Abstract
Aplastic anemia (AA) is a life-threatening bone marrow failure disorder, if untreated, is associated with very high mortality. Allogenic bone marrow transplantation (BMT) is the standard of care for severe aplastic anemia (SAA) patients those who are younger than 40 years of age. The development of secondary malignancies in post-BMT setting for AA is a rare, however, well documented phenomenon. Among the secondary malignancies, development of acute myeloid leukemia is even rarer entity. Here we report a case of acute myeloid leukemia following human leucocyte antigen (HLA) matched sibling peripheral blood stem cell transplant (PBSCT) in a case of SAA. The patient achieved complete remission (CR) following chemotherapy and in CR1, a second HLA matched PBSCT from a different donor was offered. The patient is presently in remission at day +180 post-PBSCT.
Introduction
The various modalities of treatment available for severe aplastic anemia (SAA) are immunosuppressive therapy (IST) and allogenic bone marrow transplantation (BMT). Among these modalities, BMT is often proven to be successful treatment for very severe aplastic anemia (VSAA) [1]. The major complications that can occur in the post-BMT setting include graft failure and graft versus host disease (GVHD). Leukemic transformation in aplastic anemia (AA) is a known phenomenon, especially after IST [2]. However, occurrence of leukemia in post-BMT setting for AA is a very rare phenomenon [3, 4]. Here, we report such a case of acute myeloid leukemia (AML) following human leucocyte antigen (HLA) identical sibling matched peripheral blood stem cell transplant (PBSCT) performed for SAA.
Case details
A 15 year-old-male presented with chief complaints of weakness, easy fatiguability and skin bleeds for two years duration in April 2011. There was no history of fever, jaundice, bony pains, bleeding from other sites and swelling anywhere in the body. There was no history of exposure to drugs or chemicals. He had received six units of packed red cell transfusion previously. On clinical examination, he had pallor and petechiae. There was no icterus, lymphadenopathy, organomegaly, bony tenderness, gum hypertrophy or congenital malformations. Systemic examination was within normal limits.
Baseline complete hemogram showed hemoglobin (Hb) of 57 g/L, total leucocyte count (TLC) of 2.88 × 109/L with lymphocytic preponderance (80 %) and absolute neutrophil count (ANC) of 0.46 × 109/L and platelet count of 90 × 109/L. Bone marrow aspiration (BMA) was paucicellular and showed predominantly lymphocytes and plasma cells. Its corresponding bone marrow biopsy (BMB) was hypocellular for age (<10 % cellularity) and there were no abnormal cells, granuloma or parasites (Fig. 1). A diagnosis of severe aplastic anemia (SAA) was made as per Camitta et al. [4] criteria and adopted by British Committee for Standards in Hematology (BCSH) [5]. Flow cytometry for paroxysmal nocturnal hemoglobinuria (PNH) clone and a chromosomal breakage study for Fanconi’s anemia (FA) were negative.
Fig. 1.
a, b—Paucicellular bone marrow aspirate (BMA) shows predominantly mature lymphocytes (Jenner-Giemsa stain, 100× and 400× respectively). c, d—Hypocellular bone marrow biopsy (BMB) with less than 10 % cellularity (Hematoxylin and Eosin stain, 100× and 400× respectively)
Patient was started on IST with cyclosporine (CSA) (5 mg/kg/day) and anabolic steroid (Danazol) for initial five months with supportive care and blood products. However, no response was obtained with these drugs. He had 10/10 HLA-matched elder brother and planned for SCT. He received conditioning regimen with fludarabine, cyclophosphamide and anti-thymocyte globulin (ATG) and peripheral blood was used as a stem cell source. For GVHD prophylaxis, he had received cyclosporine and methotrexate. His initial post-transplant period was uneventful and engrafted on day +11 and later discharged in a stable condition.
