Abstract
Although total body perfusion with extracorporeal life support (ECLS) can be maintained for weeks, individual organ perfusion beyond 12 hours has yet to be achieved clinically. Normothermic ex situ heart perfusion (ESHP) offers the potential for prolonged cardiac preservation. We developed an ESHP system to study the effect of perfusate variables on organ preservation, with the ultimate goal of extending organ perfusion for ≥ 24 hours.
Forty porcine hearts were perfused for a target of 12 hours. Hearts that maintained electromechanical activity and had a <3× increase in vascular resistance were considered successful preservations. Perfusion variables, metabolic byproducts, and histopathology were monitored and sampled to identify factors associated with preservation failure.
Twenty-two of 40 hearts were successfully preserved at 12 hours. Successful 12-hour experiments demonstrated lower potassium (4.3±0.8 vs. 5.0±1.2 mmol/L, p=0.018) and lactate (3.5±2.8 vs. 4.5±2.9 mmol/L, p=0.139) levels, and histopathology revealed less tissue damage (p=0.003) and less weight gain (p=0.072).
Results of these early experiments suggest prolonged ESHP is feasible, and that elevated lactate and potassium levels are associated with organ failure. Further studies are necessary to identify the ideal perfusate for normothermic ESHP.
Keywords: Heart, ex situ normothermic perfusion, organ preservation
Introduction
Cold immersion has been the standard preservation technique for solid organ transplantation since the 1960s; however, this only provides effective preservation of up to 6 hours.1 A technique able to preserve hearts for 12 hours or longer would allow for better donor matching and recipient need prioritization, the potential for medical and immunologic organ optimization, and a wider range of organ transport.
Isolated heart perfusion for purposes of physiologic study has been performed for over a century.2,3 Many groups have shown that hypothermic perfusion can preserve hearts for hours with satisfactory outcomes.4,5 More recently, ESHP has been performed at normothermia (37°C) as a means of preserving hearts for transplantation. The advantage of perfusion at normothermia is that the functioning heart can be evaluated at near physiologic conditions, while maintaining normal cell metabolism.6 Several groups have developed normothermic ESHP systems and have demonstrated successful preservation of porcine hearts for 8–12 hours. The perfused hearts showed less cell damage and superior function after transplantation than standard cold preserved controls.6,9 Building on this work TransMedics™ developed a clinical system, Organ Care System™ (OCS), designed for 6 hours of cardiac preservation and transportation. The OCS gained European Conformity (CE Mark) rk approval in 2007 and is currently being tested in clinical trials in the U.S., but it is not being marketed for long-term normothermic perfusion.
Total body perfusion using ECLS can be achieved for weeks with normal organ function. However, individual ex situ perfused organs typically fail before 12 hours of perfusion is achieved.10 Ascertaining why individually perfused organs fail so rapidly will allow us to perfuse organs for days or weeks. . Our laboratory has a long history of developing extracorporeal perfusion techniques, leading to clinical applications such as extracorporeal membrane oxygenation (ECMO), ECLS for donation after cardiac death (eDCD), ECMO for cardiopulmonary resuscitation (ECPR), and continuous renal replacement therapy (CRRT). Based on this experience, we seek to develop reliable and reproducible techniques for prolonged organ perfusion. In this study of hearts, our goal was to first achieve routine twelve-hour preservation, and secondly to identify differences in the metabolic or hemodynamic milieus between successful and failed heart preservations. This work provides a platform for future experiments that will investigate perfusion parameters and various perfusate modifications, and may offer guidance for the continued development of a long-term normothermic cardiac preservation system.
Materials and Methods
Surgical Procedure
All animals received humane care in accordance with the NIH Guide for the Care and Use of Laboratory Animals. Animal protocols were approved by the University of Michigan Committee on Use and Care of Animals.
Healthy 50±10 Kg swine were anesthetized, and vascular access was obtained according to our established laboratory protocols.11 500 mL of blood was harvested and used to create initial perfusate. Midline sternotomy was performed, and unfractionated heparin 200 U/kg (Sagent Pharma, Schaumburg, IL) was administered. A perfusion cannula was placed in the innominate artery (all cannula used are products of Terumo CVS, Ann Arbor MI). 500 mL of high potassium cold cardioplegia (CAPS Inc. Detroit, MI) was infused via the arterial cannula after ligating the distal aorta. Once venous return to the heart was interrupted the remaining blood was harvested. To be consistent with current procurement practices, standard techniques were used to achieve topical cooling and remove the heart, which was then placed on ice bath. This also allowed for cannulation of the heart chambers in preparation for the perfusion system. Cold ischemia times did not exceed 1.5 hours.
Back Table Preparation
The pulmonary artery (PA) was cannulated with a 28Fr venous drainage cannula. A 10 Fr coronary sinus catheter, a temperature probe (ADI instruments, Dunedin NZ), and an 18 Fr right atrial drainage cannula were placed via a right atriotomy. Two 18 Fr drainage cannulas were placed via open pulmonary veins into the left atrium (LA) and left ventricle (LV). Electrodes were placed on the apex and on the LA and right atrium (RA) for continuous electrocardiogram (ECG) monitoring. The pericardium was preserved as much as possible and repaired where needed. The heart was drained of fluid and weighed. It was then de-aired and the cannulas were connected to the perfusion apparatus.
