Abstract
Background
Renal transplantation is the treatment modality of choice for patients with end stage kidney failure. We present our experience of graft and patient survival of initial 500 renal transplants performed between May 1991 and July 2006, at Army Hospital (R&R).
Material and Methods
All patients received triple drug immunosuppression with cyclosporine/tacrolimus, azathioprine/ mycophenolate mofetil and steroids. Patients in high risk group received induction therapy with IL-2 receptor blockers/anti-thymocyte globulin.
Results
Majority of the recipients (79%) were males, whereas majority of the donors (59.4%) were females. In the donor profile, 385 (77%) transplants were live related, 108 (21.6 %) were spousal and 7 (1.4%) were cadaveric transplants. Mean age of the donors and recipients was 42.11 ± 11.53 years (range 19–72 years) and 33 ± 9.39 years (range 5–60 years) respectively. Eighty two patients (16.4%) were lost to follow up and the present data on rejections, patients and graft survival pertains to 418 patients. These patients have been followed up for a mean period of 2.63 years (SE, 0.122; median 1.8 years; range 0–13.36 years). Acute rejection episodes occurred in 115 (27.3%) patients and 95% of these could be reversed with steroids/ATG. Sixty eight patients (16%) have died on follow-up. Our one-year, 5 year and 10 year estimated graft survival is 95.4% (SE, 0.01), 80.5% (SE, 0.03) and 53.1% (SE, 0.09) respectively and patient survival at one year is 93.2% (SE, 0.01). The estimated graft and patient survival in our series is 9.83 (95% CI, 8.92–10.73) and 9.80 (8.93–10.67) years respectively.
Conclusion
This centre's short-term graft survival of 95.4% is comparable to the best centres of the world.
Key Words: Kidney transplant, Graft survival, Patient survival
Introduction
Transplantation is the treatment modality of choice for patients of end stage kidney disease. Most of the centres around the world report a short-term graft survival of 90-95% [1, 2]. In an analysis of 93,934 patients transplanted in the United States between 1988 and 1996, Hariharan et al [2], reported a one-year graft survival in living related transplantation of 93.9% and half-life of graft to be 21.6 years. With better and potent immunosuppressive regimens, the incidence of acute rejection has decreased over time and is presently around 15-20% [3, 4]. However, chronic allograft nephropathy which is the commonest cause of graft loss, still remains a major issue of concern for the transplant physicians. In terms of patient survival, the three most common causes of death in transplant patients are cardiovascular disease, infection and cancer [5], but in India infections are the commonest cause of mortality. There is paucity of data on graft and patient survival in renal transplant recipients from India. We present our experience of initial 500 renal transplants performed at Army Hospital Research & Referral (AHRR).
Material and Methods
This institution performs only cadaveric or live related transplantation with first degree relatives i.e. father, mother, brother, sister, son, daughter or spouse as donors. The initial 500 renal transplants performed at this centre between May 1991 and July 2006 are included in this study. Eighty two patients (16.4%) were lost to follow up after their mandatory visit at three months and the data on rejections, patient and graft survival of the remaining 418 patients have been analysed. A limitation of this study is the lack of data of these 82 patients who were lost to follow-up.
A donor was considered as marginal if either the donor's age was more than 60 years, or the donor had an abnormality e.g. hypertension controlled on a single drug without target organ damage, presence of cyst or silent non-obstructive stone etc. In all patients DJ stent was placed at time of neoureterostomy which was removed by the end of two weeks. Urinary catheter was removed on third day and all prophylactic antibiotics for a period of 3 to 5 days were given.
Immunosuppressives were started a day before transplantation and all patients received cyclosporine, azathioprine/mycophenolate mofetil (MMF) and steroids. Patients with high (>25%) panel reactive antibodies (PRA) or cadaveric transplants, received induction therapy with antithymocyte/antilymphocyte globulin while other high-risk groups (second transplant, spousal transplants, historical high PRA) received Interleukin-2 (IL-2) receptor blockers. Starting dose of cyclosporine was 8 mg/kg body weight which was reduced to 3 mg/kg body weight by the end of six months. During initial month following transplantation, cyclosporine levels were maintained at CO level of 250-350 ng/ml or C2 level of 1200-1700 ng/ml. The initial and maintenance dose of azathioprine was 1.5-2 mg/kg body weight. Prednisolone was started at a dose of 0.5 mg/kg body weight and gradually tapered to 7.5-10 mg/day by the end of three months. Since 2006, all transplant recipients were started on MMF de novo in a dose of 1 gm twice a day. Earlier MMF was used in high-risk group only.
