Abstract
T cell large granular lymphocytic leukemia is a clonal proliferation of cytotoxic large granular T cells positive for CD3 and CD8. It is a chronic lymphoproliferative disorder with an indolent course. Therapeutic options include observation and low dose chemotherapy. Rarely, they have an aggressive course. Such cases have expression of NK cell associated antigens like CD56 in the T cells. These cases require more aggressive therapy with acute lymphoblastic leukemia regimens. We report a case of fatal CD56 negative T cell large granular lymphocytic leukemia in a 38 year old lady.
Keywords: Large granular lymphocytes, Aggressive variant, CD56 negative
Introduction
Large granular lymphocytes (LGL) are of two types: cytotoxic T cells (T-LGL) and NK cells. T-LGL proliferation can be reactive as in autoimmune diseases or clonal with aberrant immunophenotype. T-LGL leukemias have an indolent behaviour as compared to NK cell type of LGL leukemia. Uncommonly T-LGL leukemia can have aggressive course.
Case Report
A 38-year-old lady presented with abdominal pain and fever for 15 days. On examination, she had pallor, subconjunctival hemorrhage, hepatomegaly (4 cm) and splenomegaly (8 cm). Investigations showed haemoglobin of 6 g/dl, platelet count of 50 × 109/L and total leukocyte count of 90 × 109/L. The peripheral smear showed 90 % atypical lymphoid cells. These cells were 2–3 times larger than mature lymphocyte with clumped nuclear chromatin, moderate to abundant pale blue cytoplasm and prominent cytoplasmic granules, characteristic of LGL (Fig. 1). The possibility of large granular lymphocytic leukemia was considered and bone marrow aspiration was done. She was started on prednisolone 1 mg/kg for high counts but the patient progressively worsened and died within a week. The bone marrow aspiration showed suppression of normal hematopoiesis with infiltration by the LGL. Subsequently, the post-mortem liver, lung and splenic biopsies were done. Liver biopsy showed a prominent sinusoidal and mild portal infiltration. There were foci of macrovesicular fatty change (Fig. 2). Splenic biopsy showed red pulp infiltration with attenuation of the white pulp (Fig. 3). Lung biopsy showed interstitial and alveolar infiltration by these cells. Some of the alveoli showed the presence of hyaline membranes and features of diffuse alveolar damage(Fig. 4). The marrow biopsy showed an interstitial pattern of infiltration by atypical cells forming tiny nodules and linear pattern within the sinusoids (Fig. 5). There was no hemophagocytosis. On immunohistochemistry (IHC), these cells were strongly positive for CD3 and CD16, (Figs. 6, 7) with focal positivity for CD2, CD8 (Fig. 8) and negative for CD4, CD5, CD7, CD56, CD57, CD34, EBV, CD20, MPO and CD117. The atypical cell infiltration of other organs had the same CD marker expression as that of the marrow. The final diagnosis was CD56 negative aggressive T-LGL leukemia positive for CD3, CD16, CD2 and CD8.
Fig. 1.
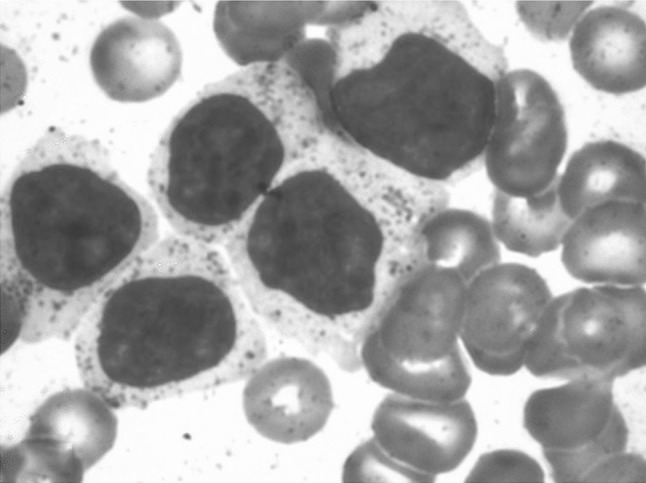
Peripheral smear shows large granular lymphocytes with abundant cytoplasm and azurophilic granules. (Giemsa stain ×400)
Fig. 2.