Blood Derived Perfusate
Given known advantages over non-sanguineous perfusates, a blood-based perfusate was utilized.6,8 The harvested blood was centrifuged (Thermo IEC Centra GP8R, Rockaway, NJ) at 3,500 RPM’s for 15 minutes to separate blood components and remove the white blood cell and platelet fractions. A total of approximately 1000 mL perfusate was created consisting of 50% plasma, 25% dextran 40 (Hospira, Lake Forest IL), 25% cell culture media (DMEM-Lonza, Walkersville, MD), and red blood cells titrated to a hemoglobin of 3–5 g/dl to limit viscosity. 10 U regular Insulin (Lilly, Indianapolis, IN), 80 mg Gentamycin, and 250 mg Nafcillin (APP Shaumberg, IL) were added to the perfusate. Dextran was intermittently titrated to a goal colloid osmotic pressure (COP) of 20–30 mmHg (Figure 1).
Figure 1. Blood Derived Perfusate Preparation Process.
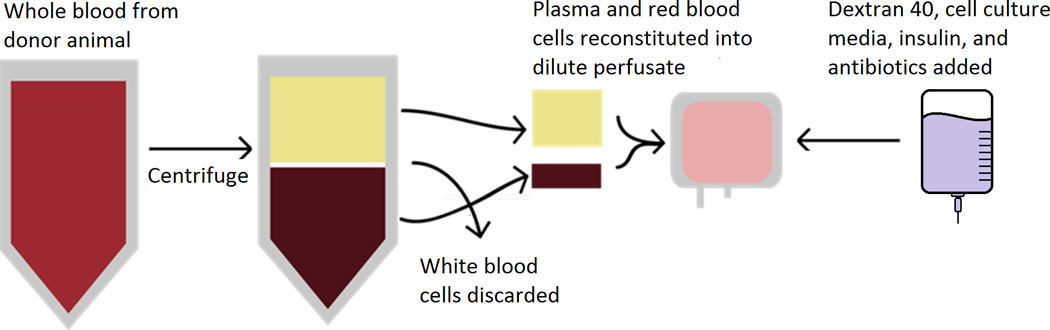
Heart Perfusion Circuit
We used commercially available components that are known to provide successful perfusion when used for extracorporeal life support. Perfusate traveled through ¼ inch Tygon™ tubing (Tygon, Lima, OH) via roller pump (Stockert Instruments Caps Roller Pump, Munich, Germany) from a cardiotomy reservoir, through a heat exchanger, to a pediatric oxygenator, and through a bubble trap prior to reaching the heart (all products Terumo Corp. Capiox RX05, Ann Arbor, MI). It was then infused retrograde into the aorta. Perfusate was returned to an open reservoir to allow full decompression of the heart chambers (Figure 2). A closed reservoir would minimize air/blood interface, however this would require the right ventricle to be actively pumping blood.12
Figure 2. ESHP Circuit.
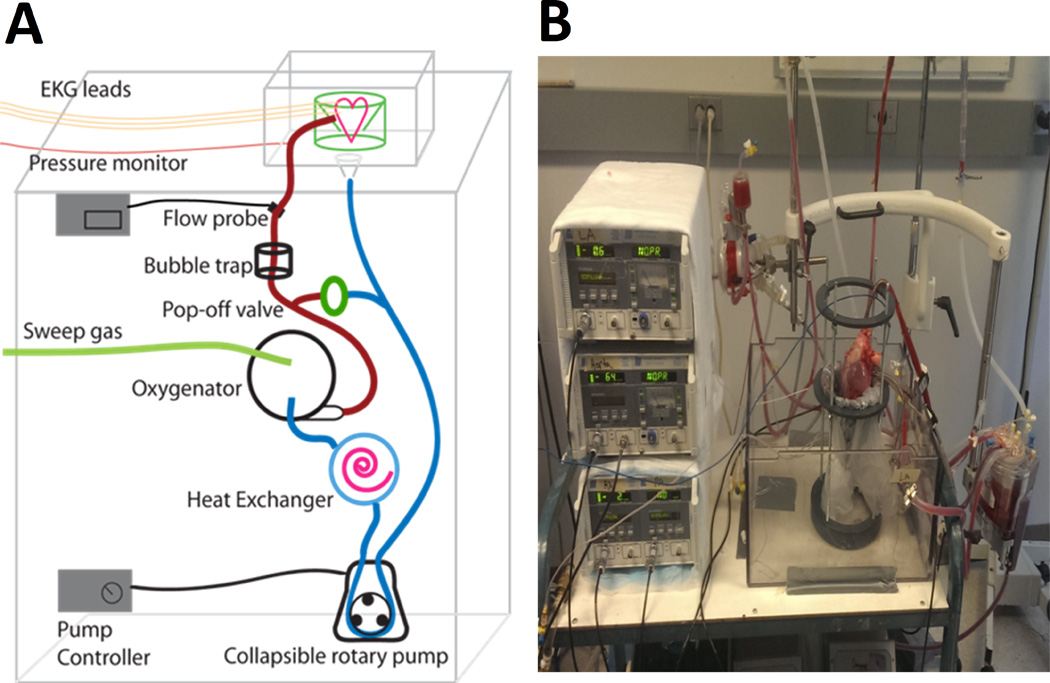
(A) Schematic of the ESHP Circuit; (B) Photograph of the ESHP Circuit
Perfusion Parameters
The benefits of hypothermic perfusion over cold storage are known, and some authors suggest that initial reperfusion with a mildly hypothermic solution may be beneficial for myocardial recovery.13,14 Thus we did not rapidly rewarm the heart, instead allowing the circuit to warm to goal temperature during the first hour. Perfusion began at a pressure of 20 mmHg and at 20°C, and was increased slowly over the hour to the goal of 40–60 mmHg, and 37°C. These parameters were targeted for the remainder of the experiment. After one hour the heart was defibrillated with up to 20 J using internal defibrillation paddles if needed (Philips, Andover MA). Following restoration of electromechanical activity, the contents of the reservoir were drained and replaced with fresh perfusate in order to eliminate toxins that may have accumulated during reperfusion, or residual cardioplegia. Subsequent perfusate exchanges of 100–200mL were performed every two hours. Arterial and venous blood gases, electrolytes, and hemoglobin (Hb) were measured every half hour. Chamber pressures, perfusion pressures, aortic flow, ECG, and temperature were recorded continuously. Experiments were terminated after 12 hours or if rhythmic ventricular contraction or organized electrical activity ceased, and/or if vascular resistance increased to >3× baseline. After termination of experiments the hearts were drained, weighted, and sent to pathology in 10% neutral buffered formalin for histologic analysis.
