (34.2) |
| ≥95 | 476 (4.1) |
| Race/ethnicity—n (%) | |
| White, non-Hispanic | 8,905 (77.2) |
| Black, non-Hispanic | 1,816 (15.8) |
| Hispanic | 522 (4.5) |
| Other | 288 (2.5) |
| Body mass index, last MDS assessment in prior year—mean (SD) | 27.9 (7.3) |
| Inpatient hospitalization, past year—n (%) | 3,487 (30.1) |
| Comorbidities, last MDS assessment in prior year— n (%) | |
| Hypertension | 8,967 (77.8) |
| Congestive heart failure | 3,338 (29.0) |
| Ischemic heart disease | 3,007 (26.1) |
| Stroke | 2,995 (26) |
| Peripheral vascular disease | 2,073 (18) |
| Dysrhythmia | 2,149 (18.6) |
| Coronary atherosclerosis | 1,912 (16.6) |
| End-stage renal disease | 1,440 (12.5) |
| Deep vein thrombosis | 336 (2.9) |
| Transient ischemic attack | 319 (2.8) |
| Diabetic retinopathy | 236 (2.1) |
| Geriatric Conditions, last MDS assessment in past year —n (%) | |
| MDS-ADL Score Categories—n (%)† | |
| Independent | 969 (8.4) |
| Supervision required to limited impairment | 1,730 (15.0) |
| Extensive assistance required | 4,429 (38.5) |
| Dependent to totally dependent | 4,403 (38.1) |
| Bladder incontinence | 8,544 (74.1) |
| Dementia | 4,844 (42) |
| Fell, past 180 days | 3,646 (31.6) |
| Weight loss, ≥5% in prior 30 days or ≥10% in prior 180 days—n (%) |
1,009 (8.8) |
| Antipsychotic medication use, last MDS assessment | 2,575 (22.3) |
|
| |
| Nursing Home Characteristics‡ | |
|
| |
| Multi-facility corporation member—n (%) | 3,935 (55.3) |
| For profit—n (%) | 5,201 (72.7) |
| Hospital-based—n (%) | 234 (3.3) |
| Number of beds—n (%) | |
| <100 | 2,349 (32.8) |
| 100-200 | 4,058 (56.7) |
| >200 | 751 (10.5) |
| Direct care hours/day/resident, mean (SD) § | 3.5 (0.9) |
| Quality measures | |
| Percent residents physically restrained, median (IQR) | 1.8 (0-4.7) |
| Percent residents on psychoactive medications, mean (SD) ∥ | 65.8 (13.5) |
| ≥1 quality-of-life survey deficiency | 3,057 (42.7) |
| More than 50% of residents on Medicaid—n (%) | 5,928 (82.8) |
Abbreviations: SD, standard deviation; MDS, Minimum Data Set; IQR, interquartile range.
Prior to initiation of a diabetes medication
Minimum Data Set Activities of Daily Living Self-Performance Hierarchy Scale
Measured at last Online Survey Certification and Reporting assessment in prior 15 months
Includes registered nurse, licensed practical nurse, and certified nursing assistant hours
Includes antipsychotic, antidepressant, antianxiety, and hypnotic medications
The individuals in our study cohort resided in 7,158 NHs during the study period. The majority of these facilities were part of a multi-facility corporation (55.3%), for profit (72.7%), or had at least 100 beds (67.2%). On average, the facilities provided 3.5 hours of direct nursing care daily to each resident. Facilities had a median of 1.8% of residents physically restrained, an average of 65.8% of residents on psychoactive medications, and 42.7% had at least one quality-of-life survey deficiency. A majority of the NH facilities (82.8%) had more than 50% of their residents on Medicaid. We document the number (i.e., count) of individuals initiated on a glucose-lowering medication in each quarter in Appendix Table A3.
