Abstract
While many phase I trials report tumor response, formal analysis of efficacy is deferred to phase II. We reviewed paired phase I and II pediatric oncology trials to ascertain the relationship between phase I and II objective response (OR%). Single-agent phase I trials were paired with corresponding phase II trials (comparable study drug, dosing schedule, and population). Phase I trials without efficacy data or a matching phase II trial were excluded. OR% was tabulated for all trials, and phase II authors' subjective conclusions regarding efficacy were documented. 35 pairs of trials were analyzed. The correlation between phase I and II OR% was 0.93. Between phase II studies with a “positive” conclusion versus a “negative” one, there was a statistically significant difference in mean phase I OR% (32.0% vs. 4.5%, p < 0.001). Thirteen phase II studies were undertaken despite phase I OR% of 0%; only one had a “positive” conclusion, and none exceeded OR% of 15%. Objective response rates are highly correlated between phase I and II pediatric oncology trials. Though not a formal measure of drug efficacy, phase I OR% may provide an estimate of phase II response, inform phase II study design, and should be given greater consideration.
Keywords: Pediatric oncology, phase I, phase II, Drug development
Introduction
Phase I clinical trials are a critical step in the evaluation of novel anti-cancer agents. They typically enroll small cohorts of patients with refractory cancers, the primary goals being to characterize the frequency and severity of toxicities associated with the drug, and to identify a maximally tolerated dose (MTD) or biologically relevant dose for use in further studies. Tumor response in the context of a phase I trial, if reported, is appropriately relegated to a secondary/exploratory objective. This is due to factors such as low sample size, a wide range of doses tested, heterogeneity of tumors treated, and prior chemotherapeutic history of study participants. The first formal analysis of the novel drug's clinical activity against specific cancers is usually reserved for subsequent phase II trials, which enroll a larger and more homogenous study population. Because of this stepwise process, it is not uncommon for drugs that demonstrate minimal clinical activity in phase I to nevertheless proceed to further evaluation in a phase II study.
Clinical drug trials thus raise important ethical objectives for the field of oncology – namely, to efficiently and accurately evaluate a multitude of new anti-cancer drugs and reap their maximum potential benefit for the study population and for society at large, but also minimize the drug-related toxicities and study-inherent risks/costs borne by trial participants. These considerations become all the more salient in the study of rare conditions, as the scarcity of eligible patients demands that new therapeutic options are evaluated as efficiently as possible.
This is highly relevant to pediatric cancers, of which there were approximately 15,780 new cases in the United States in 2014, compared to the 1.6 million projected new cases of adult cancer in 2015.[1,2] Because the same anti-cancer agents can cause different toxicities and clinical effects in pediatric patients, pediatric clinical trials are almost always conducted separately from adult trials. As a result, efficiency is even more critical for pediatric phase I and phase II trials; safe, efficacious drugs must be identified through studying a very limited number of children. Previous reviews of pediatric oncology trials have identified useful approaches to this dilemma, such as by highlighting the importance of multi-institutional collaboration in the study of rare pediatric conditions, and by presenting evidence that a narrower range of doses is needed in pediatric phase I evaluation.[3,4]
However, prior studies have not examined the possibility that clinical responses reported in pediatric phase I trials may provide predictive information regarding the drug's clinical efficacy. Though formal conclusions about efficacy are precluded by phase I study design, it remains to be seen whether anti-tumor responses in phase I are at all correlated with (or predictive of) clinical outcomes in phase II. Such a relationship could better inform phase II evaluation of certain drugs and potentially increase the overall efficiency of pediatric clinical trials, accelerating the identification of clinically active agents.
We reviewed the published literature of pediatric oncology trials from 1990-2012, identifying matched pairs of single-agent, phase I and phase II clinical trials. Our primary goal was to explore the relationship, if any, between the response rates observed in phase I trials of those agents, and the response rates observed in the corresponding phase II studies.
Materials and Methods
Literature review
Phase I pediatric clinical oncology trials published from 1990 to 2012 were identified by National Library of Medicine Gateway searches using the key words “pediatric”, “phase I/1”, and “cancer”. This was supplemented by a search in the NIH Clinical Trials database (clinicaltrials.gov), in the references of select articles reviewing pediatric clinical oncology trials, and in the Children's Oncology Group's listing of published clinical trials. Search results were screened; duplicates, adult trials, and non-cancer trials were filtered. Combination chemotherapy or chemo-radiotherapy trials were not included in the analysis to guard against the possibility that response might reflect the activity of known active agents or the use of radiation.
Single-agent trials were paired with their phase II counterparts using references from the phase II article (to locate a matching phase I study) or NLM/Google Scholar citations for the phase I article (to locate a matching phase II study). We defined matching pairs as phase I and II trials with identical drug regimen and dosing schedule, as well as matching eligible age range and tumor types under study (solid, hematologic, and/or primary CNS tumors). Combined phase I/II trials were excluded unless they enrolled patients in separate phase I and phase II cohorts, and tumor response data was reported from both cohorts. Studies enrolling both pediatric and adult patients were only included if the subset of pediatric data could be extracted.
Phase I trials that could not be matched to a corresponding study (i.e. a phase II study was never conducted, or was conducted with a combinatorial regimen or different dosing schedule) were excluded from further analysis.
Clinical responses
For each phase I and phase II study, we tabulated the number of children enrolled, the number evaluated for response, and the objective response rate (OR%). For solid tumors, we defined OR% as the proportion of evaluated children who achieved complete or partial response (CR + PR). For hematologic malignancies, we utilized each study's criteria for complete and partial response, which generally included M1 and M2 marrow respectively (most studies had additional criteria such as recovery of peripheral counts, no circulating blasts, and/or no clinical signs of disease). Cases of stable disease and minor responses that did not meet the criteria for PR were not counted as objective responses. In cases where the phase II trial assessed one subtype of tumor (and the phase I trial enrolled a population with more heterogeneous tumor types), tumor-specific phase I response data was extracted when possible.