Histologic Analysis
Routine hematoxylin and eosin (H&E) staining was performed. Myocardial injury was graded based on the presence and severity of myofiber degeneration, myocardial hemorrhage, interstitial edema, and endothelial alterations. A myocardial injury grading scale was developed by a certified veterinary pathologist and used to score each hearts (Table 1). The average score was compared for each category between successful and unsuccessful groups.
Table 1.
Myocardial injury grading scale
| Myofiber Degeneration | |
| 0 | Absent |
| 1+ | Single to multiple foci of vacuolated, shrunken, or fragmented, hypereosinophilic myofibers |
| 2+ | More frequent multifocal foci to larger zones of vacuolated, shrunken, or fragmented hypereosinophilic myofibers |
| 3+ | Large coalescing to regionally extensive zones of vacuolated, shrunken, or fragmented hypereosinophilic myofibers |
| Myocardial hemorrhage | |
| 0 | Absent |
| 1+ | Focal to multifocal mild myocardial, epicardial, or endocardial hemorrhage |
| 2+ | Moderate multifocal to regionally extensive myocardial, epicardial, or endocardial hemorrhage |
| 3+ | Severe regionally extensive hemorrhage involving large portions of heart section |
| Interstitial edema | |
| 0 | Absent |
| 1+ | Mild and multifocal separation of myofiber bundles or expansion of perivascular spaces |
| 2+ | Moderate and multifocal separation of myofiber bundles or expansion of perivascular spaces |
| 3+ | Marked regionally extensive or multifocal separation of myofiber bundles and perivascular spaces |
| Endothelial changes | |
| 0 | Absent |
| 1+ | Plump endothelial cells, separation of endothelium from underlying basal lamina |
| 2+ | Cellular infiltration of vessel wall or disruption of layers of vessel wall |
| 3+ | Necrosis or severe vascular cellular infiltration |
Statistical Analysis
Statistical analyses were performed using Microsoft Excel 2013 (Microsoft, Redmond, WA) and SPSS (IBM, Armonk NY). Binary logistic regression (one-tailed) was performed to assess the predictive value of continuously measured variables and one-tailed Student’s t-tests were performed to assess differences in overall means between successful and unsuccessful groups. Test results are expressed as means ± standard deviations. Graphs are presented as overall means ± standard errors. Simple linear regression was utilized to examine the relationship between perfusion pressures, lactate, or potassium and myocardial injury scores.
Results
Perfusion
Twenty-two of 40 (55%) perfused hearts maintained organized contractility, electromechanical association, and acceptable resistance for at least 12 hours and were considered successful experiments. The remaining eighteen (45%) of the hearts lost rhythmic contractility or spontaneous electrical activity (n=11), or had a >3× increase in resistance (n=7) prior to the 12 hour mark and were grouped as “Unsuccessful”. The mean perfusion length in unsuccessful experiments was 8.3±2.2 hours (range: 3–11 hours).
On average, successful experiments had lower potassium (4.3±0.8 vs. 5.0±1.2 mmol/L, p=0.018) and lactate (3.5±2.8 vs. 4.5±2.9 mmol/L, p=0.139) levels (Figure 3). There were no significant differences between oxygen delivery and oxygen consumption, temperature, perfusion flow, perfusion pressures, glucose, Hb, pH, pCO2, or pO2 (Figures 4–6). Given that the majority of experiments did not fail before 6 hours, we re-examined the data using a 1-tailed t-test to compare mean values from hours 6–12. No additional significant factors were revealed, though lactate came closer to approaching significance (p=0.09).
Figure 3. Metabolic Factors in Perfusate Solution During ESHP (Averages: Successful v Unsuccessful).