Graft dysfunction was defined as rise in creatinine by 25% above the baseline. Unless an obvious cause of graft dysfunction was present, all patients with acute rise in creatinine were subjected to renal biopsy. A rejection episode was treated with 500 mg of methyl prednisolone for 3-5 days and non-responders were given antithymocyte globulin (ATG) for 7-14 days. In patients with creeping creatinine and with no obvious infection e.g. cytomegalo virus (CMV), BK virus etc. immunosuppression was reviewed. In patients with chronic allograft nephropathy, the dose of cyclosporine was reduced or it was withdrawn. Some of these patients were switched over to sirolimus to slow the ongoing damage.
Kaplan Meier estimate of survival function was carried out using SPSS version 13.0.1. Time between date last seen during follow up and date of transplant was taken as time followed up. Death/graft rejection and loss to follow up were taken as events. A total of 500 transplant patients were analysed with a total of 68 events (deaths) in patients, thus a total of 432 patients were censored at various time intervals. Review after three months of transplant is mandatory, thus all loss to follow up cases were taken as followed up for three months only. There was a group of 82 cases which have been censored at three months while analyzing the survival function. Details of patients remaining at various time intervals have been provided along with survival graphs. Patients and graft survivals were calculated using the Kaplan Meier survival analysis.
Results
Five hundred and sixteen renal transplants have been performed at AHRR since 1991 and the data till November 2006 is shown in Fig. 1. Majority of recipients, (79%, n=395) were males with a mean age of 33 ± 9.39 years (range 5-60 years). In the donor pool 297 (59.4%) donors were females, 196 (39.2%) males and seven (1.4%) patients received cadaveric transplants. Mean age of donors was 42.11 ± 11.53 years (range 19-72 years). Donor relationship profile is given in Fig. 2. There were 81 donors (16%) who qualified as marginal donors and their details are given in Table 1.
Fig. 1.
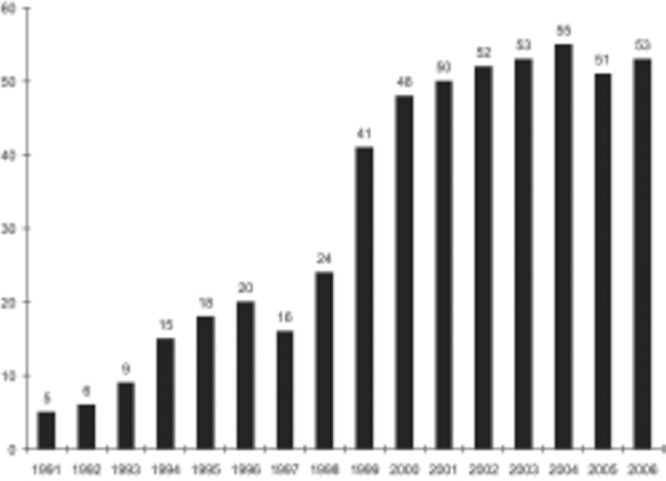
Yearly transplant data
Fig. 2.
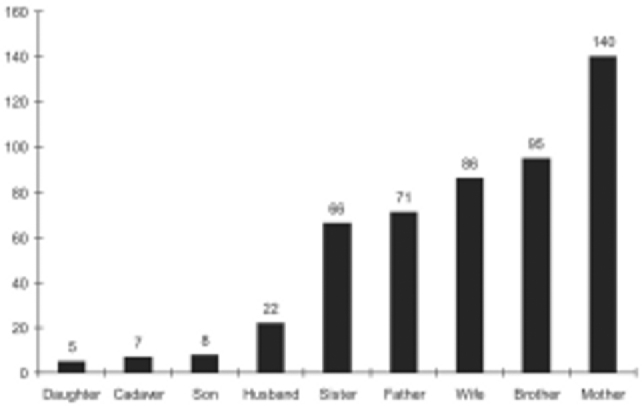
Donor relationship profile
Table 1.