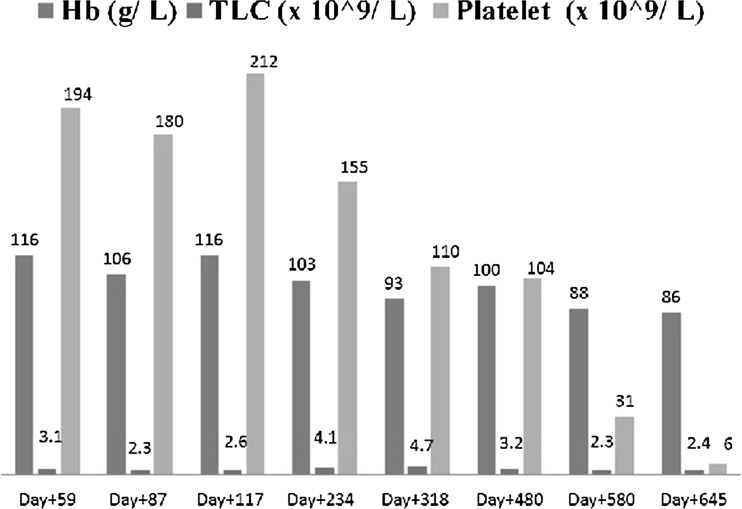
On subsequent follow-up, he had cytomegalovirus (CMV) reactivation on day +59, managed with ganciclovir. Serial monitoring with polymerase chain reaction (PCR) studies showed CMV titers to be negative. On day +318, his chimerism was 95 % and further dropped to 78 % on day +645. The serial follow-up data on complete hemogram, CMV titers and chimerism were shown in Figs. 2 and 3 respectively. Because of persistent cytopenia, IST was continued for more than a year after transplant.
Fig. 2.
Bar diagram showing serial hemogram values
Fig. 3.
Line diagram showing CMV titers and dropping chimerism levels
Patient was re-admitted on day +660 post-transplant with history of fever, weakness, easy fatiguability and skin bleeds for 15 days with cough and mild respiratory distress for 4 days. There were petechiae, pallor and oral temperature of 102 °F. Systemic examination was within normal limits. He started requiring blood products support. His electrolytes, renal and liver function tests were within normal limits. With a possible differential diagnosis of late graft rejection or relapse, bone marrow examination was done. BMA were cellular showed increase in blast (25 %) with monocytoid morphology. These cells showed positivity for non-specific esterase (NSE) and negative for myeloperoxidase (MPO). Its corresponding BMB was hypercellular and showed interstitial infiltration by immature cells (Fig. 4). Flow cytometry showed positivity for CD34, HLA-DR, CD13, CD33, and CD11c and negative for cCD3, CD19 and cMPO. Cytogenetic analysis showed complex karyotype [46XY (80 %)/45XY, del (7q), −20 (10 %)/hyperdiploidy (10 %)]. Hence, the final diagnosis of acute myeloid leukemia (AML-M4, FAB classification) in the setting of PBSCT for AA with clonal evolution was made.
Fig. 4.
a, b—Paucicellular BMA with 25 % blasts (Jenner-Giemsa stain, 100× and 400× respectively) and insets showing MPO negativity (1000×) and NSE positivity (arrow head, 1000×). c, d—BMB shows infiltration by immature cells (Hematoxylin and Eosin stain, 100× and 400× respectively)
Patient was treated with standard 3 + 7 induction chemotherapy with cytarabine and daunorubicin. He achieved complete remission (CR) post induction, taken up for second PBSCT and had 10/10 HLA match with his father. Conditioning was done with busulfan and cyclophosphamide; cyclosporine and methotrexate prophylaxis for GVHD. He had engraftment on day +12 and remaining post-transplant period was uneventful. Patient was discharged on day +31 and presently he was on regular follow up with stable haematological parameters as on day +180 and 100 % Chimerism with second donor.
Discussion
Allogenic BMT is the standard of care in younger SAA patients of less than 40 years of age [1].The development of secondary malignancies in post-BMT setting for AA is rare, however well documented phenomenon [2]. Deeg et al. [1] analyzed 700 patients with SAA in post-BMT setting for subsequent development of second malignancy. They had documented five cases of lymphoid malignancies (two acute lymphoblastic leukemias and three lymphoproliferative disorders) and 18 cases of solid organ malignancies. However, no AML was reported in this largest series available to date.
