Abstract
Introduction
Patients with Cystic Fibrosis (CF) are reported to have limited exercise capacity. There is no consensus about a possible abnormality in skeletal muscle oxidative metabolism in CF. Our aim is to test the hypothesis that abnormalities in oxygenation and/or muscle oxidative metabolism contribute to exercise intolerance in adolescents with mild CF.
Methods
Ten adolescents with CF (12–18 years of age, FEV1>80%pred, resting oxygen saturation > 94%) and ten healthy age-matched controls (HC) were tested with supine cycle ergometry using near-infrared spectroscopy (NIRS) and 31Phosphorus magnetic resonance spectroscopy (31P MRS) to study skeletal muscle oxygenation and oxidative metabolism during rest, exercise and recovery.
Results
No statistically significant (p>0.1) differences in peak workload and peak oxygen uptake per kilogram lean body mass were found between CF and HC. No differences were found between CF and HC in bulk changes of quadriceps phosphocreatine (PCr) (p = .550) and inorganic phosphate (Pi) (p = .896) content and pH (p = .512) during symptom limited exercise. Furthermore, we found statistically identical kinetics for PCr resynthesis during recovery for CF and HC (p = .53). No statistically significant difference in peak exercise arbitrary unit for total haemoglobin content (tHb_AU) was found between CF and HC (p = .66).
Discussion
The results of this study provide evidence that in patients with mild CF and a stable clinical status (without signs of systemic inflammation and/or chronic PA colonisation), no intrinsic metabolic constraints and/or abnormalities in oxygenation and/or muscle oxidative metabolism contribute to exercise intolerance.
Keywords: oxygenation, skeletal muscle function, exercise training
INTRODUCTION
Several mechanisms, as pulmonary, cardiac and peripheral skeletal muscle function, contribute to the reported limited exercise capacity in patients with Cystic Fibrosis (CF) (Almajed & Lands, 2012). Impaired skeletal muscle function may be caused by poor oxygenation of the skeletal muscles, possibly due to impaired blood flow during exercise with excessive ventilatory demands (Harms et al., 1997). Other studies however reported that Cystic Fibrosis Transmembrane conductance Regulator (CFTR)-deficient skeletal muscles demonstrate functional abnormalities primarily during periods of (increased) inflammation, leading to increased muscle weakness (Divangahi et al., 2009). This is in agreement with the impaired exercise capacity in patients with CF colonized with Pseudomonas aeruginosa (Van de Weert-van Leeuwen et al., 2012).
A number of studies reported evidence for intrinsically impaired skeletal muscle function, independent of lung function and/or muscle mass (de Meer et al., 1995; Moser et al., 2000; Rosenthal et al., 2009; Wells et al., 2011; Erickson et al., 2015). Recently, the CFTR chloride channel gene is expressed in human skeletal muscle cells (Divangahi et al., 2009; Lamhonwah et al., 2010). However, there is no consensus about either its exact localization in the muscle cell or any potential impact of a mutated CFTR channel on muscular contractile performance during exercise (Hjeltnes et al., 1984; de Meer et al., 1995; Moser et al., 2000; Hebestreit et al., 2005; Rosenthal et al., 2009; Wells et al, 2011). There is some evidence for altered proton handling and reduced mitochondrial function in CF muscle (Wells et al., 2011). A recent study reported attenuated mitochondrial function in non-skeletal muscle cells (Valdivieso et al., 2012). In muscle, 31P magnetic resonance spectroscopy (31P MRS) studies of oxidative metabolism during exercise in CF patients revealed slight abnormalities in oxidative work performance and phosphocreatine (PCr) recovery (de Meer et al., 1995; Moser et al., 2000; Rosenthal et al., 2009; Wells et al., 2011). Besides, Erickson found evidence for impaired skeletal muscle metabolism using near-infrared spectroscopy (NIRS) in patients with CF in a broad age range (Erickson et al, 2015). This finding could not be confirmed by Saynor focusing only on adolescents with CF (Saynor et al., 2014).
The present study was performed to test the hypothesis that abnormalities in oxygenation and/or muscle oxidative metabolism contribute to exercise intolerance in CF. We studied the oxidative metabolism in upper leg muscles in children and adolescents with moderate CF performing two incremental bicycle exercise tests. Two complementary non-invasive techniques (NIRS and 31Phosphorus magnetic resonance spectroscopy (31P MRS)) were used during two separate test sessions.
METHODS
Ethical approval
The medical ethics committee of the University Medical Center Utrecht approved the study, which was performed following the ethical guidelines of the Declaration of Helsinki. A total of 62 patients with CF were potentially eligible. These patients were informed about the study. When included, patients were asked to inform a HC out of their social environment to participate in the study. All participants, CF and HC, and (when < 18 years of age) their parents gave written informed consent. Patient and HC data were anonymized using codes.
Study design
Ten patients with CF and ten healthy age-matched controls (HC) were studied. Patients with CF were between twelve and eighteen years of age and all in Class I–III CFTR mutation. The patients with CF were free from acute exacerbation (no extra medication or in-hospital treatment for pulmonary of gastro-intestinal exacerbations < last three weeks) with the forced expiratory volume in one second (FEV1 (L·min−1) above 80% of predicted (Zapletal et al, 1987), and the oxygen saturation in rest (SpO2 (%)) above 94%.
For both the CF and the HC (age-matched) participants, needed to be free from constraints in performing a maximal exercise test in a magnetic resonance (MR) scanner. Possible contra-indications for in magnet cycling were identified prior to testing by standardized questionnaires.
The participants visited the hospital twice separated by at least two days. In the first session the thigh muscles of patients with CF and HC were measured with Near-Infrared Spectroscopy (NIRS) during rest, incremental cycling exercise, and recovery. In the second session they performed the same incremental cycling exercise protocol during a 31Phosphorus magnetic resonance spectroscopy (31P MRS). The tests were done in the University Medical Center Utrecht, at the Child Development and Exercise Center and the Department of Radiology. Patients were recruited and tested between August and December 2011. Sample size was based on the study of Wells et al., (2011). With our symptom limited exercise protocol, we expect to find a bigger difference between patients with CF and HC. Desired alpha and power levels of 0.05 and 0.8 respectively resulted in an estimated sample size of ten subjects in each group.
Spirometry, anthropometrics and laboratory measurements
Lung function (MicroLoop, PT-Medical, Leek, the Netherlands) and anthropometric values, using an electronic scale (Seca, Birmingham, United Kingdom) and a stadiometer (Ulmer stadiometer, Prof. E. Heinze, Ulm, Germany), were measured before both test sessions. Percentage body fat and subsequent lean body mass (LBM) were determined by measuring subcutaneous fat of the biceps, triceps, subscapular, and supra-iliac regions with a Harpenden skinfold caliper. Body density and percentage body fat were then calculated as recently described by Bongers et al., (2013). Participants completed the Habitual Activity Estimation Scale questionnaire two weeks before the first test session (Wells et al., 2008).