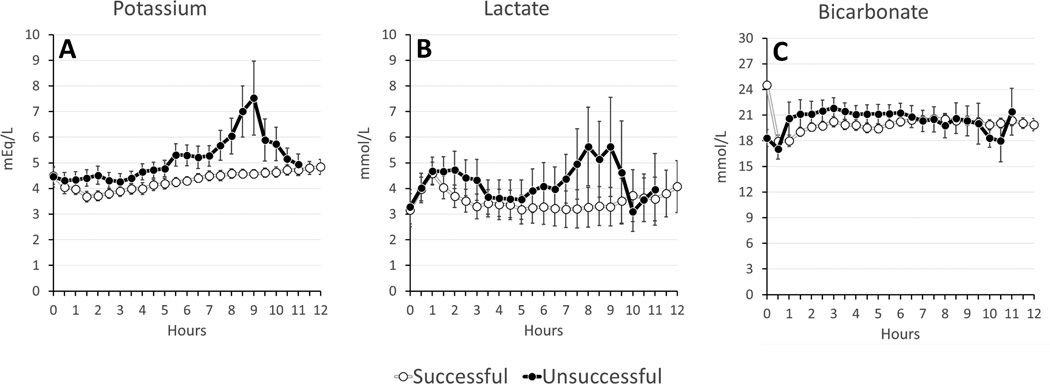
(A) Potassium; (B) Lactate; (C) Bicarbonate
Figure 4. Perfusion Parameters (Averages: Successful v Unsuccessful).
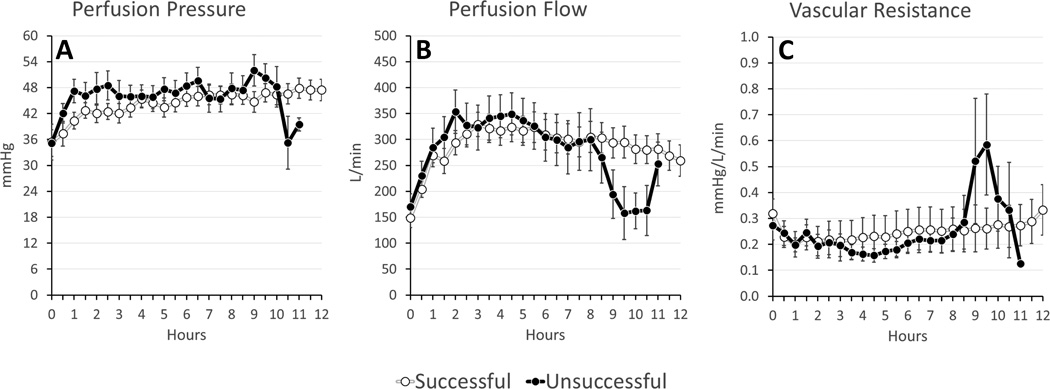
(A) Perfusion Pressure; (B) Perfusion Flow; (C) Coronary Vascular Resistance (calculated from perfusion pressure/perfusion flow)
Figure 6. Post-oxygenator Perfusion Gases.
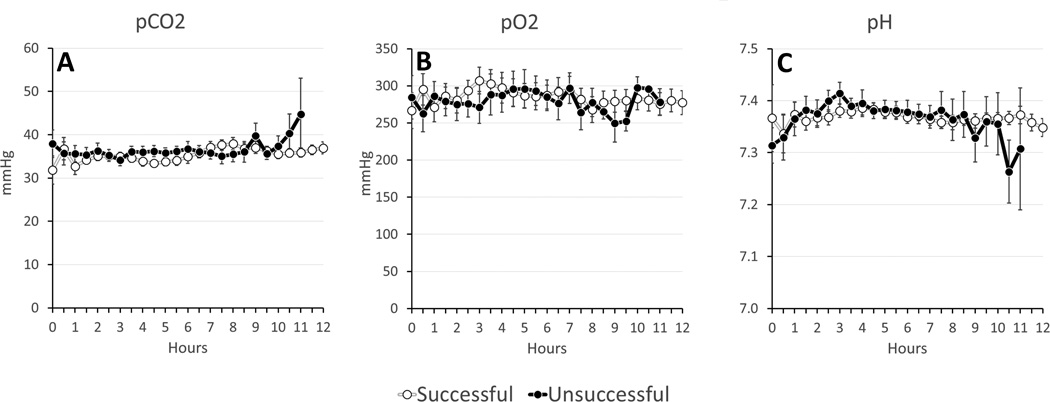
(A) Partial Pressure of CO2 (pCO2); (B) Partial Pressure of O2 (pO2); (C) pH
Binary logistic regression examined potassium, lactate, oxygen consumption, and vascular resistance as predictors of successful perfusion. Only potassium was found to be a significant variable (p=0.044) (Table 2).
Table 2.
Logistic Regression
| Odds Ratio | 95% CI | Significance | ||
|---|---|---|---|---|
| Lower | Upper | |||
| Potassium | 0.484 | 0.241 | 0.971 | 0.044 |
| Lactate | 0.978 | 0.782 | 1.224 | 0.437 |
| Oxygen Consumption | 1.187 | 0.790 | 1.784 | 0.225 |
| Vascular Resistance | 1.372 | 0.122 | 15.477 | 0.415 |
Pathology
Heart weight was used as a surrogate marker of tissue edema accumulated during perfusion. Successful hearts showed less percent weight gain (11.6±8.5 vs. 17.9±16.9 %, p=0.072) (Figure 7). Successful hearts had lower pathologic injury scores (Figure 8), with statistically significant differences in the severity of myofiber degeneration (p=0.001), myocardial hemorrhage (p=0.002), and endothelial changes (p=0.022). On linear regression analysis there was a significant association between myocardial injury score and other variables including perfusion pressure (p<0.001, R2 0.309), lactate (p<0.001, R2 0.281), and potassium (p<0.001, R2 0.351), though with high variability (Figure 9).
Figure 7. Organ Edema.