Noninsulin Glucose-lowering Medication Use
Figure 1 depicts the use of noninsulin glucose-lowering medications by class over the study period in all U.S. NH residents. In the first quarter of 2008, 25.4% of residents with diabetes initiated sulfonylureas, 15.2% metformin, 4.7% thiazolidinediones, 1.5% DPP-4 inhibitors, and 1.5% meglitinides. Less than 0.1% of residents initiated an alpha-glucosidase inhibitor or GLP-1 agonist and no residents initiated an amylin mimetic. Between the first quarter of 2008 and the last quarter of 2010, the percentage of patients initiating sulfonylureas decreased from 25.4% to 11.7%, an average decrease of 1.0% per quarter (−1.0%, 95% CLs: −1.5, −0.5). Over the same period, the percentages of initiations of metformin and DPP-4 inhibitors did not show linear trends (0.0%, 95% CLs: −0.0, 0.0 for both), but the percentage of initiations of thiazolidinediones decreased from 4.7% to 1.9%, an average decrease of 0.3% per quarter (−0.3%, 95% CLs: −0.4, −0.2). The percentage of initiations of meglitinides declined from 1.5% to 0.3%, an average decrease of 0.1% in new use per quarter (−0.1%, 95% CLs: −0.1, −0.1).
Figure 1.
National trends in the use of non-insulin glucose-lowering medication classes in the nursing home, 2008-2010.
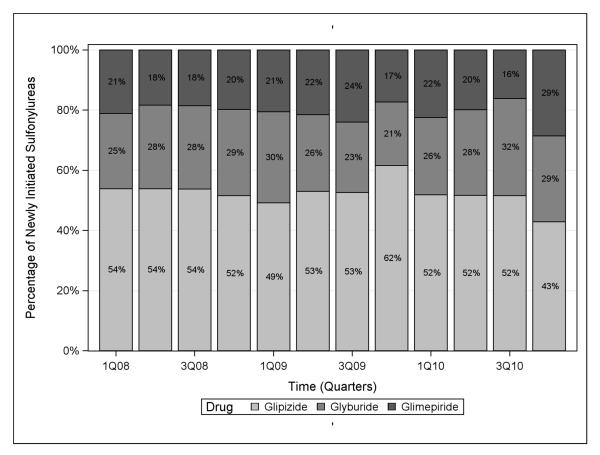
Sulfonylureas
Second-generation sulfonylureas (glimepiride, glipizide, and glyburide) were all frequently-dispensed through the study period (Figure 2), though their use declined over the study period. Between the first quarter of 2008 and the last quarter of 2010, the percentage of patients initiating glyburide decreased from 6.3% to 3.3%, an average decrease in use of 0.3% per quarter (−0.3%, 95% CLs: −0.4, −0.1). The percentage of initiations of glipizide declined from 13.6% to 5.0%, an average decrease of 0.6% per quarter (−0.6%, 95% CLs: −0.9, −0.3), and initiations of glimepiride declined from 5.3% to 3.3%, an average decrease of 0.2% per quarter (−0.2%, 95% CLs: −0.3, −0.0). Glyburide accounted for 54% of all sulfonylurea initiations in the first quarter of 2008, in contrast to 43% in the last quarter of 2010.
Figure 2.
Choice of sulfonylurea prescribed among sulfonylurea initiators over time in the nursing home, 2008-2010.