Laboratory data of sputum cultures of P. aeruginosa (PA) colonization, venous blood sampling, of C-reactive protein (CRP), total IgG and haemoglobin (Hb) levels, measured and collected during regular patient controls, were retrieved from the hospital electronic database. We used the samples which have been obtained the most recent before the exercise tests data. PA colonisation was graded classified as described by Pressler et al (2011). Acute low-grade systemic inflammation was defined as C-reactive protein (CRP) levels above 0.5 mg·dL (Fischer et al., 2007), whereas high IgG-total levels were used as a representative of chronic inflammation (Van de Weert-van Leeuwen et al., 2012). Anaemia was defined as hemoglobine (Hb) levels below 8.6 mmol/l for male and below 7.4 mmol/l for female participants. We used the cut-off values and reference values from the Clinical Chemistry Laboratory of the University Medical Center Utrecht.
Exercise testing
Both exercise tests were performed on a specialized MR-compatible bicycle ergometer (Jenseson et al., 2010). Subjects were asked to lay supine on the bicycle ergometer, and their feet were fixated onto the pedals of the ergometer. The upper body position was under maximal upright angle (typically 40°), using a wedge-shaped support cushion. Before the cycling started, subjects rested in supine position for 5 minutes to ensure a stable resting muscle metabolism. The actual measurement started with one minute of unloaded cycling to familiarize the subject with the exercise, including stabilizing his upper body position by pulling on the handlebar ropes and testing proper function of all components. The desired cycling rate (80 revolutions per minute) was communicated to the subject by audio feedback of a metronome signal. Subsequently, subjects performed an incremental exercise protocol (estimated duration 8–10 minutes), which step increments protocol was tailored to provide exhaustion between 8–10 minutes. The workload increments were 0.3 kg/min for males and 0.2 kg/min for females. Recovery measurements were obtained for 5 minutes after exercise had ceased.
31P-MRS protocol
Subjects were positioned supine and feet-first in the MR scanner (1.5 T Intera, Philips Healthcare, Best, the Netherlands) and attached to the MR-compatible cycle ergometer. A 6-cm 31P surface coil (P-60; Philips Healthcare, Best) was fixated over the medial head of the quadriceps muscle by Velcro strips. Subjects then performed a short bout of unloaded cycling to familiarize them with the exercise regimen. First, a set of scout 1H images of the upper leg was obtained using the body 1H coil for localized shimming of the magnet over the medial head of the quadriceps for 31P spectroscopic acquisitions. Next, resting 31P MR spectra were acquired with a 90° adiabatic excitation (AHP) pulse (16 free induction decays; FIDs) and repetition times (TR) of 20,000 and 3,000 ms, respectively, to establish saturation correction factors for the TR of 3,000 ms that was used in all ensuing acquisitions. During incremental exercise, 31P MR spectroscopic data acquisition was synchronized with the cyclic motion of the upper leg as described by Jeneson et al., (2010). Two FIDs were averaged per spectrum yielding a time resolution of 6 s in the dynamic datasets acquired during exercise and subsequent recovery.
31P MRS Data processing and analysis
PCr, Pi, and ATP resonances were fitted in the time domain using the AMARES algorithm in the jMRUI software package. Absolute concentrations were calculated after correction for partial saturation and assuming adenine nucleotide and creatine pool sizes of 8 and 42 mM, respectively (Jeneson et al., 1997). Intracellular pH was calculated from the chemical shift difference between the Pi and PCr resonances (Jeneson et al., 1997). Free ADP concentrations ([ADP]) and the molar Gibbs free energy of cytosolic ATP hydrolysis (ΔGp) at rest and at maximal exercise were calculated as described by Jeneson et al (1997). The mitochondrial response function to ADP concentration changes and the thermodynamic flow-force function of ATP free energy transduction were determined by nonlinear 3-parameter curve-fitting (Microcal Origin 6.0, OriginLab, Northampton MA, USA) of sigmoidal functions to the covariation of [ADP] and ΔGp with exercise workload, respectively, as described elsewhere (Jeneson et al., 1996). In the analysis, a proportional relation between workload and mitochondrial ATP synthesis rate was assumed (Wu et al., 2007). The kinetics of PCr recovery following exercise were determined by nonlinear curve-fitting of a mono-exponential function to the PCr time course (Meyer et al., 1988).
NIRS protocol
During NIRS measurements, the participants lay supine on a MR-compatible cycle ergometer. The measurements were performed according to a previously used protocol in our exercise laboratory (Habers et al., 2013). The probes of a single distance continuous wave photometer with two channels (OXYMON; Artinis, Zetten, the Netherlands) were fixed on the VM of the dominant leg of the participant. The VL probe was located on one third of the distance from the lateral epicondyle to the greater trochanter of the femur. The VM probe was located in the same transversal plane, but at the medial part of the leg. The light source and detector was housed in a holder, with a constant distance of 3.0 centimetres between, which an average measurement depth of approximately 1.5 centimetres. Near-infrared light was emitted at two wavelengths (775 nm and 850 nm).
Since the length of the light beam that travels through the tissue is longer than the distance between the source and the detector due to the scattering effects of different tissue layers (skin, adipose tissue thickness, and muscle), a differential path-length factor (DPF) set at 4.0 had to be included to calculate the path-length. To prevent the probes shifting on the skin, the probes were fixed by tape on the skin. To reduce the intrusion of stray light and loss of transmitted light from the field of examination, a black cloth was placed at the location of the NIRS device on the leg. Thereafter, an elastic bandage was wrapped around the leg a few times to further minimize movement of the probes. Changes in deoxyhemoglobin, oxyhemoglobin and total haemoglobin concentrations ([HHb], [O2Hb] and [tHb]) from baseline were expressed in μM. Data were sampled and displayed in real time at a frequency of 50 Hz from the start of the rest to the end of the recovery period. The data were analysed after filtering by a Gaussian filter and NIRS outcome measures were normalized to arbitrary units (AU), as described by Habers et al, to allow comparisons between groups (Habers et al., 2013). This normalization procedure involved averaging the values of Δ[O2Hb], Δ[HHb] and Δ[tHb] over the last 30 s of the resting period before exercise and assigning them a value of 0 arbitrary units (AU). Next, Δ[O2Hb], Δ[HHb] and Δ[tHb] during the recovery phase were averaged over a 10-s period, and the maximal values were assigned a value of 1 AU. All values between these two time-points were normalized to this scale. Normalized Δ[O2Hb], Δ[HHb] and Δ[tHb] values were thus dependent on their representative maximum values reached during peak exercise (Δ[HHb]) and/or recovery (Δ[tHb] and Δ[O2Hb]).