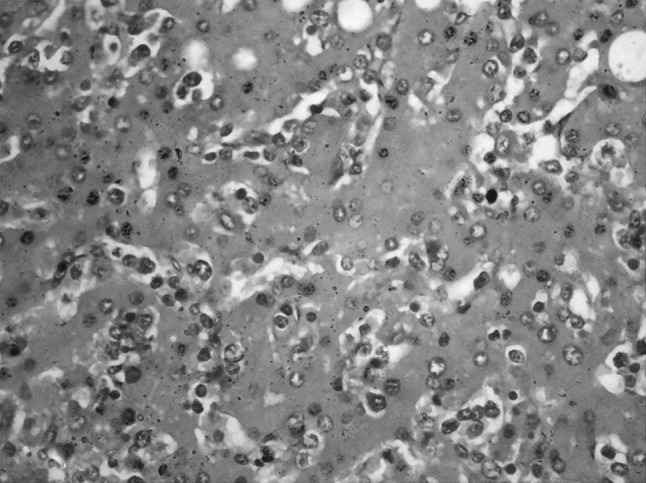
Liver biopsy shows sinusoidal infiltration by atypical lymphoid cells. (H&E ×400)
Fig. 3.
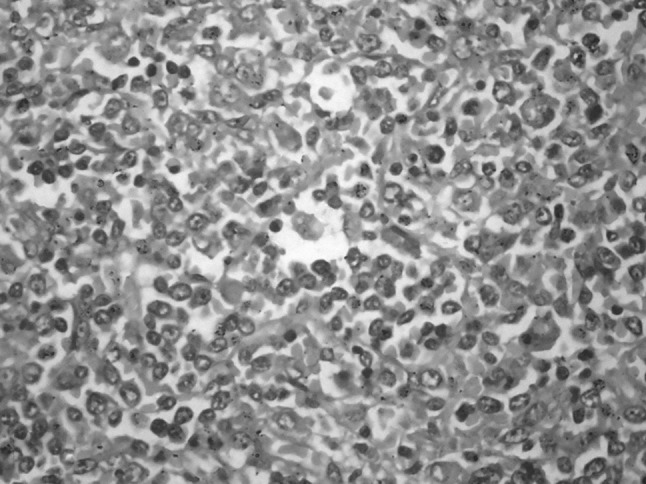
Spleen biopsy shows atypical lymphocytes infiltrating the red pulp. (H&E ×400)
Fig. 4.
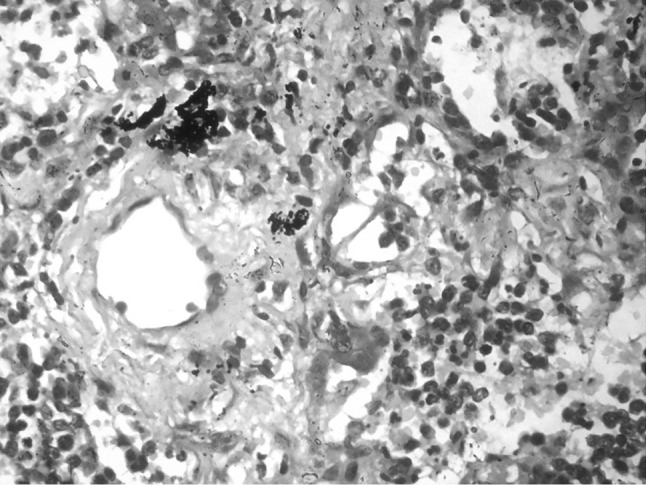
Lung biopsy with alveolar infiltration by the LGL. (H&E ×400)
Fig. 5.