Details of Marginal donors
| Number of patients | |
|---|---|
| Elderly donors | 46 |
| Renal anomaly or disease | 19 |
| Low GFR | 8 |
| Renal cyst | 3 |
| Renal calculi | 2 |
| Ureteric calculi | 2 |
| Retrocaval ureter | 1 |
| Adrenal tumours | 2 |
| Ectopic kidneys | 1 |
| Nonrenal anomalies | 21 |
| Hypertension | 18 |
| Cholelithiasis and choledocholithiasis | 1 |
| Incisional hernia | 1 |
| Benign prostatic hypertrophy | 1 |
Eighty two patients could not be followed up after their mandatory visit at three months. The remaining 418 (83.6%) patients have been followed for a mean period of 2.63 years (SE+0.122, median 1.8 years, range 0-13.36 years). Immunosuppressive protocol of these patients is shown in Table 2. Sixty-eight (16%) patients have died and 33 (7.8%) patients have lost their graft while on follow-up. 115 of 418 patients (27.5%) developed acute rejection episode, which was reversed with steroids/ATG in all except six cases (5.2%) where rejection was irreversible and resulted in graft loss. 10% (7/72) of all rejection episodes were due to drug non-compliance. 35% of acute rejections occurred in the first month after transplantation, another 35% during the next two months and the rest occurred after three months. A total of 33 grafts (7.8%) have been lost, the causes of graft loss being irreversible acute rejection in six, graft artery thrombosis in 11, persistent post biopsy haematuria necessitating graft nephrectomy in one and chronic rejection in 15 cases.
Table 2.
Immunosuppression protocol (n=420)
| Immunosuppression regime | Number of patients |
|---|---|
| Cyclosporine + Azathioprine + Prednisolone | 162 |
| Cyclosporine + Prednisolone | 17 |
| Azathioprine + Prednisolone | 25 |
| Cyclosporine + MMF + Prednisolone | 188 |
| Cyclosporine + Rapamycin + Prednisolone | 18 |
| MMF + Prednisolone | 10 |
In a prospective analysis of 259 patients transplanted between Jan 2002 and July 2006, 71 (27%) patients developed biopsy proven rejection. Subgroup analysis revealed that 12/66 (18.2%) spousal transplant recipients with 0 HLA match who had received IL-2 receptor blockers and MMF developed rejection, while the recipients in the HLA haplomatch group (donors being father, mother, brother, sister, son or daughter) who received azathioprine, 59/193 patients (30.56%) suffered from a rejection episode (p<0.05).
Our estimates for one-year graft and patient survival are 95.5% (SE, 0.01) and 93.2% (0.01) respectively (Fig. 3, Fig. 4). Our estimated mean graft and patient survival is 9.83 (95% CI 8.92 - 10.73) and 9.80 years (8.93 - 10.67) respectively. Our 5-year and 10-year estimated graft survival is 80.5% (0.03) and 53.1% (SE, 0.09) respectively. If patient death is taken as graft loss, then one-year graft survival reduces to 93.2% (SE, 0.01) and estimated 5-year and 10-year graft survival becomes 80.1% (SE, 0.03) and 56.2% (SE, 0.08) respectively.
Fig. 3.
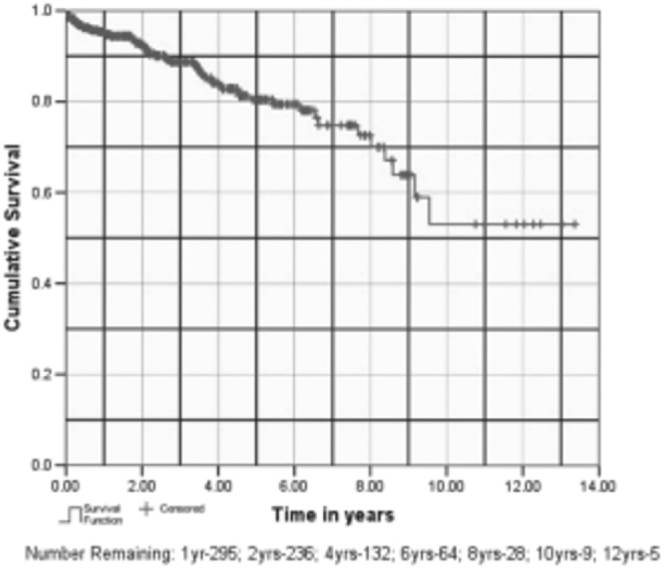
Graft survival
Fig. 4.