Finally, because a drug's clinical promise may not always be fully described by its objective response rate, we also evaluated authors' subjective conclusions about phase II efficacy. For each phase II study, the study conclusion was documented and categorized as positive or negative, as illustrated by the example statements below: Positive: “May be efficacious”, “Showed significant response with [a specific tumor type]”, “Further studies are warranted”. Negative: “No/limited objective response”, “No effect”, “Did not meet pre-determined criteria for clinical activity”, “Further study not warranted”. We also documented whether trials were cooperative group-sponsored, industry-sponsored, or investigator-initiated.
Results
Literature review
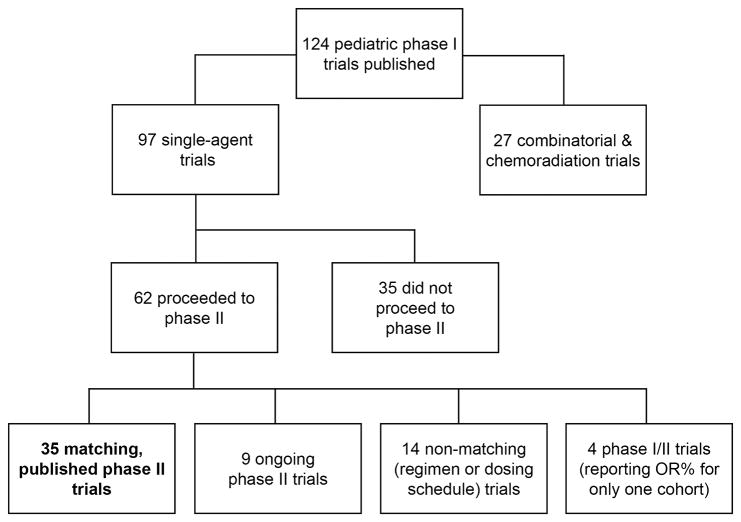
The search process (Figure 1) yielded a total of 124 pediatric phase I trials published between January 1, 1990 and December 31, 2012. These included 97 single-agent trials and 27 combinatorial or chemo-radiation trials. Of the 97 single-agent trials, 35 had published phase II studies that met matching criteria and were included in the final analysis. Of these 35 phase I trials, 2 were industry-sponsored, 19 were cooperative group-sponsored, and 14 were investigator-initiated.
Figure 1.
Phase I Trials Published in Pediatric Oncology, 1990-2012. This flowchart illustrates the results of our literature search, which found 124 published phase I trials from 1990 to 2012, 97 of which were single-agent trials. 35 of these trials (bolded) had published phase II trials that met matching criteria and were ultimately included in analysis. The other trials were excluded from analysis for the reasons listed above. OR% = objective response rate.
Of the remaining 62 trials, 35 did not proceed to phase II (due to toxicity and/or lack of response), 14 proceeded to phase II trials utilizing a combination regimen or a different dosing schedule, 4 were phase I/II studies that only reported OR% for a single cohort, and 9 have phase II trials ongoing but as of yet unpublished. Of the 35 trials that did not proceed to phase II, 3 were industry-sponsored, 23 were cooperative group-sponsored, and 9 were investigator-initiated. 34 of these 35 trials reported response data: the overall response rate in these 34 trials was 2.9% (25 of 848 evaluable children), and 24 of 34 trials had no objective responses.
In total, 35 matched pairs of single-agent phase I and phase II trials were included in analysis. Each pair of studies, the agent and dosing schedule under study, phase I OR%, and phase II OR% are listed in Table 1. For most study pairs, both phase I and phase II enrolled patients with mixed solid tumors or hematologic malignancies. However, some phase II studies enrolled patients with only a specific tumor type. For these pairs, tumor-specific OR% was extracted from phase I, as described in Table 1. Significant discrepancies between the distribution of tumor types in phase I and phase II are also noted in the legend. Otherwise, OR% is reported “as-published” in each trial.
Table 1. Drugs Evaluated in Phase I and Phase II Pairs.
| Drug(s) | Schedule | Phase II Dose | Phase I OR% | Phase II OR% | Refs |
|---|---|---|---|---|---|
| Solid tumor | |||||
| ABT-751 | PO qd × 7d q 21d | 200 mg/m2 | 0 | 7 | [5,6] |
| Cilengitide | IV 1hr twice/week | 1800 mg/m2 | 3 | 4 | [7,8] |
| Cixutumumab* | IV 1hr/wk q 28d | 6-9 mg/kg | 0a | 9a | [9] |
| Docetaxel | IV 1hr q 21d | 125 mg/m2 | 5 | 5 | [10,11] |
| Etoposide | PO qd × 21d q 28d | 50 mg/m2/d | 29 | 18 | [12,13] |
| Fenretinide* | PO tid × 7d q 21d | 2475 mg/m2/d | 2 | 2 | [14,15] |
| Gemcitabine | IV 30 min/wk × 3wk | 1200 mg/m2 | 3 | 0 | [16,17] |
| hu14.18-IL2* | IV 4hr qd × 3d q 28d | 12 mg/m2/d | 0b | 14b | [18,19] |
| Ifosfamide | IV 1hr qod × 3 | 3 g/m2 | 15 | 7 | [20,21] |
| Indicine-N-oxide | IV 15min q 28d | 2000 mg/m2/d | 0c | 0c | [22,23] |
| Interleukin-2* | 24hr CI 4d/wk × 3wk | 3 × 106 U/m2/d | 0 | 3 | [24,25] |
| Irinotecan | IV 1hr qd × 5d q 21d | 50 mg/m2/d | 7 | 5 | [26,27] |
| Irinotecan | IV 1hr qd × 5d/wk × 2wk | 20 mg/m2/d | 22 | 23 | [28,29] |
| Ixabepilone | IV 1hr qd × 5d q 21d | 8 mg/m2/d | 0 | 0 | [30,31] |
| Oxaliplatin | IV 2hr q 21d | 130 mg/m2 | 0 | 1 | [32,33] |
| Paclitaxel | 24h CI q 21d | 350 mg/m2 | 10d | 6 | [34,35] |
| Pemetrexed | IV 10min q 21d | 1910 mg/m2 | 0 | 0 | [36,37] |
| Pirfenidone | PO tid × 28d | 500 mg/m2 | 0 | 0 | [38,39] |