Statistical analysis
Data were analysed with SPSS 15.0.for Windows. Data are presented as means ± SD unless otherwise noted. In all quantitative variables the Kolmogorov-Smirnov-test for normality was performed. An alpha level of p< 0.05 was set as statistical significant. Two-tailed unpaired Student’s t-tests were used to compare both groups (HC and CF) (Portney & Watkins, 2009). For the end-exercise PCr and pHi data, obtained during MR cycling, we only used the datasets of participants who cycled “maximal” (> 75% of Wpeak) attained in the first test with NIRS. Statistical analysis was performed by the single-blinded researcher (MW).
RESULTS
All 20 subjects completed both exercise tests without any adverse events. We found no significant differences in anthropometric and baseline values between patients with CF and HC. (Table 1) Patients with CF and HC were comparably physically active during daily life, except for the HAES domain “very active” (CF 212±109 minutes versus HC 335±149 minutes; p = .049).
Table 1.
Baseline characteristics
| Variable | CF (mean ± SD) | HC (mean ± SD) | p-value |
|---|---|---|---|
| Age (years) | 13.8±1.3 | 13.7±1.1 | .944 |
| Height (cm) | 163.0±9.3 | 165.6±10.1 | .584 |
| Weight (kg) | 49.0±6.9 | 56.2±10.8 | .118 |
| BMI (kg/m2) | 18.3±0.9 | 20.3±2.7 | .054 |
| Fat percentage (%) | 17.5±2.7 | 20.8±6.2 (n=9) | .156 |
| Lean body mass (kg) | 41.2±5.3 | 44.7±8.4 (n=9) | .292 |
| FEV1 (%pred.) | 92.8±14.6 | 90.3±13.4 | .717 |
| Gender | 5♀ 5♂ | 5♀ 5♂ | 1.0* |
| Hb (mmol·L) | Female: 8.7±0.7 [7.1–8.7] Male: 8.7±0.7 [7.5–9.4] |
n.a. | n.a. |
| CF mutation | ΔF508 homozygote n= 3 ΔF508 heterozygote n= 7 (Class I n= 5; Class II n=3; Class III n=2; Class IV and V n=0) |
n.a. | n.a. |
| PA colonisation |
Free of infection n=8 Intermittent n=2 |
n.a. | |
| Habitual daily activity level (min/day) | Inactive 40±42 Somewhat inactive 719±292 Somewhat active 439±263 Very active 212±109 |
Inactive 100±167 Somewhat inactive 560±293 Somewhat active 544±391 Very active 335±149 |
.276 .241 .488 .049 |
BMI = body mass index (kg/m2); FEV1 (l/min) = forced expiratory volume in one second; Hb = haemoglobin; PA = Pseudomonas aeruginosa.
Tested with Chi-square
All patients with CF were non anaemic (Hb 8.4±0.7 mmol/l), without chronic PA colonisation at the time of testing, and showed no signs of low grade inflammation or chronic systemic inflammation (serum CRP < 0.5 mg/dl IgG-total 10.8±3.4 g/l [6.6–15.3]. (see Table 2). There was no statistically significant difference in Wpeak/kgLBM, VO2peak/kgLBM, HFpeak, VEpeak, RERpeak or SpO2peak during the exercise test between the HC and CF groups.
Table 2.
Exercise results
| Variable | CF (mean ± SD) | Healthy (mean ± SD) | p-value |
|---|---|---|---|
| Rest | |||
| [PCr] (mM) | 27.5±4.6 (n=9) | 26.4±3.6 | .620 |
| [Pi] (mM) | 3.3±.5 (n=9) | 2.9±.5 | .143 |
| [pHi] (mM) | 7.1±.03 (n=9) | 7.1±0.01 | .844 |
| End Exercise | |||
| [PCr] (mM) | 11.6±8.7 (n=7) | 9.4±3.1 (n=7) | .550 |
| [Pi] (mM) | 19.2±8.2 (n=7) | 19.7±2.9 (n=9) | .896 |
| [pHi] (mM) | 6.9±.2 (n=7) | 6.9±.1 (n=9) | .512 |
| AU_tHb | .64±.20 | .59±.32 | .778 |
| HRpeak (bpm) | 162±12 | 164±9 | .690 |
| VEpeak (l·min) | 56.7±14.1 | 55.7±18.7 | .894 |
| RERpeak | 0.99±0.07 | 0.97±0.07 | .544 |
| VO2peak/kgLBM (ml·min·kg) | 44.6±8.7 | 44.5±8.0 (n=9) | .968 |
| Wpeak/kgLBM (gramm·kg) | 50.1±9.0 | 49.0±12.7 (n=9) | .828 |
| Recovery | |||
| [PCr]_T (sec) | 32.3 ± 11.3 (n=6) (range: 11 – 87 s) | 28.8 ± 3.7 (n=5) (range: 18 – 36 s) | .526 |
PCr = phosphocreatine; Pi = inorganic phosphate; pHi = inorganic pH; AU_tHb = total haemoglobin concentration in arbitrary units; HRpeak = peak heart rate; VEpeak = peak ventilation; RERpeak = peak VCO2/VO2 ratio; VO2peak/kgLBM = peak oxygen update per kilogram lean body mass; Wpeak/kgLBM = peak workload per kilogram lean body mass; PCr_T = time constant of phosphocreatine recovery
31P MRS studies
Figure 1 shows a typical series of 31P MR spectra recorded serially from the quadriceps during the in-magnet maximal exercise test.
Fig. 1.
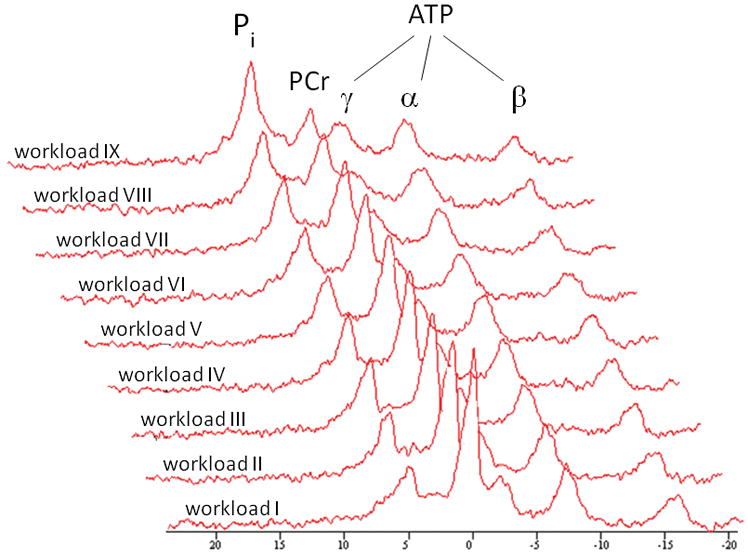
Typical time series of 31P NMR spectra acquired from the medial head of the quadriceps muscle of the right leg of a study participant performing incremental exercise to exhaustion. Each spectrum corresponds to three summed free induction decays collected during the final 36 s of each 1 minute workload. A 10 Hz line broadening filter was applied prior to Fourier transformation. Pi = inorganic phosphate; PCr = phosphocreatine; ATP = adenosine triphosphate.