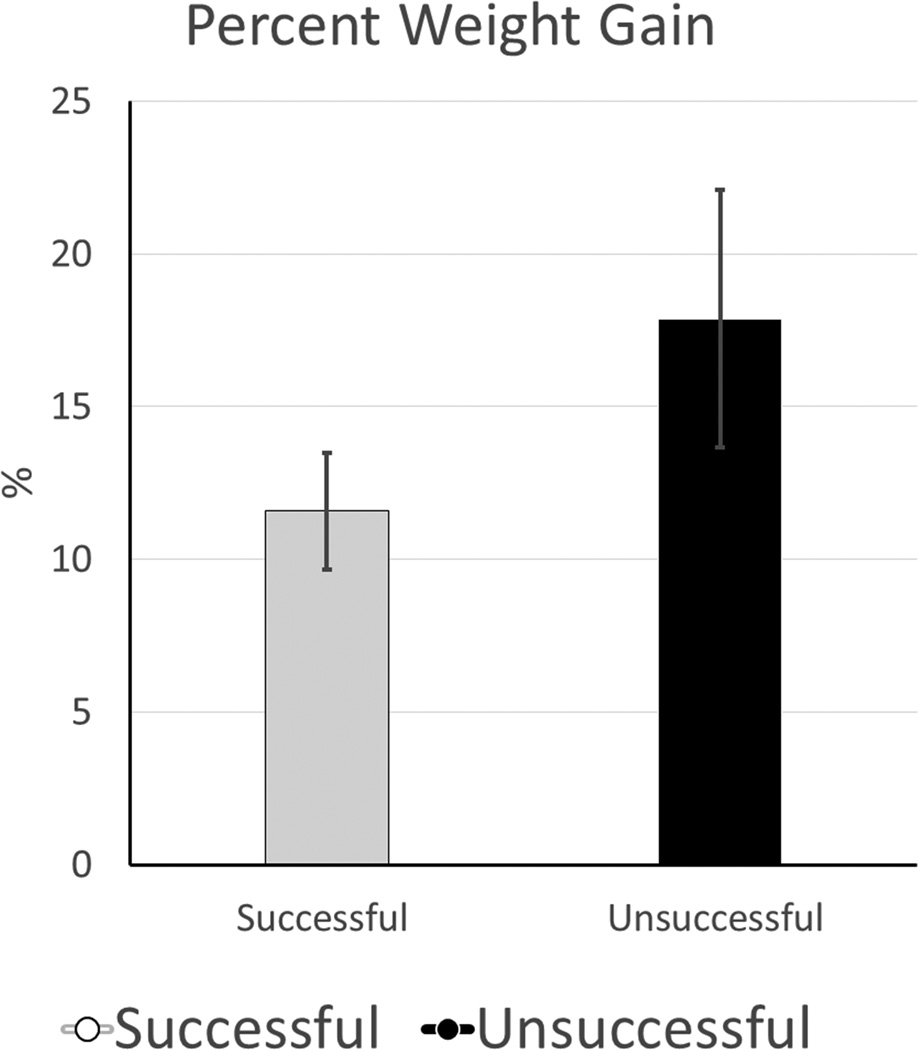
Percentage Weight Gain- (Post Perfusion Weight - Pre Perfusion Weight) / Pre Perfusion Weight
Figure 8. Myocardial Injury Score.
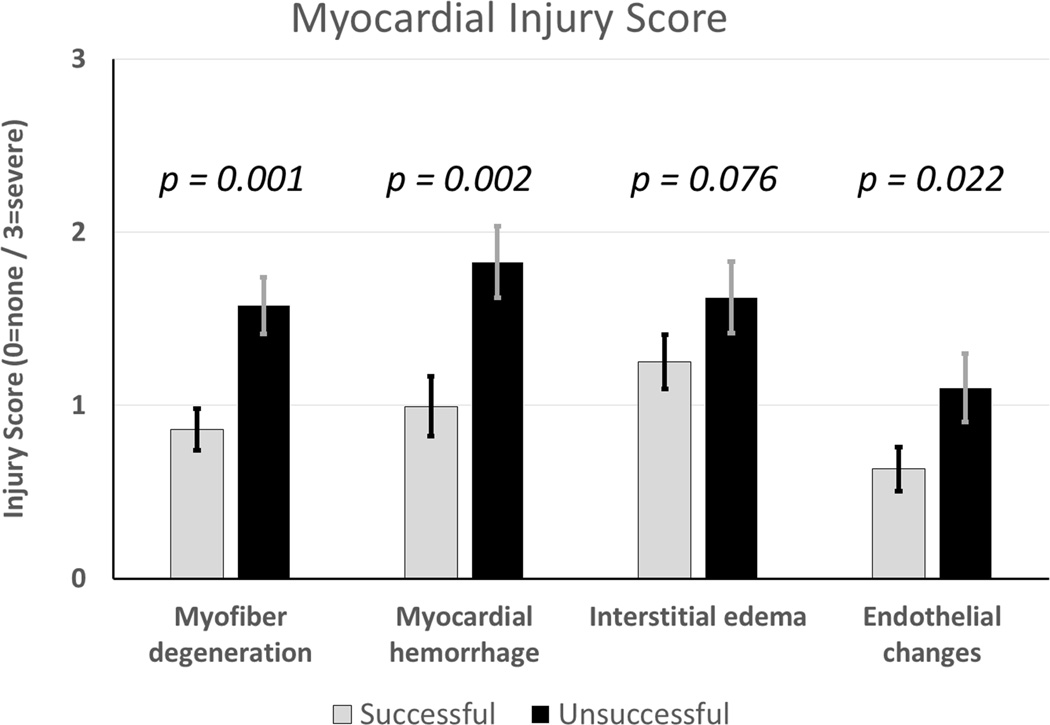
Each heart specimen scored by the same veterinary pathologist in four categories: myofiber degeneration, myocardial hemorrhage, interstitial edema, and endothelial changes.