| Pyrazoloacridine | IV 3hr q 21d | 640 mg/m2 | 0 | 0 | [40,41] |
| Rebeccamycin analog | IV 1hr q 21d | 650 mg/m2 | 0 | 3 | [42,43] |
| Temozolomide | PO qd × 5d q 28d | 180-200 mg/m2/d | 6 | 5 | [44,45] |
| Temsirolimus | IV 1hr/wk × 3wk | 75 mg/m2 | 8 | 2 | [46,47] |
| Tipifarnib* | PO bid × 21d q 28d | 200 mg/m2 | 0 | 4 | [48,49] |
| Topotecan | 21d CI q 28d | 0.3 mg/m2/d | 13 | 4 | [50,51] |
| Topotecan | IV 30min/d × 5d q 21d | 2 mg/m2/d | 8 | 4 | [52,53] |
| Trabectedin | 24hr CI q 21d | 1.5 mg/m2 | 9 | 3 | [54,55] |
| Vinblastine | IV/wk × 52wk | 6 mg/m2 | 22 | 22 | [56,57] |
| Vinorelbine | IV 20min/wk × 6 wk | 33.75 mg/m2 | 5e | 12e | [58,59] |
| Leukemia | |||||
| Arsenic trioxide | IV 2-4hr qd | 0.16-0.20/mg/kg/d | 85f | 89 | [60,61] |
| Carboplatin | 24hr CI × 5d q 28d | 216 mg/m2/d | 9 | 29 | [62,63] |
| Cladribine (2-CDA) | 24hr CI × 5d q 28d | 8.9 mg/m2/d | 14g | 46g | [64,65] |
| Clofarabine | IV 2hr qd × 5d q 2-6wk | 52 mg/m2/d | 29h | 30 | [66,67] |
| Gemcitabine | IV 6hr/wk × 3wk | 3600 mg/m2/wk | 0 | 3 | [68,69] |
| Imatinib* | PO qd × 28d | 260-340 mg/m2 | 91i | 78 | [70,71] |
| Nelarabine | IV 1hr qd × 5d q 21d | 650 mg/m2 | 55J | 41k | [72,73] |
Abbreviations: IV =intravenously; CI = continuous infusion; OR% = objective response rate (as published, unless indicated below)
Indicates a biologic agent
Combined phase I/II trial with OR% reported from independent phase I (mixed solid tumors) and phase II (Ewing sarcoma) arms
Phase II had 5 responders (all among 23 patients with non-bulky neuroblastomas, of 36 patients overall). Phase I did not stratify into bulky and non-bulky disease, documenting only stable disease in 15 of 28 patients with neuroblastoma
Phase II enrolled patients with mixed solid tumors, while phase I included solid tumors and leukemias. No objective responses were observed in either trial
Phase II enrolled patients with CNS tumors, while phase I included mixed solid and CNS tumors. OR% reported here is for the entire cohort, as OR% in the subset of phase I patients with CNS tumors could not be determined
Both studies enrolled patients with mixed solid and CNS tumors. OR% is reported here for the entire cohort, but responders primarily had soft-tissue sarcomas (Phase I: 1 responder among 7 = 14%; Phase II: 4 responders among 20 = 20%)
OR% in subset of evaluable patients with APML (11 of 13; 24 enrolled children overall)
Both studies enrolled patients with AML and ALL. OR% is reported here for the entire cohort, but responders primarily had AML (Phase I: 3 responders among 12 = 25%; Phase II: 10 responders among 17 = 59%)
OR% in subset of evaluable patients with ALL (5 of 17; 25 enrolled children overall)
OR% in subset of evaluable patients with Ph+ CML (11 of 12; 31 enrolled children overall)
OR% in subset of evaluable patients with pediatric T-ALL (11 of 20; 34 enrolled children overall)
OR% in subset of evaluable patients with pediatric T-ALL (26 of 63; 153 enrolled children overall)
Overall, these studies evaluated 34 unique agents, including 25 cytotoxic and 7 biologic agents. 28 pairs of studies enrolled patients with solid or brain tumors, and 7 enrolled patients with leukemia only. The phase I studies enrolled a total of 973 patients (median = 26 per study), of whom 840 (86%) were assessable for response. The phase II studies enrolled a total of 2314 patients (median = 51 per study), of whom 2144 (93%) were assessable for response.
Phase I and Phase II Response Rates
In the 35 pairs of single-agent studies, the median objective response rate (OR%) in phase I was 5.0% (mean = 13.2 ± 22.1%), and 22 trials reported at least one OR. 79 ORs were reported in total, for an overall phase I OR% of 9.4%. In phase II, the median OR% was also 5.0% (mean = 13.0 ± 21.0%), with 29 trials reporting at least one OR. 184 ORs were reported in total, for an overall phase II OR% of 8.6%.
In Figure 2, each phase II study's OR% was plotted graphically against its corresponding phase I study's OR%, revealing a positive correlation between phase I and phase II objective response rates. Without adjusting for different sample sizes among trials, the correlation between the two OR% was 0.8525. After adjusting for different sample sizes, the correlation increased to 0.9269. If influential points (aka “outliers” – defined as those trials with OR in Phase I and II exceeding 0.7) are excluded, the calculated correlation is 0.8751 (see Appendix for derivation).
Figure 2.
