Abstract
Although much progress is being made in understanding the molecular pathways in the placenta involved in the pathophysiology of pregnancy related disorders, a significant gap exists in utilizing this information for developing new drug therapies to improve pregnancy outcome. On March 5–6, 2015, the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health sponsored a two day workshop titled Placental Origins of Adverse Pregnancy Outcomes: Potential Molecular Targets to begin to address this gap. Particular emphasis was given in the identification of important molecular pathways that could serve as drug targets and the advantages and disadvantages of targeting these particular pathways. This article is a summary of the proceedings of this workshop. A broad number of topics were covered ranging from basic placental biology to clinical trials. This included research in the basic biology of placentation, such as trophoblast migration and spiral artery remodeling, and trophoblast sensing and response to infectious and non-infectious agents. Research findings in these areas will be critical for formulating developing future treatments and developing therapies for the prevention of a number of pregnancy disorders of placental origin including preeclampsia, fetal growth restriction, and uterine inflammation. Research was also presented summarizing ongoing clinical efforts in the U.S. and in Europe testing novel interventions for preeclampsia and fetal growth restriction, including agents such as oral arginine supplementation, sildenafil, pravastatin, gene therapy using virally-delivered vascular endothelial growth factor, and oxygen supplementation therapy. Strategies were also proposed to improve fetal growth by enhancing nutrient transport to the fetus by modulating their placental transporters, as well as targeting placental mitochondrial dysfunction and oxidative stress to improve placental health. The roles of microRNAs and placental-derived exosomes, as well as messenger RNAs, were also discussed in the context of their use for diagnostics and as drug targets. The workshop discussed the aspect of safety and pharmacokinetic profiles of potential existing and new therapeutics that will need to be determined especially in the context of the unique pharmacokinetic properties of pregnancy, as well as the hurdles and pitfalls of translating research findings into practice. The workshop also discussed novel methods of drug delivery and targeting during pregnancy using macromolecular carriers, such as nanoparticles and biopolymers, to minimize placental drug transfer and hence fetal drug exposure. In closing, a major theme that developed from the workshop was that the scientific community needs to change their thinking of the pregnant women and her fetus as a vulnerable patient population for which drug development should be avoided, but rather thought of as a deprived population in need of more effective therapeutic interventions.
Keywords: Pregnancy, placenta, drugs, therapeutics, trials
INTRODUCTION
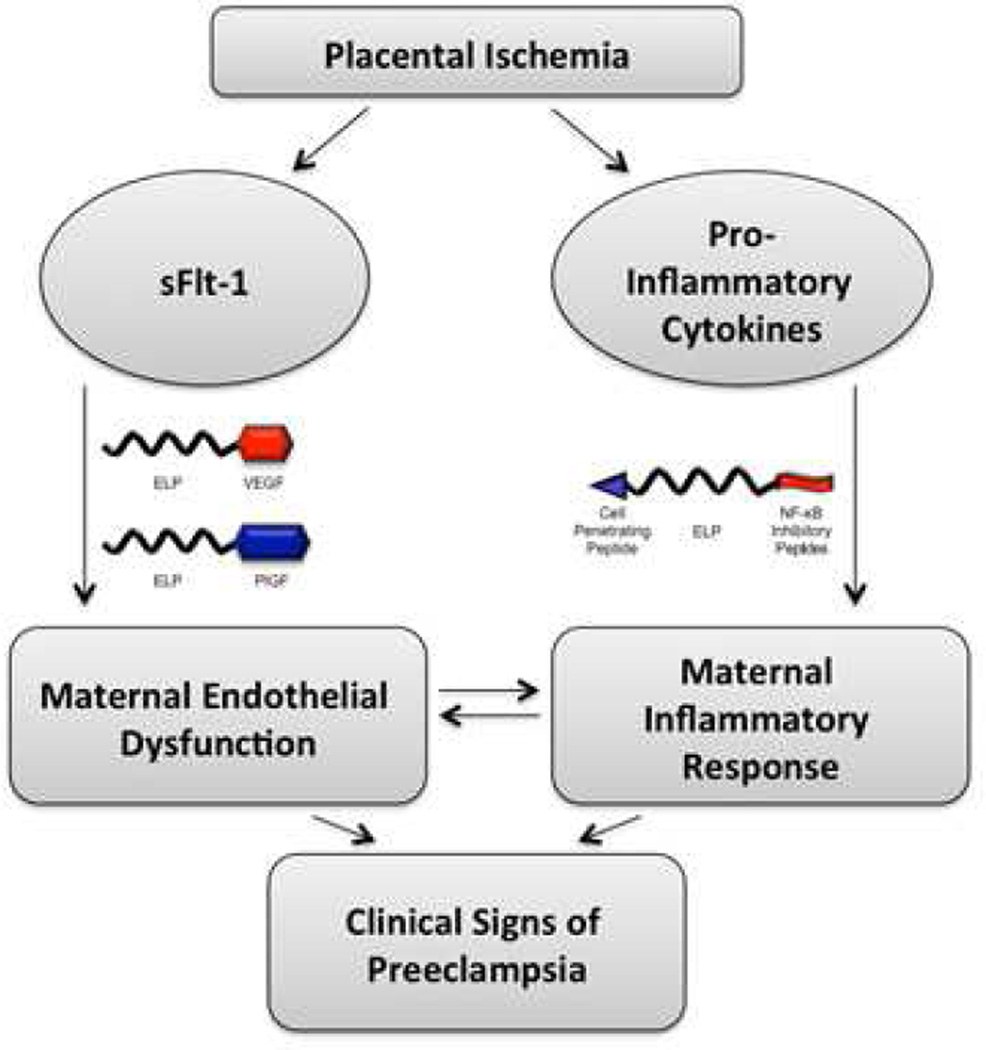
The majority of adverse pregnancy outcomes can trace their origin to the placenta. Preeclampsia (PE) and fetal growth restriction (FGR) are disorders that are rooted in defects of early placental development.1,2 These defects include poor trophoblast uterine invasion and impaired transformation of the uterine spiral arteries to high capacity and low impedance vessels and/or abnormalities in the development of chorionic villi. A number of poor pregnancy outcomes are associated with placental inflammation due to infectious or noninfectious causes and include early pregnancy loss, stillbirth and FGR.3 Significant progress is being made in understanding the molecular basis of these disorders to begin contemplating targeting the molecular pathways involved in their pathophysiology. Several potential targets could be readily envisioned. In the case of PE, an altered balance of circulating angiogenic/anti-angiogenic factors derived from the placenta are believed to responsible for the systemic vascular dysfunction observed in PE.4 These include an increase in the anti-angiogenic proteins such as soluble fms-like tyrosine kinase 1 (sFlt-1) and soluble endoglin, whose pathways can serve as targets for inhibition, or a decrease in the pro-angiogenic proteins such as placental growth factor (PlGF), whose pathway can serve as a target for stimulation. In the case of FGR, the stimulation of the PlGF pathway could also be targeted as a means to increase the number of terminal villi and thus increase the available surface area for improving nutrient transfer between the maternal blood and the growing fetus.5 Another potential treatment to increase nutrient transfer to the malnourished fetus is the stimulation of the mammalian target of rapamycin (mTOR) pathway as a means to increase nutrient transporters.6 In the case of placental inflammation, the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) pathway, a major pathway involved in mediating the inflammatory response, could be targeted to decrease placental inflammation.7,8 A number of drugs to target these pathways and many others already exist in the market place or are available at the experimental stage. A listing of these drugs can be easily obtained through a number of accessible databases.9,10,11,12,13 In addition, a promising pipeline of novel therapeutics are on the horizon that include natural or synthetic antibodies, synthetic small binding molecules (e.g., peptides and nucleic acid aptamers), and nucleic acid therapies (e.g., DNA gene therapy and small RNAs (sRNA), such as microRNAs, (miRNAs) and silencing RNAs (siRNA).14,15,16,17
A major obstacle in introducing novel pharmaceutical interventions to improve pregnancy outcomes is based on the general fear of inflicting potential harm, particularly to the fetus that may result in either short or long term deleterious effects. Understandably, a very cautious direction is taken and most studies involve either evaluating off-label drugs with a very safe history or dietary supplementation for use in pregnancy. Although extreme caution is warranted, the current challenge is to overcome the overbearing reticence of doing harm that unduly hinders the development and testing of new and novel approaches to improve pregnancy outcomes. The first step is to test potential drug therapies for their safety and efficacy in animal models, which then, in turn, can lead to human studies. A number of important factors need to be considered to improve the chance of a successful therapeutic. These include that the ideal therapeutic agent should be highly specific to a key step in the targeted pathway, and that it acts as far down stream as possible to produce the desired effect, thus minimizing unfavorable upstream mediated cascading events. Furthermore, the ideal therapeutic should avoid or minimize maternal and fetal systemic effects. Thus, selectively targeting the placenta and optimizing the dosage would be important considerations. In this regard, placental homing molecules coupled to a delivery system containing the therapeutic (such as nanoparticles, synthetic peptides, liposomes, exosomes) as well as cell-specific DNA expression vectors, show exciting promise to eliminate or minimize any deleterious collateral effects for either the mother or fetus.18 The timing of the delivery of the therapeutic agent is also another important consideration since the placenta is a developing organ with certain pathways taking critical roles at different developmental stages. Thus the modulation of a particular molecular pathway at an inappropriate time window may result in deleterious effects by interfering with the normal developmental trajectory. For example, villous maturation undergoes an orderly developmental process orchestrated by the angiogenic factors vascular endothelial growth factor (VEGF) and PlGF.1, 5, 19 VEGF is involved in early villous formation and drives primary and secondary branching angiogenesis. This is followed by non-branching angiogenesis and the formation of the tertiary terminal villi, principally under the control of PlGF. Primary and secondary branching angiogenesis is generally complete by about 20 weeks, after which tertiary terminal villi formation predominates and continues to term.1 Thus in a hypothetical situation for the treatment of FGR, stimulating the PlGF pathway too early, i.e., prior to the adequate completion of primary and secondary branching angiogenesis, could conceivably result in malformation of normal villous structure and function. Another factor to consider is the required exposure time to the therapeutic agent to obtain the desired effect. Will the therapeutic agent be required to be administered continuously or only for a short duration of time? In the above mentioned FGR treatment scenario, how long of a time period is required to increase and maintain the number of terminal villi? Once formed, will the morphological change remain permanent or will the induced morphological change regress if the stimulus is not continued? Thus specificity, dosage, delivery, timing and length of exposure are some of the key factors in the development of a successful therapeutic.
Although much progress is being made in understanding the molecular pathways in the placenta involved in the pathophysiology of pregnancy related disorders, a significant gap exists in utilizing this information for developing new drug therapies to improve pregnancy outcome. To address this concern, the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health sponsored a two day workshop on March 5–6, 2015 titled Placental Origins of Adverse Pregnancy Outcomes: Potential Molecular Targets to discuss and reflect on placental drug targeting to improve pregnancy outcomes. The workshop brought together leaders in the field to present and discuss their particular area of research and stimulate dialogue in the context of the theme of the workshop. The goals of the workshop were (1) present the state of the science with respect to the molecular mechanisms involved in placentation; (2) identify potential molecular pathways and developmental time windows for targeting effective "drug" interventions to avoid placentation defects in early pregnancy and circumvent placental defects later in pregnancy; and (3) identify major research gaps in our understanding of placental molecular pathways leading to adverse pregnancy outcomes. This article summarizes the proceedings of this workshop. The overall objective of the workshop was to stimulate the research community to better apply the knowledge obtained from the lab bench for use at the bedside. A summary of the research topic covered by each participant is presented in this article along with their opinions on current and future opportunities and research gaps. The article is organized according to four session themes. The session themes and their respective topics are listed in Table 1. Table 2 is a key to abbreviated scientific terms commonly used throughout the article.
Table 1.
Workshop session themes and their respective topics
|
Table 2.
Abbreviations for commonly used scientific terms
| Abbreviation | Description |
|---|---|
| AB | apoptotic bleb |
| aPL | antiphospholipid antibodies |
| APOA4 | apolipoprotein a-iv |
| C19MC | chromosome 19 miRNA cluster |
| CaO2 | arterial oxygen content |
| CARD | caspase activation and recruitment domain |
| Ca++ | calcium |
| CFP | cell-free plasma |
| CO | cardiac output |
| CTB | cytotrophoblast |
| CYP | cytochrome P |
| DAMP | damage associated molecular pattern |
| DNA | deoxyribonucleic acid |
| dsRNA | double-stranded ribonucleic acid |
| EDH | endothelium derived hyperpolarizing |
| EGFR | epidermal growth factor receptor |
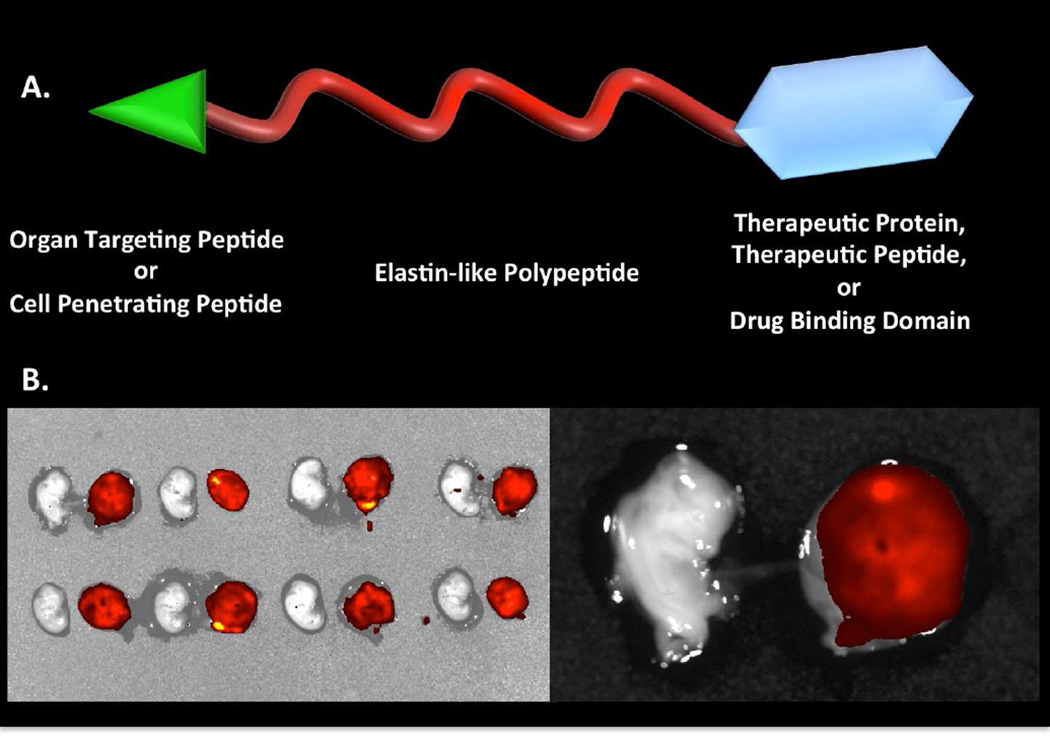
| ELP | elastin-like polypeptide |
| EPO | erythropoietin |
| FDA | Food and Drug Administration |
| FGR | fetal growth restriction; also known as IUGR |
| FOA | funding opportunity announcement |
| GDM | gestatoinal diabetes mellitus |
| GLUT | glucose transporter |
| GPx | glutathione peroxidase |
| GRO-α | melanoma growth stimulating activity, alpha |
| HIF | hypoxia inducible factor |
| HIV | human immunodeficiency virus |
| HLA | human leukocyte antigen |
| HMGB1 | high mobility group B1 |
| iE-DAP | gamma-D-glutamyl-meso-diaminopimelic acid |
| IFNGR | interferon-gamma receptor |
| IGF | insulin-like growth factor |
| IL | interleukin |
| IL | interferon |
| IRAK | interleukin-1 receptor associated kinase |
| IRF | interferon regulatory factor |
| IUGR | intrauterine growth restriction (also known as FGR) |
| LPS | lipopolysaccharide |
| m | meters |
| mmHg | millimeters mercury |
| MAL | myelin and lymphocyte |
| MCP-1 | monocyte chemoattractant protein-1 |
| MDP | muramyl dipeptide |
| miRNA | micro-ribonucleic acid |
| mRNA | messenger RNA |
| MR | mass restricted |
| mTOR | mammalian target of rapamycin |
| MV | microvesicle |
| MVB | multivesicular body |
| NADPH | myeloid differentiation primary response gene 88 |
| NCATS | National Center for Advancing Translational Sciences |
| NF-kB | nuclear factor kappa beta |
| NGS | next generation sequencing |
| NIH | National Institutes of Health |
| NK | natural killer |
| NLR | nod-like receptor |
| NO | nitric oxide |
| Nod | nucleotide oligomerization domain protein |
| NRP1 | neuropilin-1 |
| NTU | new therapeutic uses |
| O2 | oxygen |
| PAMP | pathogen-associated molecular pattern |
| PaO2 | partial pressure of arterial oxygen |
| PD | pharmacodynamic |
| PDG | peptidoglycan |
| PE | preeclampsia |
| PK | pharmacokinetic |
| PI3K/Akt | phosphatidylinositol-3-kinase/protein kinase B |
| PlGF | placental growth factor |
| PO2 | partial pressure of oxygen |
| PPROM | preterm premature rupture of membranes |
| Prl | prolactin |
| PRR | pattern recognition receptors |
| PTL/B | preterm labor/birth |
| PTB | preterm birth |
| K+ | potassium |
| RICK | receptor-interacting protein-like interacting caspase-like apoptosis regulatory protein kinase |
| RNA | ribonucleic acid |
| ROS | reactive oxygen species |
| sFlt-1 | soluble fms-like tyrosine kinase 1 (also known as soluble VEGFR1) |
| SOD | superoxide dismutase |
| sPTB | spontaneous preterm birth |
| STB | syncytiotrophoblast |
| sRNA | small ribonucleic acid |
| siRNA | silencing ribonucleic acid |
| ssRNA | single-stranded ribonucleic acid |
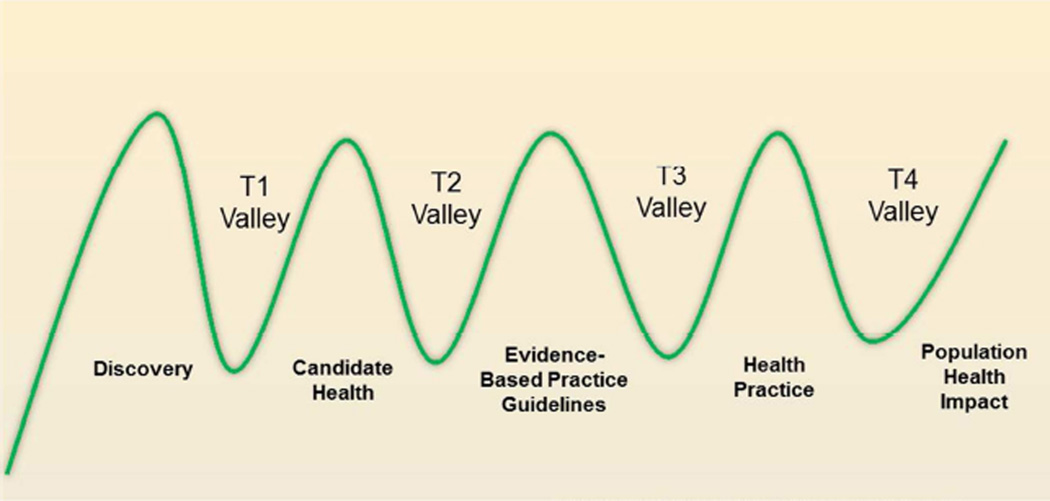
| T1 | translational spectrum 1, translation of animal and bastic research into humans |
| T2 | translational spectrum 2, translation of clinical research findings to practice |
| TBK-1 | tank-binding kinase 1 |
| TLR | toll-like receptor |
| TNFα | tumor necrosis factor; alpha |
| TRAF | TNF receptor associated factor |
| TRAM | trif-related adaptor molecule |
| TRIF | tir-domain-containing adapter-inducing interferon-β |
| TS | trophoblast stem |
| uNK | uterine natural killer |
| VEGF | vascular endothelial growth factor |
| VEGFR | VEGF receptor |
I. Review of Placental Development and Function in the Context of Molecular Mechanisms and Pathways
Human trophoblast differentiation and placentation
(Susan Fisher, University of California San Francisco)
Cytotrophoblast differentiation establishes the anatomy of the human maternal-fetal interface
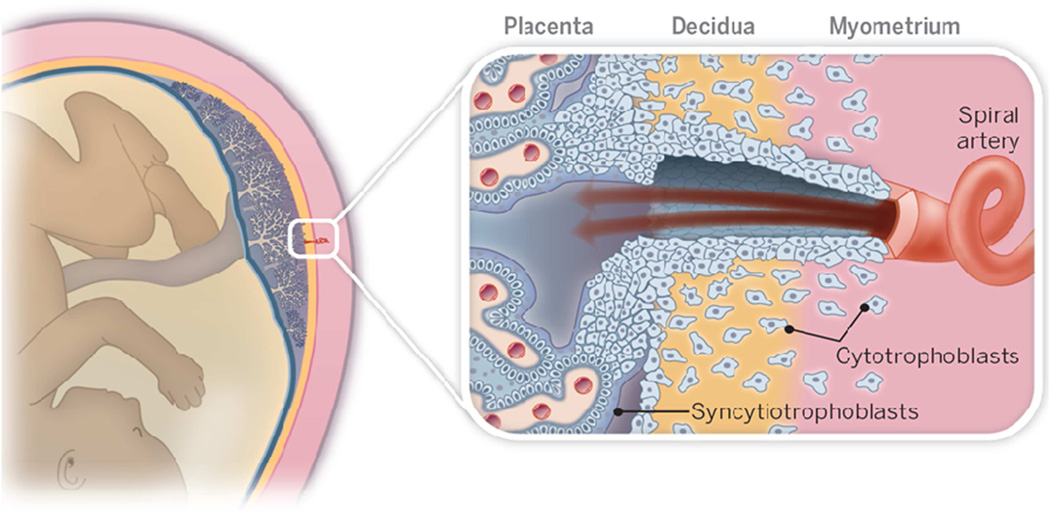
The complex cellular architecture at the boundary between the placenta and uterus is governed, in large part, by the cytotrophoblast (CTB) differentiation pathway that enables invasion.20,21 With regard to the anatomical arrangement, placental CTBs emigrate from anchoring villi and join cell columns that serve as conduits to the uterine wall (Figure 1). Within the uterus, the CTBs invade nearly its entirety, normally stopping a third of the way through the muscle layer. Within the decidua interstitial CTBs interact with specialized populations of maternal immune cells that are allowed to enter this compartment. During invasion, the cells also remodel the uterine circulation, primarily by targeting the spiral arteries. They transform the walls of these vessels. Endovascular CTBs replace the endothelium and intercalate within the smooth muscle cells of the tunica media. This process converts the originally low capacitance/high resistance uterine arteries into high capacitance/low resistance channels that perfuse the surface of the placenta, which is comprised of multinucleated syncytiotrophoblasts (STBs), a transport epithelium. Thus, they can respond to the ever-increasing demands of the offspring for maternal blood over the course of pregnancy.
Figure 1. A Schematic drawing of the maternal-fetal interface in human pregnancy.
Mononuclear placental cytotrophoblasts invade the uterine wall and its resident vasculature (right panel). During this process, they transform spiral arteries into wide bore vessels that perfuse the placenta. Its tree-like chorionic villi are covered by multinucleated syncytiotrophoblasts, which transport a variety of substances to and from the fetus, enabling normal fetal growth. Reprinted, with permission, from Romero et al.21
At a molecular level, CTB invasion of the uterus is as remarkable as the unique behaviors the cells exhibit. The progenitors, which are attached to the trophoblast basement membrane of the chorionic villi, express an adhesion molecule repertoire that is typical of epithelial cells, e.g., E-cadherin and integrin α6/β4. As they enter the columns, the emigrating CTBs undergo a stereotypical transformation. They down regulate those that are typical of an epithelial monolayer and up regulate receptors that enable invasion, e.g., αV family members, (Figure 2), VE-cadherin and integrin α1/β1. Remarkably, the end result of this transformation is vascular mimicry in which CTBs of epithelial origin express a broad repertoire of adhesion molecules, growth factors, Eph receptors and their cognate ligands (ephrins), and notch family members that are typically associated with endothelium and the muscular tunica media of vessels.
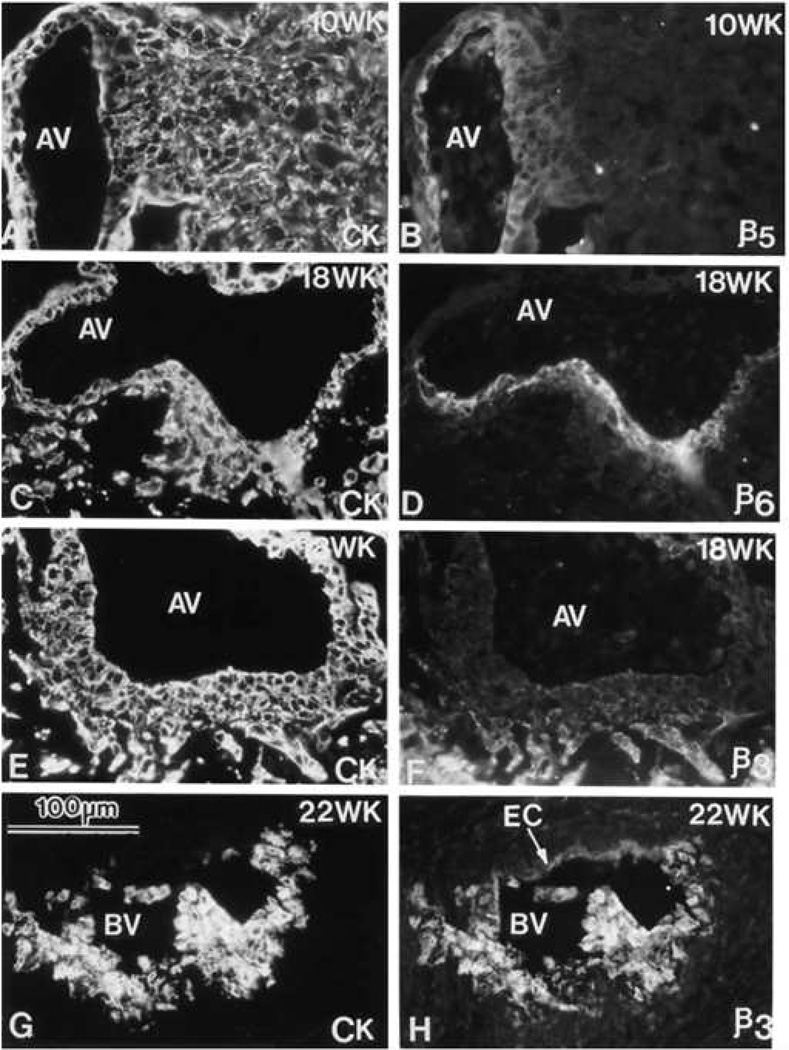
Figure 2. Phenotypic transformation of cytotrophoblast during uterine invasion.
Cytotrophoblasts (CTBs) switch their expression of integrin αVβ (αVβ) family members as they invade the uterine wall. Sections of the maternal-fetal interface at various weeks (WK) of gestation (18–22) were double stained with anti-cytokeratin (CK) to mark CTBs (panels A, C, E and G) and anti-αVβ5 (β5), anti-αVβ (β6), or anti-αVβ3 (β3) (panels B, D, F, and H, respectively). αVβ5 was detected on CTBs in floating (data not shown) and anchoring villi (AV), but not in other locations. αVβ6 was detected on villous CTBs at sites of column formation and in the first cell layer of the column. αVβ3 was upregulated in the distal portions of the columns and on endovascular CTBs that lined the maternal blood vessels (BV). EC, endothelial cell. Reprinted, with permission, from Zhou et al.134
PE is associated with abnormal cytotrophoblast invasion and differentiation
Many investigators believe that PE (the sudden onset of maternal high blood pressure, proteinuria and edema) occurs in two stages.22 The first stage involves shallow CTB invasion of the uterus, which was first described by Brosens and colleagues.23,24 Failed transformation of spiral arteries appears to be critical, leading to hypoperfusion of the placenta and oxidative stress.25 The second stage includes overactive maternal immune responses. Although these pathways are most commonly associated with PE, similar pathologies have been described in a subset of preterm labor/birth (PTL/B) cases.21 The causes are under intense investigation. Severe cases of PE are associated with failed CTB transformation into vascular-like cells coincident with shallow uterine invasion.26 For example, placental cells that enter the uterine wall fail to down regulate E-cadherin and to up regulate VE-cadherin. They also misexpress a broad array of angiogenic and/or vasculogeneic factors. These include VEGF family members. For example, invasive CTBs from PE pregnancies fail to stain with anti-VEGF A, which their normal counterparts express in abundance. In addition, they release higher amounts of soluble VEGFR1 (sFlt-1)27 as do STBs.28 Increasing circulating levels of sFlt-1 and other angiogenic factors such as endoglin causes a PE-like syndrome in animal models.29,30 Thus, there has been a great deal of interest in whether or not circulating levels of molecules that could have negative effects on the maternal vasculature can be used to predict and/or diagnose PE.31, 32, 33
Is abnormal placental production of angiogenic/vasculogenic factors a cause or consequence of PE? As yet there are no definitive answers to this question. However, alternative explanations abound. For example, particular combinations of maternal natural killer (NK) cell expression of killer-cell immunoglobulin-like receptors that recognize the certain major histocompatibility complex molecule, human leucocyte antigen (HLA) C, on invading CTBs increase the risk of PE.34 Surprisingly, a recent study showed that, upon isolation from PE placentas, CTB gene expression (e.g., growth hormone [GH] 2, corticotrophin releasing hormone, kiss-1 metastasis-supressor 1, semaphoring 3B, and several pregnancy specific beta-1-glycoproteins is normalized,35 suggesting that the defects are reversible and pursuit of therapies is warranted.
Current opportunities
As compared to other medical conditions, very little attention has been paid to therapeutic/pharmacological interventions for the great obstetrical syndromes. In this context, pregnancy complications are the equivalent of “orphan” diseases, not because they are rare conditions, but due to the fact that there is very little monetary incentive for taking on the risk that treating pregnant women entails. However, there are compelling reasons to shift this paradigm. The majority of the common pathologies that derail human pregnancy affect the placenta. Many involve either fetal or maternal cells that reside within the uterine wall. Thus, it is likely that effective therapies could be designed to target these cells without crossing the placenta and reaching the embryo/fetus. For example, many kinds of drugs (e.g., antibodies, small molecules) that target particular vulnerabilities (e.g., vascular and/or immune functions) could be formulated as derivatives that prevent syncytiotrophoblast transport, thus reducing the risk of untoward embryonic/fetal events. As a first step, this general strategy could be tried with agents that are already used to treat pregnant women, e.g., tumor necrosis factor, alpha (TNFα) inhibitors that work, in part, by blocking the activation of endothelial and immune cells that this cytokine produces. For example, Certolizumab (a pegylated fragment antigen-binding fragment of a humanized monoclonal antibody that inhibits TNFα), which does not cross the placenta, could be evaluated in women who have a high risk of pregnancy loss due to the effects of this cytokine (e.g., inflammatory and thrombotic placental lesions) in the setting of autoimmune disorders such as antiphospholipid antibody syndrome.36
Future opportunities
Until recently, it was thought that placental interactions with the mother occurred at a cellular level (e.g., invasive CTBs and maternal immune cells within the uterine wall) or involved soluble proteins (e.g., human chorionic gonadotropin). However, this paradigm is rapidly shifting. Free fetal DNA, which circulates in maternal blood,37 is being used as a noninvasive means of prenatal genetic diagnoses.38 It is possible that circulating cell-free RNA could be used as a complementary method and/or as a means of gaining additional information.39 Also, like many cancer cells, the placenta appears to release a complex repertoire of extracellular vesicles whose cargo could have major effects on numerous maternal cells, tissues and organs.40,41 Thus, obtaining an in-depth understanding of the types and content of placental extracellular vesicles will increase our understanding of their functions. For example, it would be interesting to determine how their contents and targets change over the course of gestation and the impact of the common pregnancy complications, including PE and PTL/B, on the normal trajectory. Ultimately, this important information could lead to several types of clinical applications, e.g., extracellular vesicles could be used to infer important aspects of placental functions. Other possibilities include therapies that target extracellular vesicles or take advantage of this system of intercellular communication for drug delivery.
Scientific gaps in relation to drug targeting
A myriad of questions remain to be answered about mechanisms that are central to the success of normal pregnancy and go awry in pregnancy complications. For example, maternal tolerance of hemi-allogeneic CTBs lacks a definitive explanation. Therefore, it is very difficult to devise targeted therapies for pregnancies disorders, from infertility to PE, that are thought to have an immune etiology or component. Likewise, lack of knowledge impedes strategies for dampening the maternal immune response to infections during pregnancy, which can lead to PTL/B. In cases of the latter syndrome with an unknown etiology, therapies lag because we do not understand the pathways that normally trigger normal labor and birth at the end of pregnancy. Finally, PE appears to arise due to profound miscommunication(s) between the placenta and the mother. The development of drugs that intercept or redirect these signals will require a molecular dissection of their components.
Innate immune function of human trophoblast
(Vikki M. Abrahams, Yale University)
Background
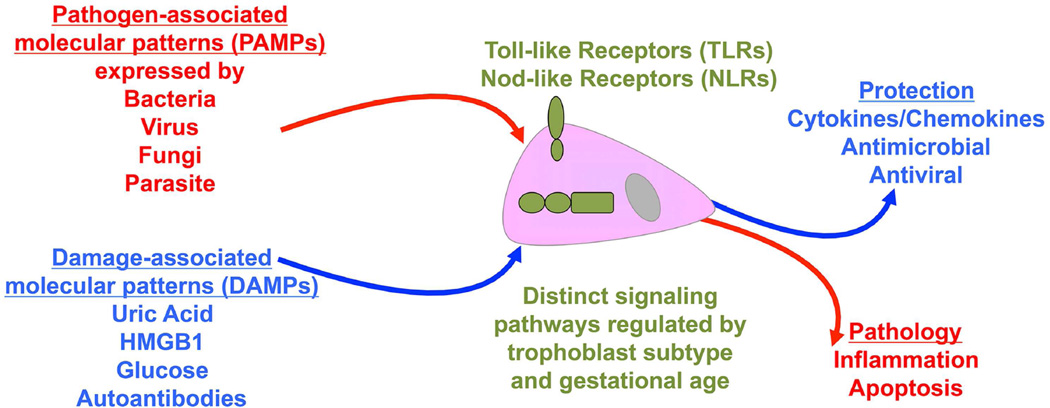
Placental trophoblast cells can sense and respond to a variety of infectious pathogen-associated molecular patterns (PAMPs) that are expressed by microbes, as well as non-infectious host-derived damage associated molecular patterns (DAMPs) through their expression of innate immune pattern recognition receptors (PRRs).42,43 Depending upon the trigger or receptor activated, the trophoblast may mount either a regulated protective response that helps to maintain and promote a healthy pregnancy, or a damaging response that might adversely impact pregnancy outcome (Figure 3). Moreover, expressions of some trophoblast PRRs are gestationally regulated and this further impacts the placental response.44
Figure 3. Innate immune sensing by the trophoblast.
