Abstract
Whereas positron emission tomography (PET) with the antagonist ligand [18F]fallypride reveals the composite of dopamine D2 and D3 receptors in brain, treatment of Parkinson's disease (PD) patients with the D3-prefering agonist pramipexole should result in preferential occupancy in the nucleus accumbens, where the D3-subtype is most abundant. To test this prediction we obtained pairs of [18F]fallypride PET recordings in a group of nine PD patients, first in a condition of treatment as usual with pramipexole (ON-Sifrol; 3 × 0.7 mg p.d.), and again at a later date, after withholding pramipexole 48–72 h (OFF-Sifrol); in that condition the serum pramipexole concentration had declined by 90% and prolactin levels had increased four-fold, in conjunction with a small but significant worsening of PD motor symptoms. Exploratory comparison with historical control material showed 14% higher dopamine D2/3 availability in the more-affected putamen of patients OFF medication. On-Sifrol there was significant (p ˂ 0.01) occupancy at [18F]fallypride binding sites in globus pallidus (8%) thalamus (9%) and substantia nigra (19%), as well as marginally significant occupancy in frontal and temporal cortex of patients. Contrary to expectation, comparison of ON- and OFF-Sifrol results did not reveal any discernible occupancy in nucleus accumbens, or elsewhere in the extended striatum; present methods should be sensitive to a 10% change in dopamine D2/3 receptor availability in striatum; the significant findings elsewhere in the basal ganglia and in cerebral cortex are consistent with a predominance of D3 receptors in those structures, especially in substantia nigra, and imply that therapeutic effects of pramipexole may be obtained at sites outside the extended striatum.
Keywords: Parkinson's disease, Pet, Fallypride, Pramipexole, Agonist, Dopamine receptors, Occupancy
Graphical abstract
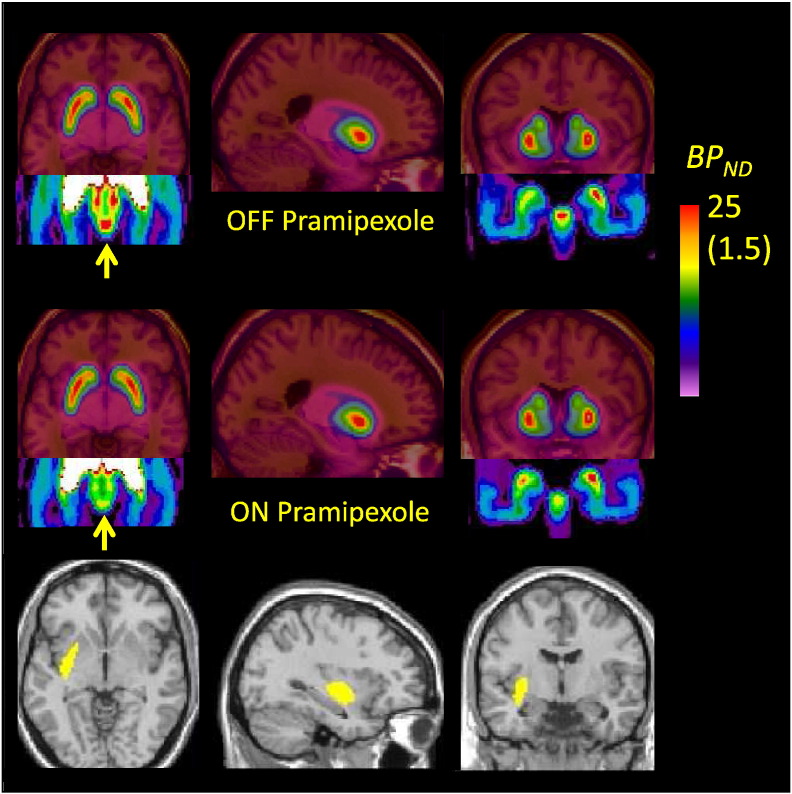
Mean parametric maps of the binding potential (BPND) of [18F]fallypride in the coronal plan of a group of nine patients with Parkinson's disease, investigated after withdrawal from Sifrol (pramipexole) and during treatment as usual, showing substantial occupancy at dopamine D2/3 receptors in the substantia nigra, but no difference in the tail of the caudate nucleus.
Highlights
-
•
Fallypride PET recordings in nine PD patients, scanned on- and off medication with pramipexole
-
•
No occupancy in the striatum, despite improved motor symptoms
-
•
Substantial occupancy in substantia nigra, thalamus and globus pallidus
1. Introduction
Levodopa and direct agonists at striatal dopamine D2/3 receptors are the mainstay treatment for motor symptoms of Parkinson's disease (PD). Among the most important dopamine agonists in clinical practice are the benzothiazole pramipexole, the dipropylaminoethyl indole ropinirole, its thiophen homologues rotigotine, piripedil, and the dibenzoquinoline apomorphine; all of these medications are characterized by partial selectivity for D3 over the more abundant D2 receptors. In the case of pramipexole, this preference is reported to be as high as 100-fold in favor of D3 receptors (Millan et al., 2002). At least in rodent models of PD, pramipexole is neuroprotective against nigrostriatal degeneration (Joyce et al., 2004, Lao et al., 2013, Kim et al., 2015), although there is little evidence of disease-modifying effects of treatment of PD patients with pramipexole (Schapira et al., 2013), or conversely that levodopa exacerbates disease progression (Fahn, 2005). Nonetheless, clinical use of pramipexole and other direct agonists is favored in younger patients with PD (< 70 years), and pramipexole in particular is considered to have an antidepressant effect in PD patients (Barone et al., 2010), in addition to its alleviation of motor symptoms, suggesting a particular link between D3 receptor activation and favorable treatment responses.
On the other hand, treatment with dopamine agonists, and to a lesser extent also levodopa, can evoke a variety of undesirable neuropsychiatric side-effects, including sleepiness and visual illusions, as well as delusions; one study reports that pramipexole is more likely than other agonists to evoke hallucinations and confusion (Kulisevsky and Pagonabarraga, 2010). Pramipexole and other direct agonists have been particularly implicated in iatrogenic impulse control disorders, including compulsive gambling, sexual behavior, buying and binge-eating. In one study of nearly 200 PD patients taking oral pramipexole, 42% developed impulse control disturbances (Garcia-Ruiz et al., 2014), versus 14% in a larger cross-sectional study (Weintraub et al., 2010). Indeed, preclinical studies show an effect of pramipexole in the probabilistic discounting paradigm, which is an index of risk-taking (Rokosik and Napier, 2012). Similarly, in a functional fMRI study of healthy young adults, pramipexole enhanced the BOLD signal change in the nucleus accumbens associated with anticipation of monetary reward, whilst reducing the apparent top-down regulation by the prefrontal cortex (Ye et al., 2011).