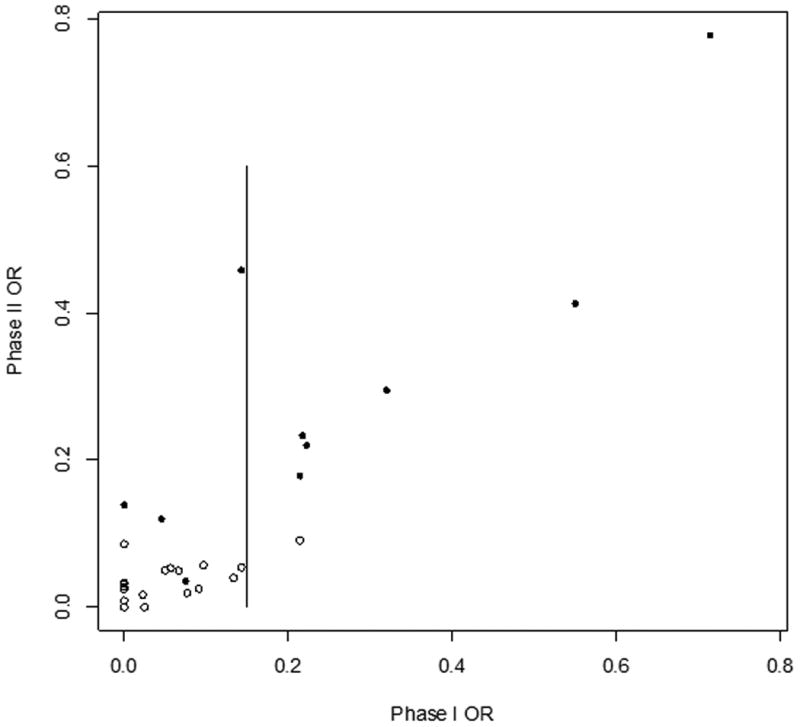
Phase II OR% vs. Phase I OR% (37 Study Pairs). Each circle represents one study pair. Open circles denote pairs in which the phase II study authors reached a “negative” conclusion about efficacy. Solid circles denote a “positive” conclusion about phase II efficacy. Vertical bar indicates phase I OR% = 0.15. Correlation between phase I OR% and phase II OR% is 0.8573 without adjustment for trial sample size, and 0.9269 with adjustment for different sample sizes.
Phase I Response Rate and Phase II Efficacy
Out of 35 phase II trials, 11 were categorized as having a “positive” conclusion (Figure 2, solid circles) regarding drug efficacy, while the other 24 had a “negative” conclusion (Figure 2, open circles). Of the 11 “positive” trials, 1 was industry-sponsored, 4 were cooperative group-sponsored, and 6 were investigator-initiated. Of the 24 “negative” trials, 2 were industry-sponsored, 21 were cooperative group-sponsored, and 1 was investigator-initiated. “Positive” phase II trials had a median phase II OR% of 23.3% (mean = 34.2 ± 27.5%), while “negative” trials had a median phase II OR% of 3.0% (mean = 3.3 ± 2.8%). More interestingly, “positive” and “negative” phase II trials also differed in the average OR% of their respective phase I studies (mean = 32.0 ± 31.4% vs. 4.5 ± 6.9%; median = 21.7% vs. 1.1%). This difference was statistically significant (p < 0.001) by Wilcoxon rank-sum test for continuous, non-parametric outcomes. Among the 13 phase I studies reporting no objective responses, only 1 of the corresponding phase II studies had a “positive” conclusion.[19] None of the phase II studies demonstrated an OR% ≥ 15%.
Lastly, for various cut-offs of phase I OR%, we tabulated the frequency at which the corresponding phase II OR% exceeded certain levels. These probabilities are shown in Table 2.
Table 2. Probability of a Phase II OR% ≥ C2, Given a Phase I OR% ≤ C1.
| Phase II OR% cut-off (C2) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Phase I OR% cut-off (C1) | 0% | 5% | 10% | 15% | 20% | 25% | 30% | 35% | 40% | 45% |
|
|
||||||||||
| 0% (n = 13) | 100% | 23% | 8% | 0% | 0% | 0% | 0% | 0% | 0% | 0% |
| 5% (n = 18) | 100% | 28% | 11% | 0% | 0% | 0% | 0% | 0% | 0% | 0% |
| 10% (n = 24) | 100% | 33% | 8% | 0% | 0% | 0% | 0% | 0% | 0% | 0% |
| 15% (n = 27) | 100% | 37% | 11% | 4% | 4% | 4% | 4% | 4% | 4% | 4% |
| 25% (n = 30) | 100% | 43% | 20% | 13% | 10% | 4% | 4% | 4% | 4% | 4% |
| 35% (n = 32) | 100% | 47% | 22% | 16% | 13% | 6% | 3% | 3% | 3% | 3% |
Percentage of phase II trials that achieved an OR% ≥ C2 when their corresponding phase I trials had an OR% ≤ C1. Sample size (number of pairs of trials) at each cut-off level C1 are displayed in the left column.
Discussion
In this study, we reviewed pairs of pediatric phase I and phase II clinical trials for single chemotherapy agents, in order to determine if phase I objective response rate (OR%) correlated with phase II OR%, and with authors' conclusions regarding phase II efficacy.
In 35 pairs of single-agent studies for which tumor response data was available, phase I OR% and phase II OR% were well-correlated. Drugs rarely demonstrated a dramatically different OR% in phase II than they did in phase I. In only 2 pairs of studies (cladribine; carboplatin) did phase I and phase II OR% differ by 15% or more, and the evaluable sample size in 3 of these studies was less than 25 children. [62-65]
Higher phase I OR% also correlated with greater probability of a positive phase II outcome for a particular drug or regimen, as rated by the study authors. Conversely, out of 17 agents with phase I OR% < 5%, only 2 had phase II trials with “positive” conclusions. In both trials, efficacy was primarily demonstrated in one type or subtype of tumor (hu14.18-IL2 fusion protein for non-bulky neuroblastomas and vinorelbine for rhabdomyosarcoma, as detailed in the legend of Table 1). [19,59] Enrichment for these tumor types in phase II may explain the discrepancy between low phase I OR% and promising phase II showing.
These findings should be viewed in the context of several important caveats. First, due to the nature of our analysis, we excluded 62 single-agent phase I trials that did not have a matching, published phase II trial. For 35 of these trials, the agents (and indications) under study have not proceeded to phase II, ostensibly because they were already deemed to show little promise of clinical efficacy. We also excluded 14 trials where a corresponding phase II trial with matching drug regimen could not be found (i.e. where the phase II trial utilized a combinatorial regimen or different dosing schedule). It should be noted that our analysis is not meant to describe the full benefit derived by participants in pediatric oncology trials, as we did not assess cases of stable disease, minor responses that did not meet standard criteria for PR, or other positive outcomes (e.g. amelioration of symptoms and improved quality of life).
The most important caveat to our findings is that we compared OR% in phase I and phase II – which enroll different study populations and assess response under different circumstances. While we compared trials with matching study regimen and tumor type (solid vs. brain vs. hematologic), for most trials (the exceptions are indicated in Table 1), we did not attempt to control for more granular patient characteristics, such as the distribution of tumor subtypes or number of previous regimens. Nevertheless, despite essentially comparing phase I and phase II OR% as-published from each study, we found that response rates were well correlated.