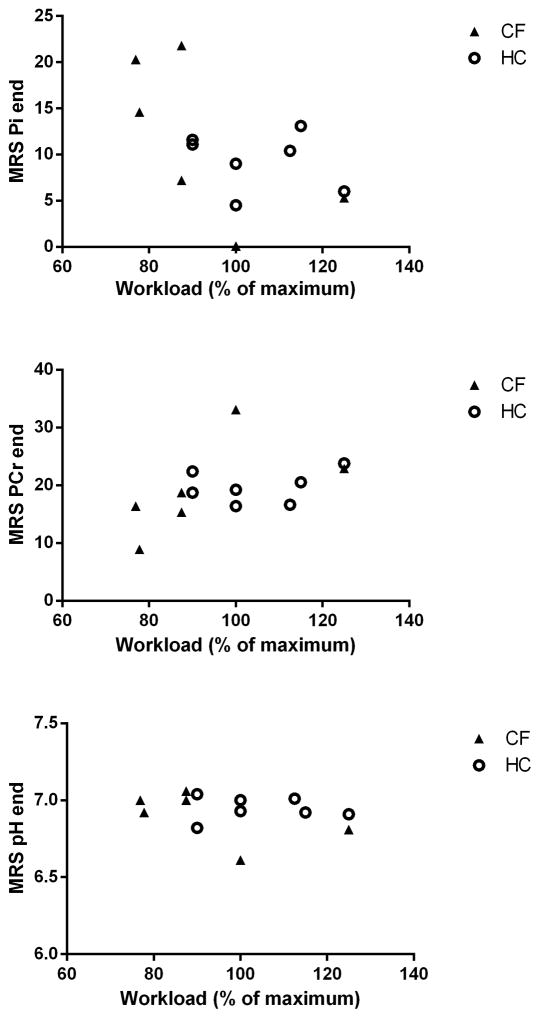
At a qualitative level, the typical inverse changes in PCr and Pi signal amplitudes at constant ATP amplitudes were observed during the exercise test in all subjects. At the quantitative level, no differences were found between controls and CF patients with respect to bulk changes in quadriceps PCr and Pi content and pH during exercise that could point to any abnormalities in mitochondrial ATP synthetic function (Table 2; Figure 2, panels A, B and C, respectively).
Fig. 2.
Metabolic endpoints of the in-magnet maximal exercise test in the CF patients (solid square symbols) and their age- and sex-matched controls (open circles). A: end-PCr concentration (mM); B: end-Pi concentration (mM); C: end-pH. Quadriceps metabolite concentrations and pH were calculated as described in Methods. The specific endpoints are shown as a function of the maximal workload attained in-magnet compared to testing outside the magnet.
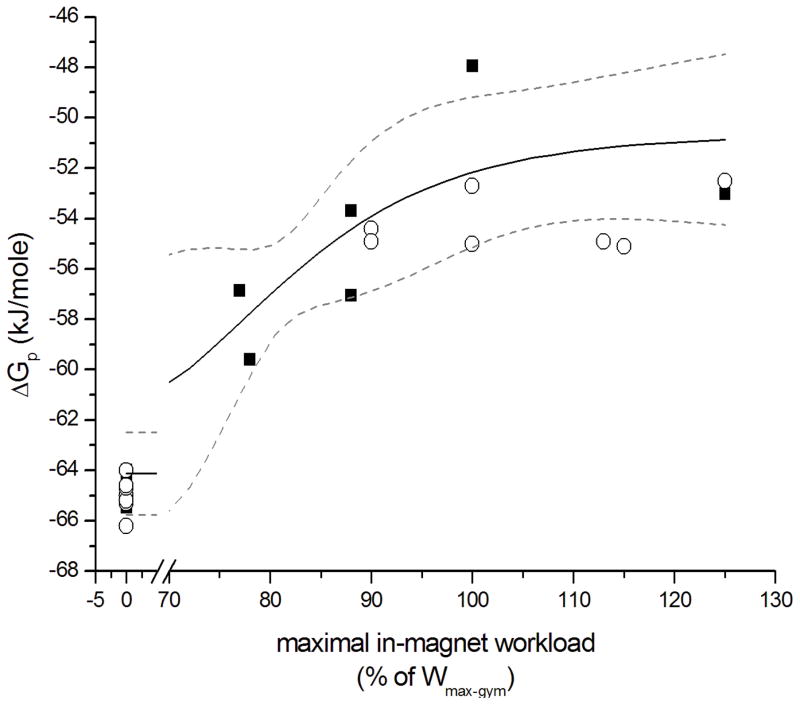
Specifically, the change in cytosolic free energy of ATP hydrolysis (ΔGp) from resting state to maximally active state during exercise in quadriceps muscle of the CF patients closely matched the empirical sigmoidal covariation of normalized workload and ΔGp in healthy controls (Figure 3). The fitted estimates of the maximal (resting state) and minimal (maximal work rate) values of ΔGp in quadriceps muscle of the CF patients were −64.1 ± 0.8 and −50.62 ± 2.1 kJ/mole, respectively (± SE from regression). These values closely matched previously reported limit values for ΔGp in quadriceps muscle of healthy subjects (Jeneson et al., 2004).
Fig. 3.
Thermodynamic flow force relation of dynamic bicycling exercise in CF patients (solid square symbols) and their age- and sex-matched controls (open circles). The solid line shows the 3-parameter fit of a sigmoidal function to the covariation of the molar Gibbs free energy of ATP hydrolysis (ΔGp; in kJ/mole) in quadriceps muscle and normalized work rate for the CF patients. Wmax-gym: maximal work rate attained on the ergometer during a separate exercise test outside the MR scanner (r2 0.84). The dotted lines show 95% confidence interval of the fit. Regression equation: y = −14/(1 + (X/79)^9) − 51.
