Abstract
Objective
To explore differences in blood cytokine profiles among distinct BPD patterns.
Study design
We evaluated blood spots collected from 943 infants born at ≤1000 grams and surviving to 28 days on postnatal days 1, 3, 7, 14, and 21 for 25 cytokines. Infants were assigned to the following lung disease patterns: (1) no lung disease (NLD), (2) respiratory distress syndrome (RDS) without BPD, (3) classic BPD (persistent exposure to supplemental oxygen until 28 days of age) or (4) atypical BPD (period without supplemental oxygen before 28 days). Median cytokine levels for infants with BPD were compared with the interquartile range (IQR) of results among infants with NLD.
Results
The distribution of enrolled infants by group was as follows: 69 (NLD), 73 (RDS), 381 (classic BPD), and 160 (atypical BPD). The remaining 260 infants could not be classified due to missing data (104) or not fitting a pre-defined pattern (156). Median levels of 3 cytokines (elevated IL-8, MMP-9; decreased GM-CSF) fell outside the IQR for at least 2 time points in both atypical and classic BPD infants. Profiles of 7 cytokines (IL-6, -10, -18, MIP-1α, CRP, BDNF, RANTES) differed between infants with classic and atypical BPD.
Conclusion
Blood cytokine profiles may differ between infants developing classic and atypical BPD. These dissimilarities suggest the possibility that differing mechanisms could explain the varied patterns of pathophysiology of lung disease in extremely premature infants.
Keywords: Premature infant, respiratory distress syndrome, chronic lung disease, inflammatory markers
Many investigators have described the association of altered lung fluid cytokine levels with the development of bronchopulmonary dysplasia (BPD), but these studies are limited by their small size and their inability to obtain samples from non-intubated infants [1–8]. Fewer studies have explored the potential association between variations in blood cytokine levels and BPD [9–12].
Some authors have described a “new” BPD associated with improved survival in smaller infants and decreased incidence of the more classic, severe form of BPD due to the availability of newer management approaches [13, 14]. We have previously described four patterns of respiratory course among infants ≤ 1250 grams birth weight, based on oxygen administration: (1) normal – no clinical or radiological evidence of lung disease; (2) respiratory distress syndrome (RDS) – typical RDS, with no supplemental oxygen administration at 28 days of age; (3) classic BPD – acute lung disease that does not resolve, leading to oxygen administration and radiologic evidence of BPD at 28 days; and (4) atypical BPD – initial resolution of RDS, if present, followed by subsequent development of oxygen requirement that persists through 28 days [13]. Although it would not be appropriate to conflate “new” BPD with the atypical group we describe, these infants may represent a unique group in which to study the pathogenesis of the “new” BPD.
The Eunice Kennedy Shriver National Institutes of Child Health and Human Development’s Neonatal Research Network (NRN) has performed cytokine assays on blood spots from over 1000 premature infants. We have previously reported that adding cytokine results to clinical predictors improved the ability of a multivariate model to predict the later development of BPD [11]. However, the modeling did not take into account potential subtypes of BPD. We re-analyzed the cytokine data, specifically dividing subjects into groups with classic and atypical BPD. We hypothesized that the patterns of expression of a limited number of blood cytokines would differ between infants with classic BPD, infants with atypical BPD, and infants without BPD. We speculated that these differences in cytokine levels could serve as biomarkers of disease type and form the focus of further, hypothesis-driven exploration of the pathogenesis of BPD.
METHODS
This study analyzed data from a prospective cohort study of blood cytokine levels among premature infants that has been reported previously [11, 15]. Briefly, whole blood spots were collected on filter paper from infants with birth weights between 401 and 1000 grams born at one of 17 NRN centers between 1999 and 2002. Samples were collected within 4 hours after birth (“day 1”) and then between 2–4 days (“day 3”), 6–8 days (“day 7”), 11–17 days (“day 14”) and 18–24 days (“day 21”). Samples were dried using a desiccator, frozen immediately and analyzed later for levels of 25 cytokines using a multiplex, bead-based luminescent immunoassay (Luminex®, Austin, TX), as previously described [16]. The cytokine levels measured included interleukin (IL)-1β, IL-2, IL-4, IL-5, IL-6, IL-8 (CXCL8), IL-10, IL-12, IL-17, IL-18, tumor necrosis factor (TNF)-α, TNF-β, RANTES, brain-derived neurotrophic factor (BDNF), C-reactive protein (CRP), granulocyte/macrophage colony-stimulating factor (GM-CSF), interferon (IFN)-γ, macrophage chemoattractant protein (MCP)-1, macrophage inflammatory protein (MIP)-1α, MIP-1β, matrix metalloproteinase (MMP)-9, neurotrophin (NT) 4, soluble IL-6 receptor (SIR), transforming growth factor (TGF)-β, and triggering receptor expressed on myeloid cells 1 (TREM1). Clinical data, including inspired concentration of oxygen (FiO2) on days 1, 3, 7, 14, 21 and 28 and at 36 weeks’ postmenstrual age (PMA), were collected from the medical record. The original study was approved by each center’s Institutional Review Board, and parents gave informed permission for the study. This analysis used only de-identified data collected at the time of the original study.
Infants were divided into groups to correspond with our previously reported patterns of lung disease [13], according to oxygen administration at 1, 4, 7, 14, 21 and 28 days of age. The means of oxygen administration was not included in the definition. A group with “no lung disease” (NLD) was defined by having FiO2 < 0.25 at days 1, 4, 7, 14, 21 and 28 days. A group was identified as having “respiratory distress syndrome without BPD” (RDS) by having FiO2 ≥ 0.25 within the first 21 days, FiO2 < 0.25 at day 3 and/or day 7 and FiO2 <0.25 at day 28. Infants with FiO2 ≥ 0.25 at days 1, 3, 7, 14, 21 and 28 (ie, persistent supplemental oxygen administration) were classified as having “classic BPD.” Infants with FiO2 <0.25 at either day 3 or day 7, but FiO2 ≥ 0.25 at day 28 (i.e., intervening period of low or absent supplemental oxygen administration) were defined as having “atypical BPD.” The BPD groups were further subdivided into “mild BPD” (FiO2 = 0.21 at 36 weeks’ PMA) or “moderate-to-severe BPD/CLD” (FiO2 > 0.21 at 36 weeks’ PMA), in a classification roughly approximating consensus guidelines on BPD severity, to allow for evaluation of the common clinical definition that considers only FiO2 at 36 weeks’ PMA [14]. The data pre-dated the use of physiological testing to determine FiO2 required at 36 weeks’ PMA [17]. Subjects receiving nasal cannula flow at FiO2 0.21 were considered as receiving room air. Results for both any severity of BPD and moderate-to-severe BPD (with mild BPD removed) were analyzed. Subjects who did not fit into one of the classifications or who died before 28 days of age were not included in the analysis.