Importantly, our analysis is not meant to suggest that phase II trials are unnecessary or redundant. Phase II trials are an efficient mechanism for assessment of a drug's efficacy often across disease types. They can further assess preliminary response data from phase I by evaluating tumor response at therapeutic doses, enriching for specific tumor types, and combining new anti-cancer agents with established treatment options. In each of these scenarios, phase II trials may uncover promising findings that cannot be predicted from phase I data.
Rather, what our analysis illustrates is that, more often than not, a drug's clinical promise after phase II does not depart significantly from the tumor response it elicits in phase I. Given the explosion of targeted therapeutics, biologics, immunotherapies and others, efficiency in the study of these agents is required. We documented 35 trials that did not proceed to phase II due to toxicity or lack of efficacy. However, we also identified 13 phase II trials (enrolling a total of 852 children) that were undertaken in the absence of any objective response (0 OR%) in phase I. Only 1 of these (hu14.18-IL2, as discussed above) was judged to have a “positive” phase II conclusion.[19] These findings suggest that more serious consideration of phase I efficacy data is warranted, in order to more efficiently evaluate new drugs and minimize exposure of pediatric trial participants to “high-risk, low-benefit” situations.
In conclusion, retrospective analysis of 35 pairs of single-agent, pediatric phase I and phase II trials demonstrated a significant correlation between phase I OR% for novel anti-cancer drugs and their efficacy in phase II. Although formal conclusions about efficacy cannot be derived from phase I study, based on our analysis, we suggest greater consideration against conducting phase II trials of drugs that show no or minimal objective response in phase I. These findings, together with more detailed reporting of phase I clinical responses and increased implementation of combined phase I/II and Phase “Ib” trials, can improve the efficiency with which pediatric anti-cancer agents are evaluated in the future.
Supplementary Material
Footnotes
Supplemental Appendix: Formula to calculate correlation between phase I trial and phase II trial objective response rates
The authors have no conflicts of interest or funding to disclose
References
- 1.Ward E, DeSantis C, Robbins A, et al. Childhood and adolescent cancer statistics, 2014. CA Cancer J Clin. 2014;64:83–103. doi: 10.3322/caac.21219. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 3.Smith M, Bernstein M, Bleyer WA, et al. Conduct of phase I trials in children with cancer. J Clin Oncol. 1998;16:966–978. doi: 10.1200/JCO.1998.16.3.966. [DOI] [PubMed] [Google Scholar]
- 4.Lee DP, Skolnik JM, Adamson PC. Pediatric phase I trials in oncology: an analysis of study conduct efficiency. J Clin Oncol. 2005;23:8431–8441. doi: 10.1200/JCO.2005.02.1568. [DOI] [PubMed] [Google Scholar]
- 5.Fox E, Maris JM, Widemann BC, et al. A phase 1 study of ABT-751, an orally bioavailable tubulin inhibitor, administered daily for 7 days every 21 days in pediatric patients with solid tumors. Clin Cancer Res. 2006;12:4882–4887. doi: 10.1158/1078-0432.CCR-06-0534. [DOI] [PubMed] [Google Scholar]
- 6.Fox E, Mosse YP, Meany HM, et al. Time to disease progression in children with relapsed or refractory neuroblastoma treated with ABT-751: a report from the Children's Oncology Group (ANBL0621) Pediatric Blood Cancer. 2014;61:990–996. doi: 10.1002/pbc.24900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.MacDonald TJ, Stewart CF, Kocak M, et al. Phase I clinical trial of cilengitide in children with refractory brain tumors: Pediatric Brain Tumor Consortium Study PBTC-012. J Clin Oncol. 2008;26:919–924. doi: 10.1200/JCO.2007.14.1812. [DOI] [PubMed] [Google Scholar]
- 8.MacDonald TJ, Vezina G, Stewart CF, et al. Phase II study of cilengitide in the treatment of refractory or relapsed high-grade gliomas in children: a report from the Children's Oncology Group. Neuro Oncol. 2013;15:1438–1444. doi: 10.1093/neuonc/not058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malempati S, Weigel B, Ingle AM, et al. Phase I/II trial and pharmacokinetic study of cixutumumab in pediatric patients with refractory solid tumors and Ewing sarcoma: a report from the Children's Oncology Group. J Clin Oncol. 2012;30:256–262. doi: 10.1200/JCO.2011.37.4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blaney SM, Seibel NL, O'Brien M, et al. Phase I trial of docetaxel administered as a 1-hour infusion in children with refractory solid tumors: a collaborative pediatric branch, National Cancer Institute and Children's Cancer Group trial. Journal of Clinical Oncology. 1997;15:1538–1543. doi: 10.1200/JCO.1997.15.4.1538. [DOI] [PubMed] [Google Scholar]
- 11.Zwerdling T, Krailo M, Monteleone P, et al. Phase II investigation of docetaxel in pediatric patients with recurrent solid tumors: a report from the Children's Oncology Group. Cancer. 2006;106:1821–1828. doi: 10.1002/cncr.21779. [DOI] [PubMed] [Google Scholar]
- 12.Mathew P, Ribeiro RC, Sonnichsen D, et al. Phase I study of oral etoposide in children with refractory solid tumors. J Clin Oncol. 1994;12:1452–1457. doi: 10.1200/JCO.1994.12.7.1452. [DOI] [PubMed] [Google Scholar]