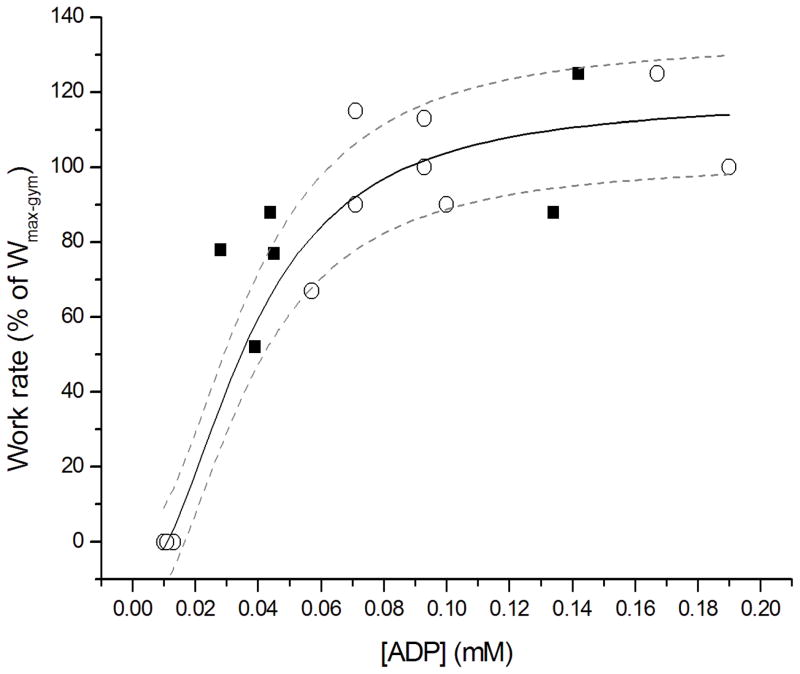
Likewise, the transduction function of [ADP] stimulation of mitochondrial ATP synthesis in quadriceps muscle determined for CF patients matched qualitatively and quantitatively well with experimental data on [ADP] and normalized exercise workload in quadriceps muscle of healthy controls (Figure 4). These outcomes were confirmed by statistically identical kinetics for post-exercise PCr resynthesis in quadriceps muscle for the CF patients and healthy controls (Table 2 and Figure 5). In one single patient with CF, we found a markedly slower estimate of the time constant for recovery (87 ± s). Interestingly, this patient with CF (mutation Class 2; ΔF508/S1251N) had an impaired glucose tolerance and was intermittently colonized with PA. Besides, as the average patients, this patient was non-anemic and showed no sign of low grade inflammation or chronic systemic inflammation.
Fig. 4.
Response function of mitochondrial ATP synthesis to ADP concentration changes in exercising quadriceps muscle of CF patients (solid square symbols) and their age- and sex-matched controls (open circles). The rate of mitochondrial ATP synthesis was taken to vary linearly with muscular work rate (Wu et al., 2007). Wmax-gym: maximal work rate attained on the ergometer during a separate exercise test outside the MR scanner. The solid line shows the 3-parameter fit of a sigmoidal Hill relation to the control dataset; dotted lines show the 95% confidence interval of the fit (r2 0.97). Regression equation: y = 127 * ((X/0.037)^2.1)/(1 + (X/0.037)^2.1) − 9.
Fig. 5.
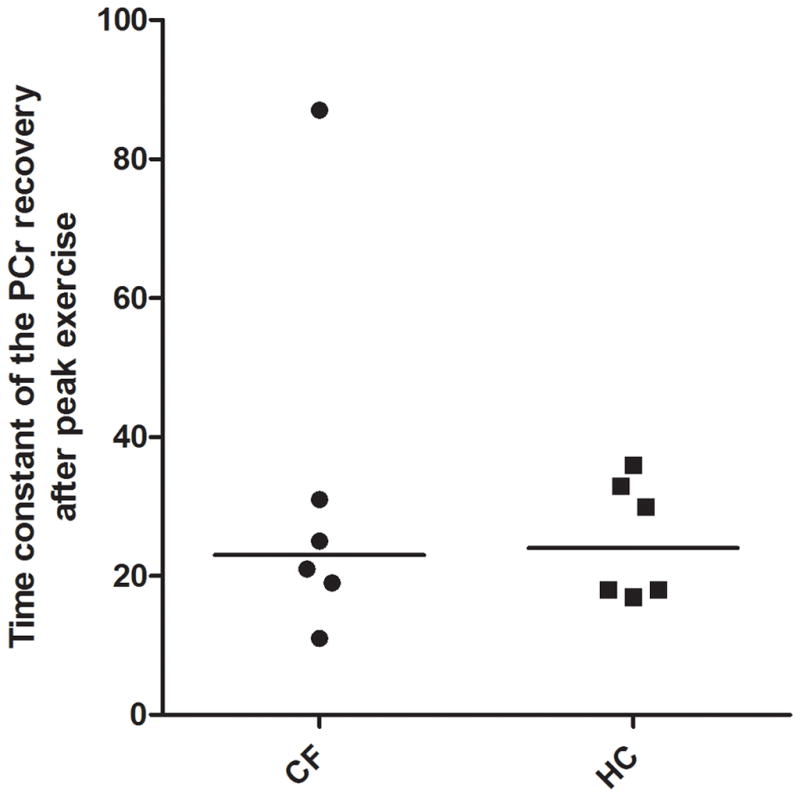
Time constant of the PCr recovery after peak exercise in patients with CF and HC. The horizontal line showing the median.
NIRS studies
During cycling combined with NIRS, no statistically significant difference in peak exercise tHb (AU) was found between patients with CF and HC (.64±.20 versus .59±.32; p = .66). (Table 2; Fig. 6)
Fig. 6.
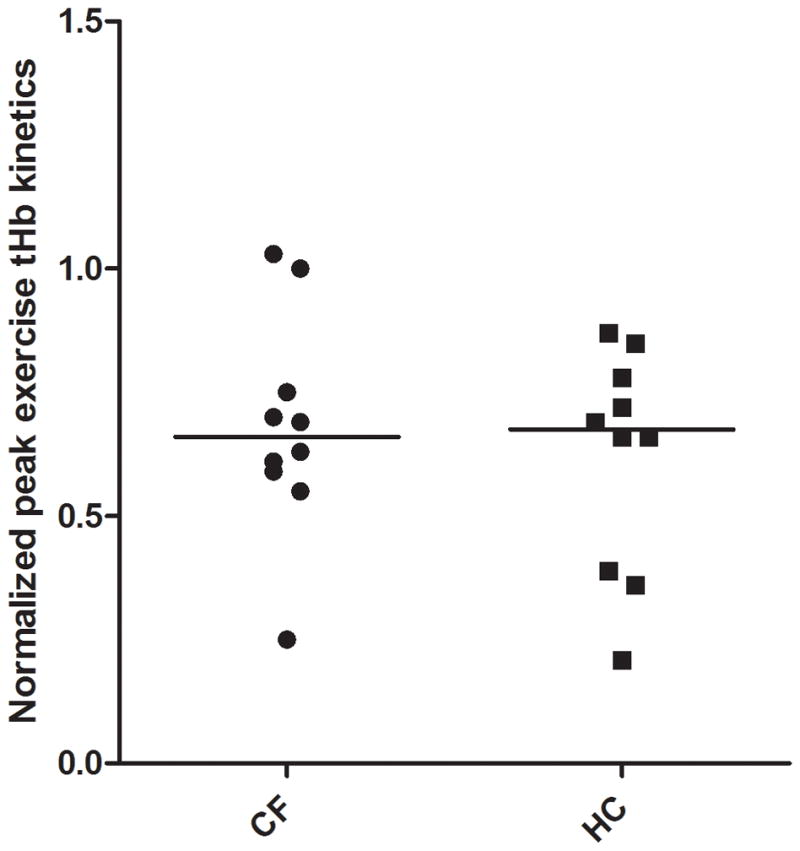
Normalized peak exercise tHb kinetics in patients with CF vs. HC. The horizontal line showing the median.