Baseline and clinical characteristics were analyzed by Kruskal-Wallis test or Median test (Apgar scores) for continuous variables, and Fisher exact test for categorical variables. The median values and interquartile range were determined for each cytokine among the NLD group at each of the sampled times. The median values for each of other groups were plotted against the NLD group. Cytokines for which two median values fell outside the interquartile range for NLD group were defined a priori as different from the pattern for the NLD group. Because the purpose of this study was to generate hypotheses about potential differences in cytokine patterns among various types of lung diseases, no statistical hypothesis testing was performed.
RESULTS
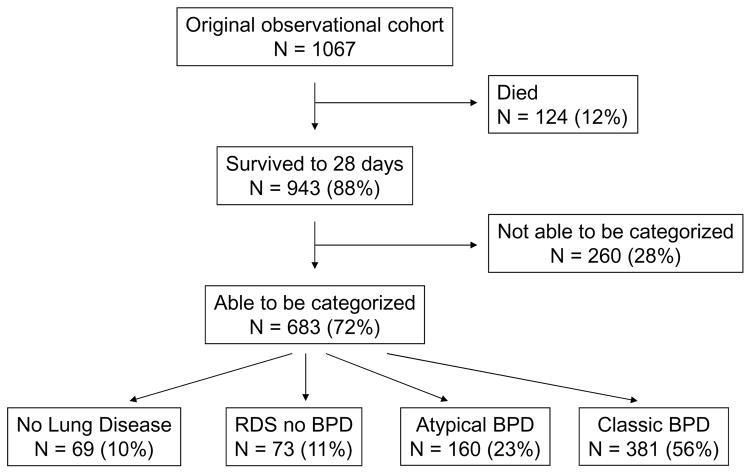
Of the original 1067 subjects in the observational cytokine cohort, 943 survived to 28 days, and 683 of these could be categorized into one of the four pre-defined groups (Figure 1; available at www.jpeds.com). Of these, 69 (10%) qualified as having no lung disease, 73 (11%) were categorized as having RDS without BPD, 160 (23%) as having atypical BPD, and 381 (56%) as having classic BPD. When analyzed at 36 weeks’ PMA, among infants with atypical BPD, 4 (3%) had died, 39 (24%) had moderate/severe BPD (continued FiO2 >0.21), 70 (44%) had mild BPD (FiO2 0.21), and 47 (29%) had missing data for FiO2 at 36 weeks’ PMA (and were not included in the 36 week data). Of infants with classic BPD assessed at 36 weeks’ PMA, 25 (7%) had died, 125 (33%) had moderate/severe BPD, 80 (21%) had mild BPD, and 151 (40%) had missing data for FiO2 at 36 weeks’ PMA (and were not included in the 36 week data). Baseline and clinical variables of the groups, including of the group of infants unable to be categorized, are described in the Table. As expected, there were several differences in baseline and clinical variables among the disease groups and between the classifiable and non-classifiable infants. Of the 260 infants who could not be classified, 156 were indeterminate (≥1 FiO2 measurement <0.25, but FiO2 ≥0.25 at both day 3 and day 7, including 120 who had FiO2 ≥0.25 at day 28) and 104 had incomplete information (missing FiO2 at 28 days or >1 missing FiO2 value).
Figure 1. Flow diagram of cohort characteristics.
Among subjects in the initial cohort, 12% died before the time of categorization (28 days of age), 64% could be assigned to one of four pre-defined patterns of lung disease (72% of survivors), and 24% could not be categorized. BPD = bronchopulmonary dysplasia.
Table 1.
Demographic and clinical characteristics.
| No Lung Disease | RDS Alone | Atypical BPD | Classic BPD | Not Classifiable | |
|---|---|---|---|---|---|
| N = 69 | N = 73 | N = 160 | N = 381 | N = 260 | |
| Gestational age (w)* | 28.3 ± 1.8 | 26.9 ± 1.5 | 25.7 ± 1.4 | 25.1 ± 1.6 | 26.6 ± 1.9 |
| Birth weight (g)* | 843 ± 129 | 843 ± 113 | 774 ± 132 | 722 ± 135 | 803 ± 124 |
| Sex (male)* | 30 (43%) | 20 (27%) | 82 (51%) | 194 (51%) | 121 (47%) |
| Race (black) | 33 (48%) | 35 (48%) | 73 (46%) | 183 (48%) | 129 (50%) |