Autoradiographic studies of human brain indicate that D3 receptors predominate over D2 receptors in the basal forebrain and in the striosome compartment of the nucleus accumbens, are of intermediate proportion in the ventral putamen, but comprise < 10% of D2/3 receptors in the caudate nucleus (Murray et al., 1994); the occurrence of this dorsal-ventral gradient of D3 distribution in human brain is confirmed by positron emission tomography (PET) displacement studies using the D3-prefering agonist [11C]-PHNO (Narendran et al., 2006). Recent autoradiographic studies in transgenic D2-knockout mice confirmed that D3-sites normally contribute to approximately 20% of the binding of the D2/3 antagonist ligand [18F]fallypide in ventral striatum, but are nearly absent in dorsal striatum (Mukherjee et al., 2015).
Given the apparent proclivity of pramipexole to influence reward and salience, and given its particular affinity for the D3 receptors in the limbic striatum, it is a matter of interest to determine the therapeutic occupancy of pramipexole at its targets in human brain. Indeed, impairment of impulse control in Parkinson's disease patients has recently been attributed to the D3-agonist selectivity of pramipexole (Seeman, 2015). Competition binding assays by PET afford estimates of the therapeutic occupancy for CNS drugs, which have proven informative in the case of antipsychotic medications for the treatment of schizophrenia. Such studies established the concept of therapeutic occupancy window at dopamine D2/3 receptors for obtaining antipsychotic efficacy (< 65%; Farde et al., 1988) without provoking extrapyramidal side effects, which typically occur when blockade exceeds 80% (Kapur et al., 1995). This competition paradigm has since been extended to PET studies of antipsychotic occupancies at multiple receptor types, in support of the concept of atypicality (Mamo et al., 2007), and to measuring antipsychotic occupancy at the relatively less abundant extra-striatal dopamine D2/3 receptors (Vernaleken et al., 2008). However, hardly anything is known about the converse case, i.e. therapeutic occupancy of dopamine receptor agonists in the treatment of PD; oral treatment with the dopamine agonist lisuride at an effective antiparkinsonian dose (1 mg p.d.) displaced 19% of [11C]raclopride binding in the putamen of PD patients (Antonini et al., 1994), whereas in another study, a low dose of the agonist apomorphine (0.03 mg/kg) decreased [11C]raclopride binding, by 9% in the more intact putamen, and by 15% on the side contralateral to the main symptoms, suggesting an increase in affinity state in the dopamine-denervated striatum (de la Fuente-Fernández et al., 2001). A [11C]FLB-457 PET study of five healthy middle-aged volunteers showed 10–20% occupancy at extra-striatal D2/3 receptors after challenge with 0.25 mg pramipexole (Ishibashi et al., 2011), but striatal binding could not be calculated with that ligand.
In the present study we undertook to measure dopamine D2/3 receptor availability in striatum and extrastriatal regions of patients with idiopathic PD using the high affinity antagonist ligand [18F]fallypride, which is equally affine at D2 and D3 sites (Mukherjee et al., 2015). We predicted that receptor availability should be higher at medication-free baseline than under treatment as usual with pramipexole, and tested our hypothesis that occupancy by pramipexole should be higher in the limbic striatum and extra-striatal regions than in the putamen and caudate nucleus, in proportion to the relative abundance of D3 sites. In an exploratory study, we also assessed disease and treatment effects by comparison with historical [18F]fallypride PET data from a healthy age-matched control group.
2. Methods
Nine patients with PD were recruited from the Department of Neurology, Ludwig-Maximilians University, Munich and from the Max Planck Institute of Psychiatry, Munich. Nine healthy control subjects were selected as an historical control group (Rominger et al., 2012, Jansen et al., 2014) so as to obtain optimal age- and demographic matching. Subjects were excluded if they had any history of substance abuse or other psychiatric disorders or any serious medical condition (other than PD) requiring treatment with CNS medications. The study protocol was approved by the local clinical institutional review board and complied with the declaration of Helsinki. Written informed consent was obtained from all participants after the procedures had been fully explained. Healthy volunteers had a single [18F]fallypride scan at the Department of Nuclear Medicine. Patients had a baseline scan at 48–72 h after cessation of pramipexole treatment and a second [18F]fallypride PET scan two weeks later, while under treatment with pramipexole as usual.
[18F]Fallypride was synthesized as described previously (Rominger et al., 2010). A 15-minute transmission scan with a rotating [68Ge] point source was followed by a 3-hour dynamic 3D emission recording, initiated immediately upon beginning the administration of [18F]fallypride (mean dose 250 MBq) as a slow intravenous bolus. The dynamic recording consisted of 39 time frames (3 × 20 s, 3 × 1 min, 3 × 2 min, 3 × 3 min, 21 × 5 min, 2 × 8 min and 4 × 10 min; 180 min in total), which were recorded with an ECAT EXACT HR + PET (Siemens/CTI, Knoxville, TN, USA). During emission and transmission scans, the subjects' heads were comfortably immobilized within the aperture using a foam cushion. The tomograph acquired 63 contiguous transaxial planes, simultaneously covering 15.5 cm in the axial field of view, with 4.0 mm centre of field resolution (full width at half maximum). Dynamic image data were reconstructed as 128 × 128 matrices of 2.1 × 2.1 × 2.4 mm voxels by 3D filtered back-projection using a Hann filter with a cut-off frequency of 0.5 Nyquist units. Final data were corrected for decay, randoms, dead time and scatter. Emission recordings were checked for motion between fraqmes, and then registered and normalized to an in-house standard brain [18F]fallypride template in MNI coordinates using the SPM5 routines (Wellcome Department of Cognitive Neurology, London, UK) implemented in MATLAB (version 7.1, MathWorks Inc., Natick, MA, USA). Details of the image analysis procedure are reported elsewhere (Rominger et al., 2012, Cumming et al., 2013). The sum of frames 9 to 13 (8–19 min) was used as source image for normalization the individual PET image using the SPM coregistration and normalization procedures. We then applied a cerebellum template (excluding the vermis) to the dynamic volumes using the program amide (http://amide.sourceforge.net) to calculate the time-activity-curve of the reference region. Using PMOD (PMOD technologies, Zurich, Switzerland) we then calculated voxelwise parametric maps of the [18F]fallypride binding potential (BPND), using a linear graphical method (Logan et al., 1996) relative to cerebellum, as previously described for [18F]fallypride (Mukherjee et al., 2002). Templates for caudate nucleus, posterior and anterior putamen, nucleus accumbens, thalamus, globus pallidus, insular and temporal cortex and the pituitary gland, were applied to the individual parametric maps in order to obtain the mean BPND by region. We used an in-house template for the striatum and its subdivisions (la Fougère et al., 2010) and the Wake Forrest University Pick Atlas for other regions (Maldjian et al., 2003).