Trophoblast cells sense infectious pathogen-associated molecular patterns (PAMPs) expressed by bacteria, viruses, fungi and parasites through their expression of Toll-like receptors (TLRs) and Nod-like receptors (NLRs). Through these receptors, trophoblast cells also mount responses to non-infectious host-derived damage associated molecular patterns (DAMPs) such as uric acid, high mobility group B1 (HMGB-1), glucose and certain autoantibodies. Trophoblast expression of some TLRs and NLRs are regulated across gestation and cell subtype. Depending upon the trigger, receptor activated, and type of signaling pathway utilized, the trophoblast may mount either a regulated protective response that helps to maintain and promote a healthy pregnancy; or a damaging pathological response that might adversely impact pregnancy outcome.
Pattern recognition receptors
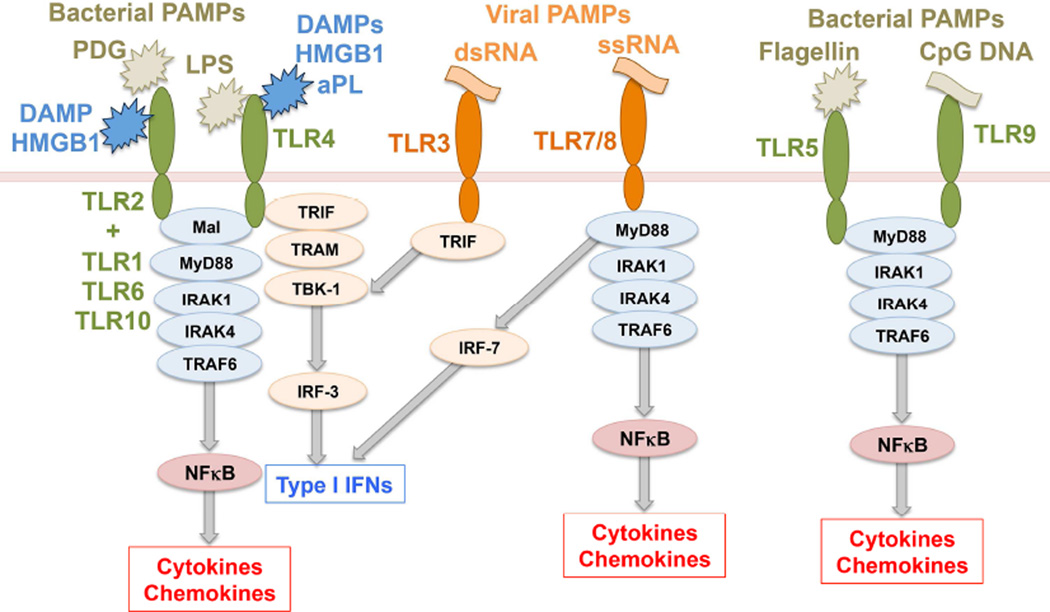
Two major families of PRRs are the Toll-like receptors (TLRs) and the Nod-like receptors (NLRs). TLRs are transmembrane receptors, allowing for the sensing of PAMPs or DAMPs either at the cell surface or within endosomal compartments.45 There are 10 human and 12 murine TLRs, each with distinct specificities.46 TLR4 recognizes Gram-negative bacterial lipopolysaccharide (LPS). TLR2, in co-operation with its co-receptors TLR1, TLR6 or TLR10, recognizes Gram-positive bacterial peptidoglycan (PDG) and lipoproteins. TLR3 senses viral double-stranded RNA (dsRNA), TLR5 senses bacterial flagellin. Mouse TLR7 and human TLR8 sense viral ssRNA, and TLR9 senses bacterial DNA.46 Four adapter proteins are involved in TLR signaling: myelin and lymphocyte protein 88 (MyD88), TIR-domain-containing adapter-inducing interferon-beta (TRIF), myelin and lymphocyte protein (Mal) and TRIF-related adaptor molecule (TRAM).47,48 TLR2 and TLR4 signal through MyD88/Mal. TLR4 can also signal through TRIF/TRAM. TLR3 signals through TRIF, while all other TLRs signal through MyD88 alone.47,48 Downstream, TLR/MyD88 signaling activates NFκB, while TLR/TRIF activates Tank-binding kinase-1 and interferon regulatory factor 3 leading to a type I interferon response (Figure 4).48, 49
Figure 4. Toll-like receptor signaling.
Toll-like receptors (TLRs) are transmembrane receptors that mediate the sensing of pathogen-associated molecular patterns (PAMPs) expressed by microorganisms. TLR2, in co-operation with its co-receptors TLR1, TLR6 or TLR10, recognizes Gram-positive bacterial peptidoglycan (PDG). TLR4 recognizes Gram-negative bacterial lipopolysaccharide (LPS). TLR3 and TLR7/TLR8 sense viral double-stranded RNA (dsRNA) and single-stranded RNA (ssRNA), respectively. TLR5 senses bacterial flagellin and TLR9 senses bacterial cytosine-guanine dinucleotide (CpG) rich DNA regions. Four adapter proteins are involved in TLR signaling: MyD88, TRIF, Mal and TRAM which trigger downstream pathways leading to either NFκB activation and subsequent cytokine/chemokine prodcution, or IRF-3/IRF-7 activation leading to a type I interferon (IFN) response. Some TLRs also sense host-derived damaged associated molecular patterns (DAMPs). TLR2 and TLR4 can sense high mobility group B1 (HMGB1) protein, while TLR4 can be activated by antiphospholipid antibodies (aPL). Refer to Table 2 for key to undefined abbreviations.
NLRs are cytoplasmic-based PRRs providing an intracellular recognition system for sensing microbe-associated PAMPs or as will be discussed, DAMPs. NLRs can synergize with TLRs for a greater response or provide a compensatory system for when TLR signaling is absent or reduced.50, 51 The NLR proteins, Nod1 and Nod2, recognize the PDG peptides-Gram-negative bacterial gamma-D-glutamyl-meso-diaminopimelic acid (iE-DAP-) and muramyl dipeptide (MDP) that is expressed by all bacteria, respectively. Both Nod1 and Nod2 signal through the common adapter protein receptor-interacting protein-like interacting caspase-like apoptosis regulatory protein kinase (RICK) to induce inflammation.52 Another NLR called NACHT, leucine rich repeat protein and the NLR, Nalp3, are involved in mediating production of the pro-inflammatory cytokine, interleukin (IL)-1β).53,54 Since IL-1β has the potential to be damaging, its regulation is tightly controlled. Indeed, IL-1β is an important mediator of preterm birth102,103,104,105, and perinatal brain injury,106,107,108,109 and can trigger the production of other pro-inflammatory cytokines and chemokines through the IL-1 receptor in a similar manner to TLRs.55 Indeed delivery of a synthetic peptide to pregnant mice that is a selective IL-1 receptor modulator, delays IL-1β delays and LPS-induced preterm birth.56 Unlike most other cytokines, IL-1β production involves a two-step process. The first step requires induction of pro-IL-1β expression. This is triggered through signals like TLRs (Signal 1). Once expressed, pro-IL-1β can be cleaved into its active secreted form.57 This second step (Signal 2) is typically mediated by the Nalp3 inflammasome a protein complex consisting of Nalp3, apoptosis-associated speck-like protein containing a caspase recruitment domain (CARD) and caspase-1.53 Once the inflammasome has assembled, caspase-1 is activated and processes pro-IL-1β into its secreted form (Figure 5).57 Although the Nalp3 inflammasome is the best characterized, there are a number of inflammasomes: Nalp1/ (apoptosis-associated speck-like protein containing a CARD; absent in melanoma 2 protein A; NLR family, CARD domain containing 4 protein; and interferon gamma-inducible protein 16.112 Furthermore, Nod proteins can mediate IL-1β production independent of the inflammasome. For example, Chlamydia trachomatis infection of human trophoblast induces IL-1β via Nod 1.113
Figure 5. Nod-like receptor signaling.
Nod-like receptors (NLRs) are cytoplasmic proteins that sense PAMPs. Nod1 recognizes bacterial iE-DAP and Nod2 senses bacterial MDP. Both Nod1 and Nod2 signal through the adapter protein RICK to induce NFκB activation and subsequent cytokine/chemokine production. Nalp3 recruits ASC and caspase-1 to form the inflammasome. Once the inflammasome has assembled, caspase-1 is activated and processes pro-IL-1β into its active, secreted form Refer to Table 2 for key to undefined abbreviations.
Trophoblast sensing of bacteria
Bacterial components such LPS, iE-DAP, and MDP, at high concentrations, trigger mild pro-inflammatory cytokines/chemokines (IL-8, IL-6, monocyte chemoattractant protein-1 [MCP-1], and melanoma growth stimulating activity, alpha [GRO-α]) responses in first trimester trophoblast cells through TLR4, Nod1, and Nod2, respectively, while lower, more physiological doses, are unable to induce this trophoblast inflammation.58,59,60,61 Third trimester trophoblast cells lack Nod2 and thus responses to MDP are altered.62 Similarly, third trimester syncytiotrophoblast cells can generate a strong inflammatory response to lower LPS doses. Thus, there are differential sensitivities of trophoblast cells to bacterial components across gestation.63 This dose dependency and role for TLRs and NLRs is reflected in vivo. In pregnant mice, high-dose LPS induce placental and uterine inflammation, and subsequent preterm birth.64 TLR4 deficient mice are protected against bacterial and LPS-induced preterm birth,65,66 and blocking TLR4 in non-human primates prevents LPS-induced preterm uterine contractility.100 High dose iE-DAP also induces inflammation at the maternal-fetal interface, and preterm birth.62 However, low-dose LPS can trigger preterm birth in mice on a pathological background, such as an active viral infection67,68 or IL-10 deficiency.69,70 Thus, while TLRs and NLRs appear to be involved in preterm birth in response to bacterial components, in normal early pregnancy there appears to be some tolerance towards certain triggers such as LPS that may always be present at the maternal-fetal interface.71,72 Furthermore, while bacteria can induce preterm birth,73,74 additional signals, such as a virus, may further sensitize the placenta to these bacterial signals.67,68
Gram-positive bacterial PDG, which activates TLR2 in association with TLR1, TLR6 or TLR10, triggers a very different response in human first trimester trophoblast cells compared to term trophoblast. At term, PDG activates these cells to produce IL-8.75 However, in first trimester trophoblast cells, instead of activating the classical inflammatory cascade, PDG through TLR1, TLR2 and TLR10, induces apoptosis and downregulates the cell’s basal cytokine/chemokine expression. Moreover, this apoptotic response is prevented by the ectopic expression of TLR6, which is absent in the first trimester trophoblast.58,76,77,78 Similarly, delivery of PDG early in gestation induces placental apoptosis without evidence of inflammation,76 while delivery later in gestation triggers preterm birth.79
Viral sensing by the trophoblast
The viral sensors, TLR3 and TLR8, which recognize viral dsRNA and ssRNA, respectively, mediate a rapid, robust chemokine/cytokine (IL-6, IL-8), type I interferon, and anti-viral response in human first trimester trophoblast.80,81,82 Furthermore, independent of TLR8, viral ssRNA also induces trophoblast expression of IL-1β, anti-viral factors, as well as inducing apoptosis.82 This placental ssRNA-induced response is mirrored in vivo without triggering preterm birth.82 In contrast, viral dsRNA induces preterm birth through TLR379,83 while other groups have reported preeclampsia-like symptoms in viral dsRNA-treated mice.84
Trophoblast activation by DAMPs
In addition to understanding how the trophoblast responds to PAMPs, the impact of DAMPs on trophoblast function has been investigated. DAMPs are host-derived factors that are either not usually released from cells or tissues, or if present in the extracellular space, are normally at low levels. The DAMP, high mobility group B1 (HMGB1) can be released, passively from damaged cells or actively in response to inflammatory triggers, and itself can mediate inflammatory responses via TLR2, TLR4 or receptor for advanced glycation end products (RAGE)85 (Figure 4). HMGB1 levels are increased in the amniotic fluid from patients with preterm birth or preterm premature rupture of membranes (PPROM) and intrauterine infection;86 in fetal membranes from patients with preterm labor and PPROM;87 and in serum from pregnancies complicated by either preeclampsia or reduced fetal movements.88,98 Term trophoblast cells treated with HMGB1 secrete significantly increased levels of IL-1β, IL-6, and MCP-1, however, mechanistic studies are needed to show which receptors and signaling pathways are activated by this DAMP.89 One type of DAMP that has been extensively characterized are antiphospholipid antibodies (aPL); autoantibodies that specifically target the trophoblast by binding surface beta2 glycoprotein 1.90 These autoantibodies activate human first trimester trophoblast TLR4 resulting in inflammatory IL-8 and IL-1β secretion (Figure 4).91,92 Downstream of TLR4, aPL induce endogenous uric acid, another DAMP that specifically activates the Nalp3 inflammasome,93 which meditates pro-IL-1β processing and secretion (Figure 5).92 In parallel, aPL via TLR4 induces the expression of the miRNA, miR-146a-3p, which drives IL-8 secretion by activating TLR8 (manuscript under review).94,95 Thus, aPL, through TLR4, induces endogenous secondary messengers that subsequently activate other trophoblast innate immune PRRs. Hyperglycemic levels of glucose have similar effects on these cells, suggesting that overt pre-gestational diabetes may impact placental inflammation and function early in pregnancy. Excess glucose induces a pro-inflammatory cytokine/chemokine profile (IL-1β, IL-6, IL-8, GRO-α, regulated on activation normal T cell expressed and secreted, chemokine, and granulocyte colony stimulating factor). Moreover, the IL-1β response is associated with elevated uric acid and is dependent upon activation of the Nalp3 inflammasome.96 Indeed elevated serum uric acid has been associated with high-risk pregnancies, such as those complicated by preeclampsia, gestational hypertension, cases of reduced fetal movements, or obstetric aPL syndrome.92,97,98,99 Thus, rather than simply correlating levels with disease, uric acid can act as a direct mediator of trophoblast inflammasome activation and placental inflammation,93 suggesting it may play a pathological role. Furthermore, those host-derived non-infectious triggers, such as uric acid, glucose and aPL can activate the inflammasome indicates that this mechanism is not only involved microbial-induced inflammation, but also in sterile-induced inflammation.
Current opportunities
Inhibiting PRR activation to prevent infection-associated preterm birth has been considered. Indeed, a TLR4 antagonist prevented LPS-induced preterm uterine contractility in non-human primates100 and knockout mice for TLR or associated adapter proteins are resistant to microbial and PAMP-induced preterm birth.83,101 Similarly, since IL-1β is a mediator of preterm birth102,103,104,105 and fetal brain injury106,107,108,109 studies have focused on using IL-1 receptor antagonists or selective IL-1 receptor modulators to prevent these adverse outcomes.110,111 Thus, preventing the upstream induction of IL-1β by inhibiting placental inflammasome activity may also serve as a potential target for preventing adverse pregnancy outcomes.
Future opportunities
Since uric acid mediates placental inflammasome function92,93,96 currently available drugs that inhibit xanthine oxidase, such as allopurinol or febuxostat, may provide potential avenues to explore. However, this is only one mechanism by which IL-1β production can arise; there are a number of other inflammasomes that uric acid may not activate,112 as well as non-inflammasome-mediated pathways,113 all of which could be possible targets. Similarly, as we expand our knowledge about the role of miRNAs in mediating and regulating placental function and PRR activity we can begin to consider these as potential therapeutic targets.
Scientific gaps
Together, the studies discussed herein demonstrate that the placenta is immunologically functional with the trophoblast able to generate specific and diverse innate immune-like responses through their expression of a range of pattern recognition receptors. However, the type of response is highly dependent upon the stimuli, the receptor(s) expressed and activated, the downstream signaling pathways involved, and the timing of gestation. Indeed, while many of the end points and impact on pregnancy outcome triggered by PAMPs and DAMPs may be common, the upstream mechanisms are often quite distinct. These challenges for drug discovery and applications highlight the need for a greater understanding of the precise molecular pathways involved in placental sensing of infectious and non-infectious triggers.
Modelling trophoblast differentiation and placentation in the rat
(Michael J. Soares, University of Kansas)
Background
Fundamental to the establishment of pregnancy and formation of the hemochorial placenta is remodeling and restructuring of uterine spiral arteries allowing for flow of nutrients to the placenta and ultimately to the fetus.114 Trophoblast cells play a central role in this vascular remodeling process. Pathologies associated with trophoblast-directed uterine spiral artery remodeling underlie some of the most significant and challenging obstetrical diseases.114,115,116 There are several research strategies for gaining insight into the process of hemochorial placentation and for the identification of potentially vulnerable molecular mechanisms leading to disease. In this section we address the merits of animal models, especially the rat, for placental research.
Placentation in the rat
The rat possesses hemochorial placentation with deep intrauterine trophoblast cell invasion and trophoblast-directed uterine spiral artery remodelling;117,118 features shared with human placentation.114,119,120,121 Recognition of these similarities spurred the establishment of in vitro and in vivo research methods using the rat as an animal model to address mechanistic questions regarding development of the hemochorial placenta and especially the role of invasive trophoblast cells in the remodeling of uterine spiral arteries. Rcho-1 trophoblast stem (TS) cells and blastocyst-derived TS cells are two rat in vitro culture systems that have been used extensively to investigate signaling pathways and mechanisms controlling trophoblast cell differentiation.122,123 Regulatory factors identified in vitro have been experimentally tested using an assortment of in vivo research strategies, including transgenesis to monitor the invasive trophoblast lineage,124 spontaneous mutant rat models possessing placental insufficiency118,125,126,127,128 and gain-of-function and loss-of-function manipulations using trophoblast-specific lentiviral gene delivery and genome editing.129,130,131,132
A fundamental property of placentation is its plasticity, which is characterized by the acquisition of structural/functional properties permitting adaptation to environmental challenges. Mechanisms underlying these adaptive processes are an important feature of placentation and represent potential therapeutic targets. Limiting oxygen delivery to the placentation site at developmentally defined phases of gestation effectively activates acquisition of the invasive trophoblast phenotype and promotes uterine spiral artery remodeling124,133 adaptive responses conserved in primates.134,135 These observations illustrate the instructive nature of oxygen delivery as a signal driving decision-making within trophoblast stem/progenitor cell populations and ultimately affecting hemochorial placentation; however, it is important to appreciate that failures in adaptive responses to hypoxia can lead to disruptions in placentation. Additionally, hypoxia can also be a pathologic response to a failed placenta resulting in disease. Dissociating these potentially confounding outcomes of hypoxia is essential. Dissection of hypoxia-guided pathways within trophoblast stem/progenitor cell populations offers an opportunity to identify molecular events in the placentation process that could be manipulated to improve and enhance placental development, preventing the spiraling consequences of a failed placenta.
Role of natural killer cells
Immune cells, especially natural killer (NK) cells, are active contributors to remodeling of uterine spiral arteries during the establishment of pregnancy. The involvement of NK cells in the hemochorial placentation process has been effectively investigated in the rat (Figure 6). NK cells directly regulate uterine spiral artery growth and progression towards the developing placenta. These critical events impact oxygen delivery to the placentation site and trophoblast differentiation.133 Failures in NK cells result in attenuated uterine spiral artery development, local hypoxia at the placentation site, and premature and exaggerated activation of endovascular invasive trophoblast cells leading to extensive uterine spiral artery remodeling. Thus NK cells control oxygen delivery to the developing placenta and regulate the timing and extent of endovascular invasive trophoblast cell differentiation and trophoblast-directed uterine spiral artery remodeling. Evidence also exists supporting a conserved role for uterine NK cell actions within the human placentation site.136 Modulation of uterine NK cell behavior represents another potential target for modulating the placentation process.
Figure 6. NK cells and endovascular trophoblast cells contribute to uterine spiral artery remodeling.
Rats were treated on E4.5 and E9.5 with normal rabbit serum (Control) or anti-asialo GM1 (NK cell depleted) and sacrificed on E13.5 (A–D). Double immunofluorescence staining for ANK61 (NK cell marker) and ACTA (smooth muscle marker; A, B) and cytokeratin and ACTA2 (C, D). Asterisks demarcate blood vessels possessing interruptions (arrowheads) in the tunica media (A, C). Asterisks identify blood vessels with intact tunica media (B). Scale bars=0.25 mm.
Reprinted, with permission, from Chakraborty et al.133
Current Opportunities
Animal models are important tools to understand human disease. Animal models provide the opportunity to study biological phenomenas not easily studied in the human. Although no model is ideal, each provides useful insight relevant to the human condition. The rat is a particularly important experimental tool for investigating regulatory processes controlling hemochorial placentation. Species differences in placental organization and gene expression patterns are evident but there are also underlying commonalities in structure, function, and especially molecular mechanisms regulating the placentation process, and when the time and effort are taken to investigate these processes it has been demonstrated that there is considerable merit for animal models in placental research.120,137,138,139,140,141
Future Opportunities
Maximal benefits for animal models in placental research will be achieved when efforts are directed to regulatory events and mechanisms that are conserved in the human. The rat has been effectively used to investigate regulatory events involving trophoblast cells, NK cells, and uterine spiral arteries, a triad of key players in hemochorial placentation.
A paradigm for investigating molecular mechanisms impacting hemochorial placentation utilizing rodent TS cell models, followed by the validation of the obtained results in human placenta and human trophoblast cell models, and then proceeding to in vivo rodent experimentation represents a powerful research approach.131,142,143 Evaluation of conservation is generally limited to utilization of primary or immortalized human trophoblast cell model systems and expression analysis in human placental tissues. A human TS cell culture system functionally equivalent to rodent TS cells would be optimal for studying the regulation of differentiation, especially the identification of conserved processes. Some progress has been made in developing this important experimental in vitro tool.144 The recent availability of genome editing strategies to generate mutations in rodents should lead to the establishment of new animal models for in vivo testing of conserved molecular mechanisms controlling hemochorial placentation. These in vivo approaches should include targeting the activities of specific trophoblast and immune cell populations in order to identify molecular pathways that could serve as sites for therapeutic intervention.
Scientific Gaps
The utilization of relevant and appropriate animal models, including rodents, to test hypotheses in vivo most importantly extends placental research beyond description, classification, in vitro analyses, and molecular phenotyping and permits a rational approach for understanding the physiology of placentation, the pathogenesis of placental disease, and importantly the identification and testing of potential drug targets for treating placental disease.
Modeling placental function and pregnancy physiology in mice
(James C. Cross, University of Calgary)
Background
Understanding the molecular, cellular and physiological functions of the placenta in humans is limited to expression studies in normal and pathological human pregnancies, and some in vitro systems. Because of this, animal models remain critical for investigation of the basic biology and assessment of biomarkers and treatments of pregnancy complications. The mouse has been a powerful model for understanding animal biology in the last 25 years with the advent of transgenic and knockout technologies. Hundreds of different gene knockouts in mice have given molecular insights into the development and function of the placenta. Many of these genes have human homologues that are expressed in the placenta.145,146 However, before zeroing in on genes and cells, if mice are to be truly useful for understanding human pregnancy complications, it is critical to first ask if mice and humans have similar physiologies of pregnancy. This is the starting point for using mice to investigate molecular mechanisms giving us testable hypotheses to assess in human studies.
Similar physiology of pregnancy in humans and mice
Mice adapt to pregnancy with major changes in the maternal cardiovascular, metabolic and immune systems. Similar to humans, mice show increased cardiac output, plasma volume, and a mid-gestation drop in blood pressure.147,148 There are also major changes in metabolism in which the mother’s fat and muscle become insulin resistant, requiring more insulin to take up glucose, which helps to shunt glucose to the fetus.149 In order to combat insulin resistance, an increase in pancreatic β cells and insulin synthesis occurs.150,151,152 Gestational diabetes occurs if there is inadequate β cell compensation.153,154 The most important change in the immune system during pregnancy is the appearance of large numbers of uterine natural killer (uNK) cells in the decidua,155 first described in mice and only later in humans. One difference between mice and humans is that mice are litter bearing whereas humans tend to have singleton pregnancies but what functional difference this has is not clear given the similarity of pregnancy physiology.
Trophoblast functions and pregnancy
Most of the research on trophoblast cell function in human pregnancy complications has focused on trophoblast cell invasion and its association with spiral artery remodeling. Cell ablation experiments in mice showed that this is not just an association and that trophoblast cell association with spiral arteries is critical for remodeling of those arteries156, though uNK in the decidua also play a role.157 Beyond just invasion of spiral arteries, however, the human placenta contains diverse extravillous trophoblast subtypes158 and mice have diverse trophoblast cells in the junctional zone expressing complex patterns of hormones.159
Several lines of evidence indicate that the endocrine function of the placenta modifies metabolism in the mother necessary to promote fetal growth. Scanning through microarray data in the public domain indicate that the human placenta expresses over 80 different hormones.160 While a few are placenta-specific hormones arising from duplication of the GH 2 gene but most are from canonical hormone genes expressed in the placenta presumably due to evolution of placenta-specific promoter and/or enhancers. The hormones include known regulators of metabolism, blood cell production and reproduction. Placental Prolactin (Prl)-related hormones can promote proliferation of pancreatic β cells and insulin synthesis.161 glucose transporter-related hormones162, progesterone,163 resistin,164 and leptin165 can promote insulin resistance. Paradoxically, the placenta also produces adiponectin166 that promotes insulin sensitivity. It is interesting that while these hormones are normally expressed by the pituitary (Prl, GH), ovary (progesterone), and fat (the ‘adipokines’: resistin, leptin, adiponectin), the human placenta is a major source during pregnancy and production from the maternal tissues is downregulated.
The mouse placenta expresses ~40 protein hormone genes.167 As with humans, Prland GH-like hormones, progesterone, resistin, leptin and adiponectin are elevated during pregnancy. However, the evolution of the system is slightly different as the Prl gene, and not the GH gene, is duplicated in mice to produce 22 placenta-specific members168 with Prl- and GH-like activities. In addition, the placenta is not the only source of the other metabolic hormones. Progesterone is produced by the ovary throughout pregnancy in response to stimulation by Prl-like hormones from the placenta.169 resistin,170 leptin171 and adiponectin172 are produced by fat during pregnancy in mice. Prl receptor signaling can regulate adipokine expression,173,174,175 suggesting the possibility that while the mouse placenta is not the direct source of all metabolic hormones, unlike humans, it may still orchestrate the network. The best evidence in mice that placental hormones regulate fetal growth is that of the pleckstrin homology-like domain, family A member 2 gene which regulates the fetal growth by influencing the number of endocrine cells in the placenta.176
Current opportunities
Despite the clear evidence that placental hormones drive fetal growth and regulate maternal metabolism, it is curious that research has not continued to understand their roles in intrauterine growth restriction (IUGR) and gestational diabetes in humans. Placental hormones are attractive targets from a diagnostic standpoint because they can be measured serially and improvements in multiplex immunoassays mean that several hormones can be assessed at the same time. Both hormone levels and polymorphisms in the placenta GH-related genes have been associated with pregnancy complications in humans,165 though the number of published studies is limited and they have often examined single hormones and not made connections with anatomical changes in the placenta.
Future opportunities
There is emerging evidence from mouse studies that the placental hormones are sensitive to maternal nutrition and changes in their levels likely reflect attempts to mitigate the impact of poor nutrition on fetal growth.177 Therefore hormone levels should have good predictive value both in reflecting stress to the pregnancy and the robustness of the placenta’s ability to mitigate the impact on the fetus. In addition to diagnostic value, it is easy to imagine therapeutic strategies in which hormone supplementation or blockade is used to treat pregnancy complications.
Scientific gaps
The complexity of the hormone network at play during pregnancy, both the number of hormones and the systems of feedback and adaptation, will require the use of animal models, particularly knockout and transgenic mice, to understand them. With the ability to study mouse physiology, it is clear that while a mouse is not a human we can certainly learn from them.
II. Potential Drug Targets of Important Placental Pathways in Relation to Pregnancy Disorders
Placental hypoxia as a molecular target
(Stacy Zamudio, Hackensack University Medical Center)
Background
Hypoxia is a pathological condition in which there is insufficient oxygen to maintain normal physiological processes. However ‘hypoxia’ is often used imprecisely in the literature, interchangeably with some of its causes, for example-hypoxemia reduced partial pressure of oxygen (PO2), anemia (insufficient hemoglobin or hemoglobinopathies that alter oxygen binding/release) or reduced environmental oxygen availability (as in high altitude, animals dwelling in burrows, diving mammals). Hypoxia is usually assumed to be present in any one of these conditions. For example, a reduction in blood flow to a specific organ or tissue, whether acute or chronic, is often assumed to be a hypoxic insult. However, hematological adaptations can compensate for lower blood flow,178 and the volume and speed with which blood travels, as well as diffusional distances, affect tissue oxygen delivery.179,180,181,182,183 Thus in the absence of direct measures of oxygenation in the tissue, cell or organ of interest, it is difficult to tell if hypoxia is present.
Oxygen levels in the human placenta and fetus
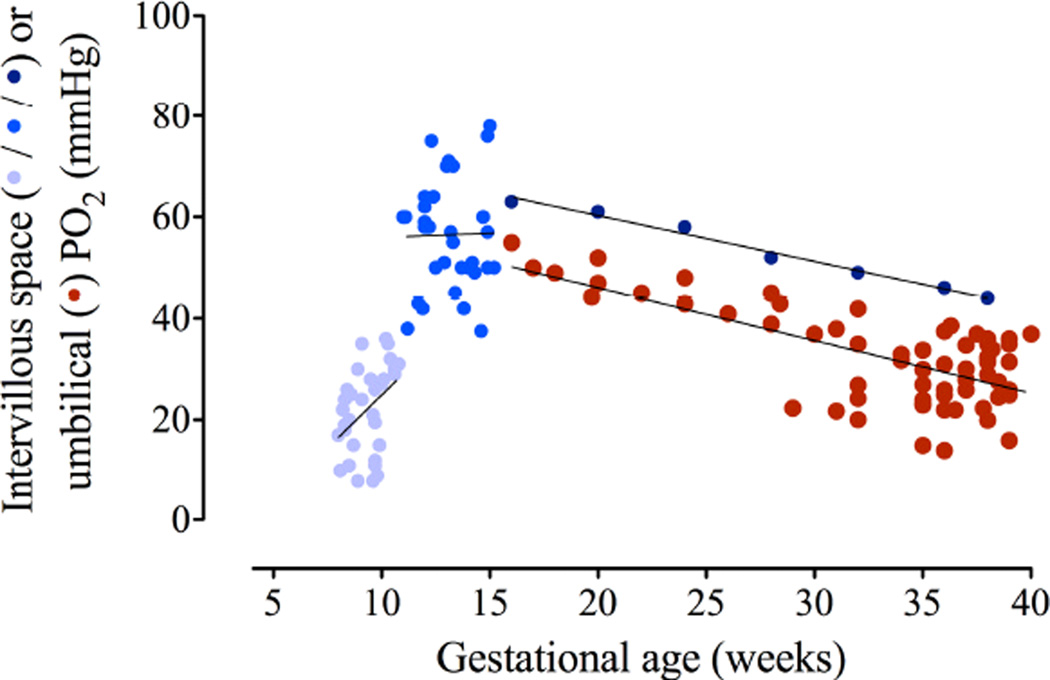
Normal oxygen levels within the intervillous space of the placenta are low early in gestation (~20 millimeters of mercurymmHg), can rise to as high as 80 mmHg in the early second trimester and then decline progressively towards term (Figure 7)184,185,186,187. Fetal umbilical venous PO2 follows a similar pattern, perhaps reaching as.188 Despite difficulty in quantifying magnitude and intensity of hypoxia, much less being able to detect its onset, the bulk of evidence has led to general acceptance that fetal growth pathologies are often associated with placental hypoxia, specifically early-onset preeclampsia, most non-genetic/syndromic IUGR, PE with IUGR, and some diabetes/gestational diabetes (GDM) with large for gestational age neonates.189,190,191,192,193, 194,195,196,197 Figure 7 shows that what might be considered abnormally low O2 levels in the placenta will vary not only by gestational age, but also with location within the placenta. The 3rd trimester placenta is exposed to PO2s that can range from < 20 mmHg where the deoxygenated blood from the umbilical artery flows back into the placenta to > 80 mmHg where the maternal arterial blood first enters the intervillous space. This equates to 1–10% ambient O2 for in vitro experimentation. Such data have given rise to a convention in which 5–8% O2 is used to mimic normoxic conditions in the 3rd trimester and < 3% for hypoxia.
Figure 7. Relationship of maternal intervillous blood PO2 to fetal umbilical venous PO2.
Oxygen tension in the intervillous space of the placenta is very low until the opening of the spiral arteries to blood flow at about 10–12 weeks of gestation. The light blue dots are individual data points obtained at 8–11 weeks gestational age. Medium blue dots are data obtained from individual pregnancies at 11–16 weeks. Dark blue dots are the mean of values obtained in only a few women between 16–38 weeks and have very wide confidence intervals (>30 mmHg). Red dots are umbilical venous PO2. Note the tight relationship and narrow diffusional gradient between intervillous and fetal PO2 late in pregnancy. Figure is a composite of data obtained from various references.
Composite of data obtained from several sources.184,185,186,187
Placenta hypoxia cannot be unambiguously diagnosed due to the inherent limitations of human experimentation and the inaccessibility of the placenta and fetus in vivo. Proxies are used instead. For example, reduction in blood flow severe enough to deprive tissues of oxygen and glucose sufficient for metabolic needs is assumed to be present where elevated maternal/fetal Doppler indices reflect increased impedance. Elevated erythropoietin (EPO) levels in mothers and babies, nucleated red blood cells in neonates, increased placental expression of hypoxia-inducible-factor (HIF) and its target gene products have all been used to demonstrate that placental hypoxia is present in fetal growth pathologies.194,198,199,200,201,202,203,204,205,206,207,208,209,210 Thus placental hypoxia is a target for therapeutic intervention, and unlike some of the topics discussed at this workshop, there have already been a number of clinical trials designed to ameliorate or prevent presumed consequences of hypoxia such as inflammation211 and oxidative stress.212,213,214,215
Chicken and egg, cause and effect
Of critical importance is distinguishing between hypoxia per se, and the hypoxia response. The hypoxia response is evidence that hypoxia is or has been present (e.g., up-regulation of HIF and HIF-regulated genes, higher fetal hemoglobin concentrations). The strength and magnitude of the response may reflect the intensity of the insult, but it is also often adaptive in that it enables mechanisms that increase delivery of oxygen to the fetus (as in metabolic reprogramming or the development of decreased vascular syncytial membrane thickness). On the other hand, we must be mindful of the possibility that failure of an appropriate hypoxia response may itself be part of the pathology. There is evidence of HIF dysregulation and consequent over-expression without adaptation in the more severe forms of preeclampsia.209,210,216 In contrast, in some severe, early-onset IUGR there often appears to be a lack of HIF-mediated responses, where all evidence suggests such response is needed.217,218,219
Studies at high altitude have revealed the importance of being able to distinguish between adaptive hypoxia response and pathology, as well as the subtlety with which O2 tension can exert an effect. Maternal partial pressure of O2 in arterial blood (PaO2) falls considerably at ≥2700 meters (m) elevation, and due to the sigmoid shape of the O2 dissociation curve, PaO2 falls precipitously at >3000 m. Even in pregnancies with entirely normal outcomes, physiologically lowered maternal PaO2 results in a progressive slowing of the 3rd-trimester fetal growth trajectory220,221 This is despite significant placental adaptation both structurally (increased angiogenesis, decreased vascular syncytial membrane thickness) and metabolically.222,223,224,225,226,227 Altitude studies in vivo showed that the human placenta subjected to hypoxic stress engages in a highly conserved process most obviously seen in solid tumors.228,229,230,231 Metabolic reprogramming is a reversible form of hypometabolism in which an effective, largely mitochondrial driven switch from oxidative phosphorylation to aerobic (i.e. glycolytic) glucose consumption by the trophoblast, results in increased cellular oxygen availability, which is then available for diffusion to the fetus.226,227 In essence the mechanism ‘spares” oxygen for the fetus, but at the same time reduces fetal access to glucose. This slows fetal growth but ensures that the fetus does not outgrow its supply line. Even when placental metabolic programming is active, a fetus with greater umbilical venous O2 tension consumes more O2.