DISCUSSION
This study, in 10 clinically stable adolescents with mild CF, did not support the hypothesis that exercise capacity in patients with mild CF is related to impaired skeletal muscle oxidative metabolism due to intrinsic mitochondrial dysfunction.
In contrast to Erickson et al, in this study, the group (n=10) of adolescents with CF achieved similar peak workloads and VO2peak per kilogram LBM during exercise testing as age- and sex-matched healthy controls (Table 2) (Erickson et al., 2015). Interestingly, an extra post-hoc subgroup analysis based on CFTR mutation Class (I–IV) revealed no differences in exercise capacity (VO2peak/kgFFM and Wpeak/kgFFM) between patients from different mutation classes. Unlike Selvadurai et al, who found differences between CFTR mutation classes in 97 participants, our analysis was only based on ten patients with CF all within mutation spectrum Class I–III (Selvadurai et al., 2002).
The experimental design of the present study was chosen specifically to obtain a highly sensitive platform to test the particular hypothesis under investigation. If present, any limitation of oxidative muscle metabolism in CF, caused by an intrinsic mitochondrial defect or oxygen supply to the muscle, would be readily detected either by altered homeostasis of ATP free energy (Figures 2A, 2B and 3), altered proton balance (Figure 2C), altered kinetics of PCr recovery (qualitatively and quantitatively; Figure 5), or altered Hb saturation during exercise (Figure 6) (Prompers et al., 2006). In addition, the evaluation of the macroscopic sensitivity of muscle mitochondria to ADP concentration changes (Figure 4) constitutes a robust screening test for any intrinsic mitochondrial defect since ADP is the dominant feedback signal in muscle respiratory control (Jeneson et al., 1996). None of the results of our in vivo 31P MRS and NIRS measurements in CF muscle during exercise and recovery provided evidence in supporting this hypothesis at any of these levels.
At a first glance, the results of our study appear to be in contrast to the findings of two previous 31P MRS studies of oxidative metabolism in skeletal muscle of CF patients (de Meer et al., 1995; Wells et al., 2011). However, we think the evidence for an intrinsic mitochondrial defect in CF provided by these two studies was questionable. De Meer and coworkers did not rule out that their finding of an altered relation between work rate and cytosolic free energy of ATP hydrolysis in non-cyclic exercising forearm muscle could simply be the result of altered contractile economics of the muscle (de Meer et al., 1995). Such a change in contractile economics of skeletal muscle may stem from a slow-to-fast change in muscle fiber type composition documented, for example, found in patients with heart failure (Mancini et al., 1997). Secondly, Wells and co-workers did not find any indication for mitochondrial dysfunction in CF muscle in two out of three different exercise experiments they conducted in CF patients. Specifically, they observed identical, normal values for the half-time of PCr recovery (HT) after a single 30 s exercise bout at maximal intensity and repetitive 30 s bouts of exercise at moderate intensity (65% of maximal; (Wells et al., 2011)). A counterpoint on the present study is that we studied a relatively small group of ten patients with CF and ten HC, which might have implications for comparisons made with the study of Wells (2011).
Furthermore, our NIRS and 31P MRS results also seem to be in contrast to the NIRS results of Erickson et al (2015). They found evidence for an impaired skeletal muscle oxidative capacity in patients with CF. However, these patients with CF were older (mean age 20.2±11.2 years [7–42] than the patients in our study. Interesting, age and thereby disease progression, seems to be a significant contributor to impaired oxidative capacity in patients with CF, which makes a comparison between both studies unfair (Erickson et al., 2015).
Based on these considerations, the outcome of the present study may help settle the debate on any (clinical) significant role for impaired oxidative metabolism as a cause of decreased exercise tolerance in CF; at least in the particular patient population of the present study. Recent studies found that hypoxia and/or hypercapnia, often present in moderate to severe patients with CF, could inhibit the mitochondrial oxidative phosphorylation (Evans et al., 2011; Vohwinkel et al., 2011). Additionally, systemic inflammation c.q. PA colonisation, which is present in many chronic diseases, is suggested to inhibit mitochondrial function (Wells et al., 2011), and has been demonstrated to affect exercise capacity (Van de Weert-van Leeuwen et al., 2012), and diaphragm contractile force in CF patients (Divangahi et al., 2009). Additionally, in a recent mouse study, acute PA colonization was demonstrated to induce insulin resistance and mitochondrial dysfunction in the skeletal muscles (Tzika et al., 2013). Additionally, no evidence is available concerning the effect of exercise training on the skeletal muscle metabolism in patients with CF with more severe disease and more systemic inflammation (Van de Weert-van Leeuwen et al., 2013). These mechanisms do not seem to apply to the particular group of non-cachexic, non-hypoxic, non-anaemic CF patients with no presence of systemic inflammation and almost normal lung function that were enrolled in the present study. In support, Saynor (2014) found no evidence for impaired peripheral oxygen extraction in adolescents with CF in a comparative study population. However, this could still differ between patients with different disease severity and comorbidities (Saynor et al., 2014). Conversely, future research may focus on the possible presence of a mitochondrial dysfunction in adolescents with CF with different CF mutations and/or in adolescents with a more progressive disease state, comorbidities, presence of hypoxia, hypercapnia and systemic inflammation c.q. PA colonisation.
Despite being less active in the vigorous activity domain, in concert with their normal oxidative metabolism and oxygenation, our patients with CF were capable of achieving workloads and VO2peak per kilogram LBM comparable to healthy controls. In that way, there seems to be no muscular energetic rationale for the previously reported less vigorous daily activity level in patients with CF versus healthy controls (Nixon et al., 1993). Additionally, this supports previous findings in adult patients with CF showing significant but moderate associations between exercise capacity parameters and levels of daily life physical activity (Savi et al., 2015).