[18F]Fallypide PET scans were obtained from nine neurologically normal control subjects of mean (SD) age 63 ± 6 years who had participated in earlier studies (Rominger et al., 2012, Jansen et al., 2014). In an exploratory study, the spatially normalized mean parametric maps from the patients with PD (OFF condition) and the healthy control group were compared (subtraction analysis). The threshold for significant difference was arbitrarily set at 0.5 units of BPND. For statistical comparisons with predictable outcomes (i.e. UPDRS III scores and prolactin levels would increase in the OFF condition) we used the Student's 1-tailed t-test
3. Results
The nine patients (7 male) were of mean (± SD) age 65 ± 6 years, and had a diagnosis of PD since 6 ± 3 years. Five were receiving pramipexole monotherapy (3 × 0.7 mg), and four received additional levodopa (from 100 mg to 400 mg per day). The baseline PET scans (ON) was obtained under conditions of pramipexole medication as usual (pramipexole 3 × 0.7 mg), except that the patients who additionally took levodopa withdrew for 18 h before the scan. Scanning was always performed at 11:30 a.m. Two weeks later, the second PET scan (OFF) was obtained after pramipexole had been paused for 48–60 h; again, levodopa was paused for 18 h before the scan. Medication as usual was resumed after conclusion of the second PET recording in each patient.
The mean UPDRS III score was 11.3 ± 4.8 in the ON condition, and deteriorated significantly to 15.0 ± 3.0 in the OFF condition (p = 0.008). Mean prolactin levels increased from 13 ± 7 IU/ml in the ON condition to 65 ± 39 IU/ml in the OFF condition (n = 7; p = 0.008). Plasma pramipexole levels declined from 2590 ± 870 (range 910–3960) ng/l in the ON condition to 300 ± 240 (range 186–689) ng/l in the OFF condition (p = 0.00002), showing a strong negative correlation between individual pramipexole and prolactin levels (R = − 0.81: p < 0.001).
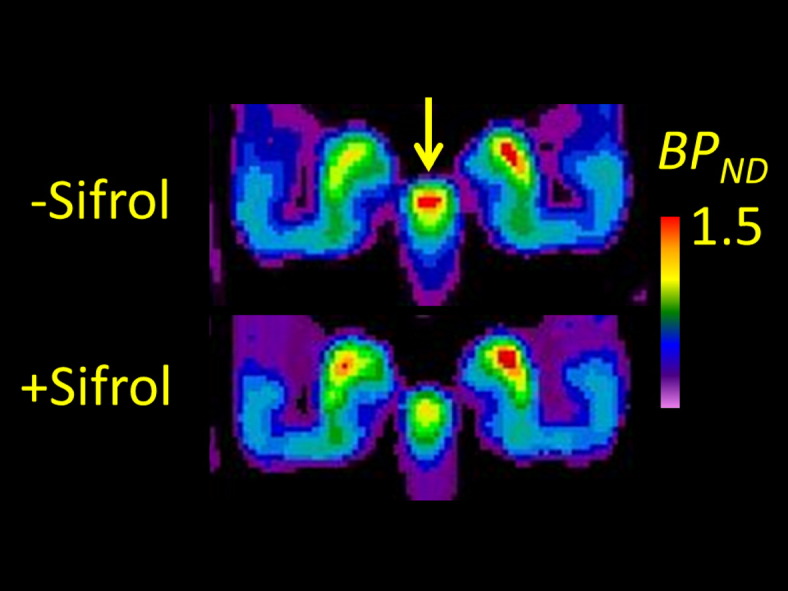
Mean parametric maps ON and OFF pramipexole (Fig. 1) are depicted with flipping of images so that the left cerebral hemisphere is contralateral to the main motor symptoms. There is no discernible difference by condition in the mean BPND anywhere in the extended striatum, although a reduction in availability was clearly discernible in the substantia nigra (insets), when the color scale was restricted for visualization of the much lower binding. Examination of individual parametric maps suggested that patients had relatively greater striatal volume loss and more pronounced ventricular dilation than did the age-matched controls. In the absence of individual MR images, registration and normalization procedures tended to result in misalignment of the VOI template set, such that binding results between patients and controls were not directly comparable. However, the exploratory voxelwise subtraction revealed a zone in the left (more affected) putamen where BPND in the patients scanned OFF pramipexole exceeded that in the healthy controls by at least 0.5 units; the mean magnitude of BPND the corresponding masked region was 10.5 ± 1.5 in the healthy controls, suggesting a 14% increase in dopamine D2/3 availability on the more-affected side in patients OFF medication.
Fig. 1.
Mean parametric maps of [18F]fallypride BPND in a group of nine patients with Parkinson's disease scanned OFF medication (upper row) and ON pramipexole monotherapy, at a dose significantly alleviating motor symptoms (middle row). The insets show the results in substantia nigra, with the BPND color scale restricted to the range 0–1.5 units (rather than 0–25 as required for depiction of striatal binding). An exploratory voxelwise subtraction of the mean OFF medication condition and results from the historical age-matched healthy controls (bottom row) show a zone in the left striatum (i.e. contralateral to body side with main symptoms) in which BPND exceeded that in the healthy controls by at least 0.5 units; the mean increase in that volume corresponded 14% relative to the control group.