How might hypoxia be ameliorated
Therapeutic strategies must necessarily depend on what is the pathological barrier to normal placental oxygen diffusion. Four ‘targets’ will be considered here: blood O2 content, uteroplacental blood flow, placental structure (angiogenesis), and hypoxia-induced metabolic reprogramming.
A. Raise arterial O2 content (CaO2) or PaO2
In women who are anemic, the obvious treatment would be increasing maternal red cell mass and thereby increasing CaO2. This is harder than it sounds, as women have less of a response to EPO than men,232 and up to 25% of pregnant women treated with EPO do not respond.233 At high altitude CaO2 is increased by raising hemoglobin concentration. This preserves maternal and fetal oxygen delivery178,234 despite a ~ 20% reduction in uterine artery blood flow in mothers, and an even greater decrement in fetal umbilical venous blood flow. Packed red blood cell transfusion might work for anemic mothers, but it is unlikely to be of value where the barrier to O2 diffusion is due to placental structural defects such as the failed tertiary villous vascular development (branching angiogenesis and elongation/dilation of terminal capillary loops) seen in idiopathic IUGR. Moreover there is a balance that must be maintained between blood viscosity and O2 carrying capacity: e.g., the optimal hematocrit for preservation of O2 delivery in the brain microcirculation is 15–40%.235 One obvious method for increasing PaO2 is O2 supplementation. Sadly, a 2003 Cochrane review found that of 10 trials of O2 therapy, only 3 were adequate for inclusion in a meta-analysis. O2 compared with no O2 was given to mothers for improvement of fetal growth. Re-review in 2009 changed none of the Cochrane conclusions since no additional trials were undertaken.236 The trials were poorly designed and involved <100 women. However, 24 hr supplemental O2 led to a decrease in perinatal mortality rate. The studies were confounded in that the O2-treated group was of greater gestational age at initiation of treatment. Concerns over possible response to “hyperoxia” such as generation of free radicals, inhibition of potentially adaptive responses ongoing in fetus and placenta, have been raised as objections to O2 therapy.
B. Blood flow
Deficits in uteroplacental or fetal blood flow are strongly associated with PE and IUGR as evidenced by Doppler ultrasound measures of impedance to blood flow in the uterine arteries and within the fetus. The studies in which blood flow in human pregnancy complications has been measured quantitatively or semi-quantitatively support this.237 Absent or reversed end diastolic blood flow velocity in the umbilical arteries is a grave sign and usually leads to emergent delivery.238 Elevated Doppler resistance indices in the maternal uterine arteries indicates there is downstream impedance to flow. This is accompanied by morphological evidence of placental structural problems such as a reduction in small arteries within the tertiatry stem villi,239 thickening of the basal lamina and erythrocyte congestion in teriary villous capillaries240,241 As with CaO2 or PaO2, how one might target blood flow depends on what is the underlying problem. The vast majority of blood entering the intervillous space is carried by the maternal uterine arteries. The uterine arteries are therefore critical to pregnancy success and a potential target. In normal pregnancy eccentric remodeling of the uterine arteries leads to doubling of uterine artery diameter by 20 weeks of pregnancy and a further, smaller increase, likely due to shear stress, in the late third trimester.242 Eccentric remodeling is characterized by changes in the composition of the vessel wall that permit greater distensibility. In addition, there is inhibition of the myogenic response (the rise in vessel tone with increasing pressure).243,244,245
In human and multiple experimental animal models endothelium-dependent vasodilator response is markedly increased in the uterine circulation during pregnancy. In rodent models, depending on what branch of the uterine artery is investigated, nitric oxide (NO) contributes 30–80% and endothelium derived hyperpolarizing (EDH) effectors 20–70% of the endothelium-dependent vasodilator response of the pregnant uterine arteries.246,247
C. Placental structure (e.g. angiogenesis)
In idiopathic IUGR the placenta frequently has a dearth of branching angiogenesis and poorly developed tertiary capillary development, leading to reduced O2 diffusion capacity.180,218 Structural placental defects in PE are less clear. An excess of fibrinoid deposition leading to, or as a result of, villous death has been attributed to excess oxidative stress or inflammation. In preeclamptics, in general, placentas are smaller (reduced weight and volume), and the volume of functional tissue (parenchyma) and villous surface area (area of nutrient and gas exchange) are reduced.248 However, unlike IUGR, they also have an increase in the fetal capillary volumes and density (indicative of a hypoxia response). Late onset preeclampsia (without fetal compromise) show no such changes and cannot be statistically differentiated from normal placentas.249 The interplay of many angiogenic growth factors and of several critical cell types (pericytes, endothelium, trophoblasts) is involved in the normal vasculogenesis of the placenta.225,250
D. Placental metabolic reprogramming
In cancer biology several recent, unique insights are relevant to the placenta and consideration of molecular pathways that might be amenable to therapeutic intervention. Cancer cells have dysregulated, Warburg-like glucose metabolism. Energy production is abnormally dependent on aerobic glycolysis; there is increased fatty acid synthesis and increased rates of glutamine metabolism.251 In fact the term “glutamine addicted” is now applied to many cancers.252 In cancer biology “glutamine addicted” refers to an extension of metabolic reprogramming in which glutamine is required for essential amino acid uptake to maintain activation of mTOR. In many, if not all cancer cells, glutamine is also the primary mitochondrial substrate. These metabolic changes are linked to therapeutic resistance in cancer treatment and hence strategies are being developed to target this altered cancer metabolism in conjunction with older treatments designed to inhibit angiogenesis or otherwise shrink the tumor.253,254 We have demonstrated the same, evolutionarily conserved mechanism of metabolic reprogramming in the hypoxic placenta, associated with stably elevated HIF-1alpha levels, which initiates metabolic reprogramming.207,226,227 However in contrast to cancer, the objective of a therapy targeting metabolic reprogramming in the placenta would be to sustain the response, which leads to increased intracellular oxygen levels and hence more oxygen for diffusion to the fetus.
Current opportunities
There is widespread acceptance that placental hypoxia, acknowledging the already low O2 environment that is normal, is associated with pregnancy pathologies. However, before a placental molecular intervention is attempted there should be a greater effort to examine relatively non-invasive means of improving oxygenation in the placenta. These should include definitive studies of maternal O2 supplementation, dietary strategies designed to increase maternal substrates for NO, and other, obviously more benign strategies that target maternal physiology rather than placental function.
Methods which might be employed to provide additional means of carrying oxygen in the circulation, e.g., oxygen filled microbubbles, are under investigation.255,256,257,258 Moreover, the opportunity exists currently to further test the effects of supplemental O2; more modern standards of clinical trials such as establishing dose/response, effects of treatment duration, minimum dosing necessary etc. should surely be attempted as a simple yet potentially effective intervention.
Beyond this are the effectors responsible for altering vascular tone that could be molecular targets, including NO by stimulating release via a number of means including: administration of VEGF, PlGF, relaxin, or stimulation of the reticular activating system, hemoxygenase – carbon monoxide, large conductance Ca2+-activated potassium (K+) channels, as well as increased dietary intact of arginine or citrulline.259,260,261 For EDH, potential effectors include K+ C-type natriuretic peptide, arachidonic acid derivatives, epoxyeicosatrienoic acids and hydrogen peroxide.
Three approaches have been or are currently being investigated to increase blood flow. The least invasive of these involves dietary arginine (or citrulline) supplementation, in theory to increase substrate for NO production. A number of small trials led to one randomized controlled clinical trial.262 High-risk women (with a history of preeclampsia in the prior pregnancy) had less than half the rate of PE as women receiving placebo. However, another trial in women already diagnosed with PE and treated acutely, rather than throughout pregnancy, showed no benefit.263 International recruitment for a trial is ongoing for the treatment of early-onset IUGR.264 This 6-year study is based on several small studies showing that low-dose sildenafil improved fetal growth and neonatal survival.265 Another trial is ongoing led by Dr. Anna David in the United Kingdom, targets women with even earlier IUGR, women whose IUGR is so severe the fetus would normally die before viability.266 Treatment consists of uterine artery catheterization and injection of adenovirally delivered VEGF-D. Proof of concept studies in sheep demonstrated that this treatment improves uterine artery blood flow for ~1 month.267
Future opportunities
With respect to angiogenesis, cancer therapies, especially for solid tumors, provide some clues. These therapies target inhibition of angiogenesis, whilst in the placenta one might wish to stimulate organ-specific angiogenesis. Effectors in this molecular pathway provide a variety of targets. Vascular Endothelial (VE)-cadherin and matrix metalloproteinases), for example, loosen gap junctions in the endothelium, which is required for tip-cell formation, a prerequisite to branching angiogenesis. However tip cell formation also requires coordinated activity by VEGFR-2, various ligands for Notch-1 receptor, (such as Delta-like ligand and jagged 1), neuropilin 1, integrins, HIF-1α and by angiogenic growth factors such as VEGF, fibroblast growth factors, angiopoietins.
Metabolic reprogramming is HIF-dependent, and, as indicated above, HIF may be dysregulated in some placental pathologies. Targets other than HIF that could be manipulated to sustain the metabolic response to hypoxia might include a variety of enzymes: hexokinase, pyruvate kinase M2, lactate dehydrogenase A, pyruvate dehydrogenase kinase, fatty acid synthase and glutaminase.
Inhibition of HIF-1α or its gene products has been a primary focus in development of cancer drugs. There are over 400 drugs that specifically target the HIF pathway, most of them inhibitory. Many of them also target specific, cancer-related mutations that are related to the HIF pathway; hence a reverse engineering approach could be applied to existing drugs to see whether they would be beneficial, as has been done in human immunodeficiency virus.268,269 A placental strategy might consist of controlled up-regulation of HIF-1α, as it is known to be stably elevated in hypoxic placentae and likely contributes to the increased angiogenesis that is a favorable adaptation where placental hypoxia is present but not associated with a pathology.222
Scientific gaps
For further advances in molecular targeting, greater information is required in a number of areas. For example, while there is a large quantity of data on the role and effects of vasodilators, the other half of this equation, the receptors for these agents are poorly understood. Although a targeted increase in receptors for vasodilators would be a possible therapeutic strategy, we clearly require more knowledge of the broader effects of receptor modulation.
Similarly for the angiogenic growth factors, what are the consequences if some, but not other angiogenic growth factors, are targeted in the placenta. What might be the response to modulating the angiogenic growth factor receptors rather than the growth factors themselves? Might it be possible to devise tissue and/or cell type selective modulation which would bypass the problems of the less-controlled distribution of the ligands? Placental vascular pericytes have been suggested as a hematopoietic stem cell or mesenchymal stem cell. They are intimately involved in vessel stability and angiogenesis.270,271,272 Pericytes can be detached from their vascular niche by angiopoietin-2, which would increase the stimulus for branching angiogenesis. Again we lack more knowledge to determine whether these supporting characters may be the key to successful vascular therapies.
Is fetal growth a feasible target for placental intervention?
(Nicholas P. Illsley, Hackensack University Medical Center)
Background
Alterations in fetal nutrient concentrations due to changes in placental transport and/or metabolism are associated with a variety of fetal growth pathologies. Deficits in oxygen are associated with hypoxia, PE, and IUGR. Deficits in glucose and/or amino acids are associated with IUGR and an excess of glucose is associated with macrosomia, obesity and diabetes. Alterations in placental nutrient transporters are associated with a variety of fetal growth pathologies; the glucose transporter (GLUT)1 in hypoxia and diabetes273,274 and amino acid transporters in IUGR and diabetes.275,276 The simplest possibility in these circumstances would be to reverse these deleterious changes by manipulation of maternal substrate levels. There are situations where action to re-balance abnormal nutrient concentrations is capable of correcting growth problems, the restoration of normal maternal glycemic status to prevent macrosomia being an example. Overall, however, the record with regard to maternal nutrient supplementation is one of minimal effect combined with potentially serious side effects.277
Another set of important questions revolves around timing. Is there a window during which intervention is possible, and perhaps more importantly, how do we know when intervention is necessary? In answer to the former, intervention is clearly preferable before structural and biochemical changes make the growth restriction process difficult to reverse, leading to the latter question – how do we detect the initial stages of growth restriction? The best indicator we have currently that fetal growth is deviating from normal is the fetal growth trajectory derived from serial ultrasound measurements. Given that this requires multiple measurements over weeks of gestation, by the time we can verify growth restriction, the fetus (and placenta) are already well advanced through pathologic changes in growth. This crude indicator signals a process that is already well underway and will, at best, allow for stabilization of growth at a much lower centile. It is clear that without a means to detect the early stages of the processes that contribute to growth restriction, intervention will lag well behind. Our own research into placental metabolic processes has shown that the early stages of growth reduction involve a switch away from oxidative metabolism toward an increased level of glycolytic metabolism (placental metabolic reprogramming).278 As a potential early indicator of growth problems, initiation of placental metabolic reprogramming may provide the crucial biomarkers needed to detect the first stages of an altered growth trajectory.
Current Opportunities
Assuming that combined biochemical/imaging methods will eventually detect at-risk pregnancies, what might we focus upon as placental targets for intervention? Is it possible to see nutrient transporters as possible molecular targets? The ones that concern us here are those that transport the essential, higher volume nutrients, including glucose, amino acids and fatty acids. As the high volume glucose transporters (i.e. GLUT1 and GLUT3) demonstrate unique polarized distributions between the maternal-facing microvillous and fetal-facing basal surfaces,279,280 altering the balance of glucose transporters within the syncytiotrophoblast raises with it the question of the selective polarized targeting needed to achieve increased transport. The polarized targeting question arises also for amino acid transporters where the asymmetric distribution acts to move sufficient quantities and types of amino acid against their gradient, into the fetus.281 In the absence of targeting information for these transporters, we need to be assured that interventions will provide the appropriate distribution between microvillous and basal faces necessary to increase flux. Moreover the effects of increased transporter density, while possibly increasing transplacental fluxes, will also pose other problems. In the case of glucose, this is likely to generate increased glycolytic metabolism and subsequent lactic acidosis, as observed in glucose supplementation interventions. In the case of amino acids, the interconnected nature of their transport and the broad substrate specificity of amino acid transporters suggest that alterations to an individual transporter will have significant and hard-to-predict ramifications for transport of other amino acids and for placental amino acid metabolism. Similarly, it is difficult to predict what the effect of altering the expression of placental lipases, fatty acid transport or fatty acid binding proteins might be on processes such as oxidative metabolism and the transport of other nutrients. The essential, high-volume nutrients have a wide range of metabolic fates and they or their metabolites play crucial roles in regulating key metabolic pathways. Not surprisingly therefore, changes in the expression of individual nutrient transporters are likely to be associated with unwanted changes in placental and/or fetal metabolism. The uncertainty engendered by the alteration of individual transporters makes them problematic candidate molecular targets.
An alternative approach to targeting individual transport or metabolic processes is the generation of alterations in a manner that combines multiple metabolic and/or transport targets. In this way the transport and metabolic processes that involve specific substrates are modulated in an integrated fashion. We already have detailed information on many of these integrated pathways. They form the traditional endocrine or growth factor pathways that mediate regulation of cellular function by extracellular factors. We know for example that insulin-like growth factor I (IGF-1) regulates the expression of placental glucose and amino acid transporters282,283 and that it is positively associated with fetal and placental growth.284,285 Judicious adjustment of the placental IGF-1 pathway might allow for an integrated regulation of growth processes. Recent research has shown that adenoviral-mediated delivery of IGF-1 to the placenta appears to stimulate glucose and amino acid transporter expression in vivo and to correct placental insufficiency.286,287 Similar types of growth factor-mediated regulation might be possible via the signaling pathways stimulated by IGF-2 or placental growth hormone. Perhaps the molecular targets for these pathways might be restricted to selected cell types by altering receptor levels rather than altering the concentration of the growth factors themselves.
Future opportunities
While the effectors described above may be able to stimulate integrated pathways for the promotion of fetoplacental growth, the signaling pathways activated in this way may be too numerous or too broadly based to avoid other less desirable effects. Growth factor signaling pathways frequently have many transduction pathways which branch off below the growth factor receptor. Another alternative is the targeting of metabolic or signaling sub-networks that control multiple components but do not extend to all of the endpoints for a signaling pathway. For example mTOR (mammalian target of rapamycin) is a serine kinase that is the focus of a signaling pathway which forms a nexus for multiple nutritional and metabolic signals. mTOR affects growth by modulating protein synthesis, lipid synthesis and energy metabolism, primarily through phosphorylation of the 4E-binding proteins which in turn have marked effects on the production of the proteins which control messenger RNA (mRNA) translation.288 In the placenta, in addition to effects on protein synthesis, mTOR appears also to modulate amino acid transporters by regulating the membrane trafficking of transporter protein.289 There is good evidence to suggest that mTOR activity is proportional to maternal body mass index,290,291 while in IUGR, syncytial mTOR, while displaying increased expression, shows decreased activity.291,292 This suggests that the mTOR pathway may be a good potential regulatory point for growth-related intervention. Is it possible, for example, to directly target the mTOR system, below the broadly regulatory phosphatidylinositol-3-kinase/protein kinase B stage (Figure 8)? Activation via agents such as the small experimental drugs, MHY 1485293 or 3BDO294 might confine the effects to a smaller set of endpoints than use of IGF-1. Will it be possible to selectively target functions below the level of mTOR, for example modulating amino acid transporter expression via changes in microtubule organization?295 Another possibility is suggested by the inhibitory actions of metformin on oxidative metabolism.296 Might it be possible to initiate or extend placental metabolic reprogramming effects through artificial stimulation of placental oxygen sparing and consequent increased glycolytic metabolism?297 Action via subsystems may avoid the lateral signaling effects of classical growth factors while providing for confined but integrated stimulation. This will however require detailed knowledge of the subsystems involved and raises the question of targeting and specificity.
Figure 8. Metabolic mTOR intervention points.
Potential intervention points (open arrows) for modulation of placental metabolism. These range from extracellular endocrine regulation by IGF-I (insulin-like growth factor I) through effects on mTOR (mechanistic Target Of Rapamycin) by activators such as 3BDO (3-benzyl-5-((2-nitrophenoxy) methyl)-dihydrofuran-2(3H)-one) or MHY 1485 (4,6-dimorpholino-N-(4-nitrophenyl)-1,3,5-triazin-2-amine) to points in the metabolic subsystems regulated by mTOR.
Scientific gaps
Like substrate supplementation, alteration of an individual transporter appears likely to distort the transport and metabolism of other substrates and metabolites. Modulation of systems that integrate multiple aspects related to nutrient transfer, whether by endocrine or intracellular sub-system approaches, is likely to be more successful. This requires not only detailed knowledge of the pathways involved but also understanding of the lateral or related pathways that may be affected to prevent generation of off-target effects.
Whether stimulation via a growth factor pathway or an intracellular sub-system, a prerequisite is to restrict the site of action to the trophoblast. Renewed attention is required to the delivery vehicles for agents of interest. Some of the vehicles for these actions are under development. Viral or homing-peptide directed nanoparticles carrying directly active agents or vectors with trophoblast-specific promoters may provide the means to overcome questions of targeting and specificity. But just as importantly, timing is everything! Unless it is possible to detect changes in nutrient transport or growth early enough to intervene, modification of nutrient transport may exacerbate rather than resolve problems. Research to determine the onset of pathological events and the means to monitor growth and the ameliorative effects of targeted interventions is an aspect of this process that is just as important as the intervention itself.
Targeting Oxidative/Nitrative Stress and Mitochondrial Dysfunction in Placenta to Relieve Adverse Pregnancy Outcomes
(Leslie Myatt, University of Texas Health Science Center San Antonio)
Background
It has long been recognized that pregnancy is a state of oxidative stress and that this is further increased in first, second and third trimesters in pregnancies resulting in adverse outcomes such as PE and diabetes298 preterm birth (PTB),299 stillbirth300 and in those complicated by maternal obesity.301 However a cause and effect relationship between increased oxidative stress and adverse pregnancy outcomes still remains to be definitively proven. Oxidative stress is defined as an imbalance between the production of reactive oxygen species (ROS), including superoxide, hydroxyl anion, hydrogen peroxide, and the ability of various antioxidant mechanisms to scavenge them. Further, ROS can also interact with reactive nitrogen species such as nitric oxide to produce the powerful pro-oxidant peroxynitrite which in turn can nitrate tyrosine residues in proteins, such as superoxide dismutase (SOD)302 (Figure 9), causing nitrative stress, which affects protein function, usually in a negative manner.303 Not all reactive oxygen and nitrogen species are equal in potency, that being determined by their cellular diffusion distance, defined as the distance moved in aqueous solution in one half life. Hence hydroxyl anion has a diffusion distance of only 5 angstroms, superoxide 0.4µm, peroxynitrite 5µm and nitric oxide 100µm.304 Therefore in the placenta their site(s) of action (intra vs extra or trans-cellular) depends on intracellular and cellular site of synthesis in relation to placental structure such as trophoblast thickness (5µm), stem villous diameter (500–1500µm) and also the presence of antioxidant molecules to scavenge them.305
Figure 9. Generation of oxidative and nitrative stress.
Superoxide generated from molecular oxygen by membrane bound (NADPH oxidase) or cytosolic (Xanthine oxidase) enzymes or the mitochondrial electron transport chain can normally be effectively dismutated to hydrogen peroxide by superoxide dismutase (SOD). Increased superoxide will attack targets leading to oxidative stress. With increasing generation of nitric oxide (NO) NO outcompetes SOD for superoxide and interacts to produce the more powerful pro-oxidant peroxynitrite (ONOO-). Peroxynitrite nitrates proteins at tyrosine residues and covalently modifies function usually in a negative manner. SOD is inactivated when nitrated by ONOO-, hence leading to a negative feedback loop increasing oxidative and nitrative stress.
Role of mitochondria
The major sources of ROS are the mitochondrial electron transport chain but also plasma membrane enzymes such as the nicotinamide adenine dinucleotide phosphate (NADPH) oxidases, cytosolic enzymes (including xanthine oxidase, flavin enzymes, and cytochrome p450’s), lipoxygenases and cyclooxygenases.298 While widely acknowledged for their pathologic effects including covalent modification of proteins, lipids and DNA, ROS also function as physiologic effectors, regulating redox sensitive genes and proteins.306 There are many methods available to measure ROS. Due to their ephemeral nature direct measurement of ROS is difficult therefore measurement of effects including lipid peroxidation, covalent modification of proteins, DNA damage and repair, levels and activity of antioxidant molecules and enzymes, and enzymes generating ROS are used. There is no gold standard measure of oxidative stress; rather one should chose a measure related to the area of interest.307
Mitochondria have many roles in cellular function in addition to energy production, including in apoptosis, steroid synthesis, calcium homeostasis and amino acid transport. Changes in mitochondrial activity can be induced by environmental factors such as nutrition, hypoxia, aging and obesity that impact cellular survival and which are associated with adverse pregnancy outcome. Mitochondria are the major source of ROS under physiologic conditions due to release of high energy electrons from complexes I and III of the electron transport chain, these electrons reduce molecular O2 to superoxide which is normally scavenged by the mitochondrial SOD isoform, mitochondrial antioxidant manganese superoxide dismutase. However mitochondrial function itself can be compromised by severe and/or prolonged oxidative stress via damage to mitochondrial DNA, proteins and lipids. We have recently shown that maternal adiposity leads to increased oxidative stress in the placenta and that this is associated with decreased trophoblast mitochondrial respiration measured in vitro using a Seahorse extracellular flux analyzer.308 This dysfunction is further evidenced by decreased mitochondria number, expression of complexes I-V of the electronic transport chain and decreased placental adenosine triphosphate generation. In addition these trophoblasts appear metabolically inflexible as they cannot switch to alternative energy sources when placed on galactose to prevent glycolysis.308
Current opportunities
Subsequent to acquiring knowledge of increased oxidative stress in pregnancies with adverse outcomes there have been many trials of antioxidant therapy to try and prevent these outcomes.309 All have failed, an outcome variously attributed to choice of antioxidant, time and duration of treatment, heterogeneity of patients studied, the fact that oxidative stress may indeed not be part of the pathophysiology or the need for targeted rather than global therapy. This begs the question what are the targets at the tissue and cellular levels? Should antioxidants targeted to placenta by use of nanoparticles or liposomes be used rather than global treatment? Cellular targets could include antioxidant enzymes such as SOD, glutathione peroxidase (GPx), thioredoxin reductase and catalase, the enzymes that synthesize ROS such as xanthine oxidase and NADPH oxidases, extracellular antioxidants (transferrin, ceruloplasmin, uric acid and bilirubin), intracellular reducing elements (glutathione, coenzyme Q10 and cytochrome c oxidase) and nutrients and supplements such as vitamins C and E and melatonin. As mitochondria are the major source of antioxidants there has been growing interest in mitochondrial targeting of antioxidants by compounds such as the mitochondria-targeted antioxidant drug, MitoQ, where coenzyme Q10 is linked to a lipophilic cation to allow adsorption through the inner mitochondrial membrane.310 Other approaches include the use of selenium311, which is found in the active site of the selenoprotein GPx, or of melatonin which functions as an antioxidant.312
Future opportunities
We have recently shown that the hypoxamir, microRNA 210 (miR210) can inhibit mitochondrial respiration in trophoblast cells,313 hence opening the door to targeted therapies to alleviate mitochondrial dysfunction and oxidative stress in the placenta. The male fetus is at greater risk of adverse pregnancy outcome, e.g., PTB and stillbirth, than the female fetus, although it is unclear if this is because the male places itself at risk by its desire for greater growth or if the female adopts a conservative strategy to ensure successful delivery and propagation of the species.314,315 Sexual dimorphism occurs in placental gene expression,316 particularly of genes involved in the inflammatory response, in response to maternal adiposity and in pathologies such as PE. We found increased expression of inflammatory and apoptotic markers in placentas of male fetuses from preeclamptic pregnancies compared to female fetuses.317 Similarly, we found differences in placental expression of miR210 and its mitochondrial target genes between male and female fetuses of lean, overweight and obese women which were mediated by differences in the transcription factor NFkB p50.308 Our current data therefore indicate that in conditions such as GDM, PE and obesity inflammatory pathways are activated in the placenta in a sexually dimorphic manner to regulate production of reactive O2 and nitrogen species leading to oxidative/nitrative stress and to mitochondrial dysfunction (Figure 10). This results in placental dysfunction and adverse pregnancy outcome. This suggests that targeting of inflammatory pathways might be a useful approach to prevent oxidative stress. Dietary manipulation (e.g., inclusion of omega 3 fatty acids and vegetables), the use of antiinflammatories (e.g., resveratrol) and immune selective anti-inflammatory derivatives, or targeting strategies involving miRNA, siRNA, viral vectors, nanoparticles and biologics may prove useful. These targeting strategies may, however, need to be different based on the sex of the placenta.
Figure 10. Putative mechanism for the involvement of inflammation-mediated placental dysfunction in fetal programming.
The adverse inflammatory maternal environments of gestational diabetes (GDM), preeclampsia or obesity can generate increased oxidative/nitrative stress and cause mitochondrial dysfunction in the placenta in a sexually dimorphic manner. This disrupts placental function and in turn may lead to alterations in placental-mediated regulation of maternal metabolism and fetal growth and differentiation and hence result in fetal programming.
Scientific gaps
Current gaps in the knowledge include absence of proof of a cause and effect relationship between oxidative stress and adverse outcome. Perhaps an animal model of induced oxidative stress with adverse outcome would facilitate this. In addition, the tissue and cellular localization and contribution of different sources of oxidative stress (i.e., including the generation and scavenging mechanisms of ROS) needs to be comprehensively studied.
Further the timing of the oxidative stress insult, either early, mid or late gestation or continuously, on outcomes needs to be considered when designing drug targeting studies.
III. Identification of Potentially Useful or Experimental Drugs to Target Important
Challenges and advantages of rescuing and repurposing
(Christine Colvis, National Center for Advancing Translational Sciences)
Background
The most common reason for an investigational drug to fail to make it through the Food and Drug Administration (FDA) approval for marketing is its inability to demonstrate clinically meaningful efficacy in the disease being studied. At the stage of phase 2 trials, another major reason for failure is simply due to business strategy decisions that result in the deprioritization of a drug.318,319 In either case, there is no scientific reason that the drug could not be pursued for an indication other than that for which it was originally being developed.
National Center for Advancing Translational Sciences (NCATS) new therapeutic uses program (NTU)
The NTU uses a novel approach to provide academic researchers with access to investigational drugs from pharmaceutical companies.320 The program leverages investments that have been made by pharmaceutical companies in a variety of drugs and biologics (agents) but for which the company has often times discontinued development. To qualify for inclusion in the program, the agent offered by the company has to have been through at least a phase 1 clinical trial, so that safety in humans has already been assessed in at least one population. By limiting the agents to only those that have been tested in humans, NTU is able to move projects quickly to phase 2a trials to test the agents for efficacy in new indications. Under the program, the pharmaceutical companies provide agents – clinical supply including matched placebo and pre-clinical material, if needed – as well as the data that will be needed to file an investigational new drug application with the FDA to study the agent for the new indication. The National Institutes of Health (NIH) provides the financial support for phase 2a trials and if needed, phase 1b trials and/or pre-clinical studies.
The NIH and the pharmaceutical companies are turning to the broader research community to identify novel uses for the agents. The value of this strategy bore out when as a pilot NCATS listed 58 agents provided by companies in June 2012 and within 2 months received almost 160 pre-applications with ideas of indications that the agents might be used to treat. Because of the limited time (12 months) allowed under the program for pre-clinical studies, pediatric trials were not encouraged in the 2012 pilot. However, in 2014, NCATS issued a new set of solicitations, or Funding Opportunity Announcements (FOAs), and this time offered an FOA specifically for pediatric indications. The companies in turn had identified those agents that they felt could be developed for a pediatric indication. In general, it was expected that pediatric trials would only be proposed when there was no adult patient population for the disease or for which the mechanism of action of the drug might only be meaningful if modulated at an earlier stage in development than in adulthood.
There are significant challenges when trying to repurpose an investigational drug for a pediatric indication. Unlike an FDA approved drug, these drugs have often been only studied in dozens or maybe a few hundred adults. So, side effects have been documented for only a small number of individuals. Furthermore, the metabolism of drugs can be very different in younger individuals than in adults. We face similar challenges when we think about exposing a pregnant woman to an investigational drug. Effects of drug exposure in pregnant women will generally remain unknown until after the drug has been on the market for years or even decades.
Current opportunities
The NCATS intramural program offers several opportunities for collaboration. While funds are not offered, there are several ways to access valuable resources and expertise at no cost to our collaborators. The intramural program that likely has the most potential for repurposing drugs for use in pregnant women is the screening of the NCATS pharmaceutical collection. Nearly 2,750 small molecular entities have been approved for clinical use by U.S. and in other countries around the world. NCATS has amassed a screening collection of 2,500 of these, along with about 1,000 additional investigational compounds. Because they have been approved for clinical use, these drugs will have been used in far more people than the investigational drugs that are used in the New Therapeutic Uses program, thereby providing a more comprehensive risk profile for the drugs. Collaborators generally approach NCATS with a high throughput screening compatible assay as a first step toward repurposing one of these approved medications. Of course, when a drug is first marketed, often, the only data available on fetal effects will generally come from preclinical data. The FDA encourages the use of pregnancy exposure registries as a first line strategy for gathering information on drug exposure during pregnancy. They have compiled a list of more than 60 registries to facilitate the gathering of valuable data.321 Cross referencing data from such registries with findings from in vitro assays that were used to screen the NCATS pharmaceutical collection may provide a strategy for repurposing drugs to address placental health indications.
Future opportunities
Repurposing both investigational and approved drugs, is a strategy that is currently pursued, in small and large pharma as well as in academia. The strategy is attractive to so many because of the significant cost-savings that result from using drugs for which so much early development has already been completed. In the case of repurposing drugs for use in pregnant women, it would be expected that the longer a drug has been on the market, the more information we would have on exposure during pregnancy. Unfortunately, the drugs that will fall in this category will often be drugs for which a generic is available, thereby eliminating an enforceable use patent or regulatory exclusivity. Currently, there are no effective incentives to pursue a new indication for a drug that is available as a generic regardless of the indication unless a unique formulation for the drug is developed.322 While this is a barrier for all indications, it is particularly unfortunate for pregnant women, a population for which the development of new drugs – investigational drugs – is extremely unlikely.
Effect of pregnancy on drugs pharmacokinetics and pharmacodynamics
(Maged M. Costantine, MD, University of Texas Medical Branch, Galveston)
Background
The use of medications in pregnancy has been progressively increasing over the past 3–4 decades (Figure 11).323,324,325,326,327 This is predominantly due to changing in the demographics of pregnant women, as more women enter pregnancy at a later age with increasing prevalence of preexisting medical co-morbidities (such as diabetes, hypertension, asthma, depression, and others) and increased risk of obstetric complications (e.g., nausea and vomiting of pregnancy, GDM, cholestasis of pregnancy, PE, etc.) that require pharmacotherapy.323,324,325,326,327 In a recent review, the average number of medications used by pregnant women is more than four.323 This increase in the use of medications is also predominant in the first trimester, where almost one third of pregnant women use at least 4 medications.323 This is concerning since the first trimester is a crucial period for organogenesis and placental development including trophoblast invasion, vascular remodeling and chorionic villi development; in addition many women are unaware of their pregnancy status.323,324,325,326,327
Figure 11. Medication use during pregnancy.
Secular patterns of use of any medication restricted to the first trimester at any time during the period of 1976–2008. Average number of medications and proportion of women taking 4 or more medications (n=25,313) is shown.