In conclusion, the results of this study provide evidence that in patients with mild CF, in a stable clinical status, without signs of systemic inflammation and/or chronic PA colonisation, there seem to be no intrinsic metabolic constraints and/or abnormalities in oxygenation and/or muscle oxidative metabolism contributing to exercise intolerance.
NEW FINDINGS.
What is the central question of this study?
Do intrinsic abnormalities in oxygenation and/or muscle oxidative metabolism contribute to exercise intolerance in adolescents with mild Cystic Fibrosis?
What is the main finding and its importance?
This paper found no evidence that in adolescents with mild Cystic Fibrosis in a stable clinical status intrinsic abnormalities in skeletal muscle oxidative metabolism seem to play a clinical significant role. Based on these results we concluded that there is no metabolic constraint to benefit from exercise training.
Acknowledgments
Funding Support: This study was funded in part by a subcontract to NIH grant HL-072011 (to JJ) and by an unrestricted research grant from the Scientific College Physiotherapy of the Royal Dutch Society for Physiotherapy. The funding source had no involvement in the study, design or manuscript.
The authors would like to thank Mrs. G. Bouwman and Mr. N. Blanken for their evident contribution to this study by performing the MR scans during cycle ergometry. Furthermore we would like to thank Dr. B. Bongers (PhD) and Mr. R. de Knikker (MSc) for their assistance during the NIRS cycle ergometry.
Footnotes
Conflict of interest: The authors have no conflicts of interest to report.
Author contributions: MW, JJ, EH and TT contributed equally to this work in writing the manuscript. JJ was primarily responsible for the MR analysis, MW for the NIRS analysis. JJ, MW, EH and TT were all responsible for statistical analysis. PH, KvdE and BA were involved in the clinical translation and implementation of the data and contributed equally to the design of the study. RAN and BV supervised the MR measurements and MR data analysis. EH and TT designed the final concept, obtained funding and supervised the study. All authors contributed to, reviewed and approved the final version of the manuscript. All persons designated as authors qualify for authorship and all those who qualify for authorship are listed.
References
- 1.Almajed A, Lands LC. The evolution of exercise capacity and its limiting factors in cystic fibrosis. Paediatr Respir Rev. 2012;13:195–199. doi: 10.1016/j.prrv.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 2.Bongers BC, de Vries SI, Helders PJM, Takken T. The steep ramp test in healthy children and adolescents: reliability and validity. Med Sci Sports Exerc. 2013;45:366–371. doi: 10.1249/MSS.0b013e31826e32c5. [DOI] [PubMed] [Google Scholar]
- 3.Divangahi M, Balghi H, Danialou G, Comtois AS, Demoule A, Ernest S, Haston C, Robert R, Hanrahan JW, Radzioch D, Petrof BJ. Lack of CFTR in skeletal muscle predisposes to muscle wasting and diaphragm muscle pump failure in cystic fibrosis mice. PLoS Genet; 2009 doi: 10.1371/journal.pgen.1000586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Erickson ML, Seigler N, McKie KT, McCully KK, Ryan AH. Skeletal Muscle Oxidative Capacity in Patients with Cystic Fibrosis. Exp Physiol. 2015;100:545–52. doi: 10.1113/EP085037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Evans AM, Hardie DG, Peers C, Mahmoud A. Hypoxic pulmonary vasoconstriction; mechanisms of oxygen sensing. Curr Opin Anesthesiol. 2011;24:13–20. doi: 10.1097/ACO.0b013e3283421201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fischer R, Simmerlein R, Huber RM, Schiffl H, Lang SM. Lung disease severity, chronic inflammation, iron deficiency, and erythropoietin response in adults with cystic fibrosis. Pediatr Pulmonol. 2007;42:1193–1197. doi: 10.1002/ppul.20717. [DOI] [PubMed] [Google Scholar]
- 7.Habers GEA, de Knikker R, van Brussel M, Hulzebos E, Stegeman DF, van Royen A, Takken T. Near-infrared spectroscopy during exercise and recovery in children with juvenile dermatomyositis. Muscle Nerve. 2013;47:108–115. doi: 10.1002/mus.23484. [DOI] [PubMed] [Google Scholar]
- 8.Harms CA, Babcock MA, McClaren SR, Pegelow DF, Nickele GA, Nelson WB, Dempsey JA. Respiratory muscle work comprises leg blood flow during maximal exercise. J Appl Physiol. 1997;82:1573–1583. doi: 10.1152/jappl.1997.82.5.1573. [DOI] [PubMed] [Google Scholar]
- 9.Hebestreit H, Hebestreit A, Trusen A, Hughson RL. Oxygen uptake kinetics are slowed in cystic fibrosis. Med Sci Sports Exerc. 2005;37:10–17. doi: 10.1249/01.mss.0000150065.97657.7b. [DOI] [PubMed] [Google Scholar]
- 10.Hjeltnes N, Stanghelle JK, Skyberg D. Pulmonary function and oxygen uptake during exercise in 16 year old boys with cystic fibrosis. Acta Paediatr Scand. 1984;73:548–553. doi: 10.1111/j.1651-2227.1984.tb09969.x. [DOI] [PubMed] [Google Scholar]
- 11.Jeneson JA, Wiseman RW, Westerhoff HV, Kushmerick MJ. The signal transduction function for oxidative phosphorylation is at least second order in ADP. J Biol Chem. 1996;271:27995–27998. doi: 10.1074/jbc.271.45.27995. [DOI] [PubMed] [Google Scholar]
- 12.Jeneson JA, Wiseman RW, Kushmerick MJ. Non-invasive quantitative 31P MRS assay of mitochondrial function in skeletal muscle in situ. Mol Cell Biochem. 1997;174:17–22. [PubMed] [Google Scholar]
- 13.Jeneson JA, Bruggeman FJ. Robust homeostatic control of quadriceps pH during natural locomotor activity in man. FASEB J. 2004 Jun;18(9):1010–2. doi: 10.1096/fj.03-0762fje. [DOI] [PubMed] [Google Scholar]
- 14.Jeneson JAL, Schmitz JPJ, Hilbers PAJ, Nicolay K. An MRI-compatible bicycle ergometer for in-magnet whole body human exercise testing. Magn Reson Med. 2010;63:257–261. doi: 10.1002/mrm.22179. [DOI] [PubMed] [Google Scholar]