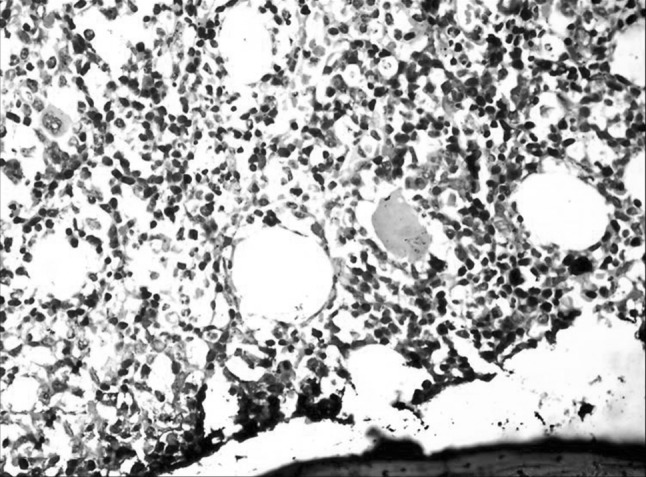
Bone marrow with interstitial infiltration by LGL. (H&E ×400)
Fig. 6.
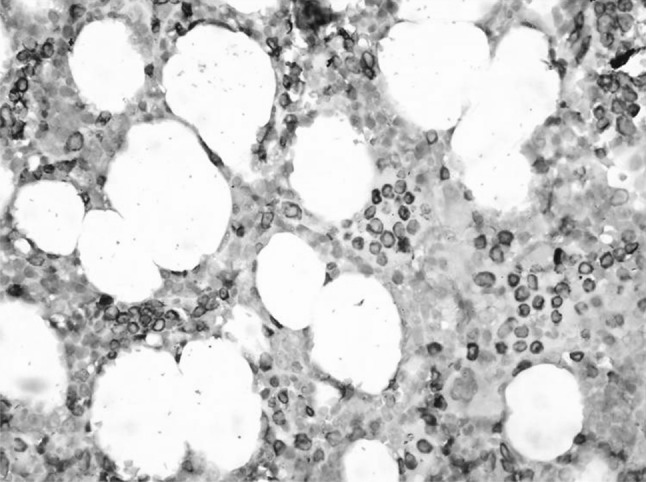
Bone marrow with interstitial and linear infiltration highlighted by CD3. (IHC ×400)
Fig. 7.
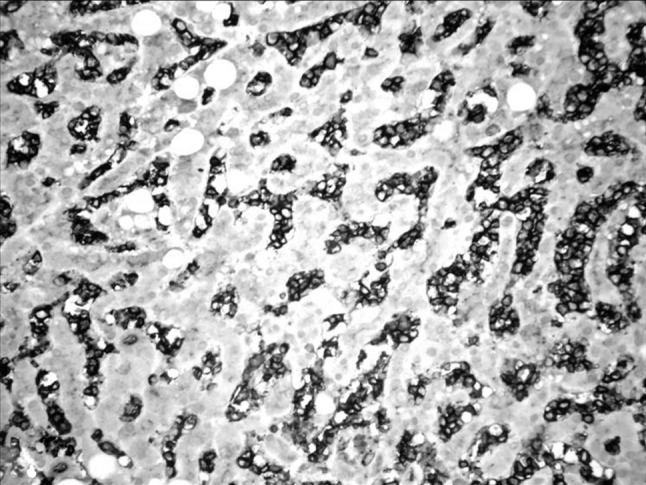
CD16 positive in the LGL showing the sinusoidal infiltration in the liver. (IHC ×400)
Fig. 8.