Reprinted, with permission, from Mitchell et al.323
Pregnancy physiologic changes & their impact on medications pharmacokinetic (PK) and pharmacodynamics properties (PD)
Pregnancy is characterized by significant changes in the anatomy and physiology of almost all systems, which affect medications PK/PD properties (Figure 12). PK generally refers to “what the body does to the drug”, and is usually described using the drug’s absorption, distribution, metabolism and elimination; whereas PD refers to “what the drug does to the body” and usually influences the drug’s efficacy and safety.328,329,330,331,332 Cardiac output (CO) increases by 30–50% due to both increase in heart rate and stroke volume. The majority of the increase occurs early in pregnancy (75% by the end of the first trimester). The increase in CO is preferential in that uterine blood flow increases 10-fold (17% of total CO compared with 2% pre-pregnancy) and renal blood flow increases 50%; whereas there is minimal alterations to the liver and brain blood flow.330,333 CO is further increased during labor, with additional 300–500 ml of blood re-entering the circulation with every contraction. Both systemic and pulmonary vascular resistances decrease by 20–30%. Blood pressure also falls toward the end of the first trimester and then rises again in the third trimester to pre-pregnancy levels. The increase in renal blood flow leads to a parallel increase in the glomerular filtration rate by approximately 40–65%.330,332,334 This can lead to significant increase (20 – 60%) in the elimination rates of renally cleared medications, leading to shorter half-lives, and risk of subtherapeutic concentrations. Examples of these medications include lithium, ampicillin, cefuroxime, cefazolin, piperacillin, atenalol, and digoxin.329,332
Figure 12. Factors affecting drug pharmacokinetics and pharmacodynamics in pregnancy.
CO= cardiac output, HR= heart rate, BP= blood pressure, SVR= systemic vascular resistance, NVP= nausea and vomiting of pregnancy
During pregnancy, maternal blood volume increases by 40–50%, and total body water increases to almost 8.5 liters by the end of pregnancy. In addition, maternal fat stores and fat mass increase by almost 10 fold. The increase in blood volume and water within the body expands the volume of distribution of hydrophilic substances.335 On the other hand, the increase in fat mass leads to a larger volume of distribution of lipophilic drugs, such as sedatives. For some drugs, a larger volume of distribution could lead to decreased peak serum concentration, which may necessitate a higher initial and maintenance dose to obtain therapeutic plasma concentrations.332,336 Additionally, because of the decrease in serum albumin during pregnancy, highly protein bound compounds (such as digoxin, midazolam, and phenytoin) may display higher free levels, which increases their peak plasma concentrations.330,332 The hypervascularity and edema of the upper respiratory mucosa may theoretically result in an increased absorption of inhaled agents such as corticosteroids.332,337 The delayed gastric emptying and prolonged small bowel transit time may alter the bioavailability (decrease maximum concentration and time to maximum concentration) and thus decrease the efficacy of oral drugs that are taken as a single dose because a rapid onset of action is desired.332,338
Drug metabolism is also altered in pregnancy in part secondary to elevated sex hormones. In general, drug metabolism occurs through phase I (oxidation, reduction or hydrolysis), and/or phase II metabolism (glucuronidation, acetylation, methylation and sulfation). Cytochrome (CYP) P450 represents a family of enzymes, and is a major route of drug metabolism that is affected by pregnancy (activity increased for CYP3A4, CYP2D6, CYP2C9, CYP2A6, and decreased for CYP1A2, CYP2C19, and CYP2D6 subtypes). For example, the increase in activity and abundance of CYP3A4 in pregnancy lead to increase in the clearance of its many substrates such as nifedipine, carbamazepine, midazolam and saquinavir, indinavir, lopinavir, ritonavir, and many others.339,340,341,342
In summary, maternal physiologic changes affect PK/PD of drugs through changes in drugs’ absorption, increasing the volume of distribution, protein binding, renal clearance, metabolism, and placental bio-disposition. Any novel drug identified to act on potential placental molecular target(s) needs to be evaluated in the context of these changes so that appropriate dosing be identified.
Current opportunities
Despite the above, pregnant women are still considered therapeutic orphans as the majority of current therapeutics were never studied during pregnancy.343,344,345 In a recent review of the literature, less than 2% of all PK studies involved pregnant women.346 This is concerning since the lack of data on pregnancy specific dosage of many medications leads physicians to extrapolate drug dosage regimens from non-pregnant subjects or men. This may lead to over or under dosing and may reduce efficacy and increase risk of toxicity. In view of these gaps, lie the opportunities to study the PK properties of many currently used medications with potential impact on placental development and use in selective targeting of molecular pathways involved in the pathophysiology of various obstetrical complications. This will lead to more effective treatment of pregnant women, while reducing the risks of adverse events.344 The new FDA rule regarding drug classification represents a huge current opportunity to encourage research in this field.
Future opportunities
A major obstacle to studying medications (current and novel) in pregnancy is the lack of systems that allow researcher to determine the safety and efficacy of these medications. Some rely on in-vitro placental transfusion models; however these placentas are typically collected after delivery, usually at term, and may not represent the changes in drugs PK and PD throughout the pregnancy. Others rely on primate animal models; however they are usually expensive and hard to work with. Future opportunities in this field include the development of novel technologies to assess the placenta in real time and applying them to study medication’s biodisposition in pregnancy. The use of novel ultrasound techniques or other novel technologies to tag drugs and then be able to image the placenta and fetus to look for their distribution would be a great future opportunity.
Scientific gaps
The majority of therapeutics was never studied in pregnancy during development, as pregnant women were excluded from such studies.344,345,346 In addition, their safety, tolerability, efficacy and dosing were extrapolated from studied conducted in men or non-pregnant women. This leads to under or over dosing and affects efficacy and toxicity of these medications. There exists a large gap in information regarding medications’ PK properties in pregnancy and during lactation. The effects of placental transporters and biodisposition enzymes on medications’ PK and PD, and transplacental transfer represent a large gap in our scientific knowledge. Moreover, the inability to study placental transport expect after delivery using delivered placentas widens this scientific gap.
From bench to bedside: Processes and pitfalls translating research findings into practice paradigms
(David M. Haas, Indiana University)
Background
When Vannevar Bush wrote “Science: the endless frontier” in 1945, it inspired that science would provide solutions to health problems. It further described how investment in scientific research could serve as a vehicle to enhance the wealth and prosperity of the nation.347 It is against that backdrop that the public holds out hope for scientific inquiry. This promise engenders a social contract that funding of scientific inquiry would be translated into better health that can elevate the national good on many levels.
The objectives of this portion of the workshop were to: describe the translational research spectrum and the difficulties with translating research into practice; to articulate additional concerns and difficulties with translational research in pregnancy, highlighting some translational successes in pregnancy research; and to propose some mechanisms aimed at improving translation of findings into practice and measuring research impact.
Translational research
The research spectrum generally begins with basic science findings which then are translated to human and clinical research (Figure 13). This is termed “T1” research on the translational spectrum- the translation of animal and basic science research into humans.348 The “T2” step in research translation is taking the clinically relevant research findings and translating them into practice guidelines and having them become routine practice in health care.349,350 Some models of the translational spectrum have included more steps that can include the population health impact and involvement of consumers. All of the models serve to help assess the success of translational research at fulfilling the initial promise to improve public health.
Figure 13. Translational research peaks and valleys.
This figure illustrates the multiple (5 hills and 4 valleys) step model of translating research into practice and public health benefit and the many “valleys of death” that can be encountered along the way. Depicted are the five phases of translational research separating four valleys: basic discovery science to research involving humans (T1), from human studies to evidence-based guidelines (T2), from guidelines to health practice (T3), and from health practices to population health programs (T4).
Reprinted, with permission, from Meslin et al.347
Globally, billions of dollars are spent on biomedical, clinical, and health services research, training of providers, quality improvement initiatives, and safety initiatives.350 However, it has been demonstrated that in the United States, only 55% of patients receive the recommended evidence-based care.351 Additionally, 20–30% of patients get care that is not needed or could be potentially harmful.352 This is a failure in translating research findings into practice. Experts have found that this can be due to system failures. Jeremy Grimshaw noted, “Systems fail to ensure that effective and cost-effective programs, services, and drugs get to all those who need them and providers fail to provide the level of care they aspire to.”350
An example of this is seen in the fact that in 2010, the NIH spent $31 billion on research. However, from 2006–2009, only 74 new drugs were approved by the FDA, only half the number approved during the prior epoch.353 One interesting study looked at 101 promising technologies published in the highest impact journals. This report found that in the 20 years after publication, only 5 of these promising technologies were licensed for clinical use, with only one angiotension-converting enzyme inhibitor being used regularly in clinical practice.354 The report found multiple steps where these promising technologies failed, were lost, refuted, or neglected along the translational spectrum. It has also been found that promising technologies take a long time to get into routine practice, at times up to 17 years.348 These areas of translational failure have been coined the translational valleys of death.347 The translational valleys of death can come from regulations that impede research or practice, costs, limited resources; commercialization issues, broad social policy implications, professional standards, governmental policy, and general science policy.347 There are many places where potential health-improving research findings can become derailed. The key is to overcome these difficulties and cross the translational valleys of death.
Translational research in pregnancy
Translational research in pregnancy holds additional potential pitfalls. These generally flow from the concerns for the impact of therapies on developing babies and potential long-term impacts that may be unrecognized for years after birth. It is for this reason that for many years, pregnant women were actively excluded from research. There are several examples where translational research failed to detect problems, leading to major public health consequences. In 1956, thalidomide was introduced as a “wonder drug” in Germany.355 It was purported to be a treatment for nausea and vomiting, insomnia, coughs, colds, and headaches. Early studies in mice and rats did not demonstrate congenital anomalies and thus it was used as a sedative and tranquilizer in early pregnancy. However, in 1961 reports surfaced of severe congenital anomalies including limb reduction defects which led to its rapid withdrawal from the market. This was a clear failure of T1 research.
Two notable examples of failure in T2 research in pregnancy have been seen recently. The withdrawal from the market of the combination drug Bendectin was done due to lawsuit pressure regarding congenital anomalies.356 The drug was used by a large proportion of pregnant women for nausea and vomiting in early pregnancy. However, no reports of defects were proven. Without an alternative, women suffered. The rates of hospitalization for severe dehydration from hyperemesis gravidarum rose dramatically. This was an example of where research demonstrated safety and practice patterns were established but fear and a lack of a supportive response to the public caused the withdrawal of an effective therapy, leading to a negative public health impact. Even more recently, the poor adoption rates of the effective human papilloma virus vaccines to prevent cervical cancer is another example of success at the T1 stage but unfortunately a failure at the T2 stage.357 Despite proven efficacy and safety and coverage by the Children’s Immunization Program, vaccination rates of the target populations remain low.
However there are some noteworthy translational successes in pregnancy. The development of Rhogam to prevent Rh isoimmunization came about from a confluence of observations along the translational spectrum. After demonstrating efficacy and safety in clinical trials, it has been universally adopted as a standard of care preventive therapy in pregnancy, saving thousands of babies every year.358,359 In similar ways, antenatal corticosteroids for accelerating fetal lung maturity in threatened preterm birth have been a translational success story in obstetrics.360 From astute observations in sheep to landmark clinical trials in humans and pivotal meta-analyses, antenatal corticosteroids are now routinely employed on labor and delivery units and clearly have reduced the rates of mortality in preterm newborns361,362,363, This was greatly aided by NIH Consensus Conferences highlighting the benefits and safety of the therapy.364
How to improve translational success
Many have proposed systems aimed at crossing the translational valleys of death. One unifying theme is aimed at getting stakeholder investment in the translational process. Practicing providers, the public, industry, and regulatory/governmental agencies must have a stake in the translational processes along the way if there is any hope of translating important research findings into practice aimed at improving the public health350, 365,366,367 Otherwise, failure at T2 and beyond can commonly occur. The formation of translational teams that involve the stakeholders can ensure involvement from the beginning and should involve the entire translational spectrum347,350 Effective dissemination of the impact of translational research can inform those providing funding and the public as to the public good that is being gained.
Psychology of change research has noted three stages of practice change: Awareness, Acceptance, and Adoption.368 Most translational research focuses on the first two stages, whereas the reason most promising therapies fail to realize their public health impact is the failure of “Adoption” into practice. A provider may know that research shows a benefit and accept that it might help their patients but there may be obstacles to getting it into a routine practice pattern for them and thus it is not adopted. This is because “Adoption” takes different pathways in the brain before these cognitive changes become embedded in practice.368
A focus on stakeholders and alignment of incentives can help with the adoption into practice. Dissemination of the impact of research findings is crucial to get stakeholder enthusiasm. For instance, the Human Genome Project turned a $4 billion government investment into $796 billion in economic growth.369 Translational teams, like those stimulated by the NIH Clinical and Translational Science Awards, focus on collaboration and development of networks of scientists, stakeholders, and regulatory agencies in an effort to propel translational science across the valleys of death and into practice.370,371
Specific to pregnancy, there is a tremendous need for public involvement, education, and advocacy. The focus of protection of mothers and infants through research instead of from research should be highlighted. Ethicists and children’s advocacy groups should also be engaged. The advent of individualized therapeutics and diagnostics are forcing this conversation to accelerate.372 In addition, the utilization of biorepositories can help stimulate this translational process by focusing on potential pooled resources and collaborations.
In conclusion, bridging the translational research gap from bench to bedside has many potential pitfalls. Pregnancy translational research has had well-documented missteps but several important success stories as well. While research to cross the translational “valleys of death” is difficult, utilizing what is known about behavior change will be important in ultimately fulfilling the promise of scientific research funding to improve public health and the public good. Thinking broadly about stakeholders and involving these stakeholder teams in the processes across the translational spectrum can help cross the valleys of death and help take promising discoveries from bench to bedside.
Current opportunities
Individualized pharmacotherapy provides opportunities currently to tie genomic and other “-omic” technologies to ongoing clinical trials. In addition, the development and utilization of saved samples from past trials and biorepositories provide a wealth of opportunities for analyses utilizing cutting edge technologies to assay for biomarkers that can serve as therapeutic monitoring or individualized pharmacotherapy model development aids for patients. The focus on diagnostic and therapeutic studies in human models and humans is a clear priority moving forward.
Future opportunities
Clinical trials in the future should have clear response biomarkers, pharmacokinetic/pharmacodynamic/pharmacogenomic outcomes, and harmonized efficacy outcome measures in this field. This will help evaluate molecular targets more robustly with fewer trials and may help us evaluate promising technologies in a more cost-effective manner. Requiring all diagnostic and therapeutic trials that receive funding to have these type of outcomes and measures will squarely focus investigators.
Scientific gaps
The plethora of basic science data accumulating about placental development and the interface between mother and fetus need to be linked clearly to diagnostic and/or therapeutic needs. In addition, as there is not perfect animal model, there needs to be a way to study these on human placental models as often as possible. Another clear gap in much of the therapeutic research in pregnancy is a lack of long-term follow up of the infant. Ensuring funding for follow-up studies, including potential “-omic” studies of the offspring should be a priority.
IV. Evolving Technologies for Placental Specific Therapeutic Drugs
Trophoblastic nanovesicles, miRNA, and their function
(Yoel Sadovsky, MD, Magee-Womens Research Institute)
Background
Coordinated communication among cells and tissues is critical for development, homeostatic function, and adaptation to change. The trafficking of information between the feto-placental and maternal compartments is essential for fetal development, growth, and pregnancy health. It also serves to reduce maternal-fetal conflicts and to balance biological resources for the benefit of the two organisms. In past years it was believed that communication between the feto-placental unit and the mother was executed by hormones and growth factors, which are soluble in the blood or are bound by protein carriers, and serve as paracrine or endocrine signals that control pregnancy health. It is now clear that nucleic acids, and particularly sRNA molecules, traffic among tissues and impact cell biology.
miRNAs and extracellular nanovesicles
A major class of these non-coding RNAs, and one of the best studied, is the family of small regulatory RNAs called miRNA. miRNAs were originally described in the nematode C. elegans, and were later found in the genome of many organisms, including humans. They are single-strand RNA molecules of 20 to 24 nucleotides that characteristically repress gene expression by guiding an RNA-induced silencing complex to a target RNA, with subsequent attenuation of gene expression by inhibition of mRNA translation and/or mRNA degradation.
It has recently become clear that in addition to the effect of miRNAs on intercellular gene regulatory networks, miRNA are also packaged within extracellular vesicles, where they can be trafficked to local and distant cell types (Figure 14). Transport within extracellular vesicles may provide substantial advantages, including stability, intravascular processing, and selective delivery to target cells.373,374,375 Diverse cell types (e.g., hematopoietic cells, mast cells, platelets, neurons) produce and release extracellular vesicles, which can be found in the blood, urine, saliva, breast milk, amniotic fluid, ascites, cerebrospinal fluid, bile, lymph, tears, and semen.376,377, 378,379 These vesicles are characterized by their size, shape, and content.380 Importantly, when released to the extracellular space, these vesicles may target local and distant cell types, and release non-hormonal signals that control physiology, determine disease risk, and even harbor therapeutic potential.381, 382,383, 384 The major types of extracellular vesicles include apoptotic blebs (ABs), microvesicles (MVs) and exosomes, each produced by different pathways. ABs (500–4000 nm in diameter) are formed during partial or complete cell death and disintegration, and contain cell organelles and cytoplasmic proteins that are subsequently cleared from the circulation by macrophages.380,385,386 MVs (100–1000 nm in diameter) originate from outward budding and fission of the plasma membrane, where microdomains and associated protein cargo are enveloped for direct cellular exit.387,388,389,390,391 Exosomes (40–150) nm in diameter) originate from the intracellular endosomal network through serial processes that are initiated by enrichment of endosomal membrane complexes by tetraspanins and other proteins.392,393 This is followed by budding of intraluminal vesicles within multivesicular bodies (MVBs) and subsequent movement to the plasma membranes, where MVBs fuse with the cell membrane to actively release intraluminal vesicles as extracellular exosomes.380,394 Among all extracellular vesicles, exosomes have been extensively studied in recent years because of their ability to modulate target cell physiology, immune function and carcinogenesis.395 Exosomes also harbor miRNAs as part of their cargo.374,396,397
Figure 14. Schematic of extracellular miRNAs derived from human trophoblasts.
MicroRNAs (miRNAs) can be released from the trophoblast layer in different forms: microvesicle-enveloped form; apoptotic body–enveloped form; nano-sized, exosome-encapsulated form; and RNA-binding, protein-bound form. Exosomes are formed by budding in intraluminal vesicles to form multivesicular bodies (MVB). MVBs fuse with the plasma membrane and release their intraluminal vesicles as exosomes into the extracellular space. In contrast, microvesicles are produced directly by budding and the detachment of membrane vesicles from the plasma membrane. Apoptotic bodies (blebs) derive from cells undergoing apoptotic fragmentation and the formation of membrane-enclosed vesicles.
Reprinted, with permission, from Ouyang et al.397
We and others have recently characterized the trophoblastic miRNA landscape, and the potential role of trophoblastic miRNA in feto-placental to maternal communication.396,397,398,399 Whereas exosomes are found in the circulation of pregnant women, little is known about trophoblastic exosomes, and many studies on extracellular vesicles in pregnancy have associated a mixture of vesicles (exosomes, MVs, ABs) with disorders of pregnancy, thus blurring some the distinct properties of these vesicles.400,401,402,403,404 Placental exosomes have been implicated in several discrete functions, such as immune modulation,405,406,407 where exosomal cargo proteins, such as galectin-3408 and HLA-G409, may perform an immune modulatory function.410 Other exosomal cargo molecules, such as Wnt/β-catenin, fibronectin, and prostaglandins, may play a role in supporting homeostasis throughout pregnancy.411,412,413,414,415,416
We have shown that the miRNA landscape within the cargo of human trophoblastic exosomes is nearly identical to that of villous trophoblasts.399 The most abundant trophoblastic miRNA species are those derived from the chromosome 19 miRNA cluster (C19MC),399,417 which is the largest miRNA cluster in the human genome, spanning ~100 kb of genomic DNA and harboring 46 intronic miRNA genes that express 58 miRNA species.399,417,418,419 Importantly, we found that, in comparison to non-trophoblastic cells, primary human trophoblasts are resistant to infection by diverse types of DNA and RNA viruses, including clinically relevant species such as herpes simplex virus type 1, rubella, HIV, and cytomegalovirus, but not the non-viral pathogens Listeria monocytogenes and Toxoplasma gondii.396,420,421,422 A remarkable finding was that the viral resistance could be transferred to other, non-placental primary cells and cell lines by exposing these cells to medium that was pre-conditioned by primary human trophoblasts or to exosomes produced in human trophoblasts (Figure 15). We also found that this effect was mediated, at least in part, by exosome-packaged C19MC miRNAs.396,399 Cells transfected with a bacterial artificial chromosome that harbors the C19MC locus or with individual, highly expressed C19MC miRNAs also became more resistant to viral infections. Our data showed that this effect of exosomal C19MC miRNAs was mediated by stimulation of autophagy in recipient cells, and pharmacologic or genomic inhibition of autophagy diminished the conferral of antiviral response to recipient cells.396 While C19MC miRNAs likely act in concert with other antiviral responses, our data pointed to a unique and transferrable antiviral response, mediated by human trophoblasts-derived exosomes.
Figure 15. Schematic depicting the exosome mediated induction of viral resistance.
C19MC miRNAs (C19) were transferred to recipient cells. Primary trophoblast cells release exosomes (EXO) containing C19MC miRNAs, which are taken up by recipient cells, thereby mediating C19MC miRNA-dependent autophagy. Incoming viral particles (in red) are likely trafficked in endocytic vesicles (EV) from the endosomal pathway into pre-existing autophagosomes (APs), which then fuse with lysosomes to form autolysosomes (AL), as a mechanism to degrade these virus-containing vesicles.
Reprinted, with permission, from Delorme-Axford et al.420
Although the mechanisms, dynamics and targets of miRNA-containing trophoblastic exosomes remain unknown, our recent human and mouse data suggest that trophoblastic C19MC miRNAs are transferred primarily from trophoblasts to maternal tissues. This is plausible, as trophoblasts are directly bathed in maternal blood, yet separated from the fetal blood by a basement membrane and endothelial cells. Together, these analyses shed new light on miRNA-based communication among the feto-placental and maternal compartments. Importantly, our data suggest an extraordinary means of non-hormonal communication between the placenta and the maternal-fetal compartments, suggesting a critical role for miRNAs in pregnancy health.
Current opportunities
While it is clear that the plasma contains several types of extracellular vesicles, their molecular and metabolic cargo are largely unknown. Moreover, our understanding of the function of these vesicles is rudimentary. The formation of the placenta during pregnancy adds a new source of extracellular vesicles to the maternal blood stream, and possibly also to the fetal compartment. Whereas C19MC miRNAs are distinctly packaged within these placental vesicles, it is not clear if there are other, placenta-specific, molecules and signals that are packaged within vesicles. Importantly, modern technology enables us to separate the different types of vesicles, and to associate them with disorders of pregnancy that reflect placental function. It is also possible that the state of pregnancy changes the repertoire of extracellular vesicles produced by maternal tissues, thus adding to the landscape of pregnancy-related extracellular vesicles. This creates a new opportunity to catalog these vesicles, assess their origin, content, and cellular targets, and define the normal landscape of extracellular vesicles (the “vesiculome”) throughout human gestation.
Future opportunities
The development of new tools to efficiently isolate extracellular vesicles may allow us to examine local and systemic targets of trophoblastic extracellular vesicles, and whether or not their cargo faithfully informs the health of trophoblasts. It may also allow us to identify target cells and interrogate the impact of pregnancy-specific extracellular vesicles on these cells in health and disease. While initial work has centered primarily on maternal tissue targets, future research may focus on intra-placental, paracrine targets, as well as fetal targets of placental extracellular vesicles. These opportunities, coupled with recent developments in understanding of miRNA function, will require the development of innovative label-free technologies to collect and sort extracellular vesicles, which will shorten the time to clinical translation and potentially offer a dramatic improvement in our ability to dynamically assess placental health throughout pregnancy using maternal blood tests. Knowledge in this field may also stimulate the development of nanovesicles as carriers of non-coding RNAs, drugs and chemicals that selectively target placental cells for therapeutic purposes.
Scientific gaps
The current and future opportunities summarized above require us to bridge pertinent knowledge gaps. We must understand the process of cargo loading unto diverse trophoblastic vesicles, and the potential selectivity of this process. This should be followed by analysis of the mechanisms utilized by trophoblastic exosomes to selectively enter target cells and impact their biology. The current state of perinatal science raises additional questions: Do surface and cargo proteins within trophoblastic extracellular vesicles play a role in this process? What determines targeting by extracellular vesicles? Can fetal vesicle-bound or free miRNAs and other types of RNA molecules cross the placenta into the maternal circulation, and inform on the health of fetal tissues? How early in gestation can we find and isolate placental extracellular vesicles and RNA from maternal blood? The answers to these and related questions will greatly impact the field of placental biology and its translation to pregnancy diagnostics and therapy.
Aberrant regulation of myometrial contractility by maternal cell free plasma (CFP) RNA of placental origin: screening and therapeutic implications
(Carl P. Weiner, MD, University of Kansas School of Medicine)
Background
The CFP transcriptome is altered by numerous disorders, often reflecting the stage of disease development.423 While the biologic roles of these coding and noncoding RNAs are unclear, they are not simply cellular debris. Synthesized and released by a range of cells including human chorionic villi, their biologic stability and target tissue specificity is thought to reflect their release within either exosomes, shedding vesicles or attached to argonaute 2 proteins.424 Cell to cell transfer and their ability to function once transferred are shown in studies using mRNA/miRNA- loaded extracellular vesicles and protein complexes.425,426,427
Based predominantly on studies of symptomatic women, spontaneous preterm birth (sPTB) is considered an inflammatory process.428 We measured both the Mass Restricted (MR) Score and IL-6 in 1004 consecutive amnionic fluid samples at 16 weeks from healthy pregnancies with known outcomes (unpublished data). Ten percent delivered <37 weeks; 4% <32 weeks. Nineteen percent had a MR Score ≥ 1, while 2% had a MR Score of 3 or 4 indicating significant inflammation. However, there was no relationship between either sPTB or preterm premature rupture of membranes and the MR Score; 23/27 deliveries <28weeks occurred to women with an MR Score = 0. IL-6 levels increased as the MR Score rose (0 = 102, 3 = 451, and 4 = 706 pg/mL), but was unrelated to subsequent sPTB. Whatever the role of inflammation in preterm or term birth, it seemingly occurs after 20 weeks.
We proposed in 2011 that mRNA and miRNA were candidate mechanisms for the regulation of myometrial quiescence activation (unpublished data), and have been testing the hypothesis that CFP RNAs enable communication between mother and pregnancy, and, that as a direct result, changes in the maternal CFP transcriptome predict both the occurrence and mechanism of sPTB.
Methodological refinements
In 2007, we developed an extraction method that yielded increased quantities of both CFP nucleic acids and proteins in a single process. The typical yield of total RNA extracted is 18–35µg per mL plasma, enabling comprehensive, transcriptome wide study using both multiple microarrays and/or next generation sequencing (NGS), plus the confirmatory -polymerase chain reaction studies all on the same patient plasma sample.
To speed marker validation and facilitate translation into the clinical arena, we developed a series of techniques, including economical, high-throughput gene quantification that allows for the running of panels of genes to predict sPTB within a few hours. The method allows us to run all controls necessary for marker quantification in a single polymerase chain reaction well.
The CFP transcriptome is altered in women destined for sPTB by 16 weeks
Using longitudinally obtained banked plasma samples from women with known pregnancy outcomes and microarray/NGS, we identified and confirmed > 90 novel CFP RNAs in the plasma of women destined for sPTB between 26–32 weeks; some of the marker expressions were altered as early as 16 weeks. In a second cohort, we found that several markers that were abnormal at 16 weeks were also abnormal at 12 weeks. We then determined that 5 CFP markers were known from studies of other cell systems to interact with 7 myometrial gene/gene products belonging to a previously identified cluster of genes- the Preterm Initiator set.429 Five of the 7 myometrial genes were involved with the regulation of intracellular calcium.
CFP RNAs originate from the placenta
We next sought the anatomic origin of the 5 CFP RNA markers- each was dramatically overexpressed in the placenta of women with <33 sPTB weeks but not the placenta of women delivered prematurely absent labor and of women delivered at term in labor.
CFP RNAs markers of sPTB can regulate myometrial contractility
We then focused on one particular CFP mRNA biomarker, apolipoprotein (APOA4), which in silica interacted with the interferon gamma receptor (IFNGR), which in turn, interacted with four additional Preterm Initiator genes. Overexpression of APOA4 in human pregnant myometrial cells increased both intracellular calcium flux and cell contractility. The addition of IFNGR siRNA blunted the stimulatory effect of APOA4 demonstrating that IFNGR was at least one of the targets of the APOA4 mRNA.
Current Opportunities
These findings support the often stated premise that sPTB is a result of placental dysfunction. Specific CFP RNAs are released from the placenta of women destined for sPTB <33 weeks by the end of the first/beginning of the second trimester that interfere with myometrial quiescence, thus potentially creating an environment favoring sPTB. The early pregnancy time frame suggests effective therapy will require early detection and initiation, which is consistent with the general failure of available tocolytic agents. It highlights the preeminent importance of elucidating the events involved in human implantation and early placentation. As such, the sPTB studies described may serve an investigational paradigm for the other great pregnancy syndromes such as PE and hypoxia associated FGR and perinatal brain damage.
The first task along the path to understanding human placentation is descriptive- the creation of an appropriately sized research medical center network for sample processing (using new and established biobanks) for the rapid construction of an ‘omics’ atlas of “normal” from the time period covering implantation through at least 16 weeks gestation. Such an atlas would serve as a basis for all future research and generate a host of new targets for hypothesis based testing in vitro using systems similar to described above.
Future Opportunities
Real-time assessment of the human placenta all but requires noninvasive methods that may not presently exist, or have not yet been applied to the study of the placenta. So much about human placental function is shrouded by darkness that the areas for investigation are almost unlimited, each with the potential for making a contribution. Yet, it is highly likely that much placental dysfunction has its foundations set by the early second trimester, and it is here that new insights are desperately needed. The application of newer organ on chip technology could allow the testing of potential interventions identified during atlas construction.430 Real-time approaches will require either new imaging modalities, or be “omics” in nature. As discrete markers of placental function linked to specific disease phenotypes emerge, it may be possible to reduce the processing from a minimum of a laboratory work day to less than a few hours, providing near real time feedback for non-imaging modalities.
Scientific gaps
There are presently no targeted pharmacologic therapies for placental abnormalities. The fact that a placental derived CFP RNA uniquely to women destined for sPTB <33 weeks modulates a myometrial gene associated with sPTB, and that its inhibition with siRNA reverses the up regulation demonstrates therapeutic targets can be identified with current transcriptomic approaches. It is likely attempts to address placental dysfunction after its establishment will be of modest impact. Future efforts should build upon the knowledge derived from atlas construction to seek targeted therapies that enhance implantation and early placental development.
Nanoparticles for placental drug delivery
(Erik Rytting, University of Texas Medical Branch, Galveston)
Nanomedicine
The field of nanomedicine includes nanoparticles for medical diagnostics, nanoparticles for therapeutic delivery, and particles playing both roles, referred to as theranostic nanoparticles (Figure 16).431 Types of nanoparticles used in therapeutic delivery include liposomes, solid lipid nanoparticles, polymeric nanoparticles, polymeric micelles, dendrimers, and polymeric DNA or siRNA complexes called polyplexes.432 Advantages of nanoparticles for drug delivery include improved bioavailability, protection of therapeutic payload, controlled release, and increased drug targeting efficiency.433 Targeting ligands such as antibodies, peptides, aptamers, or small molecules can be conjugated or adsorbed onto the surface of nanoparticles to promote the accumulation of the particles in specific regions or tissues, facilitated by binding of the targeting ligands to a particular receptor.434
Figure 16. Translational steps for the development of nanoparticles for placental drug delivery.
Nanoparticles may contain therapeutic, diagnostic, and/or targeting components. In vitro, ex vivo, and in vivo models will be used to investigate proof of concept and safety prior to clinical trials.
Nanoparticle characterization and biocompatibility
Characterization of drug-loaded nanoparticles typically includes assessments of particle size, zeta potential (surface charge), encapsulation efficiency, and drug release kinetics. Particle size and surface charge can both have a significant effect on particle distribution and cellular uptake. Encapsulation efficiency represents the mass of drug actually encapsulated within a nanoformulation as a percentage of the intended drug loading. The drug release profile will demonstrate whether sustained delivery of the drug from the nanoparticles can be anticipated, which can reduce dosing frequencies. It has been demonstrated that drugs and proteins are still pharmacologically and biologically active following their release from nanoparticles.435,436
Biocompatibility is an important consideration in the development of nanoparticles for therapeutic delivery. The nanomaterial excipients themselves must not elicit toxic responses such as inflammation or oxidative stress. Many of the polymeric nanoparticles developed in our laboratory are based on lactic acid polymer. When this polymer breaks down within the body, the so-called degradation product is lactic acid, a naturally endogenous compound.
Transport of nanoparticles across the placenta
To predict placental drug and nanoparticle transport, our laboratory focuses primarily on two experimental models: the BeWo b30 human trophoblast cell line and the dually-perfused human placental lobule. Our comparative study demonstrated excellent correlation between the in vitro BeWo cell line model and the ex vivo perfused human placenta model in predicting the transplacental transport of four model compounds.437 A separate study then expanded the comparison with placental transport data in the literature and reported a strong correlation (correlation coefficient=0.95) between the in vitro relative transfer rate and the ex vivo transfer index.438 Both experimental models appear to be suitable for nanoparticle transport studies as well. For example, the transplacental transfer of polymeric nanoparticles has been shown to be size-dependent in both models, and limited transfer of silica nanoparticles was demonstrated in both BeWo cell and placental perfusion experiments.439,440,441
Additional studies have shown that gold nanoparticles do not cross the term human placenta; the transplacental transport of poly-amidoamine dendrimers is limited; and liposome transport across the placenta is size-dependent.442,443,444 The observed size dependence of nanoparticle transport has been extended to demonstrate increased drug transport across placental trophoblast cells when the drug is encapsulated in smaller polymeric nanoparticles (146 nm diameter) compared to larger nanoparticles (diameter 232 nm).445 Literature reports of nanoparticles administered to pregnant rodents have included titanium dioxide nanoparticles, silica nanoparticles, zinc oxide nanoparticles, iron oxide nanoparticles, and gold nanoparticles; results vary depending on particle size, surface charge, and gestational age.446,447,448,449,450
Targeting strategies
Strategies for targeted delivery of nanoparticles to the placenta include the identification of peptides by phage display.451 Shaik et al. reported enhanced transcellular transfer of T7 bacteriophages across BeWo cells with an integrin-binding amino acid sequence peptide (unpublished data). Harris et al. used a similar approach in pregnant mice and identified two peptides with increased placental binding, designated as amino acid sequence consisting of CRGDKGPDC and KRK (unpublished data).452 Kaitu’u-Lino et al. designed bacterial-derived nanocells coated with antibodies to EGFR (epidermal growth factor receptor). The uptake of these EGFR-targeted delivery vehicles in ex vivo human placental explants was greater than that of non-targeted nanocells.453 We have synthesized polymeric nanoparticles conjugated with folic acid in order to take advantage of the relatively high expression of folate receptors in the placenta. This has resulted in greater cellular uptake and transport of the nanoparticles across BeWo cells.