Kojima et al. [6] analyzed the risk factors for the development of myelodysplastic syndrome (MDS)/AML in long term survivors of AA. In their study, twelve out of 113 patients (10.6 %) developed MDS at a median follow-up of 56 months with a cumulative incidence of 13.7 ± 3.9 % at eight years. In their multivariate analysis, number of days of granulocyte colony stimulating factor (G-CSF) and no response to IST are statistically significant risk factors for MDS. It is uncertain whether prolonged survival in these patients simply displays pre-malignant nature of the disease or whether MDS/AML secondary to therapy. In our case, IST therapy with CSA for 5 months with no response and subsequently taken for HLA matched sibling SCT.
Maciejewski et al. [7] reported an interesting finding that AA patients have constant rate of clonal evolution, with approximately 50 % of these patients show the cytogenetic changes within first 30 months of diagnosis. Trisomy 8, trisomy 6, 5q-, anomalies of chromosome 7 and 13 are the most commonly observed karyotypic abnormalities [1, 8]. The importance of these abnormalities is highlighted by their differential response to therapy. Among these abnormalities, trisomy 8 shows good hematologic response to IST. They also reported that most of the death was attributed to leukemic transformation and often associated with either complex cytogenetic aberration or chromosome 7 abnormalities or both [1, 7]. Loss of chromosome 7 is more often noted after therapy for AA and this is also commonly associated with subsequent of MDS/AML [9].Similarly in our case, del (7q) documented during clonal evolution.
There are only six case reports highlighting that leukemic transformation may originate from donor cells after BMT for SAA [3, 10–14]. Rarely, this transformation also been reported after unrelated umbilical cord blood [15, 16]. More often, these cases are associated with monosomy 7 and morphologically AML-M4/M5 by FAB classification [3, 4]. This possibility was kept in our case; however, chimerism was dropped to 78 % at the time of clonal evolution. Possible mechanisms for such transformation include stromal defect in bone marrow microenvironment, viral oncogenic material transferred from host to donor cells, inadequate immune surveillance due to profound IST, leukemic transformation of engrafted cells and occult leukemia in the donor cells [3].
Although sparse data available regarding treatment options, best possible option would be allogenic transplantation whenever HLA-matched donor available in first CR. This is beneficial that graft-versus-leukemia effect reduces the further risk of leukemia [17–19]. To conclude, although occurrence of AML in post-transplant setting for AA is extremely rare, such possibility should be kept along with graft rejection, if there is dropping chimerism levels or recurrence of symptoms. Whether or not, our case had donor derived leukemia is difficult to tell because of confounding effect of prolonged cyclosporine exposure as immunosuppressive therapy prior to and after transplant and also progressively falling Chimerism in the patient, suggesting the emergence of host clone, which may have clonal evolution to leukemia. Managing such cases on the lines of secondary leukemia with induction therapy followed by SCT seems most reasonable option.
References
- 1.Deeg HJ, Socié G, Schoch G, et al. Malignancies after marrow transplantation for aplastic anemia and Fanconi anemia: a joint Seattle and Paris analysis of results in 700 patients. Blood. 1996;87:386–392. [PubMed] [Google Scholar]
- 2.Otero L, de Souza DC, de Cássia Tavares R, et al. Monosomy 7 in donor cell-derived leukemia after bone marrow transplantation for severe aplastic anemia: report of a new case and review of the literature. Genet Mol Biol. 2012;35:734–736. doi: 10.1590/S1415-47572012005000056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lawler M, Locasciulli A, Longoni D, et al. Leukemic transformation of donor cells in a patient receiving a second allogeneic bone marrow transplant for severe aplastic anemia. Bone Marrow Transpl. 2002;29:453–456. doi: 10.1038/sj.bmt.1703372. [DOI] [PubMed] [Google Scholar]