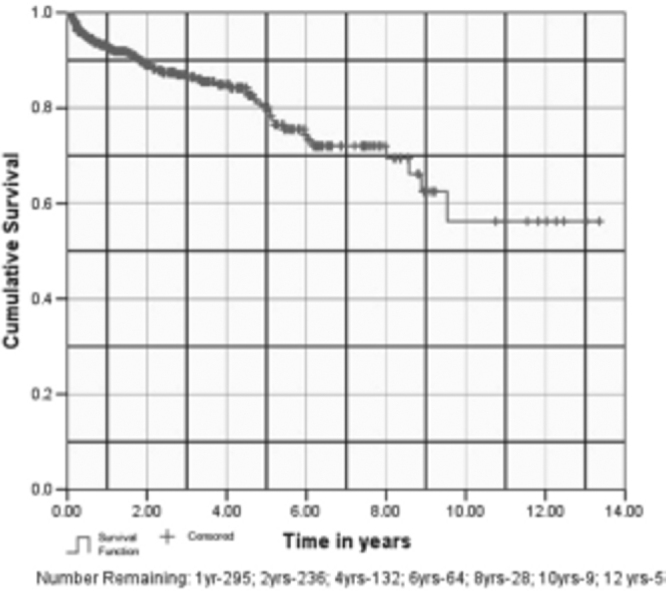
Patient survival
Among bacterial infections, urinary tract infection (UTI) was the commonest. In a prospective study of 53 patients for a year, UTI developed in 11 (20.8%) patients within the first three months. Upper UTI was also common and acute graft pyelonephritis was responsible for 16% of all acute graft dysfunctions. A six-week course of antibiotics was effective in controlling the relapse of upper UTI.
In another prospective analysis of 266 patients with follow-up period of two years, tuberculosis was encountered in 17% and in 70% of the cases, it occurred during the first year after transplantation. Patients receiving MMF and those with hepatitis C virus (HCV) infection were most susceptible to tuberculosis. Varicella was the commonest acute viral infection and affected 19% of our patients. 177 (42%) patients were either HBV or HCV positive. Of these, 39 (9.2%) had HBV infection, 93 (22.1%) had HCV and 45 (10.7%) patients had dual infection. Surgical complications encountered are shown in Table 3.
Table 3.
Surgical complications
| Complication | Number of patients | Treatment |
|---|---|---|
| Vascular | ||
| Faulty anastomosis | 5 (1) | Revision of arterial anastomosis |
| Arterial kinking | 2 (0.4) | Repositioning |
| Graft artery thrombosis | 11 (2.2) | Nephrectomy |
| Retroperitoneal haemorrhage | 5 (1) | Exploration |
| Graft laceration by drain | 1 (0.2) | Exploration |
| Urinary leak | 4 (0.8) | Conservative-1, Exploration-3 |
| Clot retention | 2 (0.4) | Conservative-1, Fulgration-1 |
| Lymphatic | ||
| Lymphocoele | 6 (1.2) | Aspiration and instillation of sclerosants |
| Prolonged drainage | 9 (1.8) | Instillation of sclerosants |
| Wound related | ||
| Wound infection | 26 (5.2) | Antibiotics and wound exploration |
| Wound dehiscence | 4 (0.8) | Secondary suturing |
(Percentage in parenthesis)
Discussion
Since the first transplant performed between identical twins in 1954, the field of transplantation has made significant strides in the immunosuppressive regimens. There has been a continuous effort to improve immunosuppressive protocols aimed at reducing the incidence of acute rejection, which is the strongest determinant of graft outcome. In 1960's and 70's, with double drug immunosuppression using azathioprine and steroids, the incidence of acute rejection was 50-60% and short-term graft survival was 60%. With the introduction of cyclosporine in early 1980's, rejection rate came down by 17% and short-term graft survival improved to over 80% [4, 6]. Over the last few years newer drugs e.g. tacrolimus, MMF, sirolimus, evrolimus etc. have reduced the rejection rate further. Tricontinental, US and European data on MMF clearly shows that when compared to azathioprine, MMF significantly reduces the biopsy proven rejection rate during initial six months by approximately 50% [7, 8, 9]. Recent studies on tacrolimus reveal that the drug is superior to cyclosporine in terms of graft survival, rejection rates and preservation of GFR [10, 11, 12]. Most of the centres using tacrolimus based immunosupression report a rejection rate of 10-15% and one-year graft survival of 90-95% [4, 13]. Use of induction therapy with ATG/IL-2 receptor blockers have further brought down the rejection rate [14, 15]. Recent data reveals that 72% of renal transplant recipients today have tacrolimus, 81% MMF, 21% cyclosporine and 12 % sirolimus on their discharge prescription [1, 16]. However, improvement in short term graft survival with tacrolimus has not transformed into long term graft or patient survival. A recent analysis of five year follow up shows that Cyclosporine + MMF + Prednisolone regimen offers survival benefit, over both Tacrolimus + MMF + Prednisolone and Cyclosporine + Azathioprine + Prednisolone regimes [17].