| Prenatal steroids* | 57 (83%) | 55 (75%) | 137 (86%) | 285 (75%) | 205 (79%) |
| Surfactant* | 14 (20%) | 47 (64%) | 130 (81%) | 349 (92%) | 212 (82%) |
| Apgar score 1 min* | 6 [3–7] | 5 [3–7] | 5 [3–7] | 4 [2–6] | 5 [3–7] |
| Apgar score 5 min* | 8 [7–8] | 7 [6–8] | 7 [6–8] | 7 [5–8] | 7 [6–8]† |
| At 28 days: | |||||
| Days on mechanical ventilation* | 0.7 ± 1.2 | 4.5 ± 6.3 | 15.9 ± 9.9 | 22.5 ± 8.0 | 12.5 ± 10.1† |
| Days of respiratory support*‡ | 2.2 ± 4.2 | 10.6 ± 9.6 | 23.2 ± 8.0 | 26.1 ± 5.1 | 18.4 ± 9.9† |
| Days of supplemental oxygen* | 1.9 ± 2.9 | 11.7 ± 7.6 | 23.2 ± 5.0 | 27.7 ±0.9 | 23.5 ± 6.3 |
| At 36 weeks: | |||||
| Days on mechanical ventilation* | 1.3 ± 2.5 | 6.2 ± 8.0 | 26.4 ± 19.0 | 41.2 ± 22.6 | 20.7 ± 20.7† |
| Days of respiratory support*‡ | 3.1 ± 4.9 | 14.1 ± 16.0 | 42.8 ± 20.3 | 53.5 ± 20.9 | 32.2 ± 23.4† |
| Days of supplemental oxygen* | 4.1 ± 9.2 | 17.4 ± 16.0 | 54.0 ± 18.7 | 67.9 ± 15.3 | 48.4 ± 21.9† |
| Early-onset sepsis (≤72 hours) | 0 (0%) | 0 (0%) | 2 (1%) | 10 (3%) | 2 (1%) |
| Late-onset sepsis (>72 hours)* | 16 (23%) | 24 (33%) | 81 (51%) | 175 (46%) | 94 (36%) |
| Severe IVH* | 2 (3%) | 6 (8%) | 15 (9%) | 75 (20%) | 22 (9%)† |
Data are presented as mean ± standard deviation, median [interquartile range] or N (%)
P<0.05 for significant differences between groups
P<0.05 for significant differences between infants that could not be classified (N=260) and infants that were classified into one of the four groups (N=683)
Respiratory support = mechanical ventilation or CPAP
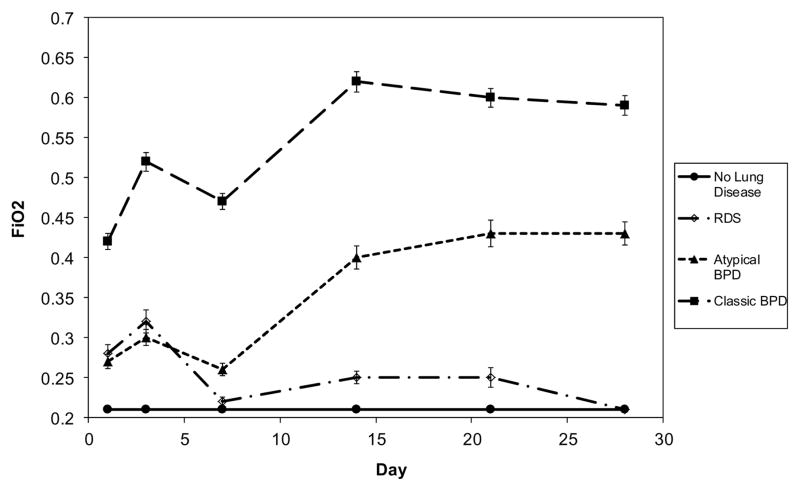
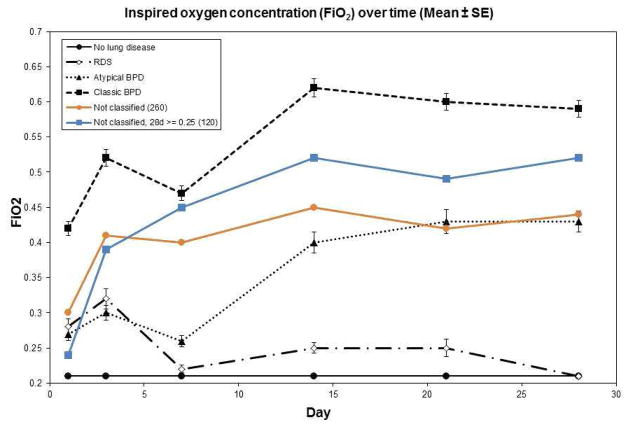
As expected from the definitions, infants with BPD of either type had higher FiO2 at 28 days (Figure 2), with mean FiO2 in the NLD and RDS groups of 0.21. Infants with atypical BPD had FiO2 at days 1 and 3 more similar to the RDS group than to the classic BPD group. Thereafter, the atypical BPD group had FiO2 values intermediate between those of the classic BPD group and the other groups. Also as expected from the group definitions, non-classified infants’ FiO2 values fell between those of the BPD groups (Figure 3; available at www.jpeds.com).
Figure 2. FiO2 among study subjects.
As expected from the lung disease pattern definitions, children with classic BPD had higher initial FiO2 than those with either atypical BPD or RDS. Children in the atypical BPD group had lower FiO2 at 28 days than did children with classic BPD. Values are shown as mean ± standard error of the mean.
Figure 3.
Inspired oxygen concentration (FiO2) for non-classified groups. Non-classified infants are shown including all infants (orange line) and only infants with FiO2 ≥0.25 at 28 days (blue line), who would qualify as having BPD. As expected from the group definitions, the FiO2 means for infants with non-classifiable BPD (≥1 FiO2 measurement <0.25, but FiO2 ≥0.25 at both day 3 and day 7 and at day 28) fell between those of the classic (FiO2 ≥ 0.25 at days 1, 3, 7, 14, 21 and 28) and atypical (FiO2 <0.25 at either day 3 or day 7, but FiO2 ≥ 0.25 at day 28) BPD groups.