Figure 9. Scattergrams show the results of linear regression analysis between myocardial injury score (MIS) and perfusion pressure (PP), lactate (L), and potassium (P).
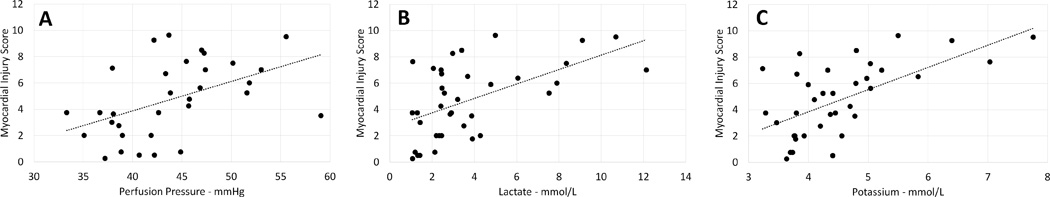
(A), MIS and PP p<0.001, R2 0.309); (B) MIS and L p<0.001, R2 0.281), and (C) MIS and P p<0.001, R2 0.351).
Discussion
While offering promising theoretical advantages over current heart preservation techniques, routine normothermic ESHP preservations lasting longer than 12 hours have not been achieved. This is a frustrating disconnect from clinical ECLS, which is able to support total body perfusion for weeks at a time.10 The ESHP perfusion apparatus is likely not a limiting factor; the same standard commercial components that work well in clinical ECLS systems are routinely used to create ESHP systems. The key factor in successful preservations is likely the perfusate and its management during perfusion. It is clear from numerous studies that the composition of perfusate strongly influences the viability of organs in ex situ experiments. Recommendations for the ideal hypothermic perfusate exist, however it has not been determined what the ideal perfusate for normothermic systems should be.3 This study was designed to evaluate outcomes after ESHP with a blood-based perfusate to begin to answer these questions.
The limitations of current preservation methods prompted other groups to investigate extended ex-situ perfusion. Hassanein et al, reported continuous normothermic sanguineous perfusion of porcine hearts in the beating working state for 12 hours. While these perfused hearts did not experience any cold ischemia or cardioplegic arrest, the group was able to demonstrate superior contractile, metabolic, and vasomotor function of these porcine organs when compared to cold immersion controls.8 This important study established the feasibility of normothermic heart perfusion for use in the clinical realm.
The commercially available OCS uses donor blood based perfusate and has been shown to have satisfactory outcomes compared to cold storage in clinical trials of short-term preservations.2 The OCS is targeted for organ transport and is not intended for long term or indefinite perfusion at this time. To our knowledge, the longest successful reported perfusion using this system has not exceeded 12 hours.2 In our study, although 55% of the hearts reached 12 hours, many appeared subjectively weak at that time. Both successful and unsuccessful preservations consistently demonstrated decreased vascular resistance, achieving physiologic coronary blood flow (~300 mL/min) at very low perfusion pressures (~40 mmHg). It was only immediately prior to failure that we observed increased vascular resistance in unsuccessful experiments. We did not alter coronary vascular resistance, and used resistance as a metric of organ health in a manner similar to the renal resistance metric in renal transplantation.15 The OCS targets physiologic perfusion pressures and uses vasodilators in order to deliver supraphysiologic levels of blood flow.12 While the OCS group demonstrated success with these parameters, in our study we observed an association between increasing perfusion pressure and myocardial injury scores. Thus it is unclear what the ideal perfusion pressure and flow for ESHP should be, and further study into this area is warranted.
Studies performed with the OCS have shown that end perfusion lactate levels above 5 mmol/L are predictive of poor post-transplant outcome.16 While not a significantly predictive factor in our experiments, we did observe increased lactate, as well as potassium, in many unsuccessful experiments. It is not difficult to extrapolate that other unmeasured toxins may accumulate and be harmful to the myocardium as suggested by other authors.17 A method of conditioning the perfusate, such as a hemofilter or dialysis, may be necessary in order to eliminate toxins and extend perfusion times
Increased lactate and potassium may be evidence of myocardial ischemia. This correlates with pathologic analysis of the heart specimens, which demonstrated a correlation between increased lactate and potassium levels and myocardial injury scores. As one might expect, we observed significantly more myocardial damage in the unsuccessful experiments. Garbade et al. have reported similar pathologic results, noting increased ultrastructural alteration, and decreasing ATP content, which correlated with a decline in function as perfused hearts approached twelve hours. This group hypothesized that a maximal ex-situ perfusion time may exist due to the absence of circulating factors present in the live animal.6 This theory is supported by clinical observation and experimental paradigms suggesting that neurologic regulation may be important for organ viability and vascular tone.18,19 Dr. Steen’s group has proposed a cocktail of hormones that may be important in regulating vascular tone, and were able to use this cocktail to promote hemodynamic stability in decapitated animal experiments. Others have shown that hormone therapies can improve myocardial function after brain death in similar animal experiments.19,20
In addition to maintaining vascular tone, other plasma factors may function to modulate organ response to ischemia. An Australian group demonstrated that glyceryl trinitrate, erythropoietin, and zoniporide are mediators of pre and post conditioning at the enzymatic level.21–23 They attempted to use these agents to limit the detrimental effects of warm ischemia in a porcine model of donation after circulatory death (DCD). They were able to show improvement in the tolerance to warm ischemia based on improvement in return of electromechanical function, and a decrease in biomarkers indicative of myocardial injury including lactate.9 If additional organ sustaining factor(s) exist they will need to be identified and provided in order to create the ideal perfusate for prolonged perfusion.