Insulin
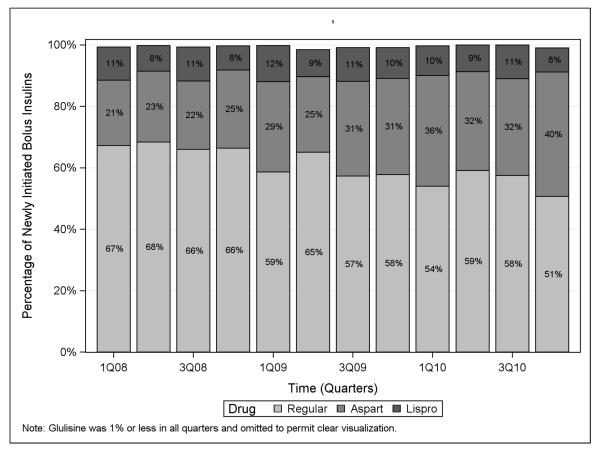
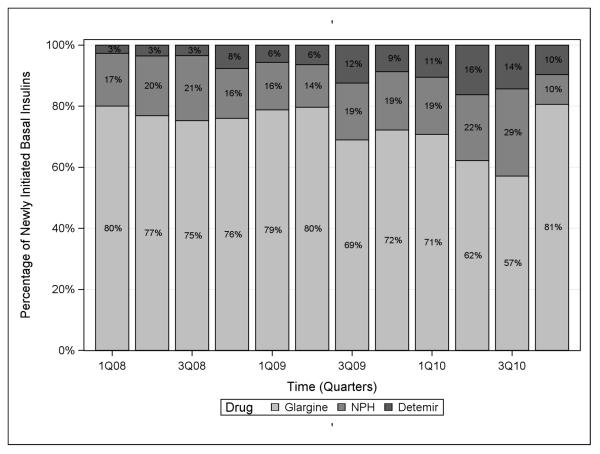
Overall, percentage use of any type of insulin in the NH increased from 51.7% in the first quarter of 2008 to 68.3% in the last quarter of 2010, an average increase of 1.4% per quarter (1.4%, 95% CLs: 0.5, 2.3). Between the first quarter of 2008 and the last quarter of 2010, the percentage of patients initiating bolus insulin increased from 33.5% to 59.7% (Figure 3), an average increase of 2.0% per quarter (2.0%, 95% CLs: 0.9, 3.2). Regular insulin remained the most commonly initiated bolus insulin throughout the study period, though insulin aspart was dispensed more frequently over time (Figure 4). During the same period, rapid-acting insulin use increased from 11% to 29.4%, an average increase of 1.4% per quarter (1.4%, 95% CLs: 0.9, 1.9), and short-acting insulin use increased from 22.6% to 30.3%, an average increase of 0.6% per quarter (0.6%, 95% CLs: −0.1, 1.3). In contrast, use of basal insulin decreased over the study period from 18.1% in 2008 to 8.6% in 2010, an average decrease of 0.6% per quarter (−0.6%, 95% CLs: −1.0, −0.2). Intermediate-acting insulin use accounted for 3.1% of new glucose-lowering medication initiations in the first quarter of 2008, but had declined to a nadir of 0.8% by the last quarter of 2010. Over the same time period, long-acting insulin use declined from 15% to a low of 7.8%, an average decrease of 0.6% per quarter (−0.6%, 95% CLs: −0.9, −0.2). As shown in Figure 5, insulin glargine was the basal insulin most commonly prescribed throughout the study period. No residents initiated lente or ultralente human insulins or animal insulins during the study period.
Figure 3.
National trends in the use of different types of insulin in the nursing home, 2008-2010.
Figure 4.
Choice of bolus insulin prescribed among bolus insulin initiators over time in the nursing home, 2008-2010.
Figure 5.
Choice of basal insulin prescribed among basal insulin initiators over time in the nursing home, 2008-2010.
Combination Products
Of all glucose-lowering medications initiated in the NH throughout the study period, 4.3% were for combination (premixed) insulin products. Use of combination insulins decreased an average of 0.3% per quarter (−0.3%, 95% CLs: −0.4, −0.2) from 6.4% at the beginning of 2008 to 2.5% at the end of 2010. The most commonly prescribed insulin combination product was insulin regular-insulin neutral protamine Hagedorn, which accounted for 73.5% of all combination insulin use in the NH. Combination oral glucose-lowering products accounted for 0.8% of all glucose-lowering medications initiated. On average, percentage use of these oral combination products decreased 0.1% per quarter (−0.1%, 95% CLs: −0.1, −0.0) from 1.4% in 2008 to 0.6% in 2010. The most commonly prescribed oral combination products were glyburide-metformin (52.8%; introduced to U.S. market in 2000), metformin-sitagliptin (16.5%; introduced in 2007), and metformin-pioglitazone (15.4%; introduced in 2005).