Having achieved low rejection rates and excellent short-term graft survival, the stress now is on improving long-term graft survival. Chronic allograft nephropathy is the commonest cause of graft loss and an average graft fails by 10 years due to chronic allograft nephropathy [17]. Available data shows the beneficial effect of sirolimus and evrolimus in slowing the progression of chronic graft nephropathy [16]. Our rejection rate of 27% is higher than reported by many centres, probably due to the use of conventional immunosuppressive regime (Cyclosporine + Azathioprine + Prednisolone) in majority of our initial cases. In our subgroup analysis of unrelated transplants where MMF and IL-2 blockers were used, our rejection rate of 18% was significantly lower (p<0.05) than conventional immunosuppressive group. Our study suggests that better anti-rejection protocol, at least on short-term basis can even undermine the effect of HLA mismatch. Majority (95%) of our rejection episodes could be reversed with steroids/ATG.
With increasing burden of chronic kidney disease and shortage of donors, more and more marginal donors are being accepted for transplantation. Our marginal donor pool of 16% is not unusual as compared to that reported in literature [18, 19]. Spousal transplantation accounted for 21.6% of our cases, a figure similar to that reported by many centres in India [20].
UTI was the commonest infection encountered, but our figure of 20% is lower than that reported by many workers. Early removal of urinary catheter on the 3rd day probably lowers the incidence of UTI, a fact that has been documented by other authors also [21, 22]. But the incidence of upper UTI (acute pyelonephritis) was higher in our cases, being responsible for acute graft dysfunction in 16% of cases. The higher incidence of upper UTI in our cases may be related to DJ stent placement in all cases, which has been reported to be a risk factor for UTI [23].
Tuberculosis (TB) was encountered in 17% of our patients and in 70% the disease occurred within one year after transplantation. Patients receiving MMF and those with HCV infection were found to be the vulnerable groups. Similar susceptibility and higher incidence of TB in HCV infected patients has been reported by other centres from India [24, 25]. Varicella affected 19% (chickenpox in 13% and herpes zoster in 6%) of our patients and was the commonest acute viral infection. In all the cases, it occurred during the first three months after transplantation. All patients responded to acyclovir, as reported by others workers [26].
A large number of our patients (42%, n=177) are infected with either hepatitis B or C virus. Whereas some studies have shown lower patient survival in HCV infected recipients [27, 28], others have shown similar survival rates [29]. We did encounter rapid progression to fulminant hepatitis in some of our patients [30].
Our one-year graft survival of 95.4% is comparable to the recent US and European data showing a 1-year graft survival in living related transplantation of 93.9% and 95% respectively [1, 2, 3]. However, our one-year patient survival of 93.2% is slightly inferior to the European data of 95% [3]. This is largely due to infection related deaths in our patients. Our surgical complications are comparable to that reported in literature [31].
Conflicts of Interest
None identified
Acknowledgements
The authors acknowledge the contribution of the following members of the transplant team: Maj Gen P Madhusoodanan, VSM, Brig Yashpal, VSM (Retd), Col A Mishra, VSM (Retd), Brig N Raychaudhury (Retd), Col SR Gedela, Col AS Narula, Col A Rajvanshi, Col KV Baliga, Col UK Sharma, Lt Col HS Gill (Retd), Col SV Kotwal (Retd), Lt Col HS Bhatyal (Retd), Lt Col R Sood, VSM (Retd), Lt Col AS Sandhu, Lt Col SK Gupta, Col KK Upadhyaya, VSM, Gp Capt R Chaturvedi, Lt Col N Sethi, Col Chander Mohan, SM and Lt Col Lavan Singh.