Limitations and Future Directions
This study was designed to evaluate heart preservation with a blood-based perfusate, and to begin to elucidate the ideal perfusate management necessary for prolonged preservation. It was not designed to evaluate cardiac function, and not intended as a method to prepare hearts for transplantation. The results are not meant for comparison to commercial systems designed to preserve hearts for rapid transplantation; however some comparison to transplant-based studies are included in the discussion. These results provide a baseline against which future studies evaluating perfusate modifications can be performed. Additional future studies will use cross circulation between ex situ organs and a live animal to: compare the excretory effect of a live animal versus hemofiltration and dialysis of the circuit, determine how the neurologic and hormonal milieu influences preservation, and evaluate the influence of cellular and humoral components of blood based perfusate. Preliminary cross circulation studies have shown great promise (Abstract ASAIO Chicago 2015).
Monitoring of cardiac function included in future experiments will help us to linearly quantify the performance of each heart as perfusate conditions change. More frequent histopathologic samples taken at baseline and at regular intervals can allow correlation between changes in function with changes at the tissue level.
Conclusion
Our results support that 12 hours of cardiac preservation can be achieved with ESHP. This work provides a foundation for the design of future experiments that will help determine what is necessary for prolonged ESHP. Eventually all hearts do fail despite adequate oxygenation suggesting the need for further investigation into the metabolic effects of the perfusate at the cellular and molecular level. With future work targeting the identification of essential perfusate components and system optimization, ESHP may become a viable approach for long-term cardiac preservation.
Figure 5. Oxygen Kinetics during ESHP (Averages: Successful v Unsuccessful).
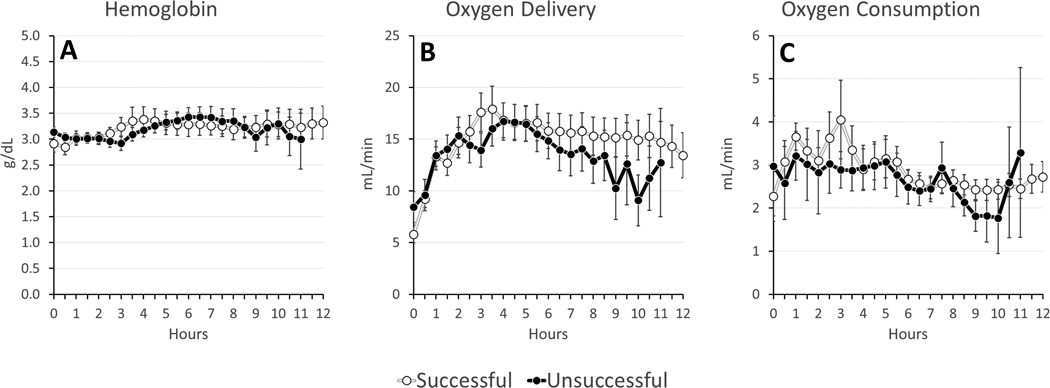
(A) Hemoglobin; (B) Oxygen Delivery; (C) Oxygen Consumption
Acknowledgments
We thank: The Frankel Family Foundation and the Charles Woodson Research Fund for their generous support without which this work would not be possible. Terumo Cardiovascular Group for contribution of vascular and perfusion materials, The University of Michigan Undergraduate Research Opportunity Program (UROP), and the Cardiovascular Center Summer Undergraduate Research Fellowship. Finally, the authors acknowledge the efforts of Adina Robinson PhD for her help with our statistical methods, and Cindy Cooke for review and preparation of the manuscript.
Footnotes
Disclosures: The authors declare no conflicts of interest.