- 13.Needle MN, Molloy PT, Geyer JR, et al. Phase II study of daily oral etoposide in children with recurrent brain tumors and other solid tumors. Med Pediatr Oncol. 1997;29:28–32. doi: 10.1002/(sici)1096-911x(199707)29:1<28::aid-mpo5>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 14.Villablanca JG, Krailo MD, Ames MM, et al. Phase I trial of oral fenretinide in children with high-risk solid tumors: a report from the Children's Oncology Group (CCG 09709) J Clin Oncol. 2006;24:3423–3430. doi: 10.1200/JCO.2005.03.9271. [DOI] [PubMed] [Google Scholar]
- 15.Villablanca JG, London WB, Naranjo A, et al. Phase II study of oral capsular 4-hydroxyphenylretinamide (4-HPR/fenretinide) in pediatric patients with refractory or recurrent neuroblastoma: a report from the Children's Oncology Group. Clin Cancer Res. 2011;17:6858–6866. doi: 10.1158/1078-0432.CCR-11-0995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reid JM, Qu W, Safgren SL, et al. Phase I trial and pharmacokinetics of gemcitabine in children with advanced solid tumors. J Clin Oncol. 2004;22:2445–2451. doi: 10.1200/JCO.2004.10.142. [DOI] [PubMed] [Google Scholar]
- 17.Wagner-Bohn A, Paulussen M, Vieira Pinheiro JP, et al. Phase II study of gemcitabine in children with solid tumors of mesenchymal and embryonic origin. Anticancer Drugs. 2006;17:859–864. doi: 10.1097/01.cad.0000217426.82702.74. [DOI] [PubMed] [Google Scholar]
- 18.Osenga KL, Hank JA, Albertini MR, et al. A phase I clinical trial of the hu14. 18-IL2 (EMD 273063) as a treatment for children with refractory or recurrent neuroblastoma and melanoma: a study of the Children's Oncology Group Clin Cancer Res. 2006;12:1750–1759. doi: 10.1158/1078-0432.CCR-05-2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shusterman S, London WB, Gillies SD, et al. Antitumor activity of hu14. 18-IL2 in patients with relapsed/refractory neuroblastoma: a Children's Oncology Group (COG) phase II study J Clin Oncol. 2010;28:4969–4975. doi: 10.1200/JCO.2009.27.8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pratt CB, Douglass EC, Kovnar EH, et al. A phase I study of ifosfamide given on alternate days to treat children with brain tumors. Cancer. 1993;71:3666–3669. doi: 10.1002/1097-0142(19930601)71:11<3666::aid-cncr2820711132>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 21.Heideman RL, Douglass EC, Langston JA, et al. A phase II study of every other day high-dose ifosfamide in pediatric brain tumors: a Pediatric Oncology Group Study. J Neurooncol. 1995;25:77–84. doi: 10.1007/BF01054726. [DOI] [PubMed] [Google Scholar]
- 22.Whitehead VM, Bernstein ML, Vega R, et al. Phase I trial of indicine-N-oxide in children with leukemia and solid tumors: a Pediatric Oncology Group study. Cancer Chemother Pharmacol. 1990;26:377–379. doi: 10.1007/BF02897298. [DOI] [PubMed] [Google Scholar]
- 23.Miser JS, Smithson WA, Krivit W, et al. Phase II trial of indicine N-oxide in relapsed pediatric solid tumors. A report from the Childrens Cancer Study Group Invest New Drugs. 1991;9:339–342. doi: 10.1007/BF00183576. [DOI] [PubMed] [Google Scholar]
- 24.Ribeiro RC, Rill D, Roberson PK, et al. Continuous infusion of interleukin-2 in children with refractory malignancies. Cancer. 1993;72:623–628. doi: 10.1002/1097-0142(19930715)72:2<623::aid-cncr2820720248>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 25.Bauer M, Reaman GH, Hank JA, et al. A phase II trial of human recombinant interleukin-2 administered as a 4-day continuous infusion for children with refractory neuroblastoma, non-Hodgkin's lymphoma, sarcoma, renal cell carcinoma, and malignant melanoma. A Childrens Cancer Group study Cancer. 1995;75:2959–2965. doi: 10.1002/1097-0142(19950615)75:12<2959::aid-cncr2820751225>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 26.Blaney S, Berg SL, Pratt C, et al. A phase I study of irinotecan in pediatric patients: a pediatric oncology group study. Clin Cancer Res. 2001;7:32–37. [PubMed] [Google Scholar]
- 27.Bomgaars LR, Bernstein M, Krailo M, et al. Phase II trial of irinotecan in children with refractory solid tumors: a Children's Oncology Group Study. J Clin Oncol. 2007;25:4622–4627. doi: 10.1200/JCO.2007.11.6103. [DOI] [PubMed] [Google Scholar]
- 28.Furman WL, Stewart CF, Poquette CA, et al. Direct translation of a protracted irinotecan schedule from a xenograft model to a phase I trial in children. J Clin Oncol. 1999;17:1815–1824. doi: 10.1200/JCO.1999.17.6.1815. [DOI] [PubMed] [Google Scholar]
- 29.Bisogno G, Riccardi R, Ruggiero A, et al. Phase II study of a protracted irinotecan schedule in children with refractory or recurrent soft tissue sarcoma. Cancer. 2006;106:703–707. doi: 10.1002/cncr.21629. [DOI] [PubMed] [Google Scholar]
- 30.Widemann BC, Goodspeed W, Goodwin A, et al. Phase I trial and pharmacokinetic study of ixabepilone administered daily for 5 days in children and adolescents with refractory solid tumors. J Clin Oncol. 2009;27:550–556. doi: 10.1200/JCO.2008.17.6644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jacobs S, Fox E, Krailo M, et al. Phase II trial of ixabepilone administered daily for five days in children and young adults with refractory solid tumors: a report from the children's oncology group. Clin Cancer Res. 2010;16:750–754. doi: 10.1158/1078-0432.CCR-09-1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spunt SL, Freeman BB, Billups CA, et al. Phase I clinical trial of oxaliplatin in children and adolescents with refractory solid tumors. J Clin Oncol. 2007;25:2274–2280. doi: 10.1200/JCO.2006.08.2388. [DOI] [PubMed] [Google Scholar]