Discussion
Our study, which employed a nationally-representative sample of all U.S. NHs, indicates the changing landscape of diabetes drug therapy in the NH setting. Dramatic changes in the prescribing of insulin were observed, with rapid-acting insulin driving much of that new uptake. Although second-generation sulfonylureas were once the mainstay of pharmacologic diabetes therapy, the market share of all sulfonylureas declined over time. A similar pattern was observed for thiazolidinediones. This trend followed the widely-publicized 2007 U.S. Food and Drug Administration Boxed Warning that cautioned against cardiovascular adverse events including myocardial infarction with the thiazolidinedione rosiglitazone, plus concerns about increased heart failure risk with both rosiglitazone and pioglitazone.18-22 Similar shifts in prescribing for other classes of medications have been observed when the FDA issued boxed warnings that were widely disseminated among physicians.23-25 We found little use or increase in the market share of newer glucose-lowering medications DPP-4 inhibitors (introduced in 2006), GLP-1 agonists (exenatide, introduced in 2005, was the only one used despite availability of liraglutide starting in January 2010), and amylin mimetics (introduced in 2005). This low uptake is perhaps due to limited prescriber experience with the drugs, cost, route of administration, Medicare Part D plan formulary inclusion and status, lack of studies in older patients, and concern about undesirable side effects in a frail population.4, 7
Previously published literature on trends in glucose lowering medications only focused on young, ambulatory patients or among NH residents, those residing in the Veterans Affairs (VA) NHs.6 Our study adds to the literature by focusing on a nationally representative sample of nursing home residents, on the new initiation of prescribing of glucose-lowering medications, and on trends in individual and combination medication products. In addition, we examine the use of insulins by type and individual product.
Lee and colleagues focused on 123 VA NHs using administrative data linked to laboratory, pharmacy, and MDS data.6 As in our study, they found stable use of metformin, which is often considered first-line therapy for diabetes in older adults due to its low risk for hypoglycemia and low cost, as well as decreasing use of the sulfonylurea glyburide and thiazolidinedione medications from 2005 to 2011.6, 7 In contrast, we found that decreases in sulfonylurea initiations were driven by all medications in the class, not only glyburide. At the end of our study period, the decrease in glyburide use as a proportion of all sulfonylurea initiations suggested a shift in practice toward use of sulfonylureas with a more favorable adverse effect profile. Evidence of hypoglycemia risk associated with glyburide began mounting in 1996 and the class-wide decrease in use of sulfonylureas likely is due to an increasing general concern about the risk of hypoglycemia in geriatric individuals with all members of this drug class. 26-31 Given the high prevalence of diabetes in the NH (25 to 35% of all residents), using the percentage of NH residents with diabetes receiving glyburide or sulfonylureas in general is of interest as a potential process quality measure to assess both prescriber and facility quality of care.10 A medication-related measure would complement process measures from other domains of care, such as appropriate diagnosis of diabetes, diet orders, and monitoring via hemoglobin A1c (HbA1c).32 Future studies should aim to document the use of glyburide in the wake of the 2012 and 2015 Beers List recommendations to avoid use of glyburide and its absence from the American Medical Directors Association guidelines.2, 28, 29, 32
We found a large increase in the new use of insulin therapy among NH residents. Viewed in aggregate, it appears that prescribers progressively favored bolus insulin therapy and ceased initiating patients on sulfonylureas, thiazolidinediones, and basal insulin therapy during the study period. In particular, prescribers started more patients on rapid-acting insulins. The use of rapid-acting insulin over regular insulin may have been due in part to the dissemination of evidence that suggested rapid-acting insulins were associated with a modest decrease in hypoglycemia compared to regular insulin.33, 34 Guidelines recommend mealtime bolus insulin to control post-prandial hyperglycemia, especially as an addition when basal insulin with or without oral agents is no longer adequate to achieve glycemic targets, but basal insulin is preferred to maintain constant insulin levels and suppress hepatic glucose production between meals. 4, 32, 35 Increased use of insulin may therefore partially reflect efforts to achieve stricter blood glucose control in NH residents during the study period. It will be of interest in future studies to determine whether this pattern changes with the adoption of current clinical guidelines recommending less stringent glycemic targets in NH residents, especially for those who are frail or with limited life expectancy.7, 9, 36 Use of combination products containing two glucose-lowering medications in our NH sample was similar to that documented in studies of younger individuals in the community.37