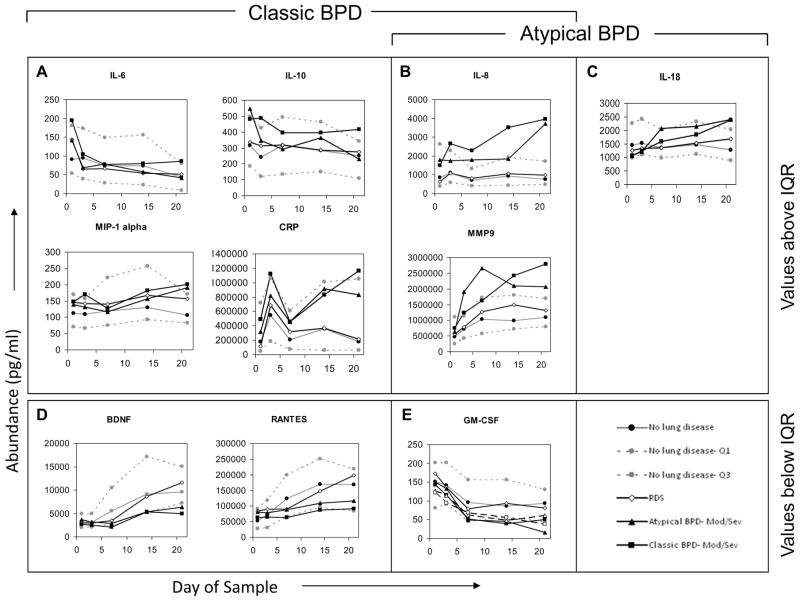
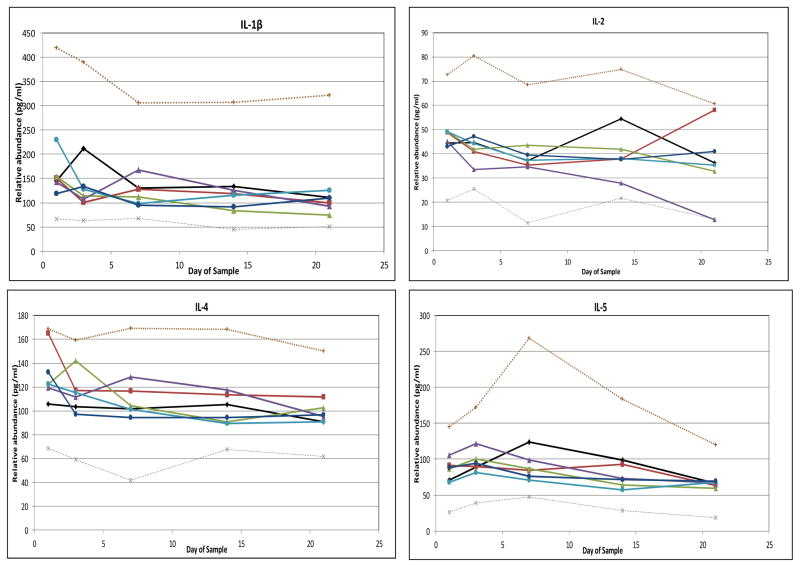
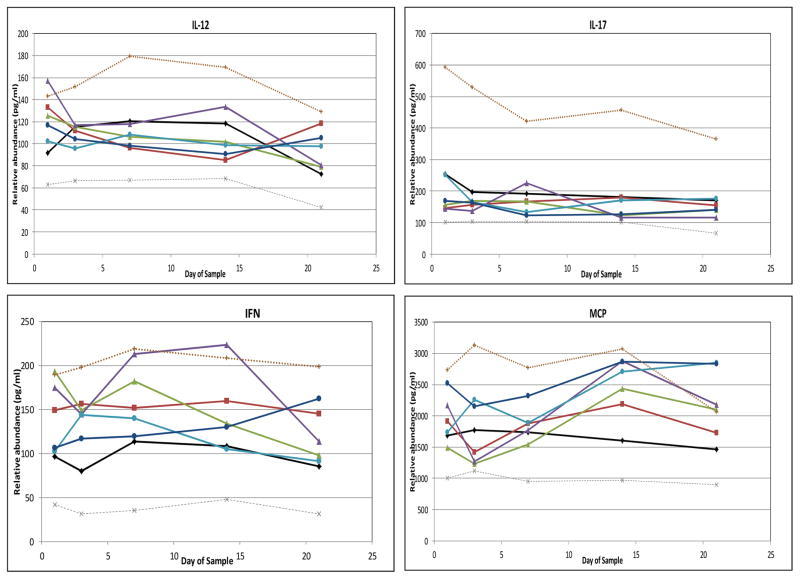
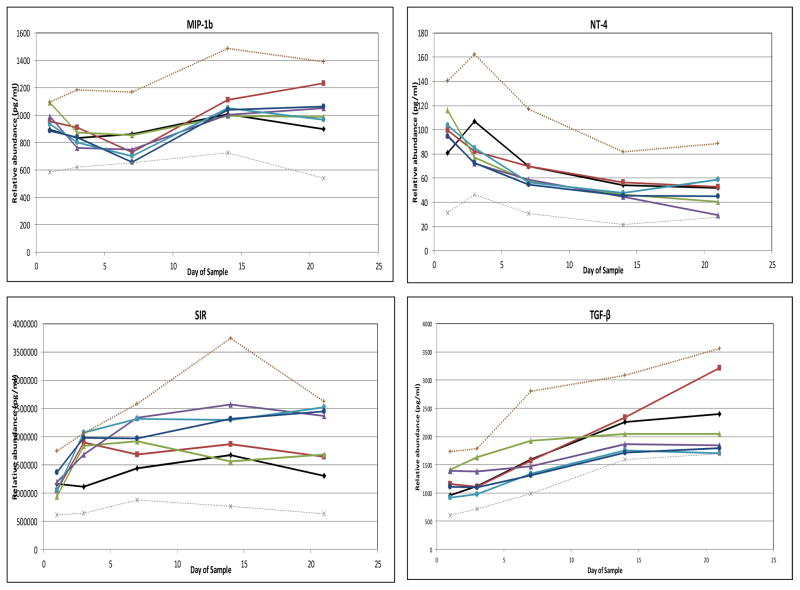
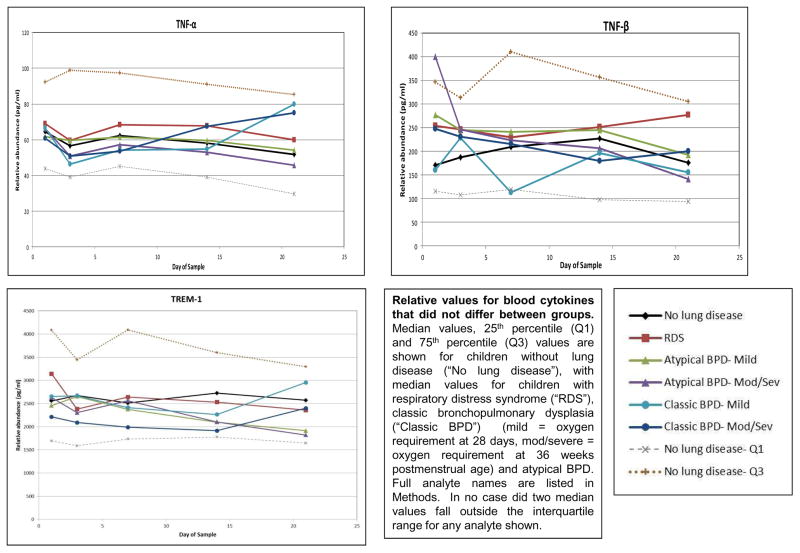
No median cytokine levels in the RDS group fell outside the interquartile range (IQR) of infants in the NLD group (Figure 4). To simplify presentation, Figure 4 depicts only values for infants in the moderate-to-severe BPD groups, except in the case of GM-CSF. For the majority of cytokines, median cytokine levels in neither the atypical nor the classic BPD groups (whether all BPD or only moderate-to-severe BPD) fell outside the IQR of the NLD group. However, median levels of IL-8 and MMP-9 fell above the IQR of the NLD infants for at least two determinations in both the atypical BPD and classic BPD groups. Median levels of CRP, IL-6, IL-10 and MIP-1α had at least two determinations above the IQR of the NLD infants in the classic BPD group, and median levels of IL-18 were above the IQR of the NLD group at least twice in the atypical BPD groups. In addition, GM-CSF median levels were below the IQR of the NLD group at least twice in both the atypical and classic BPD groups. Median BDNF and RANTES levels were below the IQR of the NLD group at least twice in the classic BPD group. In all cases, except that of GM-CSF, cytokine concentrations among the groups with moderate- to-severe BPD were more extreme than values among the BPD group as a whole and accounted for the two values outside the IQR of the NLD group (data not shown). None of the other analytes displayed two values outside the IQR of the NLD infants (Figure 5; available at www.jpeds.com). Sensitivity analyses using FiO2 0.21 (rather than <0.25) at 28 days as the definition of BPD or including the non-classified infants with FiO2 ≥0.25 as a separate “BPD” group in the analysis did not uncover any new analytes that departed from the IQR of the NLD group.