- 33.Beaty O, Berg S, Blaney S, et al. A phase II trial and pharmacokinetic study of oxaliplatin in children with refractory solid tumors: a Children's Oncology Group study. Pediatric Blood Cancer. 2010;55:440–445. doi: 10.1002/pbc.22544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hurwitz CA, Relling MV, Weitman SD, et al. Phase I trial of paclitaxel in children with refractory solid tumors: a Pediatric Oncology Group Study. J Clin Oncol. 1993;11:2324–2329. doi: 10.1200/JCO.1993.11.12.2324. [DOI] [PubMed] [Google Scholar]
- 35.Hurwitz CA, Strauss LC, Kepner J, et al. Paclitaxel for the treatment of progressive or recurrent childhood brain tumors: a pediatric oncology phase II study. J Pediatr Hematol Oncol. 2001;23:277–281. doi: 10.1097/00043426-200106000-00008. [DOI] [PubMed] [Google Scholar]
- 36.Malempati S, Nicholson HS, Reid JM, et al. Phase I trial and pharmacokinetic study of pemetrexed in children with refractory solid tumors: the Children's Oncology Group. J Clin Oncol. 2007;25:1505–1511. doi: 10.1200/JCO.2006.09.1694. [DOI] [PubMed] [Google Scholar]
- 37.Warwick AB, Malempati S, Krailo M, et al. Phase 2 trial of pemetrexed in children and adolescents with refractory solid tumors: a Children's Oncology Group study. Pediatric Blood Cancer. 2013;60:237–241. doi: 10.1002/pbc.24244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Babovic-Vuksanovic D, Widemann BC, Dombi E, et al. Phase I trial of pirfenidone in children with neurofibromatosis 1 and plexiform neurofibromas. Pediatr Neurol. 2007;36:293–300. doi: 10.1016/j.pediatrneurol.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 39.Widemann BC, Babovic-Vuksanovic D, Dombi E, et al. Phase II trial of pirfenidone in children and young adults with neurofibromatosis type 1 and progressive plexiform neurofibromas. Pediatric Blood Cancer. 2014;61:1598–1602. doi: 10.1002/pbc.25041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berg SL, Blaney SM, Adamson PC, et al. Phase I trial and pharmacokinetic study of pyrazoloacridine in children and young adults with refractory cancers. J Clin Oncol. 1998;16:181–186. doi: 10.1200/JCO.1998.16.1.181. [DOI] [PubMed] [Google Scholar]
- 41.Berg SL, Blaney SM, Sullivan J, et al. Phase II trial of pyrazoloacridine in children with solid tumors: a Pediatric Oncology Group phase II study. J Pediatr Hematol Oncol. 2000;22:506–509. doi: 10.1097/00043426-200011000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Langevin AM, Weitman SD, Kuhn JG, et al. Phase I trial of rebeccamycin analog (NSC #655649) in children with refractory solid tumors: a pediatric oncology group study. J Pediatr Hematol Oncol. 2003;25:526–533. doi: 10.1097/00043426-200307000-00006. [DOI] [PubMed] [Google Scholar]
- 43.Langevin AM, Bernstein M, Kuhn JG, et al. A phase II trial of rebeccamycin analogue (NSC #655649) in children with solid tumors: a Children's Oncology Group study. Pediatric Blood Cancer. 2008;50:577–580. doi: 10.1002/pbc.21274. [DOI] [PubMed] [Google Scholar]
- 44.Nicholson HS, Krailo M, Ames MM, et al. Phase I study of temozolomide in children and adolescents with recurrent solid tumors: a report from the Children's Cancer Group. J Clin Oncol. 1998;16:3037–3043. doi: 10.1200/JCO.1998.16.9.3037. [DOI] [PubMed] [Google Scholar]
- 45.Nicholson HS, Kretschmar CS, Krailo M, et al. Phase 2 study of temozolomide in children and adolescents with recurrent central nervous system tumors: a report from the Children's Oncology Group. Cancer. 2007;110:1542–1550. doi: 10.1002/cncr.22961. [DOI] [PubMed] [Google Scholar]
- 46.Spunt SL, Grupp SA, Vik TA, et al. Phase I study of temsirolimus in pediatric patients with recurrent/refractory solid tumors. J Clin Oncol. 2011;29:2933–2940. doi: 10.1200/JCO.2010.33.4649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Geoerger B, Kieran MW, Grupp S, et al. Phase II trial of temsirolimus in children with high-grade glioma, neuroblastoma and rhabdomyosarcoma. Eur J Cancer. 2012;48:253–262. doi: 10.1016/j.ejca.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Widemann BC, Salzer WL, Arceci RJ, et al. Phase I trial and pharmacokinetic study of the farnesyltransferase inhibitor tipifarnib in children with refractory solid tumors or neurofibromatosis type I and plexiform neurofibromas. J Clin Oncol. 2006;24:507–516. doi: 10.1200/JCO.2005.03.8638. [DOI] [PubMed] [Google Scholar]
- 49.Fouladi M, Nicholson HS, Zhou T, et al. A phase II study of the farnesyl transferase inhibitor, tipifarnib, in children with recurrent or progressive high-grade glioma, medulloblastoma/primitive neuroectodermal tumor, or brainstem glioma: a Children's Oncology Group study. Cancer. 2007;110:2535–2541. doi: 10.1002/cncr.23078. [DOI] [PubMed] [Google Scholar]
- 50.Frangoul H, Ames MM, Mosher RB, et al. Phase I study of topotecan administered as a 21-day continous infusion in children with recurrent solid tumors: a report from the Children's Cancer Group. Clin Cancer Res. 1999;5:3956–3962. [PubMed] [Google Scholar]
- 51.Hawkins DS, Bradfield S, Whitlock JA, et al. Topotecan by 21-day continuous infusion in children with relapsed or refractory solid tumors: a Children's Oncology Group study. Pediatric Blood Cancer. 2006;47:790–794. doi: 10.1002/pbc.20739. [DOI] [PubMed] [Google Scholar]
- 52.Tubergen DG, Stewart CF, Pratt CB, et al. Phase I trial and pharmacokinetic (PK) and pharmacodynamics (PD) study of topotecan using a five-day course in children with refractory solid tumors: a pediatric oncology group study. J Pediatr Hematol Oncol. 1996;18:352–361. doi: 10.1097/00043426-199611000-00004. [DOI] [PubMed] [Google Scholar]
- 53.Nitschke R, Parkhurst J, Sullivan J, et al. Topotecan in pediatric patients with recurrent and progressive solid tumors: a Pediatric Oncology Group phase II study. J Pediatr Hematol Oncol. 1998;20:315–318. doi: 10.1097/00043426-199807000-00006. [DOI] [PubMed] [Google Scholar]