- 4.Camitta BM, Rappeport JM, Parkman R, et al. Selection of patients for bone marrow transplantation in severe aplastic anemia. Blood. 1975;45:355–363. [PubMed] [Google Scholar]
- 5.Marsh JC, Ball SE, Cavenagh J, et al. British Committee for Standards in Haematology. Guidelines for the diagnosis and management of aplastic anaemia. Br J Haematol. 2009;147:43–70. doi: 10.1111/j.1365-2141.2009.07842.x. [DOI] [PubMed] [Google Scholar]
- 6.Kojima S, Ohara A, Tsuchida M, Japan Childhood Aplastic Anemia Study Group et al. Risk factors for evolution of acquired aplastic anemia into myelodysplastic syndrome and acute myeloid leukemia after immunosuppressive therapy in children. Blood. 2002;100:786–790. doi: 10.1182/blood.V100.3.786. [DOI] [PubMed] [Google Scholar]
- 7.Maciejewski JP, Risitano A, Sloand EM, et al. Distinct clinical outcomes for cytogenetic abnormalities evolving from aplastic anemia. Blood. 2002;99:3129–3135. doi: 10.1182/blood.V99.9.3129. [DOI] [PubMed] [Google Scholar]
- 8.Keung YK, Pettenati MJ, Cruz JM, et al. Bone marrow cytogenetic abnormalities of aplastic anemia. Am J Hematol. 2001;66:167–171. doi: 10.1002/1096-8652(200103)66:3<167::AID-AJH1040>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 9.Varma N, Varma S, Movafagh A, et al. Unusual clonal cytogenetic abnormalities in aplastic anemia. Am J Hematol. 1995;49:256–257. doi: 10.1002/ajh.2830490319. [DOI] [PubMed] [Google Scholar]
- 10.Klingemann HG, Storb R, Sanders J, et al. Acute lymphoblastic leukaemia after bone marrow transplantation for aplastic anaemia. Br J Haematol. 1986;63:47–50. doi: 10.1111/j.1365-2141.1986.tb07493.x. [DOI] [PubMed] [Google Scholar]
- 11.Haltrich I, Müller J, Szabó J, et al. Donor-cell myelodysplastic syndrome developing 13 years after marrow grafting for aplastic anemia. Cancer Genet Cytogenet. 2003;142:124–128. doi: 10.1016/S0165-4608(02)00804-X. [DOI] [PubMed] [Google Scholar]
- 12.Hashino S, Fujisawa F, Kondo T, et al. Donor-type myelodysplastic syndrome with t(2;3) and monosomy 7 after allogeneic peripheral blood stem cell transplantation and liver transplantation in a patient with severe-type aplastic anemia. Int J Hematol. 2006;84:363–366. doi: 10.1532/IJH97.06057. [DOI] [PubMed] [Google Scholar]
- 13.Hughes RT, Milligan DW, Smith GM, et al. A second bone marrow transplant for acute myeloid leukaemia after transplantation for aplastic anaemia. Br J Haematol. 1988;68:391. doi: 10.1111/j.1365-2141.1988.tb04220.x. [DOI] [PubMed] [Google Scholar]
- 14.Sevilla J, Querol S, Molines A, et al. Transient donor cell-derived myelodysplastic syndrome with monosomy 7 after unrelated cord blood transplantation. Eur J Haematol. 2006;77:259–263. doi: 10.1111/j.1600-0609.2006.00716.x. [DOI] [PubMed] [Google Scholar]
- 15.Hamaki T, Kajiwara K, Kami M, et al. Donor cell-derived acute monoblastic leukemia involving MLL gene translocation in an adult patient who received umbilical cord blood transplantation. Bone Marrow Transplant. 2008;41:91–92. doi: 10.1038/sj.bmt.1705836. [DOI] [PubMed] [Google Scholar]
- 16.Browne PV, Lawler M, Humphries P, et al. Donor-cell leukemia after bone marrow transplantation for severe aplastic anemia. N Engl J Med. 1991;325:710–713. doi: 10.1056/NEJM199109053251007. [DOI] [PubMed] [Google Scholar]
- 17.Tallman MS, Rowlings PA, Milone G, et al. Effect of post-remission chemotherapy prior to HLA-identicalsibling transplantation for acute myelogenous leukemia in first complete remission. Blood. 2000;96:1254–1258. [PubMed] [Google Scholar]
- 18.Rowe JM. Therapy of secondary leukemia. Leukemia. 2002;16:748–750. doi: 10.1038/sj.leu.2402456. [DOI] [PubMed] [Google Scholar]
- 19.Pagano L, Pulsoni A, Vignetti M, et al. Secondary acute myeloid leukaemia: results of conventional treatments. Experience of GIMEMA trials. Ann Oncol. 2005;16:228–233. doi: 10.1093/annonc/mdi051. [DOI] [PubMed] [Google Scholar]