Our study has several limitations. First, we lacked laboratory data such as HbA1c levels, which would have allowed us to confirm the presence of diabetes in our study population. However, prior studies confirm the validity of Medicare claims and the MDS for identifying diabetes.15 Second, our study covers only a three-year period. While a longer duration may have been preferable, three years of calendar time is nonetheless sufficient for describing trends. Third, we were unable to differentiate those with type 1 from those with type 2 diabetes to determine whether trends differed by diabetes type. Approximately 4% of all diabetes in the U.S. is type 1, and the relative prevalence of type 2 diabetes increases with advancing age. 38, 39 Thus, the vast majority of individuals in our study undoubtedly have type 2 diabetes.38, 39 Our study also has several strengths. First, our dataset is a large, nationally-representative sample that allows us to describe trends for, essentially, the entire long-term care U.S. NH population across thousands of NH facilities. In particular, we included female NH residents, who comprise 70% of the national long-term care NH population and are virtually excluded from the VA studies. Second, we studied incident rather than prevalent use of glucose-lowering medications, allowing us to isolate the prescribing decision in the NH population and to capture trends in initiation rather than just continuation of treatment. Third, we had patient-specific (rather than unit- or ward-stock) basal and bolus insulin use measured in our data, allowing us to forgo the imputation procedures required in other data. Fourth, we describe trends in individual glucose-lowering medications, including those that are not included on the VA formulary (e.g., glimepiride). Lastly, we assessed use of combination glucose-lowering medication products, which have not been previously described for the NH population.
In summary, we documented the incident use of glucose-lowering medications in all U.S. NHs between 2008 and 2010. There were shifts in glucose-lowering treatment use for diabetes in the NH over this relatively short time period. This work will be useful for informing future cost-effectiveness analyses and identifying prescribing-related quality of care measures, such as the proportion of residents with diabetes prescribed glyburide. Our study may also inform the prioritization of new comparative pharmaceutical effectiveness research questions to identify the role of newer glucose-lowering therapies in the treatment of diabetes in NH residents.
Supplementary Material
Figure A1—Flow diagram of study cohort creation.
Acknowledgments
The authors would like to thank Vincent Mor, PhD for his comments on an earlier version of this manuscript. This work was funded by an Agency for Healthcare Research and Quality (AHRQ) award (5K12HS022998-02).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Centers for Medicare and Medicaid Services [Accessed May 25, 2015];Nursing Home Compendium. 2012 Available at: http://www.aanac.org/docs/reference-documents/nursinghomedatacompendium_508.pdf%7B?%7Dsfvrsn=2.
- 2.Resnick HE, Heineman J, Stone R, et al. Diabetes in U.S. nursing homes, 2004. Diabetes care. 2008;31:287–288. doi: 10.2337/dc07-1425. [DOI] [PubMed] [Google Scholar]
- 3.Zarowitz B, Allen C, O'Shea T, et al. Type 2 diabetes mellitus treatment patterns in U.S. nursing home residents. Postgraduate medicine. 2015;127:429–437. doi: 10.1080/00325481.2015.1035621. [DOI] [PubMed] [Google Scholar]
- 4.Zarowitz BJ, Tangalos EG, Hollenack K, et al. The application of evidence-based principles of care in older persons (issue 3): management of diabetes mellitus. Journal of the American Medical Directors Association. 2006;7:234–240. doi: 10.1016/j.jamda.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 5.Feldman SM, Rosen R, DeStasio J. Status of diabetes management in the nursing home setting in 2008: a retrospective chart review and epidemiology study of diabetic nursing home residents and nursing home initiatives in diabetes management. Journal of the American Medical Directors Association. 2009;10:354–360. doi: 10.1016/j.jamda.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 6.Lee SJ, Stijacic-Cenzer I, Barnhart C, et al. Changing Patterns of Glucose-Lowering Medication Use in VA Nursing Home Residents With Diabetes, 2005 to 2011. Journal of the American Medical Directors Association. 2015;16:898 e899–898 e814. doi: 10.1016/j.jamda.2015.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kirkman MS, Briscoe VJ, Clark N, et al. Diabetes in older adults: a consensus report. Journal of the American Geriatrics Society. 2012;60:2342–2356. doi: 10.1111/jgs.12035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.American Diabetes Association Standards of medical care in diabetes--2015: summary of revisions. Diabetes care. 2015;38(Suppl):S4. doi: 10.2337/dc15-S003. [DOI] [PubMed] [Google Scholar]
- 9.American Geriatrics Society Expert Panel on Care of Older Adults with Diabetes M. Moreno G, Mangione CM, et al. Guidelines abstracted from the American Geriatrics Society Guidelines for Improving the Care of Older Adults with Diabetes Mellitus: 2013 update. Journal of the American Geriatrics Society. 2013;61:2020–2026. doi: 10.1111/jgs.12514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O'Connor PJ, Bodkin NL, Fradkin J, et al. Diabetes performance measures: current status and future directions. Diabetes care. 2011;34:1651–1659. doi: 10.2337/dc11-0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Briesacher BA, Soumerai SB, Field TS, et al. Nursing home residents and enrollment in Medicare Part D. Journal of the American Geriatrics Society. 2009;57:1902–1907. doi: 10.1111/j.1532-5415.2009.02454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Centers for Medicare and Medicaid Services [Accessed May 27, 2015];Long-Term Care Facility Resident Assessment Instrument 2.0 User's Manual. 2009 Available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/NursingHomeQualityInits/NHQIMDS20.html.