Figure 4. Relative blood cytokine values among study subjects.
Median values, 25th percentile and 75th percentile values are shown for children without lung disease (light lines), with median values for children with respiratory distress syndrome (RDS, open diamonds), classic bronchopulmonary dysplasia (classic BPD, filled squares) and atypical BPD (filled triangles) superimposed. In all cases, except GM-CSF, values for moderate/severe BPD (oxygen administration at 36 weeks’ postmenstrual age [PMA]) were more pronounced than those for children with mild BPD (oxygen administration at 28 days of age, but not 36 weeks’ PMA), so only results for children with moderate/severe BPD are shown. For GM-CSF, mild BPD values are also shown (open triangle = atypical BPD, open square = classic BPD). A–C, Cytokines for which at least one BPD type had at least 2 values above the interquartile range (IQR) determined from children with no lung disease. D and E, Cytokines with values below the IQR. A, B, D, and E, Cytokines outside the IQR for children with classic BPD. B, C, and E, Cytokines outside the IQR for children with atypical BPD. Children with RDS had no cytokine two values outside the IQR in either direction.
Figure 5.
DISCUSSION
This analysis of cytokine data from an observational cohort of infants born at 401–1000 grams detected qualitative differences in the pattern of blood cytokine expression for infants developing classic BPD (prolonged high oxygen administration) or atypical BPD (late development of oxygen requirement after period of low or absent oxygen administration). In a previous analysis of 1062 infants from the same dataset, we reported that when controlled for multiple clinical factors, elevated peak levels of IL-1, IL-6, IL-8, IL-10 and IFNγ were associated with death or presence of BPD (defined by supplemental oxygen administration at 36 weeks PMA) when compared with infants without those outcomes [11]. The same was true of depressed peak levels of IL-17, RANTES and TNFβ. However, the predictive characteristics of the multivariate regression model was improved only marginally by the addition of the cytokine data to the model containing clinical variables.
The current analyses differ from the previous analysis in that it divided BPD into two types (classic and atypical), which differed in the pattern (continued supplemental oxygen exposure vs. period without supplemental oxygen) and degree of supplemental oxygen exposure (higher among infants with classic BPD). The cohort included in the current analysis was smaller for two reasons. Children who did not survive to the time of classification and those who could not be categorized into one of the pre-defined groups were excluded, which left 2/3 of the initial cohort available for analysis. The analysis also differed in that the previous study studied peak cytokine values in a multivariate analysis; the current evaluation was qualitative in its approach (two median determinations outside interquartile range of values from infants without evidence of RDS or BPD).
Despite these differences in population, definitions and methods, our data were consistent with the previous analysis in identifying altered levels of IL-6, IL-8, IL-10 and RANTES among infants who subsequently developed BPD [11]. The findings, particularly for IL-8, are consistent with other reports demonstrating elevated levels of IL-8 in the blood [12] or tracheal aspirate [1, 4] of infants who develop BPD or in animal models of BPD [18]. Other cytokines, such as BDNF, GM-CSF and MMP9, were altered both in our analysis and the unadjusted analysis of the previous study. Some, however, such as CRP, IL-18 and MIP-1α, were altered only in the current analysis.
The current analyses revealed groups of cytokines outside the interquartile range of NLD infants among infants with classic and atypical BPD, with some distinct patterns of deviation (e.g., consistent depression of BDNF among infants with classic BPD, but little deviation from IQR among those with atypical BPD). In addition, even when both groups departed from the IQR, the patterns could differ substantially. IL-8 among infants with classic BPD differed markedly from the IQR of children without lung disease at nearly every time point, and the only marked difference among infants with atypical BPD occurred at 21 days. MMP9 showed a crisscross pattern with elevation noted early among children with atypical BPD, but late among those with classic BPD. The differences in the cytokines associated with varying BPD types and the patterns of those alterations may yield important hypotheses for further evaluation of factors that may be involved in the pathogenesis of BPD.
Laughon et al [12] recently reported results of a similar analysis of cytokines measured on blood spots from 908 infants born at <28 weeks’ gestation who participated in the ELGAN study [19]. The investigators divided children among groups similar to those reported here: “low FiO2” (FiO2 < 0.23 on all days between days 3 and 7 after birth and on day 14 after birth), “normal pulmonary function followed by pulmonary deterioration” (“PD,” FiO2 <0.23 on any of the days between days 3 and 7, but FiO2 > 0.25 on day 14, similar to our “atypical BPD” definition) and “early and persistent pulmonary disease” (“EPPD,” FiO2 ≥ 0.23 on all days between days 3 and 7 and > 0.25 on day 14, similar to our “classic BPD” definition). The investigators used a time-oriented logistic regression model for analysis [12]. In that dataset, collected from different children and evaluated with a different statistical analysis, elevated IL-8 was associated with EPPD. Elevations in RANTES were associated with decreased risk for both EPPD and PD, which is consistent with our data. They also reported associations for three molecules not included in our cytokine set: Increased VEGF was associated with decreased risk of both PD and EPPD, elevated MMP-1 was associated with decreased risk of EPPD, and elevated ICAM-1 with increased risk of EPPD [12]. Their cytokine set did not include IL-10, IL-18, MIP-1α, BDNF or GM-CSF, among which we found alterations from the normal range in our analysis. Their complementary data strengthens the argument that blood cytokine analysis is a potentially useful biomarker for generating hypotheses regarding differing patterns of prematurity-related lung disease.