VOI analysis revealed no significant left-right differences in the regional [18F]fallypride BPND, either at Scan 1 or Scan 2, irrespective of flipping the maps, so that main motor symptoms were contralateral to the left cerebral hemisphere; for simplicity, we therefore report the mean BPND measurements for bilateral structures in Table 1. Based on the hypothesis that [18F]fallypride BPND should decrease ON pramipexole, the 1-tailed t-test showed significant decreases in bilateral globus pallidus (8%; p = 0.007), thalamus (9%; p = 0.002) substantia nigra (19%; p = 0.0004), as well as frontal cortex (28%; p = 0.043) and temporal cortex (9%; p = 0.023). There were no significant correlations between individual estimates of occupancy (BPND changes between scans) and level of pramipexole or prolactin OFF medication (data not shown).
Table 1.
The binding potential (BPND) for [18F]fallypride in various brain regions in a group of Parkinson's disease patients aged 67 ± 6 years. The patients were scanned during their regular pramipexole treatment (ON, Scan 1) and after withdrawal for 48–72 h (OFF, Scan 2). Each result is the mean (± SD) of determinations in nine subjects. There were significant differences between the ON and OFF conditions in thalamus, frontal cortex and temporal cortex. Occupancies in the ON condition are estimated from the reduction in BPND ON pramipexole relative to that OFF pramipexole.
| ON pramipexole | OFF pramipexole | |
|---|---|---|
| Caudate nucleus | 10.4 ± 1.1 (2 ± 5%) | 10.3 ± 1.3 |
| Putamen, anterior | 16.9 ± 1.1 (1 ± 5%) | 17.1 ± 1.3 |
| Putamen, posterior | 11.4 ± 0.9 (3 ± 6%) | 11.8 ± 1.3 |
| Nucleus accumbens | 8.6 ± 1.4 (1 ± 5%) | 8.7 ± 1.3 |
| Globus pallidus | 5.5 ± 2.3 (8 ± 6%; p = 0.007) | 5.9 ± 2.0 |
| Substantia nigra | 1.04 ± 0.35 (19 ± 12%; p = 0.0004) | 1.24 ± 0.29 |
| Thalamus | 0.88 ± 0.25 (9 ± 6%; p = 0.002) | 0.96 ± 0.04 |
| Hippocampus | 0.57 ± 0.20 (0 ± 0%) | 0.59 ± 0.21 |
| Amygdala | 1.61 ± 0.67 (5 ± 12%) | 1.72 ± 0.72 |
| Frontal cortex | 0.12 ± 0.07 (28 ± 31%; p = 0.043) | 0.14 ± 0.05 (p = 0.043) |
| Temporal cortex | 0.29 ± 0.12 (9 ± 23%; p = 0.023) | 0.32 ± 0.13 |
| Pituitary gland | 1.20 ± 0.43 (7 ± 28%) | 1.34 ± 0.37 |
4. Discussion
Our main finding was the discovery of significant therapeutic occupancy by pramipexole at [18F]fallypride binding sites in bilateral globus pallidus, thalamus substantia nigra, and in the temporal and frontal cortex; contrary to expectation, there was no discernible occupancy in nucleus accumbens, or elsewhere in the extended striatum. Our finding of 9% occupancy by pramipexole at thalamic dopamine D2/3 sites, is consistent the distribution of binding sites for the D3-prefering agonist [11C]-PHNO (Narendran et al., 2006), and concurs closely with the 10–20% displacement of [11C]FLB 457 in human thalamus by acute pramipexole challenge (Ishibashi et al., 2011). Our thalamus VOI encompasses the entire thalamus, thus reflecting [18F]fallypride binding not just in motor structures (ventrolateral and ventro-anterior thalamus), but also in sensory thalamus. The relevance of this occupancy by thalamic pramipexole is uncertain, since post mortem investigation shows that the dopamine content of thalamus is relatively preserved in idiopathic PD (Gerlach et al., 1996). There was a 17% reduction in [11C]FLB-457 binding to thalamic D2/3 receptors in patients with advanced PD (Kaasinen et al., 2000, Kaasinen et al., 2003), but similar thalamic decreases have also been seen in a meta-analysis of studies of schizophrenia (Kambeitz et al., 2014). We can only speculate that present findings may imply a rectification by pramipexole of sensorimotor deficits related to impaired thalamic dopamine signalling in PD patients.
We found 8% occupancy by therapeutic pramipexole in the globus pallidus, with the caveat that [18F]fallypride BPND in that brain region might be vulnerable to spill-over arising in the adjacent putamen. However, the absence of discernible occupancy in putamen, where BPND is highest, suggests that we have observed a real occupancy by therapeutic pramipexole at dopamine D2/3 receptors in globus pallidus. In a previous [11C]PHNO study in healthy volunteers with pharmacological dopamine depletion, increasing (D3) receptor availability in globus pallidus correlated with scores in a self-report of vigor (Caravaggio et al., 2014). The present data are thus consistent with a therapeutic action of pramipexole through activation of D3 receptors in the globus pallidus of PD patients.
The most highly pronounced difference in [18F]fallypride binding to emerge in this study was in the substantia nigra, where pramipexole occupancy was approximately 19%. While [18F]fallypride BPND is of low magnitude in substantia nigra (i.e. Slifstein et al., 2010), the contrast between ON and OFF pramipexole was highly significant (p = 0.0004), and would survive much more stringent statistical criteria. This is notable given the exclusive D3 nature of [18F]fallypride binding expected in that region based on studies cited above, and also considering the sedative property of low dose pramipexole, which may preferentially act at presynaptic autoreceptors, i.e. in the substantia nigra, to attenuate dopamine release (McCormick et al., 2015). Earlier [11C]PHNO PET observations of elevated D3 binding in the substantia nigra of pathological gamblers (Boileau et al., 2013) and increased displacement of D3 binding in substantia nigra of PD patients during performance of a gambling task (Boileau et al., 2014, Ray et al., 2012), call for further consideration of the effects of pramipexole treatment on the functional state or occupancy at somatodendritic autoreceptors in the substantia nigra. This may prove to be especially interesting in relation to the reports of impulse control disorders in PD patients treated with pramipexole (Seeman et al., 2015), and in consideration of the somewhat ambiguous role of D3 receptors in autoreceptor regulation in the substantia nigra (Koweltzow et al., 1998).