Current opportunities
Immediate applications of these technological advances include fetal drug therapy and placental therapy. Although medication during pregnancy is most often prescribed to treat maternal conditions, there are a number of fetal diseases for which a targeted, transplacental delivery strategy could potentially attenuate the adverse maternal side effects associated with current treatment modalities. These include fetal cardiac arrhythmias, congenital adrenal hyperplasia, and fetal thyroid disorders.454 Targeted drug delivery to the placenta itself could also improve therapeutic options for malaria-infected placenta or prevent mother-to-child transmission of HIV.455
Future opportunities
Nanotechnology has also been suggested for placental diagnostic applications.456 Opportunities abound for the development of nanoparticle-mediated biosensors and imaging agents, and it is likely that many recent advances in cancer diagnostics, for example, can be utilized to monitor the structure and function of the human placenta.457 As progress towards these goals continues, it is imperative that the biocompatibility of such nanomaterials is thoroughly investigated in order to prevent unintended consequences of fetal exposure to any potentially harmful imaging agents. For example, gadolinium-based magnetic resonance imaging contrast agents are not recommended for use during pregnancy due to concerns for nephrogenic systemic fibrosis.458
Scientific gaps
Steps to translate the preclinical promises of this technology towards clinical trials and clinical practice will include scaling proof-of-concept results from in vitro and small animal studies to larger animal models with placental structure and function more similar to human placenta. For example, recent progress has been made to establish the pregnant baboon as a non-human primate model for pharmacokinetic investigations.459,460,461,462,463,464 There is also a need to refine the pool of potential placental targeting moieties in order to minimize off-target nanoparticle distribution. Side effects can be averted when the disbursement of medication to off-target sites is reduced. A necessary but exciting task will be to determine the magnitude of dose reductions possible when targeted therapeutic strategies are applied. Nanoparticle-mediated delivery to the placenta offers tremendous benefits for maternal-fetal medicine.
Maternally sequestered delivery systems for prevention of fetal drug exposure
(Gene Bidwell, University of Mississippi Medical Center)
Background
PE is a common hypertensive disorder of pregnancy and is one of the leading causes of maternal and fetal morbidity and mortality. There is currently no effective intervention for PE short of induced delivery, which makes it a leading cause of premature birth. Improvements in PE management have been largely stifled due to deleterious effects of proposed small-molecule therapeutics on the developing fetus. The objective of our studies is to develop a drug delivery system capable of stabilizing novel therapeutic agents in the maternal circulation while protecting them from entering the fetal circulation.
The use of a biopolymer drug carrier to prevent drug transfer across the placenta
The major focus of our research group is to develop drug carriers capable of preventing placental transfer and fetal exposure to therapeutic agents. Protein transport across the placental barrier is highly regulated, and proteins which are not substrates for active transporters rarely cross into fetal circulation. To take advantage of this, we are utilizing a protein-based biopolymer to fuse to therapeutic agents and sequester them in the maternal circulation (Figure 17). This biopolymer, termed elastin-like polypeptide (ELP), is based on a repeating sequence found in human elastin.465 ELP is an ideal drug carrier for several reasons. Due to its origins in human elastin, it is not recognized as foreign by the immune system, does not induce an inflammatory response or immunogenicity, and is likely slowly broken down into individual amino acids in vivo, making it completely biocompatible. Because the ELP sequence is encoded at the DNA level, modification of the polymer sequence (either to tune the polymer size, to add reactive sites for drug conjugation, or to fuse targeting agents, therapeutic peptides, or therapeutic proteins) is a matter of simple molecular biology.466,467,468 Also, because it is protein-based, ELP can be produced by recombinant expression in bacterial or eukaryotic systems,466,467,469 and it is easily purified by a non-chromatographic procedure called inverse transition cycling.467,470 This makes scale-up to production levels required for therapeutic development very feasible. Finally and most importantly, because of its large size, ELPs can protect small peptide or drug cargo from rapid degradation and extend their plasma half-life and bioavailability.
Figure 17. Model of the ELP drug delivery vector.
Elastin-like Polypeptide (ELP) is a biocompatible protein polymer used to protect attached cargo from degradation, rapid renal clearance, and immunogenicity to prevent transfer of cargo across the placenta. ELP is modified with targeting agents or cell penetrating peptides to enhance organ specificity or to mediate target cell uptake. ELP is also modified with cargo therapeutic peptides, therapeutic proteins, or with reactive sites for covalent attachment of small molecule drugs. B. Placental transfer of ELP. Quantitative fluorescence analysis revealed that the ELP carrier accumulated highly in the placenta (red and yellow color), but did not penetrate into the fetal circulation following bolus injection or chronic infusion in a rat pregnancy model.
Reprinted, with permission, from George et al.471
Our strategy involves the use of a core ELP biopolymer as a drug carrier, and this biopolymer is modified at its amino and/or carboxy-termini with targeting agents, cell penetrating agents, peptide or protein therapeutics, or reactive sites for drug attachment (Figure 17A). In a recent study, we examined the PKs and biodistribution of ELPs in a rat pregnancy model. The ELP carrier accumulated at very high levels in the maternal kidney, liver, and placenta after systemic intravenous infusion, but little to no ELP crossed into the fetus after either a bolus administration or five days of continuous infusion (Figure 17B).471 This study indicates that the ELP drug carrier may be a powerful means of sequestering fused therapeutics to the maternal circulation and preventing fetal drug exposure.
Mechanisms of symptom onset in PE and potential therapeutic interventions
PE is a disease of placental insufficiency rooted in a poorly understood process of impaired spiral artery remodeling during early placental development.472,473,474 Because the molecular basis for this failed remodeling is poorly understood, and because a robust biomarker to predict patients who will become preeclamptic is lacking, it is not yet practical to design targeted therapeutics to correct spiral artery remodeling and prevent PE. However, preeclampsia symptoms do not present until later in pregnancy, and unlike the initiating factors in spiral artery remodeling, the molecular pathways that lead to symptom precipitation are much better understood. Therefore, now is the optimal time to develop therapeutics targeted to progression of PE with the goal of extending pregnancies and improving fetal outcomes.475,476 The overall goal of our approach is to target the pathways leading to hypertension, impaired renal function, neurological complications, and other components of the PE syndrome in hopes of delaying the need for induced delivery for as long as possible, and we aim to do so in a manner that protects the fetus from exposure to the therapeutic agents.
The molecular pathways that lead to the symptom precipitation of symptoms in PE are a consequence of placental hypoperfusion and hypoxia. In response, the hypoxic placenta releases a multitude of factors that affect the mother at nearly all organ systems.477 Briefly, the major players in the progression of the preeclampsia syndrome can be divided into three groups, anti-angiogenic factors, pro-inflammatory factors, and inducers of reactive oxygen species (Figure 18).477 These factors induce endothelial dysfunction and systemic inflammation in systemic vascular beds of the maternal circulation which ultimately lead to the clinical manifestations of preeclampsia.
Figure 18. Model for the role of anti-angiogenic factors and inflammatory cytokines in preeclampsia.
The ischemic placenta is known to be a source of the VEGF antagonist protein sFlt-1 as well as many pro-inflammatory cytokines. These factors cooperate with one another to produce systemic inflammation and endothelial dysfunction in the mother, which is manifested clinically in multiple organ systems. Illustrated are our proposed strategies for interfering with these processes by either supplementing VEGF or PlGF levels by administration of exogenous ELP-fused proteins or by giving ELP-stabilized inhibitors of NF-κB.
Adapted, with permission, from Bidwell and George.477
One of the major proteins produced by the hypoxic placenta thought to drive maternal PE symptoms is sFlt-1. sFlt-1 levels were found to be dramatically elevated in preeclamptic patients with a corresponding reduction in the plasma levels of its ligands-VEGF and PlGF.478 VEGF is known to be important in endothelial cell health, and its sequestration by sFlt-1 binding is hypothesized to lead to many of the renal and vascular effects of preeclampsia.479 This makes sFlt-1 one of the major targets for preeclampsia drug development. One strategy we are employing is to supplement VEGF levels by administering an exogenously prepared ELP-VEGF fusion protein.477 We have previously found that VEGF signaling activity is maintained after ELP fusion,467 and our goal with this strategy is to restore the angiogenic balance that was lost when sFlt-1 production was increased. In addition to reduction of systemic endothelial dysfunction, this strategy might have the added benefit of increasing placental perfusion by enhancing uterine or spiral artery blood flow. We are also examining a related strategy of supplementing PlGF by administering an ELP-PlGF fusion protein. This has the potential to be a safer and easier to dose strategy than direct VEGF administration. The hypothesis is that the exogenous ELP-PlGF will bind sFlt-1 as well as the full length cell surface receptor Flt-1,480 thereby displacing VEGF and making it available to bind the more active receptor VEGFR2. Since sFlt-1 has no known signaling function and VEGFR1 is much less active than VEGFR2,481 supplementation of PlGF may be less prone to side effects compared to direct administration of VEGF.
Other major drivers of PE symptoms include the chronic systemic inflammatory response (exacerbated by placental production of inflammatory cytokines).482 The inflammatory response in preeclampsia is known to be mediated by TNF-α,483 pro-inflammatory interleukins,484 and agonists of TLR signaling485 produced both in the placenta and possibly by systemic macrophages and/or endothelial cells. A common mediator of all these inflammatory signaling pathways is the transcription factor NF-κB. Another strategy we are exploring is the examination of NF-κB as a potential drug target for PE. As a first attempt, we have generated an ELP-delivered peptide inhibitor of NF-κB signaling. The ELP fusion protects this peptide from rapid renal clearance after intravenous administration, enhances its placental deposition over 30-fold relative to free peptide, and prevents its transfer across the placenta (unpublished data). We hypothesize that a maternally-sequestered ELP-fused NF-κB inhibitor will be effective as an anti-inflammatory agent and may slow the progression of PE.
Current opportunities
The use of ELP or related macromolecular carriers to prevent fetal drug exposure represents a promising new strategy for drug development for disorders of pregnancy. As described above, we are employing ELP fusions with therapeutic peptides or proteins targeted to pathways of importance in PE, but this strategy is not limited to preteinacious therapeutics or to PE. ELP is amenable to fusion with small molecule drugs, and other projects in our lab are examining the ability of ELP to prevent transfer of known-toxic or teratogenic drugs across the placenta, thus potentially opening the door for safe use of drugs currently avoided in the pregnant patient population.
Future opportunities
Our current strategy is to utilize the ELP system to intervene in late gestation with therapeutics targeted to pathways of currently known importance in PE. Future work will expand this strategy as the list of potential drug targets continues to grow. The ELP system is easily adapted for the delivery of nearly any type of therapeutic, so expanding this strategy to target newly identified causative pathways is highly feasible. We view the ELP system as a platform for maternal sequestration of therapeutics, and its attractive properties as a drug carrier make it a promising platform to be applied for delivery of novel therapeutics.
Scientific gaps
Though we are focused on treating symptoms of PE during late gestation, the ideal scenario would be to intervene early in pregnancy, during trophoblast invasion and placentation, to prevent the onset of PE. However, to do that, we need to gain a better understanding of the causative factors hampering proper placental formation in PE, and we need robust biomarkers to identify patients who will develop the disorder.
Another area that currently stands as a hurdle to developing therapeutics for PE and other disorders of pregnancy is the high level of risk aversion when treating pregnant mothers. This risk aversion is well justified given the vulnerability of the patient population, and we have to address these risks by doing all we can pre-clinically to insure the safety of developmental therapeutics. One strategy for this is to prevent fetal drug exposure using drug carriers, as described above. In addition, we must also strive to insure that our therapeutics is not adversely affecting normal placental function. To address these issues, we must use the best pre-clinical models at our disposal to study placental drug transport, fetal exposure, and drug effects on normal placental function, and we must follow offspring in our pre-clinical models to insure normal physical, mental, and physiological development. Only after collecting robust data in these areas should we attempt to begin testing in human pregnancies. Though we should proceed with caution and utilize every means at our disposal to insure safety, we should not let fear of doing harm stifle innovation in developing diagnostics and therapeutics for use in pregnant patients.
COMMENT
Exciting research was presented at the workshop in the area of placentation, trophoblast migration, and spiral artery remodeling. Research findings in these areas will be critical for developing future treatments, and ultimately prevention, of adverse pregnancy outcomes of placental origin including PE and FGR. Knowledge of the basic biology of trophoblast invasion and spiral artery remodeling is critical to understanding the etiology of many pregnancy disorders, and once the molecular pathways that go awry are identified, it will be possible to design therapeutics to intervene in these pathways. However, this goal also elucidates another major gap in the field, the need for robust biomarkers to predict the onset of the disease early in pregnancy. Future research in these areas will be critical for filling this gap.
Data were also presented regarding ongoing clinical efforts in the U.S. and in Europe testing novel interventions for PE and FGR, including agents such as oral arginine supplementation, sildenafil, pravastatin, a virally-delivered VEGF for local administration into the uterine artery to improve blood flow, and oxygen supplementation therapy. Proposals were also made to improve fetal health not by targeting maternal blood flow, but by enhancing nutrient transport to the fetus by modulating glucose and amino acid placental transporters. Strategies were presented for inhibiting mitochondrial dysfunction and oxidative stress to improve placental health, and the roles of inflammation on both trophoblast function and in the advanced stages of PE were discussed. The roles of miRNAs and placentally-derived exosomes were discussed in the context of their use for diagnostics and as drug targets. These diverse pathways illustrate many opportunities for drug intervention. Although a number of potential drugs to target these pathways are available and have been shown to be relatively safe, the therapeutic benefits of the majority of these drugs were never studied in pregnancy. Hence, the safety and pharmacokinetic profiles of these and new therapeutics will need to be determined before their use in pregnancy. The workshop discussed this aspect highlighting the unique pharmacokinetic properties of pregnancy, as well as the hurdles and pitfalls of translating research findings into practice. The workshop concluded with discussions of drug delivery during pregnancy. The potential for using nanoparticles or protein biopolymers during pregnancy was presented, either to prevent fetal drug exposure by blocking placental transfer or to encourage drug delivery to the fetus for in utero therapy by attaching agents actively transported by the placenta. The use of macromolecular carriers to prevent placental drug transfer and fetal drug exposure represents an exciting new strategy for drug development in pregnancy. Knowledge that the fetus will not be exposed to the therapeutic agent will open the door for use of many drugs currently avoided during pregnancy, and it will help lessen the regulatory burden on drug development for pregnancyrelated disorders.
The pregnant patient population may be one of the most challenging cohorts for which to develop drugs due to concerns regarding drug effects on fetal outcome. Placental drug transfer is a very real risk for many small molecule drugs and even for some actively-transported biologics. The potential for adverse fetal effects not only represents a concern to pregnant mothers, it also stifles drug development due to risk aversion from the pharmaceutical industry and from regulatory bodies. For many therapeutics, we simply do not know the risk to pregnant mothers or to their developing offspring because pregnancy is most commonly excluded from clinical trials. Of course, intervention during this vulnerable developmental period must be approached with extreme care. We must use the best preclinical models at our disposal to maximize the chance of elucidating adverse drug effects for novel test agents. Also, utilizing drug delivery strategies to specifically target the placenta and/or prevent placental drug transfer and fetal drug exposure is an exceptionally promising method of developing new and safe therapeutics for use in pregnant women.
In closing, a major theme that clearly developed from the workshop was that we, as a scientific community, need to stop thinking of pregnant women as a vulnerable patient population for which drug development should be avoided and begin to appreciate our opportunity to improve both maternal and fetal health. This strategy could not only have an immediate benefit on pregnancy outcome but also have long-term benefit on the future health of the infant, as recent evidence has made it clear that risk for many health outcomes, including development of cardiovascular diseases,486 metabolic diseases,487 and cognitive developmental disorders,488 just to name a few, are strongly programmed by the in utero environment.
Acknowledgments
Dr. Bidwell is owner of Leflore Technologies, LLC, a private company working to develop biopolymer-delivered therapeutics.
We wish to acknowledge Tamika Turner-Graydon for her major contribution in the preparation and editing of the manuscript and Rosalina Bray for her excellent assistance in organizing the workshop meeting.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Workshop held in North Bethesda, MD 20852
All other authors report no conflict of interest.
Comments and views of the author(s) do not necessarily represent the views of the NICHD.
References
- 1.Chaddha V, Viero S, Huppertz B, Kingdom J. Developmental biology of the placenta and the origins of placental insufficiency. Semin Fetal Neonatal Med. 2004;9:357–369. doi: 10.1016/j.siny.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 2.Fisher SJ. The placental problem: linking abnormal cytotrophoblast differentiation to the maternal symptoms of preeclampsia. Reprod Biol Endocrinol. 2004;2:53. doi: 10.1186/1477-7827-2-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Redline RW. Placental inflammation. Semin Neonatol. 2004;9:265–274. doi: 10.1016/j.siny.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 4.Andraweera PH, Dekker GA, Roberts CT. The vascular endothelial growth factor family in adverse pregnancy outcomes. Hum Reprod Update. 2012;18:436–457. doi: 10.1093/humupd/dms011. [DOI] [PubMed] [Google Scholar]
- 5.Kingdom J, Huppertz B, Seaward G, Kaufmann P. Development of the placental villous tree and its consequences for fetal growth. Eur J Obstet Gynecol Reprod Biol. 2000;92:35–43. doi: 10.1016/s0301-2115(00)00423-1. [DOI] [PubMed] [Google Scholar]
- 6.Jansson T, Aye IL, Goberdhan DC. The emerging role of mTORC1 signaling in placental nutrient-sensing. Placenta. 2012;33(Suppl 2):e23–e29. doi: 10.1016/j.placenta.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perkins ND. Integrating cell-signalling pathways with NF-kappaB and IKK function. Nat Rev Mol Cell Biol. 2007;8:49–62. doi: 10.1038/nrm2083. [DOI] [PubMed] [Google Scholar]
- 8.Macintyre DA, Sykes L, Teoh TG, Bennett PR. Prevention of preterm labour via the modulation of inflammatory pathways. J Matern Fetal Neonatal Med. 2012;25(Suppl 1):17–20. doi: 10.3109/14767058.2012.666114. [DOI] [PubMed] [Google Scholar]
- 9.The NCGC/NCATS Pharmaceutical Collection (vol 2015) [Accessed on June 3, 2015]; Available at http://tripod.nih.gov/npc.
- 10.PubChem. [Accessed on June 3, 2015]; Available at http://pubchem.ncbi.nlm.nih.gov/
- 11.The DrugBank database. [Accessed on June 3, 2015]; Available at http://www.drugbank.ca. [Google Scholar]
- 12.KEGG DRUG. [Accessed on June 3, 2015]; Available at http://www.genome.jp/kegg/drug. [Google Scholar]
- 13.GeneCards. [Accessed on June 3, 2015]; Available at http://genecards.org. [Google Scholar]
- 14.Toporkiewicz M, Meissner J, Matusewicz L, Czogalla A, Sikorski AF. Toward a magic or imaginary bullet? Ligands for drug targeting to cancer cells: principles, hopes, and challenges. Int J Nanomedicine. 2015;10:1399–1414. doi: 10.2147/IJN.S74514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silva AC, Lopes CM, Lobo JM, Amaral MH. Nucleic Acids Delivery Systems:A Challenge for Pharmaceutical Technologists. Curr Drug Metab. 2015 doi: 10.2174/1389200216666150401110211. [DOI] [PubMed] [Google Scholar]
- 16.Burnett JC, Rossi JJ. RNA-based therapeutics: current progress and future prospects. Chem Biol. 2012;19:60–71. doi: 10.1016/j.chembiol.2011.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Rooij E, Kauppinen S. Development of microRNA therapeutics is coming of age. EMBO Mol Med. 2014;6:851–864. doi: 10.15252/emmm.201100899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Svenson S, Prud'homme RE. Multifunctional Nanoparticles for Drug Delivery Applications: Imaging, Targeting, and Delivery. New York: Springer-Verlag; [Google Scholar]
- 19.Vrachnis N, Kalampokas E, Sifakis S, et al. Placental growth factor (PlGF): a key to optimizing fetal growth. J Matern Fetal Neonatal Med. 2013;26:995–1002. doi: 10.3109/14767058.2013.766694. [DOI] [PubMed] [Google Scholar]
- 20.Maltepe E, Bakardjiev AI, Fisher SJ. The placenta: transcriptional, epigenetic, and physiological integration during development. J Clin Invest. 2010;120:1016–1025. doi: 10.1172/JCI41211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Romero R, Dey SK, Fisher SJ. Preterm labor: one syndrome, many causes. Science. 2014;345:760–765. doi: 10.1126/science.1251816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Redman CW. Preeclampsia: a multi-stress disorder. Rev Med Interne. 2011;32(Suppl 1):S41–S44. doi: 10.1016/j.revmed.2011.03.331. [DOI] [PubMed] [Google Scholar]
- 23.Brosens IA, Robertson WB, Dixon HG. The role of the spiral arteries in the pathogenesis of pre-eclampsia. The Journal of pathology. 1970;101:Pvi. [PubMed] [Google Scholar]
- 24.Brosens I, Pijnenborg R, Vercruysse L, Romero R. The "Great Obstetrical Syndromes" are associated with disorders of deep placentation. Am J Obstet Gynecol. 2011;204:193–201. doi: 10.1016/j.ajog.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fisher SJ. Why is placentation abnormal in preeclampsia? Am J Obstet Gynecol. 2015;213:S115–S122. doi: 10.1016/j.ajog.2015.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou Y, Damsky CH, Fisher SJ. Preeclampsia is associated with failure of human cytotrophoblasts to mimic a vascular adhesion phenotype. One cause of defective endovascular invasion in this syndrome? J Clin Invest. 1997;99:2152–2164. doi: 10.1172/JCI119388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou Y, Mcmaster M, Woo K, et al. Vascular endothelial growth factor ligands and receptors that regulate human cytotrophoblast survival are dysregulated in severe preeclampsia and hemolysis, elevated liver enzymes, and low platelets syndrome. Am J Pathol. 2002;160:1405–1423. doi: 10.1016/S0002-9440(10)62567-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clark DE, Smith SK, He Y, et al. A vascular endothelial growth factor antagonist is produced by the human placenta and released into the maternal circulation. Biol Reprod. 1998;59:1540–1548. doi: 10.1095/biolreprod59.6.1540. [DOI] [PubMed] [Google Scholar]
- 29.Maynard SE, Min JY, Merchan J, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111:649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Venkatesha S, Toporsian M, Lam C, et al. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med. 2006;12:642–649. doi: 10.1038/nm1429. [DOI] [PubMed] [Google Scholar]
- 31.Levine RJ, Lam C, Qian C, et al. Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N Engl J Med. 2006;355:992–1005. doi: 10.1056/NEJMoa055352. [DOI] [PubMed] [Google Scholar]
- 32.Hirashima C, Ohkuchi A, Takahashi K, Suzuki H, Matsubara S, Suzuki M. A novel three-step approach for predicting the imminent onset of preeclampsia within 4 weeks after blood sampling at 19–31 weeks of gestation. Hypertension research : official journal of the Japanese Society of Hypertension. 2014;37:519–525. doi: 10.1038/hr.2014.31. [DOI] [PubMed] [Google Scholar]
- 33.Chaiworapongsa T, Romero R, Whitten AE, et al. The use of angiogenic biomarkers in maternal blood to identify which SGA fetuses will require a preterm delivery and mothers who will develop pre-eclampsia. J Matern Fetal Neonatal Med. 2015:1–15. doi: 10.3109/14767058.2015.1048431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakimuli A, Chazara O, Hiby SE, et al. A KIR B centromeric region present in Africans but not Europeans protects pregnant women from pre-eclampsia. Proc Natl Acad Sci U S A. 2015;112:845–850. doi: 10.1073/pnas.1413453112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou Y, Gormley MJ, Hunkapiller NM, et al. Reversal of gene dysregulation in cultured cytotrophoblasts reveals possible causes of preeclampsia. J Clin Invest. 2013;123:2862–2872. doi: 10.1172/JCI66966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berman J, Girardi G, Salmon JE. TNF-alpha is a critical effector and a target for therapy in antiphospholipid antibody-induced pregnancy loss. J Immunol. 2005;174:485–490. doi: 10.4049/jimmunol.174.1.485. [DOI] [PubMed] [Google Scholar]
- 37.Lo YM, Tein MS, Lau TK, et al. Quantitative analysis of fetal DNA in maternal plasma and serum: implications for noninvasive prenatal diagnosis. American journal of human genetics. 1998;62:768–775. doi: 10.1086/301800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Norton ME, Jacobsson B, Swamy GK, et al. Cell-free DNA analysis for noninvasive examination of trisomy. N Engl J Med. 2015;372:1589–1597. doi: 10.1056/NEJMoa1407349. [DOI] [PubMed] [Google Scholar]
- 39.Koh W, Pan W, Gawad C, et al. Noninvasive in vivo monitoring of tissue-specific global gene expression in humans. Proc Natl Acad Sci U S A. 2014;111:7361–7366. doi: 10.1073/pnas.1405528111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Redman CW, Sargent IL. Circulating microparticles in normal pregnancy and preeclampsia. Placenta. 2008;29(Suppl A):S73–S77. doi: 10.1016/j.placenta.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 41.Mincheva-Nilsson L, Baranov V. Placenta-derived exosomes and syncytiotrophoblast microparticles and their role in human reproduction: immune modulation for pregnancy success. Am J Reprod Immunol. 2014;72:440–457. doi: 10.1111/aji.12311. [DOI] [PubMed] [Google Scholar]
- 42.Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007;449:819–826. doi: 10.1038/nature06246. [DOI] [PubMed] [Google Scholar]
- 43.Jin C, Flavell RA. Innate sensors of pathogen and stress: linking inflammation to obesity. J Allergy Clin Immunol. 2013;132:287–294. doi: 10.1016/j.jaci.2013.06.022. [DOI] [PubMed] [Google Scholar]
- 44.Chaouat G, Sandra O, Ledee N. Immunology of Implantation, Implantation Failure and Pregnancy. In: Abrahams VM, editor. Immunology of Pregnancy. Bentham e-books; 2013. [Google Scholar]
- 45.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 46.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 47.Song DH, Lee JO. Sensing of microbial molecular patterns by Toll-like receptors. Immunol Rev. 2012;250:216–229. doi: 10.1111/j.1600-065X.2012.01167.x. [DOI] [PubMed] [Google Scholar]
- 48.Kondo T, Kawai T, Akira S. Dissecting negative regulation of Toll-like receptor signaling. Trends Immunol. 2012;33:449–458. doi: 10.1016/j.it.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 49.O'neill LA. When signaling pathways collide: positive and negative regulation of toll-like receptor signal transduction. Immunity. 2008;29:12–20. doi: 10.1016/j.immuni.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 50.Shaw MH, Reimer T, Kim YG, Nunez G. NOD-like receptors (NLRs): bona fide intracellular microbial sensors. Curr Opin Immunol. 2008;20:377–382. doi: 10.1016/j.coi.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Franchi L, Warner N, Viani K, Nunez G. Function of Nod-like receptors in microbial recognition and host defense. Immunol Rev. 2009;227:106–128. doi: 10.1111/j.1600-065X.2008.00734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Caruso R, Warner N, Inohara N, Nunez G. NOD1 and NOD2: signaling, host defense, and inflammatory disease. Immunity. 2014;41:898–908. doi: 10.1016/j.immuni.2014.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jin C, Flavell RA. Molecular mechanism of NLRP3 inflammasome activation. J Clin Immunol. 2010;30:628–631. doi: 10.1007/s10875-010-9440-3. [DOI] [PubMed] [Google Scholar]
- 54.Martinon F, Tschopp J. Inflammatory caspases and inflammasomes: master switches of inflammation. Cell Death Differ. 2007;14:10–22. doi: 10.1038/sj.cdd.4402038. [DOI] [PubMed] [Google Scholar]
- 55.Cohen P. The TLR and IL-1 signalling network at a glance. J Cell Sci. 2014;127:2383–2390. doi: 10.1242/jcs.149831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nadeau-Vallee M, Quiniou C, Palacios J, et al. Novel Noncompetitive IL-1 Receptor-Biased Ligand Prevents Infection- and Inflammation-Induced Preterm Birth. J Immunol. 2015;195:3402–3415. doi: 10.4049/jimmunol.1500758. [DOI] [PubMed] [Google Scholar]
- 57.Netea MG, Simon A, Van De Veerdonk F, Kullberg BJ, Van Der Meer JW, Joosten LA. IL-1beta processing in host defense: beyond the inflammasomes. PLoS Pathog. 2010;6:e1000661. doi: 10.1371/journal.ppat.1000661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Abrahams VM, Bole-Aldo P, Kim YM, et al. Divergent trophoblast responses to bacterial products mediated by TLRs. J Immunol. 2004;173:4286–4296. doi: 10.4049/jimmunol.173.7.4286. [DOI] [PubMed] [Google Scholar]
- 59.Abrahams VM, Visintin I, Aldo PB, Guller S, Romero R, Mor G. A role for TLRs in the regulation of immune cell migration by first trimester trophoblast cells. J Immunol. 2005;175:8096–8104. doi: 10.4049/jimmunol.175.12.8096. [DOI] [PubMed] [Google Scholar]
- 60.Costello MJ, Joyce SK, Abrahams VM. NOD protein expression and function in first trimester trophoblast cells. Am J Reprod Immunol. 2007;57:67–80. doi: 10.1111/j.1600-0897.2006.00447.x. [DOI] [PubMed] [Google Scholar]
- 61.Mulla MJ, Yu AG, Cardenas I, Guller S, Panda B, Abrahams VM. Regulation of Nod1 and Nod2 in first trimester trophoblast cells. Am J Reprod Immunol. 2009;61:294–302. doi: 10.1111/j.1600-0897.2009.00694.x. [DOI] [PubMed] [Google Scholar]
- 62.Cardenas I, Mulla MJ, Myrtolli K, et al. Nod1 activation by bacterial iE-DAP induces maternal-fetal inflammation and preterm labor. J Immunol. 2011;187:980–986. doi: 10.4049/jimmunol.1100578. [DOI] [PubMed] [Google Scholar]
- 63.Ma Y, Kadner SS, Guller S. Differential effects of lipopolysaccharide and thrombin on interleukin-8 expression in syncytiotrophoblasts and endothelial cells: implications for fetal survival. Annals of the New York Academy of Sciences. 2004;1034:236–244. doi: 10.1196/annals.1335.025. [DOI] [PubMed] [Google Scholar]
- 64.Elovitz MA, Mrinalini C. Animal models of preterm birth. Trends Endocrinol Metab. 2004;15:479–487. doi: 10.1016/j.tem.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 65.Wang H, Hirsch E. Bacterially-induced preterm labor and regulation of prostaglandin-metabolizing enzyme expression in mice: the role of toll-like receptor 4. Biol Reprod. 2003;69:1957–1963. doi: 10.1095/biolreprod.103.019620. [DOI] [PubMed] [Google Scholar]
- 66.Elovitz MA, Wang Z, Chien EK, Rychlik DF, Phillippe M. A new model for inflammation-induced preterm birth: the role of platelet-activating factor and Toll-like receptor-4. Am J Pathol. 2003;163:2103–2111. doi: 10.1016/S0002-9440(10)63567-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cardenas I, Means RE, Aldo P, et al. Viral infection of the placenta leads to fetal inflammation and sensitization to bacterial products predisposing to preterm labor. J Immunol. 2010;185:1248–1257. doi: 10.4049/jimmunol.1000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cardenas I, Mor G, Aldo P, et al. Placental viral infection sensitizes to endotoxin-induced pre-term labor: a double hit hypothesis. Am J Reprod Immunol. 2011;65:110–117. doi: 10.1111/j.1600-0897.2010.00908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Murphy SP, Fast LD, Hanna NN, Sharma S. Uterine NK cells mediate inflammation-induced fetal demise in IL-10-null mice. J Immunol. 2005;175:4084–4090. doi: 10.4049/jimmunol.175.6.4084. [DOI] [PubMed] [Google Scholar]
- 70.Robertson SA, Skinner RJ, Care AS. Essential role for IL-10 in resistance to lipopolysaccharide-induced preterm labor in mice. J Immunol. 2006;177:4888–4896. doi: 10.4049/jimmunol.177.7.4888. [DOI] [PubMed] [Google Scholar]