In our analysis, the cytokines and proteins that deviated from the interquartile range of infants without significant lung disease included CRP, IL-6, IL-8, GM-CSF and MIP-1α, associated with attraction and activation of innate inflammatory cells. IL-6 and IL-10 have been associated with stimulating Th2 responses and the development of lung disease among premature infants [8, 20], and RANTES also is associated with T-cell responses. IL-18, a member of the IL-1 superfamily, has pleiotropic effects on both innate and acquired immunity [21]. BDNF has been associated with the immunopathogenesis of inflammatory disorders both within and outside the nervous system [22]. IL-8 is perhaps the best characterized cytokine and has the best evidence for biological plausibility and possible causality. Blocking IL-8 or homologous cytokines in animal models inhibits some changes associated with lung injury or BPD [23, 24], and limiting tidal volume in adult humans decreases plasma IL-8 values in adult humans with acute lung injury [25]. However, preliminary investigations such as ours may be most useful for generating hypotheses and identifying potential biomarkers, rather than for generating complete mechanistic models.
This study has several limitations. The dataset is limited in scope and elements due to the initial decisions of the investigators. The analysis also represents practice and outcome at the turn of the 21st century, as neither the physiological requirement for oxygen nor the administration of room air by nasal cannula was used in the definition of BPD. Furthermore, the use of a 1000 g birth weight cutoff for the group likely enriches it for small-for-gestational-age infants. The available cytokine panel included primarily pro-inflammatory cytokines, and other mediators and growth factors potentially important in the development of lung disease were not evaluated. Measurements of cytokine values on blood spots is many steps removed from events in the lung, thus events distant from the lung and/or unrelated to lung disease, including intracellular events in circulating leukocytes, may explain some of the alterations we observed. The definitions of the “normal” range for cytokine values and significant deviations from normal were chosen a priori but were nonetheless arbitrary. Similarly, the definitions for the lung disease groups, although they resulted in groups that closely approximated previously described patterns of lung disease [13], were chosen pragmatically. The lung disease definitions excluded a large proportion of infants with indeterminate or incomplete data and included potentially dissimilar infants (e.g. infants off all support and infants receiving FiO2 0.21 but also respiratory support) into one of four groups. The population chosen for “normal” values were also exposed to stresses inherent to premature birth, even if these events were not reflected in supplemental oxygen requirement. The a priori decisions made to produce the four groups may have introduced bias and could limit the generalizability of the results. A different approach to the heterogeneity in the data might yield different conclusions. Finally, a diagnosis of BPD is of only limited utility in predicting future respiratory outcomes [26]. Given the qualitative and exploratory nature of the project, results were purposely not analyzed for statistical significance or corrected for covariates and confounders, such as gestational age or illness severity markers, to avoid inadvertently missing a potentially useful result. Despite these limitations, our analysis suggests several plausible hypotheses regarding differences between subtypes of BPD. It will fall to future, rigorous, hypothesis-testing, quantitative investigations to determine which hypotheses bear fruit.
In summary, this exploratory evaluation of premature infants at risk for BPD revealed several cytokines that deviated from values in premature infants without lung disease. Further, these cytokines displayed different patterns among children developing atypical BPD and those developing classic BPD. This unbiased graphical representation of blood cytokine values should generate hypotheses about the molecular antecedents of differing patterns of lung disease among premature infants. Reasonable next steps would include returning to animal models of lung injury to assess the necessity and sufficiency of alterations in analytes not previously associated with neonatal lung disease (eg, BDNF) and specific, targeted tests of reproducibility of novel differences in presence (eg, IL-18) or pattern (eg, IL-8, MMP9) of analytes among new, prospective, validation cohorts of premature infants.
Acknowledgments
The Neonatal Research Network’s Cytokines Study was supported by the National Institutes of Health (NIH; M01 RR30, M01 RR32, M01 RR39, M01 RR70, M01 RR80, M01 RR633, M01 RR750, M01 RR997, M01 RR6022, M01 RR7122, M01 RR8084, M01 RR16587), the NICHD (U01 HD36790, U10 HD21364, U10 HD21373, U10 HD21385, U10 HD21397, U10 HD21415, U10 HD27851, U10 HD27853, U10 HD27856, U10 HD27871, U10 HD27880, U10 HD27881, U10 HD27904, U10 HD34216, U10 HD40461, U10 HD40492, U10 HD40498, U10 HD40689), and the Centers for Disease Control and Prevention (CDC; Interagency Agreement Y1-HD-5000-01). C. D. is supported by NICHD (1 U10 HD 068263). Participating sites collected data and transmitted it to RTI International, the data coordinating center for the network, which stored, managed, and analyzed the data for this study. Although NICHD and CDC staff did have input into the study design, conduct, analysis, and manuscript drafting, the content is solely the responsibility of the authors and does not necessarily represent the official views of NICHD or CDC.
We are indebted to our medical and nursing colleagues and the infants and their parents who agreed to take part in this study.
APPENDIX
The following investigators are members of the NIDHD Neonatal Research Network, National Institutes of Health, Bethesda, MD:
Abhik Das (Data Coordinating Center Principal Investigator) and Scott A. McDonald (Data Coordinating Center Statistician) had full access to all of the data in the study, and with the NRN Center Principal Investigators, take responsibility for the integrity of the data and accuracy of the data analysis.
NRN Steering Committee Chair: Alan H. Jobe, MD PhD, University of Cincinnati, Cincinnati, OH.
Alpert Medical School of Brown University and Women & Infants Hospital of Rhode Island, Providence, RI– Abbot R. Laptook, MD; William Oh, MD; Lewis P. Rubin, MD; Angelita M. Hensman, RN BSN.