- 13.Mor V. A comprehensive clinical assessment tool to inform policy and practice: applications of the minimum data set. Medical care. 2004;42:III50–59. doi: 10.1097/01.mlr.0000120104.01232.5e. [DOI] [PubMed] [Google Scholar]
- 14.Kash BA, Hawes C, Phillips CD. Comparing staffing levels in the Online Survey Certification and Reporting (OSCAR) system with the Medicaid Cost Report data: are differences systematic? The Gerontologist. 2007;47:480–489. doi: 10.1093/geront/47.4.480. [DOI] [PubMed] [Google Scholar]
- 15.Mor V, Intrator O, Unruh MA, et al. Temporal and Geographic variation in the validity and internal consistency of the Nursing Home Resident Assessment Minimum Data Set 2.0. BMC health services research. 2011;11:78. doi: 10.1186/1472-6963-11-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morris JN, Fries BE, Morris SA. Scaling ADLs within the MDS. The journals of gerontology Series A, Biological sciences and medical sciences. 1999;54:M546–553. doi: 10.1093/gerona/54.11.m546. [DOI] [PubMed] [Google Scholar]
- 17.Vittinghoff E. Regression methods in biostatistics : linear, logistic, survival, and repeated measures models. Springer; New York: 2012. [Google Scholar]
- 18.Woodcock J, Sharfstein JM, Hamburg M. Regulatory action on rosiglitazone by the U.S. Food and Drug Administration. The New England journal of medicine. 2010;363:1489–1491. doi: 10.1056/NEJMp1010788. [DOI] [PubMed] [Google Scholar]
- 19.Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. The New England journal of medicine. 2007;356:2457–2471. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- 20.Tanne JH. FDA places “black box” warning on antidiabetes drugs. Bmj. 2007;334:1237. doi: 10.1136/bmj.39244.394456.DB. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nesto RW, Bell D, Bonow RO, et al. Thiazolidinedione use, fluid retention, and congestive heart failure: a consensus statement from the American Heart Association and American Diabetes Association. Diabetes care. 2004;27:256–263. doi: 10.2337/diacare.27.1.256. [DOI] [PubMed] [Google Scholar]
- 22.Graham DJ, Ouellet-Hellstrom R, MaCurdy TE, et al. Risk of acute myocardial infarction, stroke, heart failure, and death in elderly Medicare patients treated with rosiglitazone or pioglitazone. JAMA : the journal of the American Medical Association. 2010;304:411–418. doi: 10.1001/jama.2010.920. [DOI] [PubMed] [Google Scholar]
- 23.Bushnell GA, Sturmer T, Swanson SA, et al. Dosing of Selective Serotonin Reuptake Inhibitors Among Children and Adults Before and After the FDA Black-Box Warning. Psychiatric services. 2015 doi: 10.1176/appi.ps.201500088. appips201500088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ehrenpreis ED, Deepak P, Sifuentes H, et al. The metoclopramide black box warning for tardive dyskinesia: effect on clinical practice, adverse event reporting, and prescription drug lawsuits. The American journal of gastroenterology. 2013;108:866–872. doi: 10.1038/ajg.2012.300. [DOI] [PubMed] [Google Scholar]
- 25.Kales HC, Zivin K, Kim HM, et al. Trends in antipsychotic use in dementia 1999-2007. Archives of general psychiatry. 2011;68:190–197. doi: 10.1001/archgenpsychiatry.2010.200. [DOI] [PubMed] [Google Scholar]
- 26.Shorr RI, Ray WA, Daugherty JR, et al. Individual sulfonylureas and serious hypoglycemia in older people. Journal of the American Geriatrics Society. 1996;44:751–755. doi: 10.1111/j.1532-5415.1996.tb03729.x. [DOI] [PubMed] [Google Scholar]