References
- 1.Scientific registry of transplant recipients. OPTN/SRTR Annual Report. 2004 [Google Scholar]
- 2.Hariharan S, Johnson CP, Bresnahan BA. Improved graft survival after renal transplantation in United States; 1988–1996. NEJM. 2000;342:605–612. doi: 10.1056/NEJM200003023420901. [DOI] [PubMed] [Google Scholar]
- 3.Stratta RJ, Alloway RR, Hodge E, Lo A. A multicentric, open label comparative trial of two doses of daclizumab dosing strategies vs no antibody induction in combination with tacrolimus, mycophenolate mofetil and steroids for the prevention of acute rejection in simultaneous kidney-pancreas transplant recipients. Clin Transplant. 2002;16:60–68. doi: 10.1034/j.1399-0012.2002.00108.x. [DOI] [PubMed] [Google Scholar]
- 4.Webster AC, Woodroff RC, Taylor RS, Chapman JR, Craig JC. Tacrolimus versus cyclosporine as primary immunosuppressive for kidney transplant recipients: meta-analysis and metaregression of randomized trial data. BMJ. 2005;331:810–815. doi: 10.1136/bmj.38569.471007.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kasiske BL. Long term post transplantation complications and management. In: Danovitch GM, editor. Handbook of kidney transplantation. 3rd ed. Williams and Wilkins; Philadelphia: 2001. pp. 183–184. [Google Scholar]
- 6.Canafox DM, Ascher NL. Cyclosporin immunosuppression. Clin Pharm. 1983;2:515–524. [PubMed] [Google Scholar]
- 7.Mathew TH. A blinded long term randomized multicentric study of mycophenolate mofetil in cadaveric renal transplantation: results at 3 years. Tricontinent MMF renal transplant study group. Transplantation. 1998;65:1450–1454. doi: 10.1097/00007890-199806150-00007. [DOI] [PubMed] [Google Scholar]
- 8.Placebo controlled study of mycophenolate mofetil combined with cyclosporine and steroids for prevention of acute rejection: European MMF study group. Lancet. 1995;345:1321–1325. [PubMed] [Google Scholar]
- 9.Mycophenolate for prevention of acute rejection in primary cadaveric allograft recipients: US renal transplantation MMF study group. Transplantation. 1995;60:225–232. doi: 10.1097/00007890-199508000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Vincenti F, Jensik SC, Filo RS, Miller J, Pirsch J. A long-term comparison of Tacrolimus (FK506) and cyclosporine in kidney transplantation: Evidence for improved allograft survival at five years. Transplantation. 2002;23:775–782. doi: 10.1097/00007890-200203150-00021. [DOI] [PubMed] [Google Scholar]
- 11.Gonwa T, Johnson C, Ahsan N. Randomized trial of tacrolimus + mycophenolate mofetil or azathioprine after cadaveric kidney transplantation: results at three years. Transplantation. 2003;75:2048–2053. doi: 10.1097/01.TP.0000069831.76067.22. [DOI] [PubMed] [Google Scholar]
- 12.Sonoda T, Takahara S, Takahashi K. Outcome of 3 years of immunosuppression with tacrolimus in more than 1,000 renal transplant recipients in Japan. Transplantation. 2003;75:199–204. doi: 10.1097/01.TP.0000040867.67360.9F. [DOI] [PubMed] [Google Scholar]
- 13.Andrews PA. Renal Transplantation — Recent developments. BMJ. 2002;324:530–534. doi: 10.1136/bmj.324.7336.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nashan B, Moore R, Amlot P. Randomized trial of basiliximab versus placebo for control of acute cellular rejection in renal allograft recipients. CHIB International study group. Lancet. 1997;350:1193–1198. doi: 10.1016/s0140-6736(97)09278-7. [DOI] [PubMed] [Google Scholar]