Case Western Reserve University, Rainbow Babies & Children’s Hospital, Cleveland, OH– Avroy A. Fanaroff, MD; Michele C. Walsh, MD MS; Nancy S. Newman, RN; Bonnie S. Siner, RN.
Cincinnati Children’s Hospital Medical Center, University Hospital, and Good Samaritan Hospital, Cincinnati, OH– Edward F. Donovan, MD; Vivek Narendran, MD MRCP; Barbara Alexander, RN; Cathy Grisby, BSN CCRC; Jody Hessling, RN; Marcia Worley Mersmann, RN CCRC; Holly L. Mincey, RN BSN.
Duke University School of Medicine University Hospital, Alamance Regional Medical Center, and Durham Regional Hospital, Raleigh-Durham, NC– C. Michael Cotten, MD MHS; Kathy J. Auten, MSHS.
Emory University Children’s Healthcare of Atlanta, Grady Memorial Hospital, and Emory Crawford Long Hospital, Atlanta, GA– Ellen C. Hale, RN BS CCRC.
Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, MD – Linda L. Wright, MD; Sumner J. Yaffe, MD; Elizabeth M. McClure, MEd.
Indiana University University Hospital, Methodist Hospital, Riley Hospital for Children, and Wishard Health Services, Indianapolis, IN– Brenda B. Poindexter, MD MS; James A. Lemons, MD; Diana D. Appel, RN BSN; Dianne E. Herron, RN; Leslie D. Wilson, BSN CCRC.
RTI International, Research Triangle Park, NC– W. Kenneth Poole, PhD; Betty K. Hastings; Kristin M. Zaterka-Baxter, RN BSN; Jeanette O’Donnell Auman, BS; Scott E. Schaefer, MS.
Stanford University Lucile Packard Children’s Hospital, Palo Alto, CA– David K. Stevenson, MD; Krisa P. Van Meurs, MD; M. Bethany Ball, BS CCRC.
Statens Serum Institut, Copenhagen, Denmark – Kristin Skogstrand, PhD; David M. Hougaard, MD DSc.
University of Alabama at Birmingham Health System and Children’s Hospital of Alabama, Birmingham, AL– Monica V. Collins, RN BSN MaEd; Shirley S. Cosby, RN BSN.
University of California – San Diego Medical Center and Sharp Mary Birch Hospital for Women, San Diego, CA– Neil N. Finer, MD; Maynard R. Rasmussen, MD; David Kaegi, MD; Kathy Arnell, RNC; Clarence Demetrio, RN; Wade Rich, BSHS RRT.
University of Miami Holtz Children’s Hospital, Miami, FL – Charles R. Bauer, MD; Shahnaz Duara, MD; Ruth Everett-Thomas, RN MSN.
University of New Mexico Health Sciences Center, Albuquerque, NM – Lu-Ann Papile, MD; Conra Backstrom Lacy, RN.
University of Tennessee, Memphis, TN – Sheldon B. Korones, MD; Henrietta S. Bada, MD; Tina Hudson, RN BSN.
University of Texas Southwestern Medical Center at Dallas Parkland Health & Hospital System and Children’s Medical Center Dallas, Dallas, TX – Abbot R. Laptook, MD; Walid A. Salhab, MD; Susie Madison, RN.
University of Texas Health Science Center at Houston Medical School, Children’s Memorial Hermann Hospital, and Lyndon B. Johnson General Hospital, Houston, TX – Kathleen A. Kennedy, MD MPH; Brenda H. Morris, MD; Esther G. Akpa, RN BSN; Patty A. Cluff, RN; Claudia I. Franco, RNC MSN; Anna E. Lis, RN BSN; Georgia E. McDavid, RN; Patti Pierce Tate, RCP.
Wake Forest University Baptist Medical Center, Forsyth Medical Center, and Brenner Children’s Hospital, Winston-Salem, NC– T. Michael O’Shea, MD MPH; Nancy J. Peters, RN CCRP.
Wayne State University Hutzel Women’s Hospital and Children’s Hospital of Michigan, Detroit, MI– G. Ganesh Konduri, MD; Rebecca Bara, RN BSN; Geraldine Muran, RN BSN.
Yale University Yale-New Haven Children’s Hospital, New Haven, CT– Patricia Gettner, RN; Monica Konstantino, RN BSN; JoAnn Poulsen, RN.
Footnotes
The authors declare no conflicts of interest.
References
- 1.D’Angio CT, Basavegowda K, Avissar NE, Finkelstein JN, Sinkin RA. Comparison of tracheal aspirate and bronchoalveolar lavage specimens from premature infants. Biol Neonate. 2002;82:145–9. doi: 10.1159/000063608. [DOI] [PubMed] [Google Scholar]
- 2.Jonsson B, Tullus K, Brauner A, Lu Y, Noack G. Early increase of TNF alpha and IL-6 in tracheobronchial aspirate fluid indicator of subsequent chronic lung disease in preterm infants. Archives of Disease in Childhood Fetal & Neonatal Edition. 1997;77:F198–201. doi: 10.1136/fn.77.3.f198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Groneck P, Gotze-Speer B, Oppermann M, Eiffert H, Speer CP. Association of pulmonary inflammation and increased microvascular permeability during the development of bronchopulmonary dysplasia: a sequential analysis of inflammatory mediators in respiratory fluids of high-risk preterm neonates. Pediatrics. 1994;93:712–8. [PubMed] [Google Scholar]
- 4.Kotecha S, Chan B, Azam N, Silverman M, Shaw RJ. Increase in interleukin-8 and soluble intercellular adhesion molecule-1 in bronchoalveolar lavage fluid from premature infants who develop chronic lung disease. Archives of Disease in Childhood Fetal & Neonatal Edition. 1995;72:F90–6. doi: 10.1136/fn.72.2.f90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kotecha S. Cytokines in chronic lung disease of prematurity. Eur J Pediatr. 1996;155(Suppl 2):S14–7. doi: 10.1007/BF01958074. [DOI] [PubMed] [Google Scholar]
- 6.McColm JR, McIntosh N. Interleukin-8 in bronchoalveolar lavage samples as predictor of chronic lung disease in premature infants (letter) Lancet. 1994;343:729. doi: 10.1016/s0140-6736(94)91606-3. [DOI] [PubMed] [Google Scholar]