We have previously reported preferential blockade of cortical [18F]fallypride binding by the rather non-selective antagonist quetiapine (Vernaleken et al., 2010), and now report therapeutic occupancy of an agonist at cortical dopamine D2/3 receptors. We concede that the pramipexole occupancies (28% in frontal cortex and 9% in temporal cortex) are imprecise, in keeping with the relatively high covariance of our methods for quantitation of the low abundance cortical D2/3 receptors (Rominger et al., 2012). Present findings in cerebral cortex are in any event exploratory, since our hypothesis centred on occupancy in the limbic striatum. Nonetheless, we see a good concordance with the 10% displacement of D2/3 binding in frontal cortex of healthy volunteers by acute pramipexole in a PET study with [11C]FLB-457 (Ishibashi et al., 2011), which is arguably a fitter tracer for detecting cortical D2/3 receptors (Narendran et al., 2013) Pharmacology studies indicate that the dopamine autoreceptors controlling dopamine release from human neocortex slices are of the D2 type (Fedele et al., 1999), although this action is attributed to D3 receptors in frontal cortex of behaving rats (Gobert et al., 1996). While the proportion of D2 and D3 sites in human cerebral cortex remains unknown, a recent review has drawn attention to procognitive effects of D3 agonists, and suggested a mechanism mediated by enhanced acetylcholine release specifically in prefrontal cortex (Nakajima et al., 2013). Given the frequent occurrence of executive function deficits in patients with PD, present findings could implicate frontal cortex in the therapeutic action of pramipexole.
Contrary to expectation we did not detect any discernible occupancy in the nucleus accumbens, or elsewhere in the extended striatum; our earlier test-retest findings predicted that we should have had sufficient power to detect a 10% decline in [18F]fallypride BPND in striatum (Rominger et al., 2012), similar in magnitude to the effects reported earlier in [11C]raclopride PET studies in healthy pigs treated with LSD, inter alia, a dopamine agonist (Minuzzi et al., 2005) or in PD patients treated with a challenge dose of lisuride (1 mg: Antonini et al., 1994), or apomorphine (0.03 mg/kg; de la Fuente-Fernández et al., 2001). Indeed the apomorphine challenge study reported more extensive occupancy in the striatum contralateral to the main motor symptoms, suggesting a certain sensitization of agonist binding in the more severely dopamine denervated striatum, as is likewise reported in rat lesion studies (Palner et al., 2011). This phenomenon may have contributed to the 14% increase in [18F]fallypride binding in part of the putamen contralateral to the most symptomatic side, calculated relative to the age-matched healthy control group. Such increases are not always evident in patients with long-standing PD (Knudsen et al., 2004), but may be a transient feature of early disease, or in this case related to pause of medication.
Pharmacological displacement studies with the D3-selective antagonist GSK598809 in healthy volunteers indicated that 0% of [11C]-PHNO binding was to D3 receptors in dorsal striatum, versus 25% in ventral striatum, 50% in thalamus, and 100% in substantia nigra (Searle et al., 2010), although lesser regional D3 selectivity was seen with the antagonist ABT-925 (Graff-Guerrero et al., 2010). If one half of our [18F]fallypide binding in thalamus (BPND = 0.96) corresponds to D3 receptors, we can then estimate that up to 20% of these sites were occupied by pramipexole in the ON-medication condition. Given a 25% D3 component of [18F]fallypride binding in ventral striatum (25% of total BPND = 2.2), a 20% occupancy by pramipexole should have reduced [18F]fallypride binding by 0.4 BPND units.
The lack of discernible occupancy in the extended striatum is perplexing, and calls for consideration of some limitations of the study. Medication history or disease state may have attenuated the D3 occupancy by therapeutic pramipexole, although the possibility of a false negative result in striatum cannot be excluded, given our small sample size. For logistic reasons, we conducted the ON-pramipexole scans first, without randomization, being unaware of any reports of an ordering effect on [18F]fallypride binding. Although it might have been preferable to have obtained individual MR images, our subtraction of [18F]fallypride BPND maps within individuals, after normalization to a standard template should accommodate effects of brain atrophy on the magnitude of Δ-BPND. Although the deterioration in UPDRS score OFF-medication was less than expected, the study was not designed to confirm the clinical efficacy of pramipexole. Nonetheless, a pharmacodynamic effect is supported by the release of inhibition of prolactin secretion at the time of the second PET scan, which correlated inversely with the residual plasma concentration of pramipexole. The evidently incomplete clearance of plasma pramipexole, which remained at a mean of 10% of the therapeutic level during the OFF medication scan, is consistent with the 13 h plasma half-life reported in humans (Wright et al., 1997). As such, the possibility of prolonged retention and action of pramipexole in brain cannot be excluded.
While the occupancy paradigm is well-described in the case of antipsychotic medications, the corresponding occupancy for dopamine agonist ligands is the subject of only a few studies. The present finding of indiscernible effect of pramipexole at a therapeutic dose on [18F]fallypride binding in striatum, despite significant displacement in substantia nigra and other extra-striatal regions calls for further investigations of dopamine D3 agonist occupancy in Parkinson's disease patients and other clinical populations.
Acknowledgments
We thank Mrs. Katrin Richter for expert technical assistance in performing the [18F]fallypride PET studies.