- 71.Aagaard K, Ma J, Antony KM, Ganu R, Petrosino J, Versalovic J. The placenta harbors a unique microbiome. Sci Transl Med. 2014;6:237ra65. doi: 10.1126/scitranslmed.3008599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stout MJ, Conlon B, Landeau M, et al. Identification of intracellular bacteria in the basal plate of the human placenta in term and preterm gestations. Am J Obstet Gynecol. 2013;208:226.e1–226.e7. doi: 10.1016/j.ajog.2013.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Grigsby PL, Novy MJ, Sadowsky DW, et al. Maternal azithromycin therapy for Ureaplasma intraamniotic infection delays preterm delivery and reduces fetal lung injury in a primate model. Am J Obstet Gynecol. 2012;207:475.e1–475.e14. doi: 10.1016/j.ajog.2012.10.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gravett MG, Adams KM, Sadowsky DW, et al. Immunomodulators plus antibiotics delay preterm delivery after experimental intraamniotic infection in a nonhuman primate model. Am J Obstet Gynecol. 2007;197:518.e1–518.e8. doi: 10.1016/j.ajog.2007.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ma Y, Krikun G, Abrahams VM, Mor G, Guller S. Cell type-specific expression and function of toll-like receptors 2 and 4 in human placenta: implications in fetal infection. Placenta. 2007;28:1024–1031. doi: 10.1016/j.placenta.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Abrahams VM, Aldo PB, Murphy SP, et al. TLR6 modulates first trimester trophoblast responses to peptidoglycan. J Immunol. 2008;180:6035–6043. doi: 10.4049/jimmunol.180.9.6035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Garg M, Potter JA, Abrahams VM. Identification of microRNAs that regulate TLR2-mediated trophoblast apoptosis and inhibition of IL-6 mRNA. PLoS One. 2013;8:e77249. doi: 10.1371/journal.pone.0077249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mulla MJ, Myrtolli K, Tadesse S, et al. Cutting-edge report: TLR10 plays a role in mediating bacterial peptidoglycan-induced trophoblast apoptosis. Am J Reprod Immunol. 2013;69:449–453. doi: 10.1111/aji.12065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ilievski V, Lu SJ, Hirsch E. Activation of toll-like receptors 2 or 3 and preterm delivery in the mouse. Reprod Sci. 2007;14:315–320. doi: 10.1177/1933719107302959. [DOI] [PubMed] [Google Scholar]
- 80.Abrahams VM, Schaefer TM, Fahey JV, et al. Expression and secretion of antiviral factors by trophoblast cells following stimulation by the TLR-3 agonist, Poly(I : C) Hum Reprod. 2006;21:2432–2439. doi: 10.1093/humrep/del178. [DOI] [PubMed] [Google Scholar]
- 81.Aldo PB, Mulla MJ, Romero R, Mor G, Abrahams VM. Viral ssRNA induces first trimester trophoblast apoptosis through an inflammatory mechanism. Am J Reprod Immunol. 2010;64:27–37. doi: 10.1111/j.1600-0897.2010.00817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Potter JA, Garg M, Girard S, Abrahams VM. Viral single stranded RNA induces a trophoblast pro-inflammatory and antiviral response in a TLR8-dependent and -independent manner. Biol Reprod. 2015;92:17. doi: 10.1095/biolreprod.114.124032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Koga K, Cardenas I, Aldo P, et al. Activation of TLR3 in the trophoblast is associated with preterm delivery. Am J Reprod Immunol. 2009;61:196–212. doi: 10.1111/j.1600-0897.2008.00682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chatterjee P, Weaver LE, Doersch KM, et al. Placental Toll-like receptor 3 and Toll-like receptor 7/8 activation contributes to preeclampsia in humans and mice. PLoS One. 2012;7:e41884. doi: 10.1371/journal.pone.0041884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Van Beijnum JR, Buurman WA, Griffioen AW. Convergence and amplification of toll-like receptor (TLR) and receptor for advanced glycation end products (RAGE) signaling pathways via high mobility group B1 (HMGB1) Angiogenesis. 2008;11:91–99. doi: 10.1007/s10456-008-9093-5. [DOI] [PubMed] [Google Scholar]
- 86.Romero R, Chaiworapongsa T, Alpay Savasan Z, et al. Damage-associated molecular patterns (DAMPs) in preterm labor with intact membranes and preterm PROM: a study of the alarmin HMGB1. J Matern Fetal Neonatal Med. 2011;24:1444–1455. doi: 10.3109/14767058.2011.591460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bredeson S, Papaconstantinou J, Deford JH, et al. HMGB1 promotes a p38MAPK associated non-infectious inflammatory response pathway in human fetal membranes. PLoS One. 2014;9:e113799. doi: 10.1371/journal.pone.0113799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pradervand PA, Clerc S, Frantz J, et al. High mobility group box 1 protein (HMGB-1): a pathogenic role in preeclampsia? Placenta. 2014;35:784–786. doi: 10.1016/j.placenta.2014.06.370. [DOI] [PubMed] [Google Scholar]
- 89.Girard S, JR, Guller S, Sibley Cp, Abraham Vm. Alarmins Potently Induce Inflammation in Placental Cells and Impair Trophoblast Turnover. Reproductive Sciences. 2015;22 [Google Scholar]
- 90.Meroni PL, Raschi E, Grossi C, et al. Obstetric and vascular APS: same autoantibodies but different diseases? Lupus. 2012;21:708–710. doi: 10.1177/0961203312438116. [DOI] [PubMed] [Google Scholar]
- 91.Mulla MJ, Yu AG, Cardenas I, Guller S, Panda B, Abrahams VM. Regulation of Nod1 and Nod2 in first trimester trophoblast cells. Am J Reprod Immunol. 2009;61:294–302. doi: 10.1111/j.1600-0897.2009.00694.x. [DOI] [PubMed] [Google Scholar]
- 92.Mulla MJ, Salmon JE, Chamley LW, et al. A role for uric acid and the Nalp3 inflammasome in antiphospholipid antibody-induced IL-1beta production by human first trimester trophoblast. PLoS One. 2013;8:e65237. doi: 10.1371/journal.pone.0065237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mulla MJ, Myrtolli K, Potter J, et al. Uric acid induces trophoblast IL-1beta production via the inflammasome: implications for the pathogenesis of preeclampsia. Am J Reprod Immunol. 2011;65:542–548. doi: 10.1111/j.1600-0897.2010.00960.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gysler SM. MiR-146a Regulates Antiphospholipid Antibody-Induced IL-8 Secretion by Human Trophoblast Cells. Reproductive sciences. 2014;21:116A. [Google Scholar]
- 95.Gysler SM, Mulla MJ, Brosens JJ, et al. Antiphospholipid Antibody-Induced miR-146a Activates TLR8 in Trophoblast and Is Inhibited By Vitamin D. Reproductive Sciences. 2015;22:71A. [Google Scholar]
- 96.Han CS, Herrin MA, Pitruzzello MC, et al. Glucose and metformin modulate human first trimester trophoblast function: a model and potential therapy for diabetes-associated uteroplacental insufficiency. Am J Reprod Immunol. 2015;73:362–371. doi: 10.1111/aji.12339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Koopmans CM, Van Pampus MG, Groen H, Aarnoudse JG, Van Den Berg PP, Mol BW. Accuracy of serum uric acid as a predictive test for maternal complications in pre-eclampsia: bivariate meta-analysis and decision analysis. Eur J Obstet Gynecol Reprod Biol. 2009;146:8–14. doi: 10.1016/j.ejogrb.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 98.Girard S, Heazell AE, Derricott H, et al. Circulating cytokines and alarmins associated with placental inflammation in high-risk pregnancies. Am J Reprod Immunol. 2014;72:422–434. doi: 10.1111/aji.12274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Schmella MJ, Clifton RG, Althouse AD, Roberts JM. Uric Acid Determination in Gestational Hypertension: Is it as Effective a Delineator of Risk as Proteinuria in High-Risk Women? Reprod Sci. 2015 doi: 10.1177/1933719115572477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Adams Waldorf KM, Persing D, Novy MJ, Sadowsky DW, Gravett MG. Pretreatment with toll-like receptor 4 antagonist inhibits lipopolysaccharide-induced preterm uterine contractility, cytokines, and prostaglandins in rhesus monkeys. Reprod Sci. 2008;15:121–127. doi: 10.1177/1933719107310992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Filipovich Y, Lu SJ, Akira S, Hirsch E. The adaptor protein MyD88 is essential for E coli-induced preterm delivery in mice. Am J Obstet Gynecol. 2009;200:93 e1–93 e8. doi: 10.1016/j.ajog.2008.08.038. [DOI] [PubMed] [Google Scholar]
- 102.Gotsch F, Romero R, Chaiworapongsa T, et al. Evidence of the involvement of caspase-1 under physiologic and pathologic cellular stress during human pregnancy: a link between the inflammasome and parturition. J Matern Fetal Neonatal Med. 2008;21:605–616. doi: 10.1080/14767050802212109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Christiaens I, Zaragoza DB, Guilbert L, Robertson SA, Mitchell BF, Olson DM. Inflammatory processes in preterm and term parturition. J Reprod Immunol. 2008;79:50–57. doi: 10.1016/j.jri.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 104.Kemp MW, Saito M, Newnham JP, Nitsos I, Okamura K, Kallapur SG. Preterm birth, infection, and inflammation advances from the study of animal models. Reprod Sci. 2010;17:619–628. doi: 10.1177/1933719110373148. [DOI] [PubMed] [Google Scholar]
- 105.Adams Waldorf KM, Rubens CE, Gravett MG. Use of nonhuman primate models to investigate mechanisms of infection-associated preterm birth. BJOG. 2011;118:136–144. doi: 10.1111/j.1471-0528.2010.02728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Breen K, Brown A, Burd I, Chai J, Friedman A, Elovitz MA. TLR-4-dependent and -independent mechanisms of fetal brain injury in the setting of preterm birth. Reprod Sci. 2012;19:839–850. doi: 10.1177/1933719112438439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Burd I, Bentz AI, Chai J, et al. Inflammation-induced preterm birth alters neuronal morphology in the mouse fetal brain. J Neurosci Res. 2010;88:1872–1881. doi: 10.1002/jnr.22368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Girard S, Tremblay L, Lepage M, Sebire G. IL-1 receptor antagonist protects against placental and neurodevelopmental defects induced by maternal inflammation. J Immunol. 2010;184:3997–4005. doi: 10.4049/jimmunol.0903349. [DOI] [PubMed] [Google Scholar]
- 109.Leitner K, Al Shammary M, Mclane M, Johnston MV, Elovitz MA, Burd I. IL-1 receptor blockade prevents fetal cortical brain injury but not preterm birth in a mouse model of inflammation-induced preterm birth and perinatal brain injury. Am J Reprod Immunol. 2014;71:418–426. doi: 10.1111/aji.12216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Romero R, Tartakovsky B. The natural interleukin-1 receptor antagonist prevents interleukin-1-induced preterm delivery in mice. Am J Obstet Gynecol. 1992;167:1041–1045. doi: 10.1016/s0002-9378(12)80035-4. [DOI] [PubMed] [Google Scholar]
- 111.Rosenzweig JM, Lei J, Burd I. Interleukin-1 receptor blockade in perinatal brain injury. Front Pediatr. 2014;2:108. doi: 10.3389/fped.2014.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dowling JK, O'neill LA. Biochemical regulation of the inflammasome. Crit Rev Biochem Mol Biol. 2012;47:424–443. doi: 10.3109/10409238.2012.694844. [DOI] [PubMed] [Google Scholar]
- 113.Kavathas PB, Boeras CM, Mulla MJ, Abrahams VM. Nod1, but not the ASC inflammasome, contributes to induction of IL-1beta secretion in human trophoblasts after sensing of Chlamydia trachomatis. Mucosal Immunol. 2013;6:235–243. doi: 10.1038/mi.2012.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pijnenborg R, Vercruysse L, Hanssens M. The uterine spiral arteries in human pregnancy: facts and controversies. Placenta. 2006;27:939–958. doi: 10.1016/j.placenta.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 115.Kaufmann P, Black S, Huppertz B. Endovascular trophoblast invasion: implications for the pathogenesis of intrauterine growth retardation and preeclampsia. Biol Reprod. 2003;69:1–7. doi: 10.1095/biolreprod.102.014977. [DOI] [PubMed] [Google Scholar]
- 116.Wallace AE, Fraser R, Cartwright JE. Extravillous trophoblast and decidual natural killer cells: a remodelling partnership. Hum Reprod Update. 2012;18:458–471. doi: 10.1093/humupd/dms015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ain R, Canham LN, Soares MJ. Gestation stage-dependent intrauterine trophoblast cell invasion in the rat and mouse: novel endocrine phenotype and regulation. Dev Biol. 2003;260:176–190. doi: 10.1016/s0012-1606(03)00210-0. [DOI] [PubMed] [Google Scholar]
- 118.Konno T, Rempel LA, Arroyo JA, Soares MJ. Pregnancy in the brown Norway rat: a model for investigating the genetics of placentation. Biol Reprod. 2007;76:709–718. doi: 10.1095/biolreprod.106.056481. [DOI] [PubMed] [Google Scholar]
- 119.Pijnenborg R, Vercruysse L. Animal models of deep trophoblast invasion. New York: Cambridge University Press; 2010. pp. 127–139. [Google Scholar]
- 120.Soares MJ, Chakraborty D, Karim Rumi MA, Konno T, Renaud SJ. Rat placentation: an experimental model for investigating the hemochorial maternal-fetal interface. Placenta. 2012;33:233–243. doi: 10.1016/j.placenta.2011.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Soares MJ, Chakraborty D, Kubota K, Renaud SJ, Rumi MA. Adaptive mechanisms controlling uterine spiral artery remodeling during the establishment of pregnancy. Int J Dev Biol. 2014;58:247–259. doi: 10.1387/ijdb.140083ms. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Faria TN, Soares MJ. Trophoblast cell differentiation: establishment, characterization, and modulation of a rat trophoblast cell line expressing members of the placental prolactin family. Endocrinology. 1991;129:2895–2906. doi: 10.1210/endo-129-6-2895. [DOI] [PubMed] [Google Scholar]
- 123.Asanoma K, Rumi MA, Kent LN, et al. FGF4-dependent stem cells derived from rat blastocysts differentiate along the trophoblast lineage. Dev Biol. 2011;351:110–119. doi: 10.1016/j.ydbio.2010.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rosario GX, Konno T, Soares MJ. Maternal hypoxia activates endovascular trophoblast cell invasion. Dev Biol. 2008;314:362–375. doi: 10.1016/j.ydbio.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Konno T, Graham AR, Rempel LA, et al. Subfertility linked to combined luteal insufficiency and uterine progesterone resistance. Endocrinology. 2010;151:4537–4550. doi: 10.1210/en.2010-0440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Konno T, Rempel LA, Rumi MA, et al. Chromosome-substituted rat strains provide insights into the genetics of placentation. Physiol Genomics. 2011;43:930–941. doi: 10.1152/physiolgenomics.00069.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Goyal R, Yellon SM, Longo LD, Mata-Greenwood E. Placental gene expression in a rat 'model' of placental insufficiency. Placenta. 2010;31:568–575. doi: 10.1016/j.placenta.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 128.Goyal R, Zhang L, Blood AB, et al. Characterization of an animal model of pregnancy-induced vitamin D deficiency due to metabolic gene dysregulation. Am J Physiol Endocrinol Metab. 2014;306:E256–E266. doi: 10.1152/ajpendo.00528.2013. [DOI] [PubMed] [Google Scholar]
- 129.Lee DS, Rumi MA, Konno T, Soares MJ. In vivo genetic manipulation of the rat trophoblast cell lineage using lentiviral vector delivery. Genesis. 2009;47:433–439. doi: 10.1002/dvg.20518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Renaud SJ, Karim Rumi MA, Soares MJ. Review: Genetic manipulation of the rodent placenta. Placenta. 2011;32(Suppl 2):S130–S135. doi: 10.1016/j.placenta.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kent LN, Rumi MA, Kubota K, Lee DS, Soares MJ. FOSL1 is integral to establishing the maternal-fetal interface. Mol Cell Biol. 2011;31:4801–4813. doi: 10.1128/MCB.05780-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Rumi MA, Dhakal P, Kubota K, et al. Generation of Esr1-knockout rats using zinc finger nuclease-mediated genome editing. Endocrinology. 2014;155:1991–1999. doi: 10.1210/en.2013-2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chakraborty D, Rumi MA, Konno T, Soares MJ. Natural killer cells direct hemochorial placentation by regulating hypoxia-inducible factor dependent trophoblast lineage decisions. Proc Natl Acad Sci U S A. 2011;108:16295–16300. doi: 10.1073/pnas.1109478108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhou Y, Chiu K, Brescia RJ, et al. Increased depth of trophoblast invasion after chronic constriction of the lower aorta in rhesus monkeys. Am J Obstet Gynecol. 1993;169:224–229. doi: 10.1016/0002-9378(93)90172-f. [DOI] [PubMed] [Google Scholar]
- 135.Kadyrov M, Schmitz C, Black S, Kaufmann P, Huppertz B. Pre-eclampsia and maternal anaemia display reduced apoptosis and opposite invasive phenotypes of extravillous trophoblast. Placenta. 2003;24:540–548. doi: 10.1053/plac.2002.0946. [DOI] [PubMed] [Google Scholar]
- 136.Zhang J, Chen Z, Smith GN, Croy BA. Natural killer cell-triggered vascular transformation: maternal care before birth? Cellular & molecular immunology. 2011;8:1–11. doi: 10.1038/cmi.2010.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rossant J, Cross JC. Placental development: lessons from mouse mutants. Nat Rev Genet. 2001;2:538–548. doi: 10.1038/35080570. [DOI] [PubMed] [Google Scholar]
- 138.Hou Z, Romero R, Uddin M, Than NG, Wildman DE. Adaptive history of single copy genes highly expressed in the term human placenta. Genomics. 2009;93:33–41. doi: 10.1016/j.ygeno.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Maltepe E, Bakardjiev AI, Fisher SJ. The placenta: transcriptional, epigenetic, and physiological integration during development. J Clin Invest. 2010;120:1016–1025. doi: 10.1172/JCI41211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Maltepe E, Fisher SJ. Placenta: The Forgotten Organ. Annual review of cell and developmental biology. 2015;31:523–552. doi: 10.1146/annurev-cellbio-100814-125620. [DOI] [PubMed] [Google Scholar]
- 141.Cox B, Kotlyar M, Evangelou AI, et al. Comparative systems biology of human and mouse as a tool to guide the modeling of human placental pathology. Molecular systems biology. 2009;5:279. doi: 10.1038/msb.2009.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kent LN, Konno T, Soares MJ. Phosphatidylinositol 3 kinase modulation of trophoblast cell differentiation. BMC Dev Biol. 2010;10:97. doi: 10.1186/1471-213X-10-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Renaud SJ, Kubota K, Rumi MA, Soares MJ. The FOS transcription factor family differentially controls trophoblast migration and invasion. J Biol Chem. 2014;289:5025–5039. doi: 10.1074/jbc.M113.523746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Genbacev O, Lamb JD, Prakobphol A, Donne M, McMaster MT, Fisher SJ. Human trophoblast progenitors: where do they reside? Semin Reprod Med. 2013;31:56–61. doi: 10.1055/s-0032-1331798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Rossant J, Cross JC. Placental development: lessons from mouse mutants. Nat Rev Genet. 2001;2:538–548. doi: 10.1038/35080570. [DOI] [PubMed] [Google Scholar]
- 146.Cross JC, Baczyk D, Dobric N, et al. Genes, development and evolution of the placenta. Placenta. 2003;24:123–130. doi: 10.1053/plac.2002.0887. [DOI] [PubMed] [Google Scholar]
- 147.Davisson RL, Hoffmann DS, Butz GM, et al. Discovery of a spontaneous genetic mouse model of preeclampsia. Hypertension. 2002;39:337–342. doi: 10.1161/hy02t2.102904. [DOI] [PubMed] [Google Scholar]
- 148.Wong AY, Kulandavelu S, Whiteley KJ, Qu D, Langille BL, Adamson SL. Maternal cardiovascular changes during pregnancy and postpartum in mice. Am J Physiol Heart Circ Physiol. 2002;282:H918–H925. doi: 10.1152/ajpheart.00641.2001. [DOI] [PubMed] [Google Scholar]
- 149.Freemark M. Placental hormones and the control of fetal growth. J Clin Endocrinol Metab. 2010;95:2054–2057. doi: 10.1210/jc.2010-0517. [DOI] [PubMed] [Google Scholar]
- 150.Brelje TC, Parsons JA, Sorenson RL. Regulation of islet beta-cell proliferation by prolactin in rat islets. Diabetes. 1994;43:263–273. doi: 10.2337/diab.43.2.263. [DOI] [PubMed] [Google Scholar]
- 151.Parsons JA, Brelje TC, Sorenson RL. Adaptation of islets of Langerhans to pregnancy: increased islet cell proliferation and insulin secretion correlates with the onset of placental lactogen secretion. Endocrinology. 1992;130:1459–1466. doi: 10.1210/endo.130.3.1537300. [DOI] [PubMed] [Google Scholar]
- 152.Weinhaus AJ, Stout LE, Sorenson RL. Glucokinase, hexokinase, glucose transporter 2, and glucose metabolism in islets during pregnancy and prolactin-treated islets in vitro: mechanisms for long term up-regulation of islets. Endocrinology. 1996;137:1640–1649. doi: 10.1210/endo.137.5.8612496. [DOI] [PubMed] [Google Scholar]
- 153.Devlieger R, Casteels K, Van Assche FA. Reduced adaptation of the pancreatic B cells during pregnancy is the major causal factor for gestational diabetes: current knowledge and metabolic effects on the offspring. Acta Obstet Gynecol Scand. 2008;87:1266–1270. doi: 10.1080/00016340802443863. [DOI] [PubMed] [Google Scholar]
- 154.Zhang H, Zhang J, Pope CF, et al. Gestational diabetes mellitus resulting from impaired beta-cell compensation in the absence of FoxM1, a novel downstream effector of placental lactogen. Diabetes. 2010;59:143–152. doi: 10.2337/db09-0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Guimond MJ, Wang B, Fujita J, Terhorst C, Croy BA. Pregnancy-associated uterine granulated metrial gland cells in mutant and transgenic mice. Am J Reprod Immunol. 1996;35:501–509. doi: 10.1111/j.1600-0897.1996.tb00049.x. [DOI] [PubMed] [Google Scholar]
- 156.Hu D, Cross JC. Ablation of Tpbpa-positive trophoblast precursors leads to defects in maternal spiral artery remodeling in the mouse placenta. Dev Biol. 2011;358:231–239. doi: 10.1016/j.ydbio.2011.07.036. [DOI] [PubMed] [Google Scholar]
- 157.Ashkar AA, Di Santo JP, Croy BA. Interferon gamma contributes to initiation of uterine vascular modification, decidual integrity, and uterine natural killer cell maturation during normal murine pregnancy. J Exp Med. 2000;192:259–270. doi: 10.1084/jem.192.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Benirschke K, Burton G, Baergen R. Pathology of the Human Placenta. 6th. Vol. 5. New York: Springer; 2012. Early Development of the Human Placenta; pp. 41–54. [Google Scholar]
- 159.Simmons DG, Rawn S, Davies A, Hughes M, Cross JC. Spatial and temporal expression of the 23 murine Prolactin/Placental Lactogen-related genes is not associated with their position in the locus. BMC Genomics. 2008;9:352. doi: 10.1186/1471-2164-9-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Gene Expression Omnibus (GEO) Datasets. [Accessed on June3, 2015]; Available at http://www.ncbi.nlm.nih.gov/gds/
- 161.Brelje TC, Scharp DW, Lacy PE, et al. Effect of homologous placental lactogens, prolactins, and growth hormones on islet B-cell division and insulin secretion in rat, mouse, and human islets: implication for placental lactogen regulation of islet function during pregnancy. Endocrinology. 1993;132:879–887. doi: 10.1210/endo.132.2.8425500. [DOI] [PubMed] [Google Scholar]
- 162.Barbour LA, Shao J, Qiao L, et al. Human placental growth hormone causes severe insulin resistance in transgenic mice. Am J Obstet Gynecol. 2002;186:512–517. doi: 10.1067/mob.2002.121256. [DOI] [PubMed] [Google Scholar]
- 163.Branisteanu DD, Mathieu C. Progesterone in gestational diabetes mellitus: guilty or not guilty? Trends Endocrinol Metab. 2003;14:54–56. doi: 10.1016/s1043-2760(03)00003-1. [DOI] [PubMed] [Google Scholar]
- 164.Fasshauer M, Bluher M, Stumvoll M. Adipokines in gestational diabetes. Lancet Diabetes Endocrinol. 2014;2:488–499. doi: 10.1016/S2213-8587(13)70176-1. [DOI] [PubMed] [Google Scholar]
- 165.Newbern D, Freemark M. Placental hormones and the control of maternal metabolism and fetal growth. Curr Opin Endocrinol Diabetes Obes. 2011;18:409–416. doi: 10.1097/MED.0b013e32834c800d. [DOI] [PubMed] [Google Scholar]
- 166.Chen J, Tan B, Karteris E, et al. Secretion of adiponectin by human placenta: differential modulation of adiponectin and its receptors by cytokines. Diabetologia. 2006;49:1292–1302. doi: 10.1007/s00125-006-0194-7. [DOI] [PubMed] [Google Scholar]
- 167.Knox K, Leuenberger D, Penn AA, Baker JC. Global hormone profiling of murine placenta reveals Secretin expression. Placenta. 2011;32:811–816. doi: 10.1016/j.placenta.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Wiemers DO, Shao LJ, Ain R, Dai G, Soares MJ. The mouse prolactin gene family locus. Endocrinology. 2003;144:313–325. doi: 10.1210/en.2002-220724. [DOI] [PubMed] [Google Scholar]
- 169.Galosy SS, Talamantes F. Luteotropic actions of placental lactogens at midpregnancy in the mouse. Endocrinology. 1995;136:3993–4003. doi: 10.1210/endo.136.9.7649108. [DOI] [PubMed] [Google Scholar]
- 170.Chen D, Dong M, Fang Q, He J, Wang Z, Yang X. Alterations of serum resistin in normal pregnancy and pre-eclampsia. Clin Sci (Lond) 2005;108:81–84. doi: 10.1042/CS20040225. [DOI] [PubMed] [Google Scholar]
- 171.Tomimatsu T, Yamaguchi M, Murakami T, et al. Increase of mouse leptin production by adipose tissue after midpregnancy: gestational profile of serum leptin concentration. Biochem Biophys Res Commun. 1997;240:213–215. doi: 10.1006/bbrc.1997.7638. [DOI] [PubMed] [Google Scholar]
- 172.Combs TP, Berg AH, Rajala MW, et al. Sexual differentiation, pregnancy, calorie restriction, and aging affect the adipocyte-specific secretory protein adiponectin. Diabetes. 2003;52:268–276. doi: 10.2337/diabetes.52.2.268. [DOI] [PubMed] [Google Scholar]
- 173.Ling C, Kindblom J, Wennbo H, Billig H. Increased resistin expression in the adipose tissue of male prolactin transgenic mice and in male mice with elevated androgen levels. FEBS Lett. 2001;507:147–150. doi: 10.1016/s0014-5793(01)02961-1. [DOI] [PubMed] [Google Scholar]
- 174.Viengchareun S, Bouzinba-Segard H, Laigneau JP, et al. Prolactin potentiates insulin-stimulated leptin expression and release from differentiated brown adipocytes. J Mol Endocrinol. 2004;33:679–691. doi: 10.1677/jme.1.01563. [DOI] [PubMed] [Google Scholar]
- 175.Asai-Sato M, Okamoto M, Endo M, et al. Hypoadiponectinemia in lean lactating women: Prolactin inhibits adiponectin secretion from human adipocytes. Endocr J. 2006;53:555–562. doi: 10.1507/endocrj.k06-026. [DOI] [PubMed] [Google Scholar]
- 176.Tunster SJ, Creeth HD, John RM. The imprinted Phlda2 gene modulates a major endocrine compartment of the placenta to regulate placental demands for maternal resources. Dev Biol. 2016;409:251–260. doi: 10.1016/j.ydbio.2015.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Chen PY, Ganguly A, Rubbi L, et al. Intrauterine calorie restriction affects placental DNA methylation and gene expression. Physiol Genomics. 2013;45:565–576. doi: 10.1152/physiolgenomics.00034.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Postigo L, Heredia G, Illsley NP, et al. Where the O2 goes to: preservation of human fetal oxygen delivery and consumption at high altitude. J Physiol. 2009;587:693–708. doi: 10.1113/jphysiol.2008.163634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Faber JJ. Review of flow limited transfer in the placenta. Int J Obstet Anesth. 1995;4:230–237. doi: 10.1016/0959-289x(95)82916-x. [DOI] [PubMed] [Google Scholar]
- 180.Ansari T, Fenlon S, Pasha S, et al. Morphometric assessment of the oxygen diffusion conductance in placentae from pregnancies complicated by intra-uterine growth restriction. Placenta. 2003;24:618–626. doi: 10.1016/s0143-4004(03)00044-4. [DOI] [PubMed] [Google Scholar]
- 181.Mayhew TM. Scaling placental oxygen diffusion to birthweight: studies on placentae from low- and high-altitude pregnancies. J Anat. 1991;175:187–194. [PMC free article] [PubMed] [Google Scholar]
- 182.Mayhew TM, Joy CF, Haas JD. Structure-function correlation in the human placenta: the morphometric diffusing capacity for oxygen at full term. J Anat. 1984;139(Pt 4):691–708. [PMC free article] [PubMed] [Google Scholar]
- 183.Reshetnikova OS, Burton GJ, Milovanov AP. Effects of hypobaric hypoxia on the fetoplacental unit: the morphometric diffusing capacity of the villous membrane at high altitude. Am J Obstet Gynecol. 1994;171:1560–1565. doi: 10.1016/0002-9378(94)90402-2. [DOI] [PubMed] [Google Scholar]
- 184.Jauniaux E, Watson AL, Hempstock J, Bao YP, Skepper JN, Burton GJ. Onset of maternal arterial blood flow and placental oxidative stress. A possible factor in human early pregnancy failure. Am J Pathol. 2000;157:2111–2122. doi: 10.1016/S0002-9440(10)64849-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Jauniaux E, Watson A, Burton G. Evaluation of respiratory gases and acid-base gradients in human fetal fluids and uteroplacental tissue between 7 and 16 weeks' gestation. Am J Obstet Gynecol. 2001;184:998–1003. doi: 10.1067/mob.2001.111935. [DOI] [PubMed] [Google Scholar]
- 186.Rodesch F, Simon P, Donner C, Jauniaux E. Oxygen measurements in endometrial and trophoblastic tissues during early pregnancy. Obstet Gynecol. 1992;80:283–285. [PubMed] [Google Scholar]
- 187.Soothill PW, Nicolaides KH, Rodeck CH, Campbell S. Effect of gestational age on fetal and intervillous blood gas and acid-base values in human pregnancy. Fetal Ther. 1986;1:168–175. doi: 10.1159/000262264. [DOI] [PubMed] [Google Scholar]
- 188.Lackman F, Capewell V, Gagnon R, Richardson B. Fetal umbilical cord oxygen values and birth to placental weight ratio in relation to size at birth. Am J Obstet Gynecol. 2001;185:674–682. doi: 10.1067/mob.2001.116686. [DOI] [PubMed] [Google Scholar]
- 189.Kuzmina IY, Hubina-Vakulik GI, Burton GJ. Placental morphometry and Doppler flow velocimetry in cases of chronic human fetal hypoxia. Eur J Obstet Gynecol Reprod Biol. 2005;120:139–145. doi: 10.1016/j.ejogrb.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 190.Rajakumar A, Whitelock KA, Weissfeld LA, Daftary AR, Markovic N, Conrad KP. Selective overexpression of the hypoxia-inducible transcription factor, HIF-2alpha, in placentas from women with preeclampsia. Biol Reprod. 2001;64:499–506. doi: 10.1093/biolreprod/64.2.499. [DOI] [PubMed] [Google Scholar]
- 191.Rampersad R, Nelson DM. Trophoblast biology, responses to hypoxia and placental dysfunction in preeclampsia. Front Biosci. 2007;12:2447–2456. doi: 10.2741/2246. [DOI] [PubMed] [Google Scholar]
- 192.Soleymanlou N, Jurisica I, Nevo O, et al. Molecular evidence of placental hypoxia in preeclampsia. J Clin Endocrinol Metab. 2005;90:4299–4308. doi: 10.1210/jc.2005-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Tal R. The role of hypoxia and hypoxia-inducible factor-1alpha in preeclampsia pathogenesis. Biol Reprod. 2012;87:134. doi: 10.1095/biolreprod.112.102723. [DOI] [PubMed] [Google Scholar]
- 194.Yinon Y, Nevo O, Xu J, et al. Severe intrauterine growth restriction pregnancies have increased placental endoglin levels: hypoxic regulation via transforming growth factor-beta 3. Am J Pathol. 2008;172:77–85. doi: 10.2353/ajpath.2008.070640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Taricco E, Radaelli T, Rossi G, et al. Effects of gestational diabetes on fetal oxygen and glucose levels in vivo. BJOG. 2009;116:1729–1735. doi: 10.1111/j.1471-0528.2009.02341.x. [DOI] [PubMed] [Google Scholar]
- 196.Escobar J, Teramo K, Stefanovic V, et al. Amniotic fluid oxidative and nitrosative stress biomarkers correlate with fetal chronic hypoxia in diabetic pregnancies. Neonatology. 2013;103:193–198. doi: 10.1159/000345194. [DOI] [PubMed] [Google Scholar]
- 197.Hytinantti TK, Koistinen HA, Teramo K, Karonen SL, Koivisto VA, Andersson S. Increased fetal leptin in type I diabetes mellitus pregnancies complicated by chronic hypoxia. Diabetologia. 2000;43:709–713. doi: 10.1007/s001250051367. [DOI] [PubMed] [Google Scholar]
- 198.Todros T, Sciarrone A, Piccoli E, Guiot C, Kaufmann P, Kingdom J. Umbilical Doppler waveforms and placental villous angiogenesis in pregnancies complicated by fetal growth restriction. Obstet Gynecol. 1999;93:499–503. doi: 10.1016/s0029-7844(98)00440-2. [DOI] [PubMed] [Google Scholar]
- 199.Ayuk PT, Theophanous D, D'souza SW, Sibley CP, Glazier JD. L-arginine transport by the microvillous plasma membrane of the syncytiotrophoblast from human placenta in relation to nitric oxide production: effects of gestation, preeclampsia, and intrauterine growth restriction. J Clin Endocrinol Metab. 2002;87:747–751. doi: 10.1210/jcem.87.2.8204. [DOI] [PubMed] [Google Scholar]
- 200.Ferrazzi E, Rigano S, Bozzo M, et al. Umbilical vein blood flow in growth-restricted fetuses. Ultrasound Obstet Gynecol. 2000;16:432–438. doi: 10.1046/j.1469-0705.2000.00208.x. [DOI] [PubMed] [Google Scholar]
- 201.Aardema MW, Oosterhof H, Timmer A, Van Rooy I, Aarnoudse JG. Uterine artery Doppler flow and uteroplacental vascular pathology in normal pregnancies and pregnancies complicated by pre-eclampsia and small for gestational age fetuses. Placenta. 2001;22:405–411. doi: 10.1053/plac.2001.0676. [DOI] [PubMed] [Google Scholar]
- 202.Yu CK, Smith GC, Papageorghiou AT, Cacho AM, Nicolaides KH. An integrated model for the prediction of pre-eclampsia using maternal factors and uterine artery Doppler velocimetry in unselected low-risk women. Am J Obstet Gynecol. 2006;195:330. doi: 10.1016/j.ajog.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 203.Rigano S, Bozzo M, Ferrazzi E, Bellotti M, Battaglia FC, Galan HL. Early and persistent reduction in umbilical vein blood flow in the growth-restricted fetus: a longitudinal study. Am J Obstet Gynecol. 2001;185:834–838. doi: 10.1067/mob.2001.117356. [DOI] [PubMed] [Google Scholar]
- 204.Rigano S, Bozzo M, Padoan A, et al. Small size-specific umbilical vein diameter in severe growth restricted fetuses that die in utero. Prenat Diagn. 2008;28:908–913. doi: 10.1002/pd.2054. [DOI] [PubMed] [Google Scholar]
- 205.Bellotti M, Pennati G, De Gasperi C, Bozzo M, Battaglia FC, Ferrazzi E. Simultaneous measurements of umbilical venous, fetal hepatic, and ductus venosus blood flow in growth-restricted human fetuses. Am J Obstet Gynecol. 2004;190:1347–1358. doi: 10.1016/j.ajog.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 206.Widness JA, Susa JB, Garcia JF, et al. Increased erythropoiesis and elevated erythropoietin in infants born to diabetic mothers and in hyperinsulinemic rhesus fetuses. J Clin Invest. 1981;67:637–642. doi: 10.1172/JCI110078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Zamudio S, Wu Y, Ietta F, et al. Human placental hypoxia-inducible factor-1alpha expression correlates with clinical outcomes in chronic hypoxia in vivo. Am J Pathol. 2007;170:2171–2179. doi: 10.2353/ajpath.2007.061185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Nevo O, Soleymanlou N, Wu Y, et al. Increased expression of sFlt-1 in in vivo and in vitro models of human placental hypoxia is mediated by HIF-1. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1085–R1093. doi: 10.1152/ajpregu.00794.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Rajakumar A, Doty K, Daftary A, Harger G, Conrad KP. Impaired oxygen-dependent reduction of HIF-1alpha and -2alpha proteins in pre-eclamptic placentae. Placenta. 2003;24:199–208. doi: 10.1053/plac.2002.0893. [DOI] [PubMed] [Google Scholar]
- 210.Rajakumar A, Brandon HM, Daftary A, Ness R, Conrad KP. Evidence for the functional activity of hypoxia-inducible transcription factors overexpressed in preeclamptic placentae. Placenta. 2004;25:763–769. doi: 10.1016/j.placenta.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 211.Eltzschig HK, Carmeliet P. Hypoxia and inflammation. N Engl J Med. 2011;364:656–665. doi: 10.1056/NEJMra0910283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Combined Antioxidant and Preeclampsia Prediction Studies (CAPPS) [Accessed in 2015]; Available at https://clinicaltrials.gov/ct2/show/NCT00135707?term=NCT00135707&rank=1.