- 54.Lau L, Supko JG, Blaney S, et al. A phase I and pharmacokinetic study of ecteinascidin-743 (Yondelis) in children with refractory solid tumors. A Children's Oncology Group study Clin Cancer Res. 2005;11:672–677. [PubMed] [Google Scholar]
- 55.Baruchel S, Pappo A, Krailo M, et al. A phase 2 trial of trabectedin in children with recurrent rhabdomyosarcoma, Ewing sarcoma and non-rhabdomyosarcoma soft tissue sarcomas: a report from the Children's Oncology Group. Eur J Cancer. 2012;48:579–585. doi: 10.1016/j.ejca.2011.09.027. [DOI] [PubMed] [Google Scholar]
- 56.Lafay-Cousin L, Holm S, Qaddoumi I, et al. Weekly vinblastine in pediatric low-grade glioma patients with carboplatin allergic reaction. Cancer. 2005;103:2636–2642. doi: 10.1002/cncr.21091. [DOI] [PubMed] [Google Scholar]
- 57.Bouffet E, Jakacki R, Goldman S, et al. Phase II study of weekly vinblastine in recurrent or refractory pediatric low-grade glioma. J Clin Oncol. 2012;30:1358–1363. doi: 10.1200/JCO.2011.34.5843. [DOI] [PubMed] [Google Scholar]
- 58.Johansen M, Kuttesch J, Bleyer WA, et al. Phase I evaluation of oral and intravenous vinorelbine in pediatric cancer patients: a report from the Children's Oncology Group. Clin Cancer Res. 2006;12:516–522. doi: 10.1158/1078-0432.CCR-05-1541. [DOI] [PubMed] [Google Scholar]
- 59.Kuttesch JF, Jr, Krailo MD, Madden T, et al. Phase II evaluation of intravenous vinorelbine (Navelbine) in recurrent or refractory pediatric malignancies: a Children's Oncology Group study. Pediatric Blood Cancer. 2009;53:590–593. doi: 10.1002/pbc.22133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fox E, Razzouk BI, Widemann BC, et al. Phase 1 trial and pharmacokinetic study of arsenic trioxide in children and adolescents with refractory or relapsed acute leukemia, including acute promyelocytic leukemia or lymphoma. Blood. 2008;111:566–573. doi: 10.1182/blood-2007-08-107839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhou J, Zhang Y, Li J, et al. Single-agent arsenic trioxide in the treatment of children with newly diagnosed acute promyelocytic leukemia. Blood. 2010;115:1697–1702. doi: 10.1182/blood-2009-07-230805. [DOI] [PubMed] [Google Scholar]
- 62.Ettinger LJ, Krailo MD, Gaynon PS, et al. A phase I study of carboplatin in children with acute leukemia in bone marrow relapse. A report from the Childrens Cancer Group Cancer. 1993;72:917–922. doi: 10.1002/1097-0142(19930801)72:3<917::aid-cncr2820720342>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 63.Ettinger LJ, Ivy P, Gaynon PS, et al. A phase II study of carboplatin as a treatment for children with acute leukemia recurring in bone marrow: a report of the Children's Cancer Group. Cancer. 1997;80:311–316. [PubMed] [Google Scholar]
- 64.Santana VM, Mirro J, Jr, Harwood FC, et al. A phase I clinical trial of 2-chlorodeoxyadenosine in pediatric patients with acute leukemia. J Clin Oncol. 1991;9:416–422. doi: 10.1200/JCO.1991.9.3.416. [DOI] [PubMed] [Google Scholar]
- 65.Santana VM, Mirro J, Jr, Kearns C, et al. 2-Chlorodeoxyadenosine produces a high rate of complete hematologic remission in relapsed acute myeloid leukemia. J Clin Oncol. 1992;10:364–370. doi: 10.1200/JCO.1992.10.3.364. [DOI] [PubMed] [Google Scholar]
- 66.Jeha S, Gandhi V, Chan KW, et al. Clofarabine, a novel nucleoside analog, is active in pediatric patients with advanced leukemia. Blood. 2004;103:784–789. doi: 10.1182/blood-2003-06-2122. [DOI] [PubMed] [Google Scholar]
- 67.Jeha S, Gaynon PS, Razzouk BI, et al. Phase II study of clofarabine in pediatric patients with refractory or relapsed acute lymphoblastic leukemia. J Clin Oncol. 2006;24:1917–1923. doi: 10.1200/JCO.2005.03.8554. [DOI] [PubMed] [Google Scholar]
- 68.Steinherz PG, Seibel NL, Ames MM, et al. Phase I study of gemcitabine (difluorodeoxycytidine) in children with relapsed or refractory leukemia (CCG-0955): a report from the Children's Cancer Group. Leuk Lymphoma. 2002;43:1945–1950. doi: 10.1080/1042819021000015880. [DOI] [PubMed] [Google Scholar]
- 69.Angiolillo AL, Whitlock J, Chen Z, et al. Phase II study of gemcitabine in children with relapsed acute lymphoblastic leukemia or acute myelogenous leukemia (ADVL0022): a Children's Oncology Group Report. Pediatric Blood Cancer. 2006;46:193–197. doi: 10.1002/pbc.20419. [DOI] [PubMed] [Google Scholar]
- 70.Champagne MA, Capdeville R, Krailo M, et al. Imatinib mesylate (STI571) for treatment of children with Philadelphia chromosome-positive leukemia: results from a Children's Oncology Group phase 1 study. Blood. 2004;104:2655–2660. doi: 10.1182/blood-2003-09-3032. [DOI] [PubMed] [Google Scholar]
- 71.Millot F, Guilhot J, Nelken B, et al. Imatinib mesylate is effective in children with chronic myelogenous leukemia in late chronic and advanced phase and in relapse after stem cell transplantation. Leukemia. 2006;20:187–192. doi: 10.1038/sj.leu.2404051. [DOI] [PubMed] [Google Scholar]
- 72.Berg SL, Blaney SM, Devidas M, et al. Phase II study of nelarabine (compound 506U78) in children and young adults with refractory T-cell malignancies: a report from the Children's Oncology Group. J Clin Oncol. 2005;23:3376–3382. doi: 10.1200/JCO.2005.03.426. [DOI] [PubMed] [Google Scholar]
- 73.Kurtzberg J, Ernst TJ, Keating MJ, et al. Phase I study of 506U78 administered on a consecutive 5-day schedule in children and adults with refractory hematologic malignancies. J Clin Oncol. 2005;23:3396–3403. doi: 10.1200/JCO.2005.03.199. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.