References
- Antonini A., Schwarz J., Oertel W.H., Beer H.F., Madeja U.D., Leenders K.L. [11C]raclopride and positron emission tomography in previously untreated patients with Parkinson's disease: influence of L-dopa and lisuride therapy on striatal dopamine D2-receptors. Neurology. 1994;44(7):1325–1329. doi: 10.1212/wnl.44.7.1325. [DOI] [PubMed] [Google Scholar]
- Barone P., Poewe W., Albrecht S., Debieuvre C., Massey D., Rascol O., Tolosa E., Weintraub D. Pramipexole for the treatment of depressive symptoms in patients with Parkinson's disease: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2010;9(6):573–580. doi: 10.1016/S1474-4422(10)70106-X. [DOI] [PubMed] [Google Scholar]
- Boileau I., Payer D., Chugani B., Lobo D., Behzadi A., Rusjan P.M., Houle S., Wilson A.A., Warsh J., Kish S.J., Zack M. The D2/3 dopamine receptor in pathological gambling: a positron emission tomography study with [11C]-(+)-propyl-hexahydro-naphtho-oxazin and [11C]raclopride. Addiction. 2013;108(5):953–963. doi: 10.1111/add.12066. [DOI] [PubMed] [Google Scholar]
- Boileau I., Payer D., Chugani B., Lobo D.S., Houle S., Wilson A.A., Warsh J., Kish S.J., Zack M. In vivo evidence for greater amphetamine-induced dopamine release in pathological gambling: a positron emission tomography study with [(11)C]-(+)-PHNO. Mol. Psychiatry. 2014;19(12):1305–1313. doi: 10.1038/mp.2013.163. [DOI] [PubMed] [Google Scholar]
- Caravaggio F., Nakajima S., Borlido C., Remington G., Gerretsen P., Wilson A., Houle S., Menon M., Mamo D., Graff-Guerrero A. Estimating endogenous dopamine levels at D2 and D3 receptors in humans using the agonist radiotracer [(11)C]-(+)-PHNO. Neuropsychopharmacology. 2014;39(12):2769–2776. doi: 10.1038/npp.2014.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cumming P., Xiong G., la Fougère C., Rominger A., Bartenstein P., Buchholz H.G., Piel M., Rösch F., Gründer G., I. V. Surrogate markers for cerebral blood flow correlate with [18F]-fallypride binding potential at dopamine D(2/3) receptors in human striatum. Synapse. 2013;67(4):199–203. doi: 10.1002/syn.21630. [DOI] [PubMed] [Google Scholar]
- Fahn S. Does levodopa slow or hasten the rate of progression of Parkinson's disease? J. Neurol. 2005;252(Suppl. 4):IV37–IV42. doi: 10.1007/s00415-005-4008-5. [DOI] [PubMed] [Google Scholar]
- Farde L., Wiesel F.A., Halldin C., G. S. Central D2-dopamine receptor occupancy in schizophrenic patients treated with antipsychotic drugs. Arch. Gen. Psychiatry. 1988;45(1):71–76. doi: 10.1001/archpsyc.1988.01800250087012. [DOI] [PubMed] [Google Scholar]
- Fedele E., Fontana G., Munari C., Cossu M., Raiteri M. Native human neocortex release-regulating dopamine D2 type autoreceptors are dopamine D2 subtype. Eur. J. Neurosci. 1999;11(7):2351–2358. doi: 10.1046/j.1460-9568.1999.00651.x. [DOI] [PubMed] [Google Scholar]
- la Fougère C., Popperl G., Levin J., Wangler B., Boning G., Uebleis C., Cumming P., Bartenstein P., Botzel K., Tatsch K. The value of the dopamine D2/3 receptor ligand 18Fdesmethoxyfallypride for the differentiation of idiopathic and nonidiopathic Parkinsonian syndromes. J. Nucl. Med. 2010;51:581–587. doi: 10.2967/jnumed.109.071811. [DOI] [PubMed] [Google Scholar]
- de la Fuente-Fernández R., Lim A.S., Sossi V., Holden J.E., Calne D.B., Ruth T.J., Stoessl A.J. Apomorphine-induced changes in synaptic dopamine levels: positron emission tomography evidence for presynaptic inhibition. J. Cereb. Blood Flow Metab. 2001;21(10):1151–1159. doi: 10.1097/00004647-200110000-00003. [DOI] [PubMed] [Google Scholar]
- Garcia-Ruiz P.J., Martinez Castrillo J.C., Alonso-Canovas A., Herranz Barcenas A., Vela L., Sanchez Alonso P., Mata M., Olmedilla Gonzalez N., Mahillo F.I. Impulse control disorder in patients with Parkinson's disease under dopamine agonist therapy: a multicentre study. J. Neurol. Neurosurg. Psychiatry. 2014;85(8):840–844. doi: 10.1136/jnnp-2013-306787. [DOI] [PubMed] [Google Scholar]
- Gerlach M., Gsell W., Kornhuber J., Jellinger K., Krieger V., Pantucek F., Vock R., Riederer P. A post mortem study on neurochemical markers of dopaminergic, GABA-ergic and glutamatergic neurons in basal ganglia-thalamocortical circuits in Parkinson syndrome. Brain Res. 1996;741(1–2):142–152. doi: 10.1016/s0006-8993(96)00915-8. [DOI] [PubMed] [Google Scholar]
- Gobert A., Lejeune F., Rivet J.M., Cistarelli L., Millan M.J. Dopamine D3 (auto) receptors inhibit dopamine release in the frontal cortex of freely moving rats in vivo. J. Neurochem. 1996;66(5):2209–2212. doi: 10.1046/j.1471-4159.1996.66052209.x. [DOI] [PubMed] [Google Scholar]
- Graff-Guerrero A., Redden L., Abi-Saab W., Katz D.A., Houle S., Barsoum P., Bhathena A., Palaparthy R., Saltarelli M.D., Kapur S. Blockade of [11C](+)-PHNO binding in human subjects by the dopamine D3 receptor antagonist ABT-925. Int. J. Neuropsychopharmacol. 2010;13(3):273–287. doi: 10.1017/S1461145709990642. [DOI] [PubMed] [Google Scholar]
- Ishibashi K., Ishii K., Oda K., Mizusawa H., Ishiwata K. Binding of pramipexole to extra-striatal dopamine D2/D3 receptors in the human brain: a positron emission tomography study using 11C-FLB 457. PLoS One. 2011;6(3):e17723. doi: 10.1371/journal.pone.0017723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen N.L., Feuerecker R., Becker-Bense S., Zwergal A., Wulff M., Xiong G., Wängler B., Cumming P., Bartenstein P., Dieterich M., la Fougère C. Assessment of cerebral dopamine D 2/3 formula-receptors in patients with bilateral vestibular failure. J. Vestib. Res. 2014;24(5–6):403–413. doi: 10.3233/VES-140526. [DOI] [PubMed] [Google Scholar]