- 27.van Staa T, Abenhaim L, Monette J. Rates of hypoglycemia in users of sulfonylureas. Journal of clinical epidemiology. 1997;50:735–741. doi: 10.1016/s0895-4356(97)00024-3. [DOI] [PubMed] [Google Scholar]
- 28.American Geriatrics Society Beers Criteria Update Expert P American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. Journal of the American Geriatrics Society. 2012;60:616–631. doi: 10.1111/j.1532-5415.2012.03923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.By the American Geriatrics Society Beers Criteria Update Expert P American Geriatrics Society 2015 Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. Journal of the American Geriatrics Society. 2015 doi: 10.1111/jgs.13702. [DOI] [PubMed] [Google Scholar]
- 30.Nathan DM, Buse JB, Davidson MB, et al. Management of hyperglycemia in type 2 diabetes: A consensus algorithm for the initiation and adjustment of therapy: a consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes care. 2006;29:1963–1972. doi: 10.2337/dc06-9912. [DOI] [PubMed] [Google Scholar]
- 31.Aspinall SL, Zhao X, Good CB, et al. Intervention to decrease glyburide use in elderly patients with renal insufficiency. The American journal of geriatric pharmacotherapy. 2011;9:58–68. doi: 10.1016/j.amjopharm.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 32.American Medical Directors Association . Diabetes Management in the Post-Acute and Long-Term Care Setting. AMDA; Columbia, MD: 2015. [Google Scholar]
- 33.Anderson JH, Jr., Brunelle RL, Keohane P, et al. Mealtime treatment with insulin analog improves postprandial hyperglycemia and hypoglycemia in patients with non-insulin-dependent diabetes mellitus. Multicenter Insulin Lispro Study Group. Archives of internal medicine. 1997;157:1249–1255. [PubMed] [Google Scholar]
- 34.Singh SR, Ahmad F, Lal A, et al. Efficacy and safety of insulin analogues for the management of diabetes mellitus: a meta-analysis. CMAJ : Canadian Medical Association journal = journal de l'Association medicale canadienne. 2009;180:385–397. doi: 10.1503/cmaj.081041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramlo-Halsted BA, Edelman SV. The natural history of type 2 diabetes. Implications for clinical practice. Prim Care. 1999;26:771–789. doi: 10.1016/s0095-4543(05)70130-5. [DOI] [PubMed] [Google Scholar]
- 36.Munshi MN, Florez H, Huang ES, et al. Management of Diabetes in Long-term Care and Skilled Nursing Facilities: A Position Statement of the American Diabetes Association. Diabetes care. 2016;39:308–318. doi: 10.2337/dc15-2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alexander GC, Sehgal NL, Moloney RM, et al. National trends in treatment of type 2 diabetes mellitus, 1994-2007. Archives of internal medicine. 2008;168:2088–2094. doi: 10.1001/archinte.168.19.2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.American Diabetes Association [Accessed December 4, 2015];Statistics About Diabetes. 2015 Available at: http://www.diabetes.org/diabetes-basics/statistics/?referrer=https://www.google.com/
- 39.Menke A, Orchard TJ, Imperatore G, et al. The prevalence of type 1 diabetes in the United States. Epidemiology (Cambridge, Mass) 2013;24:773–774. doi: 10.1097/EDE.0b013e31829ef01a. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure A1—Flow diagram of study cohort creation.