- 213.Effect of Low Dose Aspirin on Birthweight in Twins: The GAP Trial. [Accessed in 2015]; Available at https://clinicaltrials.gov/ct2/show/NCT02280031?term=NCT02280031&rank=1.
- 214.Pravastatin for Prevention of Preeclampsia. [Accessed in 2015]; Available at https://clinicaltrials.gov/ct2/show/NCT01717586?term=NCT01717586&rank=1. [Google Scholar]
- 215.RCT of Antioxidant Therapy to Prevent Preeclampsia in Brazil. [Accessed in 2015]; Available at https://clinicaltrials.gov/ct2/show/NCT00097110?term=NCT00097110&rank=1.
- 216.Caniggia I, Winter J, Lye SJ, Post M. Oxygen and placental development during the first trimester: implications for the pathophysiology of pre-eclampsia. Placenta. 2000;21(Suppl A):S25–S30. doi: 10.1053/plac.1999.0522. [DOI] [PubMed] [Google Scholar]
- 217.Rajakumar A, Jeyabalan A, Markovic N, Ness R, Gilmour C, Conrad KP. Placental HIF-1 alpha, HIF-2 alpha, membrane and soluble VEGF receptor-1 proteins are not increased in normotensive pregnancies complicated by late-onset intrauterine growth restriction. Am J Physiol Regul Integr Comp Physiol. 2007;293:R766–R774. doi: 10.1152/ajpregu.00097.2007. [DOI] [PubMed] [Google Scholar]
- 218.Kingdom JC, Kaufmann P. Oxygen and placental vascular development. Adv Exp Med Biol. 1999;474:259–275. doi: 10.1007/978-1-4615-4711-2_20. [DOI] [PubMed] [Google Scholar]
- 219.Kingdom JC, Kaufmann P. Oxygen and placental villous development: origins of fetal hypoxia. Placenta. 1997;18:613–621. doi: 10.1016/s0143-4004(97)90000-x. discussion 23-6. [DOI] [PubMed] [Google Scholar]
- 220.Unger C, Weiser JK, McCullough RE, Keefer S, Moore LG. Altitude, low birth weight, and infant mortality in Colorado. JAMA. 1988;259:3427–3432. [PubMed] [Google Scholar]
- 221.Krampl E, Lees C, Bland JM, Espinoza Dorado J, Moscoso G, Campbell S. Fetal biometry at 4300 m compared to sea level in Peru. Ultrasound Obstet Gynecol. 2000;16:9–18. doi: 10.1046/j.1469-0705.2000.00156.x. [DOI] [PubMed] [Google Scholar]
- 222.Zamudio S. The placenta at high altitude. High Alt Med Biol. 2003;4:171–191. doi: 10.1089/152702903322022785. [DOI] [PubMed] [Google Scholar]
- 223.Espinoza J, Sebire NJ, Mcauliffe F, Krampl E, Nicolaides KH. Placental villus morphology in relation to maternal hypoxia at high altitude. Placenta. 2001;22:606–608. doi: 10.1053/plac.2001.0696. [DOI] [PubMed] [Google Scholar]
- 224.Mayhew TM. Thinning of the intervascular tissue layers of the human placenta is an adaptive response to passive diffusion in vivo and may help to predict the origins of fetal hypoxia. Eur J Obstet Gynecol Reprod Biol. 1998;81:101–109. doi: 10.1016/s0301-2115(98)00154-7. [DOI] [PubMed] [Google Scholar]
- 225.Mayhew TM, Charnock-Jones DS, Kaufmann P. Aspects of human fetoplacental vasculogenesis and angiogenesis. III. Changes in complicated pregnancies. Placenta. 2004;25:127–139. doi: 10.1016/j.placenta.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 226.Illsley NP, Caniggia I, Zamudio S. Placental metabolic reprogramming: do changes in the mix of energy-generating substrates modulate fetal growth? Int J Dev Biol. 2010;54:409–419. doi: 10.1387/ijdb.082798ni. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Zamudio S, Torricos T, Fik E, et al. Hypoglycemia and the origin of hypoxia-induced reduction in human fetal growth. PLoS One. 2010;5:e8551. doi: 10.1371/journal.pone.0008551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Lu H, Forbes RA, Verma A. Hypoxia-inducible factor 1 activation by aerobic glycolysis implicates the Warburg effect in carcinogenesis. J Biol Chem. 2002;277:23111–23115. doi: 10.1074/jbc.M202487200. [DOI] [PubMed] [Google Scholar]
- 229.Semenza GL. Oxygen-dependent regulation of mitochondrial respiration by hypoxia-inducible factor 1. Biochem J. 2007;405:1–9. doi: 10.1042/BJ20070389. [DOI] [PubMed] [Google Scholar]
- 230.Lisy K, Peet DJ. Turn me on: regulating HIF transcriptional activity. Cell Death Differ. 2008;15:642–649. doi: 10.1038/sj.cdd.4402315. [DOI] [PubMed] [Google Scholar]
- 231.Aragones J, Fraisl P, Baes M, Carmeliet P. Oxygen sensors at the crossroad of metabolism. Cell Metab. 2009;9:11–22. doi: 10.1016/j.cmet.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 232.Wilber RL, Stray-Gundersen J, Levine BD. Effect of hypoxic "dose" on physiological responses and sea-level performance. Med Sci Sports Exerc. 2007;39:1590–1599. doi: 10.1249/mss.0b013e3180de49bd. [DOI] [PubMed] [Google Scholar]
- 233.Sifakis S, Angelakis E, Vardaki E, Koumantaki Y, Matalliotakis I, Koumantakis E. Erythropoietin in the treatment of iron deficiency anemia during pregnancy. Gynecol Obstet Invest. 2001;51:150–156. doi: 10.1159/000052914. [DOI] [PubMed] [Google Scholar]
- 234.Zamudio S, Postigo L, Illsley NP, et al. Maternal oxygen delivery is not related to altitude- and ancestry-associated differences in human fetal growth. J Physiol. 2007;582:883–895. doi: 10.1113/jphysiol.2007.130708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Hudetz AG, Wood JD, Biswal BB, Krolo I, Kampine JP. Effect of hemodilution on RBC velocity, supply rate, and hematocrit in the cerebral capillary network. Journal of applied physiology (Bethesda, Md : 1985) 1999;87:505–509. doi: 10.1152/jappl.1999.87.2.505. [DOI] [PubMed] [Google Scholar]
- 236.Say L, Gulmezoglu AM, Hofmeyr GJ. Maternal oxygen administration for suspected impaired fetal growth. Cochrane Database Syst Rev. 2003:CD000137. doi: 10.1002/14651858.CD000137. [DOI] [PubMed] [Google Scholar]
- 237.Zamudio S. High-altitude hypoxia and preeclampsia. Front Biosci. 2007;12:2967–2977. doi: 10.2741/2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Galan HL, Ferrazzi E, Hobbins JC. Intrauterine growth restriction (IUGR): biometric and Doppler assessment. Prenat Diagn. 2002;22:331–337. doi: 10.1002/pd.311. [DOI] [PubMed] [Google Scholar]
- 239.Giles WB, Trudinger BJ, Baird PJ. Fetal umbilical artery flow velocity waveforms and placental resistance: pathological correlation. Br J Obstet Gynaecol. 1985;92:31–38. doi: 10.1111/j.1471-0528.1985.tb01045.x. [DOI] [PubMed] [Google Scholar]
- 240.Macara L, Kingdom JC, Kohnen G, Bowman AW, Greer IA, Kaufmann P. Elaboration of stem villous vessels in growth restricted pregnancies with abnormal umbilical artery Doppler waveforms. Br J Obstet Gynaecol. 1995;102:807–812. doi: 10.1111/j.1471-0528.1995.tb10847.x. [DOI] [PubMed] [Google Scholar]
- 241.Kreczy A, Fusi L, Wigglesworth JS. Correlation between umbilical arterial flow and placental morphology. Int J Gynecol Pathol. 1995;14:306–309. doi: 10.1097/00004347-199510000-00004. [DOI] [PubMed] [Google Scholar]
- 242.Palmer SK, Zamudio S, Coffin C, Parker S, Stamm E, Moore LG. Quantitative estimation of human uterine artery blood flow and pelvic blood flow redistribution in pregnancy. Obstet Gynecol. 1992;80:1000–1006. [PubMed] [Google Scholar]
- 243.Mandala M, Osol G. Physiological remodelling of the maternal uterine circulation during pregnancy. Basic Clin Pharmacol Toxicol. 2012;110:12–18. doi: 10.1111/j.1742-7843.2011.00793.x. [DOI] [PubMed] [Google Scholar]
- 244.Osol G, Mandala M. Maternal uterine vascular remodeling during pregnancy. Physiology (Bethesda) 2009;24:58–71. doi: 10.1152/physiol.00033.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Osol G, Cipolla M. Interaction of myogenic and adrenergic mechanisms in isolated, pressurized uterine radial arteries from late-pregnant and nonpregnant rats. Am J Obstet Gynecol. 1993;168:697–705. doi: 10.1016/0002-9378(93)90519-o. [DOI] [PubMed] [Google Scholar]
- 246.Senadheera S, Bertrand PP, Grayson TH, Leader L, Murphy TV, Sandow SL. Pregnancy-induced remodelling and enhanced endothelium-derived hyperpolarization-type vasodilator activity in rat uterine radial artery: transient receptor potential vanilloid type 4 channels, caveolae and myoendothelial gap junctions. J Anat. 2013;223:677–686. doi: 10.1111/joa.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Luksha L, Nisell H, Luksha N, Kublickas M, Hultenby K, Kublickiene K. Endothelium-derived hyperpolarizing factor in preeclampsia: heterogeneous contribution, mechanisms, and morphological prerequisites. Am J Physiol Regul Integr Comp Physiol. 2008;294:R510–R519. doi: 10.1152/ajpregu.00458.2007. [DOI] [PubMed] [Google Scholar]
- 248.Boyd PA, Scott A. Quantitative structural studies on human placentas associated with preeclampsia, essential hypertension and intrauterine growth retardation. Br J Obstet Gynaecol. 1985;92:714–721. doi: 10.1111/j.1471-0528.1985.tb01454.x. [DOI] [PubMed] [Google Scholar]
- 249.Teasdale F. Histomorphometry of the human placenta in maternal preeclampsia. Am J Obstet Gynecol. 1985;152:25–31. doi: 10.1016/s0002-9378(85)80170-8. [DOI] [PubMed] [Google Scholar]
- 250.Geva E, Ginzinger DG, Zaloudek CJ, Moore DH, Byrne A, Jaffe RB. Human placental vascular development: vasculogenic and angiogenic (branching and nonbranching) transformation is regulated by vascular endothelial growth factor-A, angiopoietin-1, and angiopoietin-2. J Clin Endocrinol Metab. 2002;87:4213–4224. doi: 10.1210/jc.2002-020195. [DOI] [PubMed] [Google Scholar]
- 251.Ratnikov B, Aza-Blanc P, Ronai ZA, Smith JW, Osterman AL, Scott DA. Glutamate and asparagine cataplerosis underlie glutamine addiction in melanoma. Oncotarget. 2015;6:7379–7389. doi: 10.18632/oncotarget.3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Wise DR, Thompson CB. Glutamine addiction: a new therapeutic target in cancer. Trends Biochem Sci. 2010;35:427–433. doi: 10.1016/j.tibs.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Medvetz D, Priolo C, Henske EP. Therapeutic targeting of cellular metabolism in cells with hyperactive mTORC1: a paradigm shift. Mol Cancer Res. 2015;13:3–8. doi: 10.1158/1541-7786.MCR-14-0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Vivanco I. Targeting molecular addictions in cancer. Br J Cancer. 2014;111:2033–2038. doi: 10.1038/bjc.2014.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Legband ND, Feshitan JA, Borden MA, Terry BS. Evaluation of peritoneal microbubble oxygenation therapy in a rabbit model of hypoxemia. IEEE Trans Biomed Eng. 2015;62:1376–1382. doi: 10.1109/TBME.2015.2388611. [DOI] [PubMed] [Google Scholar]
- 256.Matsuki N, Ishikawa T, Ichiba S, Shiba N, Ujike Y, Yamaguchi T. Oxygen supersaturated fluid using fine micro/nanobubbles. Int J Nanomedicine. 2014;9:4495–4505. doi: 10.2147/IJN.S68840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Matsuki N, Ichiba S, Ishikawa T, et al. Blood oxygenation using microbubble suspensions. Eur Biophys J. 2012;41:571–578. doi: 10.1007/s00249-012-0811-y. [DOI] [PubMed] [Google Scholar]
- 258.Bisazza A, Giustetto P, Rolfo A, et al. Microbubble-mediated oxygen delivery to hypoxic tissues as a new therapeutic device. Conf Proc IEEE Eng Med Biol Soc. 2008;2008:2067–2070. doi: 10.1109/IEMBS.2008.4649599. [DOI] [PubMed] [Google Scholar]
- 259.Hammond S, Mathewson AM, Baker PN, Mayhew TM, Dunn WR. Gap junctions and hydrogen peroxide are involved in endothelium-derived hyperpolarising responses to bradykinin in omental arteries and veins isolated from pregnant women. Eur J Pharmacol. 2011;668:225–232. doi: 10.1016/j.ejphar.2011.06.050. [DOI] [PubMed] [Google Scholar]
- 260.Vodstrcil LA, Tare M, Novak J, et al. Relaxin mediates uterine artery compliance during pregnancy and increases uterine blood flow. FASEB J. 2012;26:4035–4044. doi: 10.1096/fj.12-210567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Morton JS, Davidge ST. Arterial endothelium-derived hyperpolarization: potential role in pregnancy adaptations and complications. J Cardiovasc Pharmacol. 2013;61:197–203. doi: 10.1097/FJC.0b013e31827b6367. [DOI] [PubMed] [Google Scholar]
- 262.Vadillo-Ortega F, Perichart-Perera O, Espino S, et al. Effect of supplementation during pregnancy with L-arginine and antioxidant vitamins in medical food on pre-eclampsia in high risk population: randomised controlled trial. BMJ. 2011;342:d2901. doi: 10.1136/bmj.d2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Staff AC, Berge L, Haugen G, Lorentzen B, Mikkelsen B, Henriksen T. Dietary supplementation with L-arginine or placebo in women with pre-eclampsia. Acta Obstet Gynecol Scand. 2004;83:103–107. [PubMed] [Google Scholar]
- 264.Ganzevoort W, Alfirevic Z, Von Dadelszen P, et al. STRIDER: Sildenafil Therapy In Dismal prognosis Early-onset intrauterine growth Restriction--a protocol for a systematic review with individual participant data and aggregate data meta-analysis and trial sequential analysis. Syst Rev. 2014;3:23. doi: 10.1186/2046-4053-3-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Von Dadelszen P, Dwinnell S, Magee LA, et al. Sildenafil citrate therapy for severe early-onset intrauterine growth restriction. BJOG. 2011;118:624–628. doi: 10.1111/j.1471-0528.2010.02879.x. [DOI] [PubMed] [Google Scholar]
- 266.David A. A 6 Year Prospective Study to Define the Clinical and Biological Characteristic of Pregnancies Affected by Severe Early Onset Fetal Growth Restriction. London: 2015. EVERREST - Developing a Therapy for Fetal Growth Restriction. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.David AL, Torondel B, Zachary I, et al. Local delivery of VEGF adenovirus to the uterine artery increases vasorelaxation and uterine blood flow in the pregnant sheep. Gene Ther. 2008;15:1344–1350. doi: 10.1038/gt.2008.102. [DOI] [PubMed] [Google Scholar]
- 268.Hanauske-Abel HM, Popowicz AM. The HAG mechanism: a molecular rationale for the therapeutic application of iron chelators in human diseases involving the 2-oxoacid utilizing dioxygenases. Curr Med Chem. 2003;10:1005–1019. doi: 10.2174/0929867033457601. [DOI] [PubMed] [Google Scholar]
- 269.Hoque M, Hanauske-Abel HM, Palumbo P, et al. Inhibition of HIV-1 gene expression by Ciclopirox and Deferiprone, drugs that prevent hypusination of eukaryotic initiation factor 5A. Retrovirology. 2009;6:90. doi: 10.1186/1742-4690-6-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Zhang EG, Burton GJ, Smith SK, Charnock-Jones DS. Placental vessel adaptation during gestation and to high altitude: changes in diameter and perivascular cell coverage. Placenta. 2002;23:751–762. doi: 10.1016/s0143-4004(02)90856-8. [DOI] [PubMed] [Google Scholar]
- 271.Charnock-Jones DS. Soluble flt-1 and the angiopoietins in the development and regulation of placental vasculature. J Anat. 2002;200:607–615. doi: 10.1046/j.1469-7580.2002.00063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Shih SC, Ju M, Liu N, Mo JR, Ney JJ, Smith LE. Transforming growth factor beta1 induction of vascular endothelial growth factor receptor 1: mechanism of pericyte-induced vascular survival in vivo. Proc Natl Acad Sci U S A. 2003;100:15859–15864. doi: 10.1073/pnas.2136855100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Zamudio S, Baumann MU, Illsley NP. Effects of chronic hypoxia in vivo on the expression of human placental glucose transporters. Placenta. 2006;27:49–55. doi: 10.1016/j.placenta.2004.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Gaither K, Quraishi AN, Illsley NP. Diabetes alters the expression and activity of the human placental GLUT1 glucose transporter. J Clin Endocrinol Metab. 1999;84:695–701. doi: 10.1210/jcem.84.2.5438. [DOI] [PubMed] [Google Scholar]
- 275.Glazier JD, Cetin I, Perugino G, et al. Association between the activity of the system A amino acid transporter in the microvillous plasma membrane of the human placenta and severity of fetal compromise in intrauterine growth restriction. Pediatr Res. 1997;42:514–519. doi: 10.1203/00006450-199710000-00016. [DOI] [PubMed] [Google Scholar]
- 276.Jansson T, Ekstrand Y, Bjorn C, Wennergren M, Powell TL. Alterations in the activity of placental amino acid transporters in pregnancies complicated by diabetes. Diabetes. 2002;51:2214–2219. doi: 10.2337/diabetes.51.7.2214. [DOI] [PubMed] [Google Scholar]
- 277.Harding JE, Owens JA, Robinson JS. Should we try to supplement the growth retarded fetus? A cautionary tale. Br J Obstet Gynaecol. 1992;99:707–709. doi: 10.1111/j.1471-0528.1992.tb13866.x. [DOI] [PubMed] [Google Scholar]
- 278.Zamudio S, Torricos T, Fik E, et al. Hypoglycemia and the origin of hypoxia-induced reduction in human fetal growth. PLoS One. 2010;5:e8551. doi: 10.1371/journal.pone.0008551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Jansson T, Wennergren M, Illsley NP. Glucose transporter protein expression in human placenta throughout gestation and in intrauterine growth retardation. J Clin Endocrinol Metab. 1993;77:1554–1562. doi: 10.1210/jcem.77.6.8263141. [DOI] [PubMed] [Google Scholar]
- 280.Brown K, Heller DS, Zamudio S, Illsley NP. Glucose transporter 3 (GLUT3) protein expression in human placenta across gestation. Placenta. 2011;32:1041–1049. doi: 10.1016/j.placenta.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Cleal JK, Lewis RM. The mechanisms and regulation of placental amino acid transport to the human foetus. J Neuroendocrinol. 2008;20:419–426. doi: 10.1111/j.1365-2826.2008.01662.x. [DOI] [PubMed] [Google Scholar]
- 282.Baumann Mu, Schneider H, Malek A, et al. Regulation of human trophoblast GLUT1 glucose transporter by insulin-like growth factor I (IGF-I) PLoS One. 2014;9:e106037. doi: 10.1371/journal.pone.0106037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Roos S, Lagerlof O, Wennergren M, Powell TL, Jansson T. Regulation of amino acid transporters by glucose and growth factors in cultured primary human trophoblast cells is mediated by mTOR signaling. Am J Physiol Cell Physiol. 2009;297:C723–C731. doi: 10.1152/ajpcell.00191.2009. [DOI] [PubMed] [Google Scholar]
- 284.Giudice LC, Martina NA, Crystal RA, Tazuke S, Druzin M. Insulin-like growth factor binding protein-1 at the maternal-fetal interface and insulin-like growth factor-I, insulin-like growth factor-II, and insulin-like growth factor binding protein-1 in the circulation of women with severe preeclampsia. Am J Obstet Gynecol. 1997;176:751–757. doi: 10.1016/s0002-9378(97)70598-2. discussion 57–8. [DOI] [PubMed] [Google Scholar]
- 285.Sferruzzi-Perri AN, Owens JA, Pringle KG, Roberts CT. The neglected role of insulin-like growth factors in the maternal circulation regulating fetal growth. J Physiol. 2011;589:7–20. doi: 10.1113/jphysiol.2010.198622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Jones HN, Crombleholme T, Habli M. Adenoviral-mediated placental gene transfer of IGF-1 corrects placental insufficiency via enhanced placental glucose transport mechanisms. PLoS One. 2013;8:e74632. doi: 10.1371/journal.pone.0074632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Jones H, Crombleholme T, Habli M. Regulation of amino acid transporters by adenoviral-mediated human insulin-like growth factor-1 in a mouse model of placental insufficiency in vivo and the human trophoblast line BeWo in vitro. Placenta. 2014;35:132–138. doi: 10.1016/j.placenta.2013.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Jansson T, Aye IL, Goberdhan DC. The emerging role of mTORC1 signaling in placental nutrient-sensing. Placenta. 2012;33(Suppl 2):e23–e29. doi: 10.1016/j.placenta.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Jansson N, Rosario FJ, Gaccioli F, et al. Activation of placental mTOR signaling and amino acid transporters in obese women giving birth to large babies. J Clin Endocrinol Metab. 2013;98:105–113. doi: 10.1210/jc.2012-2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Aiko Y, Askew DJ, Aramaki S, et al. Differential levels of amino acid transporters System L and ASCT2, and the mTOR protein in placenta of preeclampsia and IUGR. BMC pregnancy and childbirth. 2014;14:181. doi: 10.1186/1471-2393-14-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Roos S, Jansson N, Palmberg I, Saljo K, Powell TL, Jansson T. Mammalian target of rapamycin in the human placenta regulates leucine transport and is down-regulated in restricted fetal growth. J Physiol. 2007;582:449–459. doi: 10.1113/jphysiol.2007.129676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Choi YJ, Park YJ, Park JY, et al. Inhibitory effect of mTOR activator MHY1485 on autophagy: suppression of lysosomal fusion. PLoS One. 2012;7:e43418. doi: 10.1371/journal.pone.0043418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Peng N, Meng N, Wang S, et al. An activator of mTOR inhibits oxLDL-induced autophagy and apoptosis in vascular endothelial cells and restricts atherosclerosis in apolipoprotein E(−)/(−) mice. Sci Rep. 2014;4:5519. doi: 10.1038/srep05519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Rosario FJ, Kanai Y, Powell TL, Jansson T. Mammalian target of rapamycin signalling modulates amino acid uptake by regulating transporter cell surface abundance in primary human trophoblast cells. J Physiol. 2013;591:609–625. doi: 10.1113/jphysiol.2012.238014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Andrzejewski S, Gravel SP, Pollak M, St-Pierre J. Metformin directly acts on mitochondria to alter cellular bioenergetics. Cancer Metab. 2014;2:12. doi: 10.1186/2049-3002-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Illsley NP, Caniggia I, Zamudio S. Placental metabolic reprogramming: do changes in the mix of energy-generating substrates modulate fetal growth? Int J Dev Biol. 2010;54:409–419. doi: 10.1387/ijdb.082798ni. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Myatt L, Cui X. Oxidative stress in the placenta. Histochem Cell Biol. 2004;122:369–382. doi: 10.1007/s00418-004-0677-x. [DOI] [PubMed] [Google Scholar]
- 299.Menon R. Oxidative stress damage as a detrimental factor in preterm birth pathology. Front Immunol. 2014;5:567. doi: 10.3389/fimmu.2014.00567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Abuja PM, Albertini R. Methods for monitoring oxidative stress, lipid peroxidation and oxidation resistance of lipoproteins. Clin Chim Acta. 2001;306:1–17. doi: 10.1016/s0009-8981(01)00393-x. [DOI] [PubMed] [Google Scholar]
- 301.Roberts VH, Smith J, Mclea SA, Heizer AB, Richardson JL, Myatt L. Effect of increasing maternal body mass index on oxidative and nitrative stress in the human placenta. Placenta. 2009;30:169–175. doi: 10.1016/j.placenta.2008.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Macmillan-Crow LA, Crow JP, Kerby JD, Beckman JS, Thompson JA. Nitration and inactivation of manganese superoxide dismutase in chronic rejection of human renal allografts. Proc Natl Acad Sci U S A. 1996;93:11853–11858. doi: 10.1073/pnas.93.21.11853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Ischiropoulos H. Biological tyrosine nitration: a pathophysiological function of nitric oxide and reactive oxygen species. Arch Biochem Biophys. 1998;356:1–11. doi: 10.1006/abbi.1998.0755. [DOI] [PubMed] [Google Scholar]
- 304.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiological reviews. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Myatt L. Review: Reactive oxygen and nitrogen species and functional adaptation of the placenta. Placenta. 2010;31(Suppl):S66–S69. doi: 10.1016/j.placenta.2009.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Sen CK, Packer L. Antioxidant and redox regulation of gene transcription. FASEB J. 1996;10:709–720. doi: 10.1096/fasebj.10.7.8635688. [DOI] [PubMed] [Google Scholar]
- 307.Halliwell B, Whiteman M. Measuring reactive species and oxidative damage in vivo and in cell culture: how should you do it and what do the results mean? Br J Pharmacol. 2004;142:231–255. doi: 10.1038/sj.bjp.0705776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Muralimanoharan S, Guo C, Myatt L, Maloyan A. Sexual dimorphism in miR-210 expression and mitochondrial dysfunction in the placenta with maternal obesity. Int J Obes (Lond) 2015 doi: 10.1038/ijo.2015.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Roberts JM, Myatt L, Spong CY, et al. Vitamins C and E to prevent complications of pregnancy-associated hypertension. N Engl J Med. 2010;362:1282–1291. doi: 10.1056/NEJMoa0908056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Kelso GF, Porteous CM, Coulter CV, et al. Selective targeting of a redox-active ubiquinone to mitochondria within cells: antioxidant and antiapoptotic properties. J Biol Chem. 2001;276:4588–4596. doi: 10.1074/jbc.M009093200. [DOI] [PubMed] [Google Scholar]
- 311.Khera A, Vanderlelie JJ, Perkins AV. Selenium supplementation protects trophoblast cells from mitochondrial oxidative stress. Placenta. 2013;34:594–598. doi: 10.1016/j.placenta.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 312.Reiter RJ, Tan DX, Mayo JC, Sainz RM, Leon J, Czarnocki Z. Melatonin as an antioxidant: biochemical mechanisms and pathophysiological implications in humans. Acta Biochim Pol. 2003;50:1129–1146. [PubMed] [Google Scholar]
- 313.Muralimanoharan S, Maloyan A, Mele J, Guo C, Myatt LG, Myatt L. MIR-210 modulates mitochondrial respiration in placenta with preeclampsia. Placenta. 2012;33:816–823. doi: 10.1016/j.placenta.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Vatten LJ, Skjaerven R. Offspring sex and pregnancy outcome by length of gestation. Early Hum Dev. 2004;76:47–54. doi: 10.1016/j.earlhumdev.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 315.Clifton VL. Review: Sex and the human placenta: mediating differential strategies of fetal growth and survival. Placenta. 2010;31(Suppl):S33–S39. doi: 10.1016/j.placenta.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 316.Sood R, Zehnder JL, Druzin ML, Brown PO. Gene expression patterns in human placenta. Proc Natl Acad Sci U S A. 2006;103:5478–5483. doi: 10.1073/pnas.0508035103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Muralimanoharan S, Maloyan A, Myatt L. Evidence of sexual dimorphism in the placental function with severe preeclampsia. Placenta. 2013;34:1183–1189. doi: 10.1016/j.placenta.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Arrowsmith J, Miller P. Trial watch: phase II and phase III attrition rates 2011–2012. Nat Rev Drug Discov. 2013;12:569. doi: 10.1038/nrd4090. [DOI] [PubMed] [Google Scholar]
- 319.Mullard A. Drug repurposing programmes get lift off. Nat Rev Drug Discov. 2012;11:505–506. doi: 10.1038/nrd3776. [DOI] [PubMed] [Google Scholar]
- 320.The National Center for Advancing Translational Sciences (NCATS) [Accessed in 2015]; Available at http://www.ncats.nih.gov/ntu.
- 321.Reviewer Guidance: Evaluating the Risks of Drug Exposure in Human Pregnancies. [Accessed in 2015]; Available at http://www.fda.gov/downloads/ScienceResearch/SpecialTopics/WomensHealthResearch/UCM133359.pdf.
- 322.Shineman DW, Alam J, Anderson M, et al. Overcoming obstacles to repurposing for neurodegenerative disease. Ann Clin Transl Neurol. 2014;1:512–518. doi: 10.1002/acn3.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Mitchell AA, Gilboa SM, Werler MM, Kelley KE, Louik C, Hernandez-Diaz S. Medication use during pregnancy, with particular focus on prescription drugs: 1976–2008. Am J Obstet Gynecol. 2011;205:51 e1–51 e8. doi: 10.1016/j.ajog.2011.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Mitchell AA, Hernandez-Diaz S, Louik C, Werler MM. Medication Use in pregnancy. Pharmacoepidemiol Drug Saf. 2001;10 [Google Scholar]
- 325.Andrade SE, Raebel MA, Morse AN, et al. Use of prescription medications with a potential for fetal harm among pregnant women. Pharmacoepidemiol Drug Saf. 2006;15:546–554. doi: 10.1002/pds.1235. [DOI] [PubMed] [Google Scholar]
- 326.Andrade SE, Gurwitz JH, Davis RL, et al. Prescription drug use in pregnancy. Am J Obstet Gynecol. 2004;191:398–407. doi: 10.1016/j.ajog.2004.04.025. [DOI] [PubMed] [Google Scholar]
- 327.Andrade SE, Raebel MA, Brown J, et al. Use of antidepressant medications during pregnancy: a multisite study. Am J Obstet Gynecol. 2008;198:194 e1–194 e5. doi: 10.1016/j.ajog.2007.07.036. [DOI] [PubMed] [Google Scholar]
- 328.Anderson GD. Pregnancy-induced changes in pharmacokinetics: a mechanistic-based approach. Clin Pharmacokinet. 2005;44:989–1008. doi: 10.2165/00003088-200544100-00001. [DOI] [PubMed] [Google Scholar]
- 329.Anger GJ, Piquette-Miller M. Pharmacokinetic studies in pregnant women. Clin Pharmacol Ther. 2008;83:184–187. doi: 10.1038/sj.clpt.6100377. [DOI] [PubMed] [Google Scholar]
- 330.Costantine MM. Physiologic and pharmacokinetic changes in pregnancy. Front Pharmacol. 2014;5:65. doi: 10.3389/fphar.2014.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Briggs G, Bpharm, FCCP, Freeman R, MD, Yaffe S., MD . Drugs in Pregnancy and Lactation: A Reference Guide to Fetal and Neonatal Risk. Philadelphia: Lippincott, Williams and Wilkins; [Google Scholar]
- 332.Pacheco LD, MM C, Hankins GD. Physiologic Changes during Pregnancy. In: Mattison DR, editor. Clinical Pharmacology During Pregnancy. London: Elsevier; 2013. [Google Scholar]
- 333.Robson SC, Hunter S, Boys RJ, Dunlop W. Serial study of factors influencing changes in cardiac output during human pregnancy. Am J Physiol. 1989;256:H1060–H1065. doi: 10.1152/ajpheart.1989.256.4.H1060. [DOI] [PubMed] [Google Scholar]
- 334.Clark SL, Cotton DB, Lee W, et al. Central hemodynamic assessment of normal term pregnancy. Am J Obstet Gynecol. 1989;161:1439–1442. doi: 10.1016/0002-9378(89)90900-9. [DOI] [PubMed] [Google Scholar]
- 335.Hytten FE, Paintin DB. Increase in plasma volume during normal pregnancy. J Obstet Gynaecol Br Emp. 1963;70:402–407. doi: 10.1111/j.1471-0528.1963.tb04922.x. [DOI] [PubMed] [Google Scholar]
- 336.Frederiksen MC. Physiologic changes in pregnancy and their effect on drug disposition. Semin Perinatol. 2001;25:120–123. doi: 10.1053/sper.2001.24565. [DOI] [PubMed] [Google Scholar]
- 337.Elkus R, Popovich J., JR Respiratory physiology in pregnancy. Clin Chest Med. 1992;13:555–565. [PubMed] [Google Scholar]
- 338.Parry E, Shields R, Turnbull AC. Transit time in the small intestine in pregnancy. J Obstet Gynaecol Br Commonw. 1970;77:900–901. doi: 10.1111/j.1471-0528.1970.tb03423.x. [DOI] [PubMed] [Google Scholar]
- 339.Evans WE, Relling MV. Pharmacogenomics: translating functional genomics into rational therapeutics. Science. 1999;286:487–491. doi: 10.1126/science.286.5439.487. [DOI] [PubMed] [Google Scholar]
- 340.Hellden A, Madadi P. Pregnancy and pharmacogenomics in the context of drug metabolism and response. Pharmacogenomics. 2013;14:1779–1791. doi: 10.2217/pgs.13.176. [DOI] [PubMed] [Google Scholar]
- 341.Ke Ab, Rostami-Hodjegan A, Zhao P, Unadkat JD. Pharmacometrics in pregnancy: An unmet need. Annu Rev Pharmacol Toxicol. 2014;54:53–69. doi: 10.1146/annurev-pharmtox-011613-140009. [DOI] [PubMed] [Google Scholar]
- 342.Weier N, He SM, Li XT, Wang LL, Zhou SF. Placental drug disposition and its clinical implications. Curr Drug Metab. 2008;9:106–121. doi: 10.2174/138920008783571828. [DOI] [PubMed] [Google Scholar]
- 343.Koren G. Sex dependent pharmacokinetics and bioequivalence--time for a change. J Popul Ther Clin Pharmacol. 2013;20:e358–e361. [PubMed] [Google Scholar]
- 344.Shields KE, Lyerly AD. Exclusion of pregnant women from industry-sponsored clinical trials. Obstet Gynecol. 2013;122:1077–1081. doi: 10.1097/AOG.0b013e3182a9ca67. [DOI] [PubMed] [Google Scholar]
- 345.Schonfeld T, Schmid KK, Brown JS, Amoura NJ, Gordon B. A pregnancy testing policy for women enrolled in clinical trials. IRB. 2013;35:9–15. [PubMed] [Google Scholar]
- 346.Mccormack SA, Best BM. Obstetric Pharmacokinetic Dosing Studies are Urgently Needed. Front Pediatr. 2014;2:9. doi: 10.3389/fped.2014.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Meslin EM, Blasimme A, Cambon-Thomsen A. Mapping the translational science policy 'valley of death'. Clin Transl Med. 2013;2:14. doi: 10.1186/2001-1326-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Morris ZS, Wooding S, Grant J. The answer is 17 years, what is the question: understanding time lags in translational research. J R Soc Med. 2011;104:510–520. doi: 10.1258/jrsm.2011.110180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Lenfant C. Shattuck lecture--clinical research to clinical practice--lost in translation? N Engl J Med. 2003;349:868–874. doi: 10.1056/NEJMsa035507. [DOI] [PubMed] [Google Scholar]
- 350.Grimshaw JM, Eccles MP, Lavis JN, Hill SJ, Squires JE. Knowledge translation of research findings. Implement Sci. 2012;7:50. doi: 10.1186/1748-5908-7-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.McGlynn EA, Asch SM, Adams J, et al. The quality of health care delivered to adults in the United States. N Engl J Med. 2003;348:2635–2645. doi: 10.1056/NEJMsa022615. [DOI] [PubMed] [Google Scholar]
- 352.Schuster MA, McGlynn EA, Brook RH. How good is the quality of health care in the United States? 1998. Milbank Q. 2005;83:843–895. doi: 10.1111/j.1468-0009.2005.00403.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Roberts SF, Fischhoff MA, Sakowski SA, Feldman EL. Perspective: Transforming science into medicine: how clinician-scientists can build bridges across research's "valley of death". Acad Med. 2012;87:266–270. doi: 10.1097/ACM.0b013e3182446fa3. [DOI] [PubMed] [Google Scholar]
- 354.Contopoulos-Ioannidis DG, Ntzani E, Ioannidis JP. Translation of highly promising basic science research into clinical applications. Am J Med. 2003;114:477–484. doi: 10.1016/s0002-9343(03)00013-5. [DOI] [PubMed] [Google Scholar]
- 355.Botting J. The History of Thalidomide. Drug News Perspect. 2002;15:604–611. doi: 10.1358/dnp.2002.15.9.840066. [DOI] [PubMed] [Google Scholar]
- 356.Kutcher JS, Engle A, Firth J, Lamm SH. Bendectin and birth defects. II. Ecological analyses. Birth Defects Res A Clin Mol Teratol. 2003;67:88–97. doi: 10.1002/bdra.10034. [DOI] [PubMed] [Google Scholar]
- 357.Palmer AK, Harris AL, Jacobson RM. Human papillomavirus vaccination: a case study in translational science. Clin Transl Sci. 2014;7:420–424. doi: 10.1111/cts.12166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Mittendorf R, Williams MA. Rho(D) immunoglobulin (RhoGAM): how it came into being. Obstet Gynecol. 1991;77:301–303. doi: 10.1097/00006250-199102000-00029. [DOI] [PubMed] [Google Scholar]
- 359.Hamilton EG. Rho(D) immunoglobulin (RhoGAM): how it came into being. Obstet Gynecol. 1991;77:957–958. [PubMed] [Google Scholar]
- 360.Crowley P. Prophylactic corticosteroids for preterm birth. Cochrane Database Syst Rev. 2000:CD000065. doi: 10.1002/14651858.CD000065. [DOI] [PubMed] [Google Scholar]
- 361.Liggins GC. Premature delivery of foetal lambs infused with glucocorticoids. J Endocrinol. 1969;45:515–523. doi: 10.1677/joe.0.0450515. [DOI] [PubMed] [Google Scholar]
- 362.Liggins GC, Howie RN. A controlled trial of antepartum glucocorticoid treatment for prevention of the respiratory distress syndrome in premature infants. Pediatrics. 1972;50:515–525. [PubMed] [Google Scholar]
- 363.ACOG practice bulletin no. 127: Management of preterm labor. Obstet Gynecol. 2012;119:1308–1317. doi: 10.1097/AOG.0b013e31825af2f0. [DOI] [PubMed] [Google Scholar]
- 364.Effect of corticosteroids for fetal maturation on perinatal outcomes. NIH Consensus Development Panel on the Effect of Corticosteroids for Fetal Maturation on Perinatal Outcomes. JAMA. 1995;273:413–418. doi: 10.1001/jama.1995.03520290065031. [DOI] [PubMed] [Google Scholar]
- 365.Bradley EH, Schlesinger M, Webster TR, Baker D, Inouye SK. Translating research into clinical practice: making change happen. J Am Geriatr Soc. 2004;52:1875–1882. doi: 10.1111/j.1532-5415.2004.52510.x. [DOI] [PubMed] [Google Scholar]
- 366.Bradley EH, Webster TR, Baker D, et al. Translating research into practice: speeding the adoption of innovative health care programs. Issue Brief (Commonw Fund) 2004:1–12. [PubMed] [Google Scholar]
- 367.Kahn K, Ryan G, Beckett M, et al. Bridging the gap between basic science and clinical practice: a role for community clinicians. Implement Sci. 2011;6:34. doi: 10.1186/1748-5908-6-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Green LA, Seifert CM. Translation of research into practice: why we can't "just do it". J Am Board Fam Pract. 2005;18:541–545. doi: 10.3122/jabfm.18.6.541. [DOI] [PubMed] [Google Scholar]
- 369.National Institutes of Health, IMPACT: Our Economy. [Accessed April 6, 2015]; Available at http://www.nih.gov/about/impact/economy.htm.