- Joyce J.N., Woolsey C., Ryoo H., Borwege S., Hagner D. Low dose pramipexole is neuroprotective in the MPTP mouse model of Parkinson's disease, and downregulates the dopamine transporter via the D3 receptor. BMC Biol. 2004;11:2–22. doi: 10.1186/1741-7007-2-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaasinen V., Aalto S., NAgren K., Hietala, Sonninen P., Rinne J.O. Extrastriatal dopamine D(2) receptors in Parkinson's disease: a longitudinal study. J. Neural Transm. 2003;110(6):591–601. doi: 10.1007/s00702-003-0816-x. [DOI] [PubMed] [Google Scholar]
- Kaasinen V., Någren K., Hietala J., Oikonen V., Vilkman H., Farde L., Halldin C., Rinne J.O. Extrastriatal dopamine D2 and D3 receptors in early and advanced Parkinson's disease. Neurology. 2000;54(7):1482–1487. doi: 10.1212/wnl.54.7.1482. [DOI] [PubMed] [Google Scholar]
- Kambeitz J., Abi-Dargham A., Kapur S., Howes O.D. Alterations in cortical and extrastriatal subcortical dopamine function in schizophrenia: systematic review and meta-analysis of imaging studies. Br. J. Psychiatry. 2014;204(6):420–429. doi: 10.1192/bjp.bp.113.132308. [DOI] [PubMed] [Google Scholar]
- Kapur S., Remington G., Zipursky R.B., Wilson A.A., Houle S. The D2 dopamine receptor occupancy of risperidone and its relationship to extrapyramidal symptoms: a PET study. Life Sci. 1995;57(10):PL103–PL107. doi: 10.1016/0024-3205(95)02037-j. [DOI] [PubMed] [Google Scholar]
- Kim M.K., Park H.S., Cho J.H., Kim G.S., Won C. Pramipexole protects dopaminergic neurons through paraplegin against 6-hydroxydopamine. Neuroreport. 2015;26(2):74–80. doi: 10.1097/WNR.0000000000000303. [DOI] [PubMed] [Google Scholar]
- Knudsen G.M., Karlsborg M., Thomsen G., Krabbe K., Regeur L., Nygaard T., Videbaek C., Werdelin L. Imaging of dopamine transporters and D2 receptors in patients with Parkinson's disease and multiple system atrophy. Eur. J. Nucl. Med. Mol. Imaging. 2004;31(12):1631–1638. doi: 10.1007/s00259-004-1578-x. [DOI] [PubMed] [Google Scholar]
- Kulisevsky J., Pagonabarraga J. Tolerability and safety of ropinirole versus other dopamine agonists and levodopa in the treatment of Parkinson's disease: meta-analysis of randomized controlled trials. Drug Saf. 2010;33(2):147–161. doi: 10.2165/11319860-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Lao C.L., Kuo Y.H., Hsieh Y.T., Chen J.C. Intranasal and subcutaneous administration of dopamine D3 receptor agonists functionally restores nigrostriatal dopamine in MPTP-treated mice. Neurotox. Res. 2013;24(4):523–531. doi: 10.1007/s12640-013-9408-1. [DOI] [PubMed] [Google Scholar]
- Logan J., Fowler J.S., Volkow N.D., Wang G.J., Ding Y.S., Alexoff D.L. Distribution volume ratios without blood sampling from graphical analysis of PET data. J. Cereb. Blood Flow Metab. 1996;16:834–840. doi: 10.1097/00004647-199609000-00008. [DOI] [PubMed] [Google Scholar]
- Maldjian J.A., Laurienti P.J., Kraft R.A., Burdette J.H. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Mamo D., Graff A., Mizrahi R., Shammi C.M., Romeyer F., Kapur S. Differential effects of aripiprazole on D(2), 5-HT(2), and 5-HT(1A) receptor occupancy in patients with schizophrenia: a triple tracer PET study. Am. J. Psychiatry. 2007;164(9):1411–1417. doi: 10.1176/appi.ajp.2007.06091479. [DOI] [PubMed] [Google Scholar]
- McCormick P.N., Fletcher P.J., Wilson V.S., Browne J.D., Nobrega J.N., Remington G.J. Low dose pramipexole causes D3 receptor-independent reduction of locomotion and responding for a conditioned reinforcer. Neuropharmacology. 2015;89:225–231. doi: 10.1016/j.neuropharm.2014.09.026. [DOI] [PubMed] [Google Scholar]
- Millan M.J., Maiofiss L., Cussac D., Audinot V., Boutin J.A., Newman-Tancredi A. Differential actions of antiparkinson agents at multiple classes of monoaminergic receptor. I. A multivariate analysis of the binding profiles of 14 drugs at 21 native and cloned human receptor subtypes. J. Pharmacol. Exp. Ther. 2002;303(2):791–804. doi: 10.1124/jpet.102.039867. [DOI] [PubMed] [Google Scholar]
- Minuzzi L., Nomikos G.G., Wade M.R., Jensen S.B., Olsen A.K., Cumming P. Interaction between LSD and dopamine D2/3 binding sites in pig brain. Synapse. 2005;56(4):198–204. doi: 10.1002/syn.20141. [DOI] [PubMed] [Google Scholar]
- Mukherjee J., Christian B.T., Dunigan K.A., Shi B., Narayanan T.K., Satter M., Mantil J. Brain imaging of 18F-fallypride in normal volunteers: blood analysis, distribution, test-retest studies, and preliminary assessment of sensitivity to aging effects on dopamine D-2/D-3 receptors. Synapse. 2002;46:170–188. doi: 10.1002/syn.10128. [DOI] [PubMed] [Google Scholar]