References
- 1.Hicks M, Hing A, Gao L, Ryan J, MacDonald P. Organ preservation. In: Hornick P, Rose M, editors. Transplantation immunology. Humana Press; 2006. pp. 331–373. [Google Scholar]
- 2.Messer S, Ardehali A, Tsui S. Normothermic donor heart perfusion: Current clinical experience and the future. Transplant international : official journal of the European Society for Organ Transplantation. 2014 doi: 10.1111/tri.12361. [DOI] [PubMed] [Google Scholar]
- 3.Smulowitz PB, Serna DL, Beckham GE, Milliken JC. Ex vivo cardiac allograft preservation by continuous perfusion techniques. ASAIO journal (American Society for Artificial Internal Organs : 1992) 2000;46:389–396. doi: 10.1097/00002480-200007000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Proctor E, Parker R. Preservation of isolated heart for 72 hours. Br Med J. 1968;4:296–298. doi: 10.1136/bmj.4.5626.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wicomb W, Boyd ST, Cooper DK, Rose AG, Barnard CN. Ex vivo functional evaluation of pig hearts subjected to 24 hours' preservation by hypothermic perfusion. South African medical journal = Suid-Afrikaanse tydskrif vir geneeskunde. 1981;60:245–248. [PubMed] [Google Scholar]
- 6.Garbade J, Krautz C, Aupperle H, et al. Functional, metabolic, and morphological aspects of continuous, normothermic heart preservation: Effects of different preparation and perfusion techniques. Tissue Eng Part C Methods. 2009;15:275–283. doi: 10.1089/ten.tec.2008.0475. [DOI] [PubMed] [Google Scholar]
- 7.Yang Y, Lin H, Wen Z, et al. Keeping donor hearts in completely beating status with normothermic blood perfusion for transplants. The Annals of thoracic surgery. 2013;95:2028–2034. doi: 10.1016/j.athoracsur.2013.03.014. [DOI] [PubMed] [Google Scholar]
- 8.Hassanein WH, Zellos L, Tyrrell TA, et al. Continuous perfusion of donor hearts in the beating state extends preservation time and improves recovery of function. The Journal of thoracic and cardiovascular surgery. 1998;116:821–830. doi: 10.1016/S0022-5223(98)00452-8. [DOI] [PubMed] [Google Scholar]
- 9.Iyer A, Gao L, Doyle A, et al. Increasing the tolerance of dcd hearts to warm ischemia by pharmacological postconditioning. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2014;14:1744–1752. doi: 10.1111/ajt.12782. [DOI] [PubMed] [Google Scholar]
- 10.Annich GMLW, MacLaren G, Wilson JM, Bartlett RH. Ecmo: Extracorporeal cardiopulmonary support in critical care. Extracorporeal Life Support Organization. 2012 [Google Scholar]
- 11.Rojas-Pena A, Reoma JL, Krause E, et al. Extracorporeal support: Improves donor renal graft function after cardiac death. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2010;10:1365–1374. doi: 10.1111/j.1600-6143.2010.03063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ardehali A, Esmailian F, Deng M, et al. Ex-vivo perfusion of donor hearts for human heart transplantation (proceed ii): A prospective, open-label, multicentre, randomised non-inferiority trial. The Lancet. 2015 doi: 10.1016/S0140-6736(15)60261-6. [DOI] [PubMed] [Google Scholar]
- 13.Messer S, Large S. Resuscitating heart transplantation: The donation after circulatory determined death donor. European Journal of Cardio-Thoracic Surgery : ezv357. 2015 doi: 10.1093/ejcts/ezv357. [DOI] [PubMed] [Google Scholar]
- 14.Van Caenegem O, Beauloye C, Bertrand L, et al. Hypothermic continuous machine perfusion enables preservation of energy charge and functional recovery of heart grafts in an ex vivo model of donation following circulatory death. European Journal of Cardio-Thoracic Surgery : ezv409. 2015 doi: 10.1093/ejcts/ezv409. [DOI] [PubMed] [Google Scholar]
- 15.Jochmans I, Moers C, Smits J, et al. The prognostic value of renal resistance during hypothermic machine perfusion of deceased donor kidneys. American Journal of Transplantation. 2011;11:2214–2220. doi: 10.1111/j.1600-6143.2011.03685.x. [DOI] [PubMed] [Google Scholar]
- 16.Hamed A, Tsui S, Huber J, Lin R, Poggio EC, Ardehali A. 19: Serum lactate is a highly sensitive and specific predictor of post cardiac transplant outcomes using the organ care system. The Journal of Heart and Lung Transplantation. 2009;28:S71. [Google Scholar]
- 17.Kontos GJ, Jr, Borkon AM, Adachi H, et al. Successful extended cardiopulmonary preservation in the autoperfused working heart-lung preparation. Surgery. 1987;102:269–276. [PubMed] [Google Scholar]
- 18.Bartlett RH. Vitalin: The rationale for a hypothetical hormone. J Am Coll Surg. 2004;199:286–292. doi: 10.1016/j.jamcollsurg.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 19.Steen S, Sjoberg T, Liao Q, Bozovic G, Wohlfart B. Pharmacological normalization of circulation after acute brain death. Acta anaesthesiologica Scandinavica. 2012;56:1006–1012. doi: 10.1111/j.1399-6576.2012.02721.x. [DOI] [PubMed] [Google Scholar]
- 20.Hing AJ, Hicks M, Garlick SR, et al. The effects of hormone resuscitation on cardiac function and hemodynamics in a porcine brain-dead organ donor model. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2007;7:809–817. doi: 10.1111/j.1600-6143.2007.01735.x. [DOI] [PubMed] [Google Scholar]
- 21.Hing AJ, Watson A, Hicks M, et al. Combining cariporide with glyceryl trinitrate optimizes cardiac preservation during porcine heart transplantation. American Journal of Transplantation. 2009;9:2048–2056. doi: 10.1111/j.1600-6143.2009.02736.x. [DOI] [PubMed] [Google Scholar]
- 22.Gao L, Tsun J, Sun L, et al. Critical role of the stat3 pathway in the cardioprotective efficacy of zoniporide in a model of myocardial preservation – the rat isolated working heart. British Journal of Pharmacology. 2011;162:633–647. doi: 10.1111/j.1476-5381.2010.01071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Watson AJ, Gao L, Sun L, et al. Enhanced preservation of the rat heart after prolonged hypothermic ischemia with erythropoietin-supplemented celsior solution. The Journal of Heart and Lung Transplantation. 2013;32:633–640. doi: 10.1016/j.healun.2013.03.014. [DOI] [PubMed] [Google Scholar]