- 370.Liu G, Chen G, Sinoway LI, Berg A. Assessing the impact of the NIH CTSA program on institutionally sponsored clinical trials. Clin Transl Sci. 2013;6:196–200. doi: 10.1111/cts.12029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.The Clinical and Translational Science Award (CTSA) [Accessed in 2015]; Available at https://ctsacentral.org/ [Google Scholar]
- 372.Haas DM. Pharmacogenetics and individualizing drug treatment during pregnancy. Pharmacogenomics. 2014;15:69–78. doi: 10.2217/pgs.13.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Arroyo JD, Chevillet JR, Kroh EM, et al. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc Natl Acad Sci U S A. 2011;108:5003–5008. doi: 10.1073/pnas.1019055108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 375.O'loughlin AJ, Woffindale CA, Wood MJ. Exosomes and the emerging field of exosome-based gene therapy. Curr Gene Ther. 2012;12:262–274. doi: 10.2174/156652312802083594. [DOI] [PubMed] [Google Scholar]
- 376.Thery C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9:581–593. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- 377.Simons M, Raposo G. Exosomes--vesicular carriers for intercellular communication. Curr Opin Cell Biol. 2009;21:575–581. doi: 10.1016/j.ceb.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 378.Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200:373–383. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Raposo G, Nijman HW, Stoorvogel W, et al. B lymphocytes secrete antigen-presenting vesicles. J Exp Med. 1996;183:1161–1172. doi: 10.1084/jem.183.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Akers JC, Gonda D, Kim R, Carter BS, Chen CC. Biogenesis of extracellular vesicles (EV): exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. J Neurooncol. 2013;113:1–11. doi: 10.1007/s11060-013-1084-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Pitt JM, Charrier M, Viaud S, et al. Dendritic cell-derived exosomes as immunotherapies in the fight against cancer. J Immunol. 2014;193:1006–1011. doi: 10.4049/jimmunol.1400703. [DOI] [PubMed] [Google Scholar]
- 382.Robbins PD, Morelli AE. Regulation of immune responses by extracellular vesicles. Nat Rev Immunol. 2014;14:195–208. doi: 10.1038/nri3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Fais S, Logozzi M, Lugini L, et al. Exosomes: the ideal nanovectors for biodelivery. Biol Chem. 2013;394:1–15. doi: 10.1515/hsz-2012-0236. [DOI] [PubMed] [Google Scholar]
- 384.Tan A, Rajadas J, Seifalian AM. Exosomes as nano-theranostic delivery platforms for gene therapy. Adv Drug Deliv Rev. 2013;65:357–367. doi: 10.1016/j.addr.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 385.Mevorach D, Mascarenhas JO, Gershov D, Elkon KB. Complement-dependent clearance of apoptotic cells by human macrophages. J Exp Med. 1998;188:2313–2320. doi: 10.1084/jem.188.12.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Taylor RC, Cullen SP, Martin SJ. Apoptosis: controlled demolition at the cellular level. Nat Rev Mol Cell Biol. 2008;9:231–241. doi: 10.1038/nrm2312. [DOI] [PubMed] [Google Scholar]
- 387.Heijnen HF, Schiel AE, Fijnheer R, Geuze HJ, Sixma JJ. Activated platelets release two types of membrane vesicles: microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and alpha-granules. Blood. 1999;94:3791–3799. [PubMed] [Google Scholar]
- 388.Cocucci E, Racchetti G, Meldolesi J. Shedding microvesicles: artefacts no more. Trends Cell Biol. 2009;19:43–51. doi: 10.1016/j.tcb.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 389.Leventis PA, Grinstein S. The distribution and function of phosphatidylserine in cellular membranes. Annu Rev Biophys. 2010;39:407–427. doi: 10.1146/annurev.biophys.093008.131234. [DOI] [PubMed] [Google Scholar]
- 390.Hugel B, Martinez MC, Kunzelmann C, Freyssinet JM. Membrane microparticles: two sides of the coin. Physiology (Bethesda) 2005;20:22–27. doi: 10.1152/physiol.00029.2004. [DOI] [PubMed] [Google Scholar]
- 391.Meckes DG, JR, Raab-Traub N. Microvesicles and viral infection. J Virol. 2011;85:12844–12854. doi: 10.1128/JVI.05853-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Pols MS, Klumperman J. Trafficking and function of the tetraspanin CD63. Exp Cell Res. 2009;315:1584–1592. doi: 10.1016/j.yexcr.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 393.Hemler ME. Tetraspanin functions and associated microdomains. Nat Rev Mol Cell Biol. 2005;6:801–811. doi: 10.1038/nrm1736. [DOI] [PubMed] [Google Scholar]
- 394.Fomina AF, Deerinck TJ, Ellisman MH, Cahalan MD. Regulation of membrane trafficking and subcellular organization of endocytic compartments revealed with FM1–43 in resting and activated human T cells. Exp Cell Res. 2003;291:150–166. doi: 10.1016/s0014-4827(03)00372-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Bobrie A, Colombo M, Raposo G, Thery C. Exosome secretion: molecular mechanisms and roles in immune responses. Traffic. 2011;12:1659–1668. doi: 10.1111/j.1600-0854.2011.01225.x. [DOI] [PubMed] [Google Scholar]
- 396.Delorme-Axford E, Donker RB, Mouillet JF, et al. Human placental trophoblasts confer viral resistance to recipient cells. Proc Natl Acad Sci U S A. 2013;110:12048–12053. doi: 10.1073/pnas.1304718110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Ouyang Y, Mouillet JF, Coyne CB, Sadovsky Y. Review: placenta-specific microRNAs in exosomes - good things come in nano-packages. Placenta. 2014;35(Suppl):S69–S73. doi: 10.1016/j.placenta.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Luo SS, Ishibashi O, Ishikawa G, et al. Human villous trophoblasts express and secrete placenta-specific microRNAs into maternal circulation via exosomes. Biol Reprod. 2009;81:717–729. doi: 10.1095/biolreprod.108.075481. [DOI] [PubMed] [Google Scholar]
- 399.Donker RB, Mouillet JF, Chu T, et al. The expression profile of C19MC microRNAs in primary human trophoblast cells and exosomes. Mol Hum Reprod. 2012;18:417–424. doi: 10.1093/molehr/gas013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Redman CW, Sargent IL. Circulating microparticles in normal pregnancy and preeclampsia. Placenta. 2008;29(Suppl A):S73–S77. doi: 10.1016/j.placenta.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 401.Aharon A, Brenner B. Placenta-derived microparticles. Thromb Res. 2013;131(Suppl 1):S22–S24. doi: 10.1016/S0049-3848(13)70014-8. [DOI] [PubMed] [Google Scholar]
- 402.Mayhew TM. Turnover of human villous trophoblast in normal pregnancy: what do we know and what do we need to know? Placenta. 2014;35:229–240. doi: 10.1016/j.placenta.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 403.Ishihara N, Matsuo H, Murakoshi H, Laoag-Fernandez JB, Samoto T, Maruo T. Increased apoptosis in the syncytiotrophoblast in human term placentas complicated by either preeclampsia or intrauterine growth retardation. Am J Obstet Gynecol. 2002;186:158–166. doi: 10.1067/mob.2002.119176. [DOI] [PubMed] [Google Scholar]
- 404.Salomon C, Kobayashi M, Ashman K, Sobrevia L, Mitchell MD, Rice GE. Hypoxia-induced changes in the bioactivity of cytotrophoblast-derived exosomes. PLoS One. 2013;8:e79636. doi: 10.1371/journal.pone.0079636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Taylor DD, Akyol S, Gercel-Taylor C. Pregnancy-associated exosomes and their modulation of T cell signaling. J Immunol. 2006;176:1534–1542. doi: 10.4049/jimmunol.176.3.1534. [DOI] [PubMed] [Google Scholar]
- 406.Kshirsagar SK, Alam SM, Jasti S, et al. Immunomodulatory molecules are released from the first trimester and term placenta via exosomes. Placenta. 2012;33:982–990. doi: 10.1016/j.placenta.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Sabapatha A, Gercel-Taylor C, Taylor DD. Specific isolation of placenta-derived exosomes from the circulation of pregnant women and their immunoregulatory consequences. Am J Reprod Immunol. 2006;56:345–355. doi: 10.1111/j.1600-0897.2006.00435.x. [DOI] [PubMed] [Google Scholar]
- 408.Jeschke U, Hutter S, Heublein S, et al. Expression and function of galectins in the endometrium and at the human feto-maternal interface. Placenta. 2013;34:863–872. doi: 10.1016/j.placenta.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 409.Alegre E, Rebmann V, Lemaoul TJ, et al. In vivo identification of an HLA-G complex as ubiquitinated protein circulating in exosomes. Eur J Immunol. 2013;43:1933–1939. doi: 10.1002/eji.201343318. [DOI] [PubMed] [Google Scholar]
- 410.Mincheva-Nilsson L, Baranov V. The role of placental exosomes in reproduction. Am J Reprod Immunol. 2010;63:520–533. doi: 10.1111/j.1600-0897.2010.00822.x. [DOI] [PubMed] [Google Scholar]
- 411.Gross JC, Chaudhary V, Bartscherer K, Boutros M. Active Wnt proteins are secreted on exosomes. Nat Cell Biol. 2012;14:1036–1045. doi: 10.1038/ncb2574. [DOI] [PubMed] [Google Scholar]
- 412.Atay S, Gercel-Taylor C, Suttles J, Mor G, Taylor DD. Trophoblast-derived exosomes mediate monocyte recruitment and differentiation. Am J Reprod Immunol. 2011;65:65–77. doi: 10.1111/j.1600-0897.2010.00880.x. [DOI] [PubMed] [Google Scholar]
- 413.Subra C, Grand D, Laulagnier K, et al. Exosomes account for vesicle-mediated transcellular transport of activatable phospholipases and prostaglandins. J Lipid Res. 2010;51:2105–2120. doi: 10.1194/jlr.M003657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Esser J, Gehrmann U, D'Alexandri FL, et al. Exosomes from human macrophages and dendritic cells contain enzymes for leukotriene biosynthesis and promote granulocyte migration. J Allergy Clin Immunol. 2010;126:1032–1040. 40 e1–40 e4. doi: 10.1016/j.jaci.2010.06.039. [DOI] [PubMed] [Google Scholar]
- 415.Record M. Intercellular communication by exosomes in placenta: a possible role in cell fusion? Placenta. 2014;35:297–302. doi: 10.1016/j.placenta.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 416.Sarker S, Scholz-Romero K, Perez A, et al. Placenta-derived exosomes continuously increase in maternal circulation over the first trimester of pregnancy. J Transl Med. 2014;12:204. doi: 10.1186/1479-5876-12-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Bentwich I, Avniel A, Karov Y, et al. Identification of hundreds of conserved and nonconserved human microRNAs. Nat Genet. 2005;37:766–770. doi: 10.1038/ng1590. [DOI] [PubMed] [Google Scholar]
- 418.Zhang R, Wang YQ, Su B. Molecular evolution of a primate-specific microRNA family. Mol Biol Evol. 2008;25:1493–1502. doi: 10.1093/molbev/msn094. [DOI] [PubMed] [Google Scholar]
- 419.Bortolin-Cavaille ML, Dance M, Weber M, Cavaille J. C19MC microRNAs are processed from introns of large Pol-II, non-protein-coding transcripts. Nucleic Acids Res. 2009;37:3464–3473. doi: 10.1093/nar/gkp205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Delorme-Axford E, Bayer A, Sadovsky Y, Coyne CB. Autophagy as a mechanism of antiviral defense at the maternal-fetal interface. Autophagy. 2013;9:2173–2174. doi: 10.4161/auto.26558. [DOI] [PubMed] [Google Scholar]
- 421.Bayer A, Delorme-Axford E, Sleigher C, et al. Human trophoblasts confer resistance to viruses implicated in perinatal infection. Am J Obstet Gynecol. 2015;212:71 e1–71 e8. doi: 10.1016/j.ajog.2014.07.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Mouillet JF, Ouyang Y, Bayer A, Coyne CB, Sadovsky Y. The role of trophoblastic microRNAs in placental viral infection. Int J Dev Biol. 2014;58:281–289. doi: 10.1387/ijdb.130349ys. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Wang K, Huang C, Nice EC. Proteomics, genomics and transcriptomics: their emerging roles in the discovery and validation of colorectal cancer biomarkers. Expert Rev Proteomics. 2014;11:179–205. doi: 10.1586/14789450.2014.894466. [DOI] [PubMed] [Google Scholar]
- 424.Choi DS, Kim DK, Kim YK, Gho YS. Proteomics, transcriptomics and lipidomics of exosomes and ectosomes. Proteomics. 2013;13:1554–1571. doi: 10.1002/pmic.201200329. [DOI] [PubMed] [Google Scholar]
- 425.Montecalvo A, Larregina AT, Shufesky WJ, et al. Mechanism of transfer of functional microRNAs between mouse dendritic cells via exosomes. Blood. 2012;119:756–766. doi: 10.1182/blood-2011-02-338004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Bartlett DW, Davis ME. Physicochemical and biological characterization of targeted, nucleic acid-containing nanoparticles. Bioconjugate chemistry. 2007;18:456–468. doi: 10.1021/bc0603539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Bryniarski K, Ptak W, Martin E, et al. Free Extracellular miRNA Functionally Targets Cells by Transfecting Exosomes from Their Companion Cells. PLoS One. 2015;10:e0122991. doi: 10.1371/journal.pone.0122991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Gotsch F, Romero R, Chaiworapongsa T, et al. Evidence of the involvement of caspase-1 under physiologic and pathologic cellular stress during human pregnancy: a link between the inflammasome and parturition. J Matern Fetal Neonatal Med. 2008;21:605–616. doi: 10.1080/14767050802212109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Weiner CP, Mason CW, Dong Y, Buhimschi IA, Swaan PW, Buhimschi CS. Human effector/initiator gene sets that regulate myometrial contractility during term and preterm labor. Am J Obstet Gynecol. 2010;202:474 e1–474 e20. doi: 10.1016/j.ajog.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Lee JS, Romero R, Han YM, et al. Placenta-on-a-chip: a novel platform to study the biology of the human placenta. J Matern Fetal Neonatal Med. 2016;29:1046–1054. doi: 10.3109/14767058.2015.1038518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Duncan R, Gaspar R. Nanomedicine(s) under the microscope. Mol Pharm. 2011;8:2101–2141. doi: 10.1021/mp200394t. [DOI] [PubMed] [Google Scholar]
- 432.Kabanov AV. Polymer genomics: an insight into pharmacology and toxicology of nanomedicines. Adv Drug Deliv Rev. 2006;58:1597–1621. doi: 10.1016/j.addr.2006.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Rytting E, Nguyen J, Wang X, Kissel T. Biodegradable polymeric nanocarriers for pulmonary drug delivery. Expert Opin Drug Deliv. 2008;5:629–639. doi: 10.1517/17425247.5.6.629. [DOI] [PubMed] [Google Scholar]
- 434.Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. 2007;2:751–760. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 435.Rytting E, Bur M, Cartier R, et al. In vitro and in vivo performance of biocompatible negatively-charged salbutamol-loaded nanoparticles. J Control Release. 2010;141:101–107. doi: 10.1016/j.jconrel.2009.08.021. [DOI] [PubMed] [Google Scholar]
- 436.Cai C, Bakowsky U, Rytting E, Schaper AK, Kissel T. Charged nanoparticles as protein delivery systems: a feasibility study using lysozyme as model protein. Eur J Pharm Biopharm. 2008;69:31–42. doi: 10.1016/j.ejpb.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 437.Poulsen MS, Rytting E, Mose T, Knudsen LE. Modeling placental transport: correlation of in vitro BeWo cell permeability and ex vivo human placental perfusion. Toxicol In Vitro. 2009;23:1380–1386. doi: 10.1016/j.tiv.2009.07.028. [DOI] [PubMed] [Google Scholar]
- 438.Li H, Van Ravenzwaay B, Rietjens IM, Louisse J. Assessment of an in vitro transport model using BeWo b30 cells to predict placental transfer of compounds. Arch Toxicol. 2013;87:1661–1669. doi: 10.1007/s00204-013-1074-9. [DOI] [PubMed] [Google Scholar]
- 439.Cartwright L, Poulsen MS, Nielsen HM, et al. In vitro placental model optimization for nanoparticle transport studies. Int J Nanomedicine. 2012;7:497–510. doi: 10.2147/IJN.S26601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Wick P, Malek A, Manser P, et al. Barrier capacity of human placenta for nanosized materials. Environ Health Perspect. 2010;118:432–436. doi: 10.1289/ehp.0901200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Poulsen MS, Mose T, Maroun LL, Mathiesen L, Knudsen LE, Rytting E. Kinetics of silica nanoparticles in the human placenta. Nanotoxicology. 2015;9:79–86. doi: 10.3109/17435390.2013.812259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Myllynen PK, Loughran MJ, Howard CV, Sormunen R, Walsh AA, Vahakangas KH. Kinetics of gold nanoparticles in the human placenta. Reprod Toxicol. 2008;26:130–137. doi: 10.1016/j.reprotox.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 443.Menjoge AR, Rinderknecht AL, Navath RS, et al. Transfer of PAMAM dendrimers across human placenta: prospects of its use as drug carrier during pregnancy. J Control Release. 2011;150:326–338. doi: 10.1016/j.jconrel.2010.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Bajoria R, Contractor SF. Effect of the size of liposomes on the transfer and uptake of carboxyfluorescein by the perfused human term placenta. J Pharm Pharmacol. 1997;49:675–681. doi: 10.1111/j.2042-7158.1997.tb06091.x. [DOI] [PubMed] [Google Scholar]
- 445.Ali H, Kalashnikova I, White MA, Sherman M, Rytting E. Preparation, characterization, and transport of dexamethasone-loaded polymeric nanoparticles across a human placental in vitro model. Int J Pharm. 2013;454:149–157. doi: 10.1016/j.ijpharm.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.Yamashita K, Yoshioka Y, Higashisaka K, et al. Silica and titanium dioxide nanoparticles cause pregnancy complications in mice. Nat Nanotechnol. 2011;6:321–328. doi: 10.1038/nnano.2011.41. [DOI] [PubMed] [Google Scholar]
- 447.Hong JS, Park MK, Kim MS, et al. Prenatal development toxicity study of zinc oxide nanoparticles in rats. Int J Nanomedicine. 2014;9(Suppl 2):159–171. doi: 10.2147/IJN.S57932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Di Bona KR, Xu Y, Ramirez PA, et al. Surface charge and dosage dependent potential developmental toxicity and biodistribution of iron oxide nanoparticles in pregnant CD-1 mice. Reprod Toxicol. 2014;50:36–42. doi: 10.1016/j.reprotox.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 449.Tsyganova NA, Khairullin RM, Terentyuk GS, et al. Penetration of pegylated gold nanoparticles through rat placental barrier. Bull Exp Biol Med. 2014;157:383–385. doi: 10.1007/s10517-014-2572-3. [DOI] [PubMed] [Google Scholar]
- 450.Rattanapinyopituk K, Shimada A, Morita T, et al. Demonstration of the clathrin- and caveolin-mediated endocytosis at the maternal-fetal barrier in mouse placenta after intravenous administration of gold nanoparticles. J Vet Med Sci. 2014;76:377–387. doi: 10.1292/jvms.13-0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Basha S, Vaidhyanathan S, Pauletti GM. Selection of peptide ligands for human placental transcytosis systems using in vitro phage display. Methods Mol Biol. 2011;716:141–156. doi: 10.1007/978-1-61779-012-6_8. [DOI] [PubMed] [Google Scholar]
- 452.Dragovic RA, Gardiner C, Brooks AS, et al. Sizing and phenotyping of cellular vesicles using Nanoparticle Tracking Analysis. Nanomedicine : nanotechnology, biology, and medicine. 2011;7:780–788. doi: 10.1016/j.nano.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.Kaitu'u-lino TJ, Pattison S, Ye L, et al. Targeted nanoparticle delivery of doxorubicin into placental tissues to treat ectopic pregnancies. Endocrinology. 2013;154:911–919. doi: 10.1210/en.2012-1832. [DOI] [PubMed] [Google Scholar]
- 454.Rytting E, Ahmed MS. 5 - Fetal Drug Therapy. In: Mattison DR, editor. Clinical Pharmacology During Pregnancy. Academic Press; 2013. [Google Scholar]
- 455.Reed MD, Mattison DR. Treating the Placenta: an Evolving Therapeutic Concept. London: Academic Press Elsevier; [Google Scholar]
- 456.Khlebtsov N, Bogatyrev V, Dykman L, et al. Analytical and theranostic applications of gold nanoparticles and multifunctional nanocomposites. Theranostics. 2013;3:167–180. doi: 10.7150/thno.5716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457.Engelberth SA, Hempel N, Bergkvist M. Development of nanoscale approaches for ovarian cancer therapeutics and diagnostics. Crit Rev Oncog. 2014;19:281–315. doi: 10.1615/critrevoncog.2014011455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458.Webb JA, Thomsen HS. Gadolinium contrast media during pregnancy and lactation. Acta Radiol. 2013;54:599–600. doi: 10.1177/0284185113484894. [DOI] [PubMed] [Google Scholar]
- 459.Rytting E, Wang X, Vernikovskaya DI, et al. Metabolism and disposition of bupropion in pregnant baboons (Papio cynocephalus) Drug Metab Dispos. 2014;42:1773–1779. doi: 10.1124/dmd.114.058255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Wang X, Paul JA, Nanovskaya TN, Hankins GD, Ahmed MS. Quantitative determination of telavancin in pregnant baboon plasma by solid-phase extraction and LC-ESI-MS. J Pharm Biomed Anal. 2014;98:107–112. doi: 10.1016/j.jpba.2014.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.Wang X, Abdelrahman DR, Fokina VM, Hankins GD, Ahmed MS, Nanovskaya TN. Metabolism of bupropion by baboon hepatic and placental microsomes. Biochem Pharmacol. 2011;82:295–303. doi: 10.1016/j.bcp.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462.Yan R, Nanovskaya TN, Zharikova OL, Mattison DR, Hankins GD, Ahmed MS. Metabolism of 17alpha-hydroxyprogesterone caproate by hepatic and placental microsomes of human and baboons. Biochem Pharmacol. 2008;75:1848–1857. doi: 10.1016/j.bcp.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463.Zharikova OL, Ravindran S, Nanovskaya TN, Hill RA, Hankins GD, Ahmed MS. Kinetics of glyburide metabolism by hepatic and placental microsomes of human and baboon. Biochem Pharmacol. 2007;73:2012–2019. doi: 10.1016/j.bcp.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 464.Ravindran S, Zharikova OL, Hill RA, Nanovskaya TN, Hankins GD, Ahmed MS. Identification of glyburide metabolites formed by hepatic and placental microsomes of humans and baboons. Biochem Pharmacol. 2006;72:1730–1737. doi: 10.1016/j.bcp.2006.08.024. [DOI] [PubMed] [Google Scholar]
- 465.Urry DW, Long MM, Cox BA, Ohnishi T, Mitchell LW, Jacobs M. The synthetic polypentapeptide of elastin coacervates and forms filamentous aggregates. Biochim Biophys Acta. 1974;371:597–602. doi: 10.1016/0005-2795(74)90057-9. [DOI] [PubMed] [Google Scholar]
- 466.Bidwell GL, 3RD, Raucher D. Application of thermally responsive polypeptides directed against c-Myc transcriptional function for cancer therapy. Mol Cancer Ther. 2005;4:1076–1085. doi: 10.1158/1535-7163.MCT-04-0253. [DOI] [PubMed] [Google Scholar]
- 467.George EM, Liu H, Robinson GG, Mahdi F, Perkins E, Bidwell GL., 3RD Growth factor purification and delivery systems (PADS) for therapeutic angiogenesis. Vasc Cell. 2015;7:1. doi: 10.1186/s13221-014-0026-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468.Meyer DE, Chilkoti A. Genetically encoded synthesis of protein-based polymers with precisely specified molecular weight and sequence by recursive directional ligation: examples from the elastin-like polypeptide system. Biomacromolecules. 2002;3:357–367. doi: 10.1021/bm015630n. [DOI] [PubMed] [Google Scholar]
- 469.Daniell H, Guda C, McPherson DT, Zhang X, Xu J, Urry DW. Hyperexpression of a synthetic protein-based polymer gene. Methods Mol Biol. 1997;63:359–371. doi: 10.1385/0-89603-481-X:359. [DOI] [PubMed] [Google Scholar]
- 470.Urry D, Urry C, Luan T, et al. Temperature of polypeptide inverse temperature transition depends on mean residue hydrophobicity. Journal of the American Chemical Society. 1991;113:4346–4348. [Google Scholar]
- 471.George EM, Liu H, Robinson GG, Bidwell GL. A polypeptide drug carrier for maternal delivery and prevention of fetal exposure. J Drug Target. 2014;22:935–947. doi: 10.3109/1061186X.2014.950666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472.Brosens JJ, Pijnenborg R, Brosens IA. The myometrial junctional zone spiral arteries in normal and abnormal pregnancies: a review of the literature. Am J Obstet Gynecol. 2002;187:1416–1423. doi: 10.1067/mob.2002.127305. [DOI] [PubMed] [Google Scholar]
- 473.Lyall F, Robson SC, Bulmer JN. Spiral artery remodeling and trophoblast invasion in preeclampsia and fetal growth restriction: relationship to clinical outcome. Hypertension. 2013;62:1046–1054. doi: 10.1161/HYPERTENSIONAHA.113.01892. [DOI] [PubMed] [Google Scholar]
- 474.Burke SD, Karumanchi SA. Spiral artery remodeling in preeclampsia revisited. Hypertension. 2013;62:1013–1014. doi: 10.1161/HYPERTENSIONAHA.113.02049. [DOI] [PubMed] [Google Scholar]
- 475.Palei AC, Spradley FT, Warrington JP, George EM, Granger JP. Pathophysiology of hypertension in pre-eclampsia: a lesson in integrative physiology. Acta Physiol (Oxf) 2013;208:224–233. doi: 10.1111/apha.12106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 476.Warrington JP, George EM, Palei AC, Spradley FT, Granger JP. Recent advances in the understanding of the pathophysiology of preeclampsia. Hypertension. 2013;62:666–673. doi: 10.1161/HYPERTENSIONAHA.113.00588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 477.Bidwell GL, 3RD, George EM. Maternally sequestered therapeutic polypeptides - a new approach for the management of preeclampsia. Front Pharmacol. 2014;5:201. doi: 10.3389/fphar.2014.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 478.Maynard SE, Min JY, Merchan J, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111:649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 479.Maynard SE, Venkatesha S, Thadhani R, Karumanchi SA. Soluble Fms-like tyrosine kinase 1 and endothelial dysfunction in the pathogenesis of preeclampsia. Pediatr Res. 2005;57:1R–7R. doi: 10.1203/01.PDR.0000159567.85157.B7. [DOI] [PubMed] [Google Scholar]
- 480.Sawano A, Takahashi T, Yamaguchi S, Aonuma M, Shibuya M. Flt-1 but not KDR/Flk-1 tyrosine kinase is a receptor for placenta growth factor, which is related to vascular endothelial growth factor. Cell Growth Differ. 1996;7:213–221. [PubMed] [Google Scholar]
- 481.Gerber HP, McMurtrey A, Kowalski J, et al. Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3'-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activation. J Biol Chem. 1998;273:30336–30343. doi: 10.1074/jbc.273.46.30336. [DOI] [PubMed] [Google Scholar]
- 482.Rusterholz C, Hahn S, Holzgreve W. Role of placentally produced inflammatory and regulatory cytokines in pregnancy and the etiology of preeclampsia. Semin Immunopathol. 2007;29:151–162. doi: 10.1007/s00281-007-0071-6. [DOI] [PubMed] [Google Scholar]
- 483.Kupferminc MJ, Peaceman AM, Wigton TR, Rehnberg KA, Socol ML. Tumor necrosis factor-alpha is elevated in plasma and amniotic fluid of patients with severe preeclampsia. Am J Obstet Gynecol. 1994;170:1752–1757. discussion 57–9. [PubMed] [Google Scholar]
- 484.Vince GS, Starkey PM, Austgulen R, Kwiatkowski D, Redman CW. Interleukin-6, tumour necrosis factor and soluble tumour necrosis factor receptors in women with pre-eclampsia. Br J Obstet Gynaecol. 1995;102:20–25. doi: 10.1111/j.1471-0528.1995.tb09020.x. [DOI] [PubMed] [Google Scholar]
- 485.Kim YM, Romero R, Oh SY, et al. Toll-like receptor 4: a potential link between "danger signals," the innate immune system, and preeclampsia? Am J Obstet Gynecol. 2005;193:921–927. doi: 10.1016/j.ajog.2005.07.076. [DOI] [PubMed] [Google Scholar]
- 486.Demicheva E, Crispi F. Long-term follow-up of intrauterine growth restriction: cardiovascular disorders. Fetal Diagn Ther. 2014;36:143–153. doi: 10.1159/000353633. [DOI] [PubMed] [Google Scholar]
- 487.Galjaard S, Devlieger R, Van Assche FA. Fetal growth and developmental programming. J Perinat Med. 2013;41:101–105. doi: 10.1515/jpm-2012-0020. [DOI] [PubMed] [Google Scholar]
- 488.Tuovinen S, Eriksson JG, Kajantie E, Raikkonen K. Maternal hypertensive pregnancy disorders and cognitive functioning of the offspring: a systematic review. J Am Soc Hypertens. 2014;8:832–847. e1. doi: 10.1016/j.jash.2014.09.005. [DOI] [PubMed] [Google Scholar]