- Mukherjee J., Constantinescu C.C., Hoang A.T., Jerjian T., Majji D., Pan M.L. Dopamine D3 receptor binding of (18) F-fallypride: evaluation using in vitro and in vivo PET imaging studies. Synapse. 2015;69(12):577–591. doi: 10.1002/syn.21867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray A.M., Ryoo H.L., Gurevich E., Joyce J.N. Localization of dopamine D3 receptors to mesolimbic and D2 receptors to mesostriatal regions of human forebrain. Proc. Natl. Acad. Sci. U. S. A. 1994;91(23):11271–11275. doi: 10.1073/pnas.91.23.11271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima S., Gerretsen P., Takeuchi H., Caravaggio F., Chow T., Le Foll B., Mulsant B., Pollock B., Graff-Guerrero A. The potential role of dopamine D3 receptor neurotransmission in cognition. Eur. Neuropsychopharmacol. 2013;23(8):799–813. doi: 10.1016/j.euroneuro.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narendran R., Himes M., NS M. Reproducibility of post-amphetamine [11C]FLB 457 binding to cortical D2/3 receptors. PLoS One. 2013;8(9):e76905. doi: 10.1371/journal.pone.0076905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narendran R., Slifstein M., Guillin O., Hwang Y., Hwang D.R., Scher E., Reeder S., Rabiner E., Laruelle M. Dopamine (D2/3) receptor agonist positron emission tomography radiotracer [11C]-(+)-PHNO is a D3 receptor preferring agonist in vivo. Synapse. 2006;60(7):485–495. doi: 10.1002/syn.20325. [DOI] [PubMed] [Google Scholar]
- Palner M., Kjaerby C., Knudsen G.M., Cumming P. Effects of unilateral 6-OHDA lesions on [3H]-N-propylnorapomorphine binding in striatum ex vivo and vulnerability to amphetamine-evoked dopamine release in rat. Neurochem. Int. 2011;58(3):243–247. doi: 10.1016/j.neuint.2010.12.007. [DOI] [PubMed] [Google Scholar]
- Ray N.J., Miyasaki J.M., Zurowski M., Ko J.H., Cho S.S., Pellecchia G., Antonelli F., Houle S., Lang A.E., Strafella A.P. Extrastriatal dopaminergic abnormalities of DA homeostasis in Parkinson's patients with medication-induced pathological gambling: a [11C] FLB-457 and PET study. Neurobiol. Dis. 2012;48(3):519–525. doi: 10.1016/j.nbd.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rokosik S.L., Napier T.C. Pramipexole-induced increased probabilistic discounting: comparison between a rodent model of Parkinson's disease and controls. Neuropsychopharmacology. 2012;37(6):1397–1408. doi: 10.1038/npp.2011.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rominger A., Cumming P., Xiong G., Koller G., Böning G., Wulff M., Zwergal A., Förster S., Reilhac A., Munk O., Soyka M., Wängler B., Bartenstein P., la Fougère C., Pogarell O. [18F]Fallypride PET measurement of striatal and extrastriatal dopamine D 2/3 receptor availability in recently abstinent alcoholics. Addict. Biol. 2012;17(2):490–503. doi: 10.1111/j.1369-1600.2011.00355.x. [DOI] [PubMed] [Google Scholar]
- Rominger A., Wagner E., Mille E., Böning G., Esmaeilzadeh M., Wängler B., Gildehaus F.J., Nowak S., Bruche A., Tatsch K., Bartenstein P., Cumming P. Endogenous competition against binding of [(18)F]DMFP and [(18)F]fallypride to dopamine D(2/3) receptors in brain of living mouse. Synapse. 2010;64(4):313–322. doi: 10.1002/syn.20730. [DOI] [PubMed] [Google Scholar]
- Schapira A.H., McDermott M.P., Barone P., Comella C.L., Albrecht S., Hsu H.H., Massey D.H., Mizuno Y., Poewe W., Rascol O., Marek K. Pramipexole in patients with early Parkinson's disease (PROUD): a randomised delayed-start trial. Lancet Neurol. 2013;12(8):747–755. doi: 10.1016/S1474-4422(13)70117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Searle G., Beaver J.D., Comley R.A., Bani M., Tziortzi A., Slifstein M., Mugnaini M., Griffante C., Wilson A.A., Merlo-Pich E., Houle S., Gunn R., Rabiner E.A., Laruelle M. Imaging dopamine D3 receptors in the human brain with positron emission tomography, [11C]PHNO, and a selective D3 receptor antagonist. Biol. Psychiatry. 2010;68(4):392–399. doi: 10.1016/j.biopsych.2010.04.038. [DOI] [PubMed] [Google Scholar]
- Seeman P. Parkinson's disease treatment may cause impulse-control disorder via dopamine D3 receptors. Synapse. 2015;69(4):183–189. doi: 10.1002/syn.21805. [DOI] [PubMed] [Google Scholar]
- Slifstein M., Kegeles L.S., Xu X., Thompson J.L., Urban N., Castrillon J., Hackett E., Bae S.A., Laruelle M., Abi-Dargham A. Striatal and extrastriatal dopamine release measured with PET and [(18)F] fallypride. Synapse. 2010;64(5):350–362. doi: 10.1002/syn.20734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernaleken I., Fellows C., Janouschek H., Bröcheler A., Veselinovic T., Landvogt C., Boy C., Buchholz H.G., Spreckelmeyer K., Bartenstein P., Cumming P., Hiemke C., Rösch F., Schäfer W., Wong D.F., Gründer G. Striatal and extrastriatal D2/D3-receptor-binding properties of ziprasidone: a positron emission tomography study with [18F]fallypride and [11C]raclopride (D2/D3-receptor occupancy of ziprasidone) J. Clin. Psychopharmacol. 2008;28(6):608–617. doi: 10.1097/JCP.0b013e31818ba2f6. [DOI] [PubMed] [Google Scholar]
- Vernaleken I., Janouschek H., Raptis M., Hellmann S., Veselinovic T., Bröcheler A., Boy C., Cumming P., Hiemke C., Rösch F., Schäfer W.M., Gründer G. Dopamine D2/3 receptor occupancy by quetiapine in striatal and extrastriatal areas. Int. J. Neuropsychopharmacol. 2010;13(7):951–960. doi: 10.1017/S1461145710000374. [DOI] [PubMed] [Google Scholar]
- Weintraub D., Koester J., Potenza M.N., Siderowf A.D., Stacy M., Voon V., Whetteckey J., Wunderlich G.R., Lang A.E. Impulse control disorders in Parkinson disease: a cross-sectional study of 3090 patients. Arch. Neurol. 2010;67(5):589–595. doi: 10.1001/archneurol.2010.65. [DOI] [PubMed] [Google Scholar]
- Wright C.E., Sisson T.L., Ichhpurani A.K., Peters G.R. Steady-state pharmaco-kinetic properties of pramipexole in healthy volunteers. J. Clin. Pharmacol. 1997;37(6):520–525. doi: 10.1002/j.1552-4604.1997.tb04330.x. [DOI] [PubMed] [Google Scholar]
- Ye Z., Hammer A., Camara E., Münte T.F. Pramipexole modulates the neural network of reward anticipation. Hum. Brain Mapp. 2011;32(5):800–811. doi: 10.1002/hbm.21067. [DOI] [PMC free article] [PubMed] [Google Scholar]