- 7.Baier RJ, Loggins J, Kruger TE. Increased interleukin-8 and monocyte chemoattractant protein-1 concentrations in mechanically ventilated preterm infants with pulmonary hemorrhage. Pediatric Pulmonology. 2002;34:131–7. doi: 10.1002/ppul.10141. [DOI] [PubMed] [Google Scholar]
- 8.Garingo A, Tesoriero L, Cayabyab R, Durand M, Blahnik M, Sardesai S, et al. Constitutive IL-10 expression by lung inflammatory cells and risk for bronchopulmonary dysplasia. Pediatr Res. 2007;61:197–202. doi: 10.1203/pdr.0b013e31802d8a1c. [DOI] [PubMed] [Google Scholar]
- 9.Vento G, Capoluongo E, Matassa PG, Concolino P, Vendettuoli V, Vaccarella C, et al. Serum levels of seven cytokines in premature ventilated newborns: correlations with old and new forms of bronchopulmonary dysplasia. Intensive Care Med. 2006;32:723–30. doi: 10.1007/s00134-006-0138-1. [DOI] [PubMed] [Google Scholar]
- 10.Viscardi RM, Muhumuza CK, Rodriguez A, Fairchild KD, Sun CC, Gross GW, et al. Inflammatory markers in intrauterine and fetal blood and cerebrospinal fluid compartments are associated with adverse pulmonary and neurologic outcomes in preterm infants. Pediatr Res. 2004;55:1009–17. doi: 10.1203/01.pdr.0000127015.60185.8a. [DOI] [PubMed] [Google Scholar]
- 11.Ambalavanan N, Carlo WA, D’Angio CT, McDonald SA, Das A, Schendel D, et al. Cytokines associated with bronchopulmonary dysplasia or death in extremely low birth weight infants. Pediatrics. 2009;123:1132–41. doi: 10.1542/peds.2008-0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laughon M, Bose C, Allred EN, O’Shea TM, Ehrenkranz RA, VanMarter LJ, et al. Patterns of blood protein concentrations of ELGANs classified by three patterns of respiratory disease in the first 2 postnatal weeks. Pediatr Res. 2011;70:292–6. doi: 10.1203/PDR.0b013e3182274f35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Charafeddine L, D’Angio CT, Phelps DL. Atypical chronic lung disease patterns in neonates. Pediatrics. 1999;103:759–65. doi: 10.1542/peds.103.4.759. [DOI] [PubMed] [Google Scholar]
- 14.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163:1723–9. doi: 10.1164/ajrccm.163.7.2011060. [DOI] [PubMed] [Google Scholar]
- 15.Carlo WA, McDonald SA, Tyson JE, Stoll BJ, Ehrenkranz RA, Shankaran S, et al. Cytokines and neurodevelopmental outcomes in extremely low birth weight infants. J Pediatr. 2011;159:919–25. e3. doi: 10.1016/j.jpeds.2011.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Skogstrand K, Thorsen P, Norgaard-Pedersen B, Schendel DE, Sorensen LC, Hougaard DM. Simultaneous measurement of 25 inflammatory markers and neurotrophins in neonatal dried blood spots by immunoassay with xMAP technology. Clin Chem. 2005;51:1854–66. doi: 10.1373/clinchem.2005.052241. [DOI] [PubMed] [Google Scholar]
- 17.Walsh MC, Yao Q, Gettner P, Hale E, Collins M, Hensman A, et al. Impact of a physiologic definition on bronchopulmonary dysplasia rates. Pediatrics. 2004;114:1305–11. doi: 10.1542/peds.2004-0204. [DOI] [PubMed] [Google Scholar]
- 18.D’Angio CT, LoMonaco MB, Chaudhry SA, Paxhia A, Ryan RM. Discordant pulmonary proinflammatory cytokine expression during acute hyperoxia in the newborn rabbit. Experimental Lung Research. 1999;25:443–65. doi: 10.1080/019021499270187. [DOI] [PubMed] [Google Scholar]
- 19.O’Shea TM, Allred EN, Dammann O, Hirtz D, Kuban KC, Paneth N, et al. The ELGAN study of the brain and related disorders in extremely low gestational age newborns. Early Hum Dev. 2009;85:719–25. doi: 10.1016/j.earlhumdev.2009.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laouini D, Alenius H, Bryce P, Oettgen H, Tsitsikov E, Geha RS. IL-10 is critical for Th2 responses in a murine model of allergic dermatitis. J Clin Invest. 2003;112:1058–66. doi: 10.1172/JCI18246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Biet F, Locht C, Kremer L. Immunoregulatory functions of interleukin 18 and its role in defense against bacterial pathogens. J Mol Med (Berl) 2002;80:147–62. doi: 10.1007/s00109-001-0307-1. [DOI] [PubMed] [Google Scholar]
- 22.Linker R, Gold R, Luhder F. Function of neurotrophic factors beyond the nervous system: inflammation and autoimmune demyelination. Crit Rev Immunol. 2009;29:43–68. doi: 10.1615/critrevimmunol.v29.i1.20. [DOI] [PubMed] [Google Scholar]
- 23.Folkesson HG, Matthay MA, Hebert CA, Broaddus VC. Acid aspiration-induced lung injury in rabbits is mediated by interleukin-8-dependent mechanisms. J Clin Invest. 1995;96:107–16. doi: 10.1172/JCI118009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Auten RL, Jr, Mason SN, Tanaka DT, Welty-Wolf K, Whorton MH. Anti-neutrophil chemokine preserves alveolar development in hyperoxia-exposed newborn rats. American Journal of Physiology - Lung Cellular & Molecular Physiology. 2001;281:L336–44. doi: 10.1152/ajplung.2001.281.2.L336. [DOI] [PubMed] [Google Scholar]
- 25.Parsons PE, Eisner MD, Thompson BT, Matthay MA, Ancukiewicz M, Bernard GR, et al. Lower tidal volume ventilation and plasma cytokine markers of inflammation in patients with acute lung injury. Crit Care Med. 2005;33:1–6. doi: 10.1097/01.ccm.0000149854.61192.dc. discussion 230–2. [DOI] [PubMed] [Google Scholar]
- 26.Parad RB, Davis JM, Lo J, Thomas M, Marlow N, Calvert S, et al. Prediction of respiratory outcome in extremely low gestational age infants. Neonatology. 2015;107:241–8. doi: 10.1159/000369878. [DOI] [PMC free article] [PubMed] [Google Scholar]