- 15.Nashan B, Light S, Hardie IR, Lin A, Johnson JR. Reduction in acute renal allograft rejection by daclizumab. Daclizumab double therapy study group. Transplantation. 1999;67:110–115. doi: 10.1097/00007890-199901150-00019. [DOI] [PubMed] [Google Scholar]
- 16.Meier-Kriesche HU, Li S, Gruessner RWG. Immunosuppression: Evolution in Practice and Trends, 19942004. Am J Transplant. 2006;6:1111–1131. doi: 10.1111/j.1600-6143.2006.01270.x. [DOI] [PubMed] [Google Scholar]
- 17.Goldfarb-Rumyantzev AS, Smith L, Shihab FS. Role of maintenance immunosuppressive regimen in kidney transplant outcome. JASN. 2006;1:563–574. doi: 10.2215/CJN.00640805. [DOI] [PubMed] [Google Scholar]
- 18.Srivastva AK, Sinha T, Varma PP. Experience with marginal living related kidney donors: are they becoming routine or there are still any doubts? Urology. 2005;66:971–975. doi: 10.1016/j.urology.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 19.Alexender JW, Vaughan WK. Use of marginal donors for organ transplantation. The influence of donor age on outcome. Transplantation. 1991;51:135–141. doi: 10.1097/00007890-199101000-00021. [DOI] [PubMed] [Google Scholar]
- 20.Lakshmi Kiran A, Saharian S. Spousal renal transplantation. Ind J Nephrol. 1999;9:53. [Google Scholar]
- 21.Hansen B, Rohr N, Svendsen V. Bacterial urinary tract infection in cyclosporine-A immunosuppressed renal transplant recipients. Scand J Infect Dis. 1988;20:425. doi: 10.3109/00365548809032480. [DOI] [PubMed] [Google Scholar]
- 22.Gupta S, Kher V, Gulati S. Urinary tract infection in renal allograft recipients. Ind J Nephrol. 1996;9:207–210. [Google Scholar]
- 23.Kamath NS, John GT, Neelakantan N, Kirubakaran MG, Jacob CK. Bacterial infections after renal transplantation. Indian J Nephrol. 2004;14:146. [Google Scholar]
- 24.Sakhuja V, Jha V, Varma PP, Joshi K, Chugh KS. The high incidence of tuberculosis among renal transplant recipients in India. Transplantation. 1996;61:211–215. doi: 10.1097/00007890-199601270-00008. [DOI] [PubMed] [Google Scholar]
- 25.John GT, Shankar V, Abraham AM. Risk factors for post transplant tuberculosis. Kidney Int. 2001;60:1148–1151. doi: 10.1046/j.1523-1755.2001.0600031148.x. [DOI] [PubMed] [Google Scholar]
- 26.Pandays A, Wasfy S, Herbert D, Allen OD. Varicella zoster infection in paediatric solid organ transplant recipients: a hospital based study in pre-varicella vaccine era. Pediatr Transplant. 2001;5:153–159. doi: 10.1034/j.1399-3046.2001.00048.x. [DOI] [PubMed] [Google Scholar]
- 27.Pereira BJ, Wright TL, Schmid CH, Levey S, New England Organ Study Group The impact of pre-transplant hepatitis C infection on the outcome of renal transplantation. Transplantation. 1995;60:779–785. [Google Scholar]
- 28.Narula AS, Hooda AK, Anand AC, Patrikar S. Impact of hepatitis C virus infection in renal transplant recipients. Ind J Gastroenterol. 2005;24:151–154. [PubMed] [Google Scholar]
- 29.Agarwal SK, Dash SC, Irshad M, Gupta S, Bhowmik D, Tiwari SC. Impact of hepatitis C virus infection on renal transplant outcomes in India - A single centre study. J Assoc Physicians India. 2000;48:1155–1159. [PubMed] [Google Scholar]
- 30.Seth AK, Anand AC, Gedela SR, Varma PP, Baliga KV. Rapid progression of hepatitis C induced liver failure in renal allograft recipients. Ind J Gastroentrol. 2006;25:155–156. [PubMed] [Google Scholar]
- 31.Srivastava A, Sinha T, Madhusoodanan P. Urological complications of live related donor renal transplantation: 13 years experience at a single centre. Urology Int. 2006;77:42–45. doi: 10.1159/000092933. [DOI] [PubMed] [Google Scholar]