- 15.Lamhonwah AM, Bear CE, Huan LJ, Kim Chiaw P, Ackerley CA, Tein I. Cystic fibrosis transmembrane conductance regulator in human muscle dysfunction causes abnormal metabolic recovery in exercise. Ann Neurol. 2010;67:802–808. doi: 10.1002/ana.21982. [DOI] [PubMed] [Google Scholar]
- 16.Mancini D. Application of near-infrared spectroscopy to the evaluation of exercise performance and limitations in patients with heart failure. J Biomed Opt. 1997;2:22–30. doi: 10.1117/12.263747. [DOI] [PubMed] [Google Scholar]
- 17.Meer de K, Jeneson JAL, Gulmans VAM, van der Laag J, Berger R. Efficiency of oxidative work performance of skeletal muscle in patients with cystic fibrosis. Thorax. 1995;50:980–983. doi: 10.1136/thx.50.9.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meyer RA. A linear model of muscle respiration explains monoexponential phosphocreatine changes. Am J Physiol Cell Physioll. 1988;254:548–553. doi: 10.1152/ajpcell.1988.254.4.C548. [DOI] [PubMed] [Google Scholar]
- 19.Moser C, Tirakitsoontorn P, Nussbaum E, Newcomb R, Cooper DM. Muscle size and cardiorespiratory response to exercise in cystic fibrosis. Am J Respir Crit Care Med. 2000;162:1823–1827. doi: 10.1164/ajrccm.162.5.2003057. [DOI] [PubMed] [Google Scholar]
- 20.Nixon PA, Orenstein DM, Kelsey SF, Doershuk CF. The prognostic value of exercise testing in patients with cystic fibrosis. Chest. 1993;104:1490–1497. doi: 10.1056/NEJM199212173272504. [DOI] [PubMed] [Google Scholar]
- 21.Portney LG, Watkins MP. Foundations of Clinical Research: Applications to Practice. Pearson Education, Inc; New Jersey: 2009. [Google Scholar]
- 22.Pressler T, Bohmova, Conway S, Dumcius S, Hjelte L, Høiby N, Kollberg H, Tümmler B, Vavrova V. Chronic Pseudomonas aeruginosa infection definition: EuroCareCF Working group report. J Cyst Fibros. 2011;10:75–78. doi: 10.1016/S1569-1993(11)60011-8. [DOI] [PubMed] [Google Scholar]
- 23.Prompers JJ, Jeneson JAL, Drost MR, Oomens CC, Strijkers GJ, Nicolay K. Dynamic MRS and MRI of skeletal muscle function and biomechanics. NMR Biomed. 2006;19:927–953. doi: 10.1002/nbm.1095. [DOI] [PubMed] [Google Scholar]
- 24.Rosenthal M, Narang I, Edwards L, Bush A. Non-invasive assessment of exercise performance in children with cystic fibrosis (CF) and non-cystic fibrosis bronchiectasis: is there a CF specific muscle defect? Pediatr Pulmonol. 2009;44:222–230. doi: 10.1002/ppul.20899. [DOI] [PubMed] [Google Scholar]
- 25.Savi D, Di Paolo M, Simmonds N, Onorati P, Internullo M, Quattrucci S, Winston B, Laveneziana P, Palange P. Relationship between daily physical activity and aerobic fitness in adults with cystic fibrosis. BMC Pulm Med. 2015 doi: 10.1186/s12890-015-0036-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saynor ZL, Barker AR, Oades PJ, Williams CA. Impaired aerobic function in patients with cystic fibrosis during ramp exercise. Med Sci Sports Exerc. 2014;46:2271–2278. doi: 10.1249/MSS.0000000000000369. [DOI] [PubMed] [Google Scholar]
- 27.Selvadurai H, McKay KO, Blimkie CJ, Cooper BJ, Mellis CM, van Asperen PP. The relationship between genotype and exercise tolerance in children with Cystic Fibrosis. Am J Respir Crit Care Med. 2002;165:762–765. doi: 10.1164/ajrccm.165.6.2104036. [DOI] [PubMed] [Google Scholar]
- 28.Tzika AA, Constantinou C, Bandyopadhaya A, Psychogios N, Lee S, Mindrinos M, Martyn JA, Tompkins RG, Rahme LG. A small volatile bacterial molecule triggers mitochondrial dysfunction in murine skeletal muscle. PLoS One; 2013 doi: 10.1371/journal.pone.0074528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vohwinkel CU, Lecuona E, Sun H, Sommer N, Vadász I, Chandel NS, Sznajder JL. Elevated CO2 levels cause mitochondrial dysfunction and impair cell proliferation. J Biol Chem. 2011;286:37067–37076. doi: 10.1074/jbc.M111.290056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Valdivieso AG, Clauzure M, Marín MC, Taminelli GL, Massip Copiz MM, Sánchez F, Schulman G, Teiber ML, Santa-Coloma TA. The mitochondrial complex 1 activity is reduced in cells with impaired cystic fibrosis transmembrane conductance regulator (CFTR) function. PLoS ONE. 2012 doi: 10.1371/journal.pone.0048059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van de Weert-van Leeuwen PB, Slieker MG, Hulzebos HJ, Kruitwagen CL, van der Ent CK, Arets HG. Chronic infection and inflammation affect exercise capacity in cystic fibrosis. Eur Respir J. 2012;39:893–898. doi: 10.1183/09031936.00086211. [DOI] [PubMed] [Google Scholar]
- 32.Van de Weert-van Leeuwen PB, Arets HGM, van der Ent CK, Beekman JM. Infection, inflammation and exercise in cystic fibrosis. Respir Res. 2013 doi: 10.1186/1465-9921-14-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wells GD, Wilkes DL, Schneidermann-Walker J, Elmi M, Tullis E, Lands LC, Ratjen F, Coates AL. Reliability and validity of the habitual activity estimation scale in patients with cystic fibrosis. Pediatr Pulmonol. 2008;43:345–353. doi: 10.1002/ppul.20737. [DOI] [PubMed] [Google Scholar]
- 34.Wells GD, Wilkes DL, Schneidermann JE, Rayner T, Elmi M, Selvadurai H, Dell SD, Noseworthy MD, Ratjen F, Tein I, Coates AL, et al. Skeletal Muscle Metabolism in Cystic fibrosis and Primairy Ciliary Dyskinesia. Pediatr Res. 2011;69:40–45. doi: 10.1203/PDR.0b013e3181fff35f. [DOI] [PubMed] [Google Scholar]
- 35.Wu F, Jeneson J, Beard DA. ATP synthesis in skeletal muscle is controlled by substrate feedback. Am J Physiol Cell Physiol. 2007;292:115–124. doi: 10.1152/ajpcell.00237.2006. [DOI] [PubMed] [Google Scholar]
- 36.Zapletal A, Samanek M, Paul T. Lung function in children and adolescents: methods, reference values. In: Zapletal A, editor. InProgress in respiration research. Vol. 22. Basel: Karger; 1987. pp. 114–218. [Google Scholar]