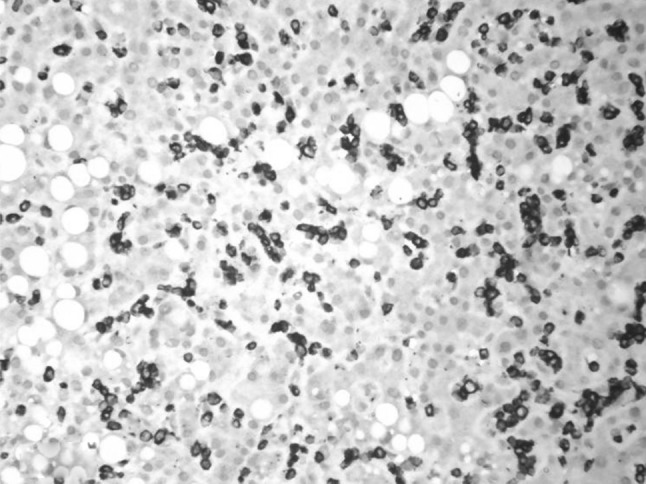
CD8 positivity (downregulated) in the LGL in the liver biopsy. (IHC ×400)
Discussion
Ten to fifteen percent of the lymphocytes in peripheral blood are large (15–18 µm) with round to reniform nucleus, abundant pale cytoplasm with azurophilic granules and are named LGL. They are of two types- cytotoxic T cells (CD3+ CD8+) and NK cells (surface CD3− CD8− CD56+). LGL play an important role in destroying the virus infected cells and tumour cells. Their proliferation is controlled by activation induced cell death [1].
Chronic antigenic stimulation leads to uncontrolled proliferation of the T-LGL. Such reactive proliferation is seen most commonly in rheumatoid arthritis. It is also seen in postsplenectomy, HIV and posttransplant [2].
WHO 2008 classifies LGL leukemias under mature T/NK cell neoplasms. They are of three types: T-LGL, Aggressive NK cell leukemia and a provisional entity chronic lymphoproliferative disorder of NK cells [3].
T-LGL leukemia is a chronic lymphoproliferative disorder. Median age of occurrence is 60 years with equal incidence in males and females. Clinical features include pancytopenia, moderate hepatosplenomegaly and characteristic absence of lymph nodes. The patients are symptomatic due to neutropenia and thrombocytopenia [4].
Chronic antigenic stimulation combined with defect in the Fas-FasL-mediated apoptosis and the activation of the survival pathways like JAK2/STAT3, RAS/MEK/ERK, and SFK/PI3K/Akt leads to leukemic proliferation of the LGL [1].
Peripheral blood examination shows Large granular lymphocytosis usually in the range of 2–20 × 109/L. These lymphocytes are clonal in T-LGL leukemia when compared to the reactive proliferation. LGL count of more than 2 × 109/L was initially required for the diagnosis of T-LGL leukemia; currently 0.4-2 × 109/L is considered compatible with diagnosis when it is associated with symptoms, cytopenias and aberrant phenotype [1]. Clonality can be established by either one of the following: abnormal immunophenotype, abnormal cytogenetics (rare), restricted Vβ expression, abnormal KIR expression or Clonal TCR rearrangements (PCR, Southern blot). [2] Our patient had large granular lymphocytosis with peripheral blood count of 81 × 109/L and abnormal immunophenotype.
Bone marrow involvement is usually subtle. It usually has an interstitial infiltrate with normal or suppressed hematopoiesis due to the cytokines released rather than the infiltrate. The presence of linear infiltrate within the vessels is considered characteristic of LGL leukemia and helps to differentiate from reactive cells. Hence IHC is required to identify these cells in the marrow [5]. The bone marrow of our patient also had similar features highlighted on IHC.
Splenic and liver involvement is usually subtle. The LGL’s infiltrate the red pulp with attenuation of the white pulp. Liver shows a prominent sinusoidal infiltration. Our patient had a massive infiltration of the spleen and liver. Such features are also seen with hepatosplenic gamma delta T cell lymphoma. However malignant hepatosplenic gammadelta T cells are small to medium size with regular or folded nuclei, inconspicuous nucleoli, mature dispersed chromatin and pale blue, agranular cytoplasm unlike the LGL.They also usually have a CD3+ CD2+ CD4− CD8− phenotype [6].
LGL infiltration of lung as seen in our patient is uncommon and is reported in four cases of aggressive T-LGL in literature [7–10].
Immunophenotyping is essential for the diagnosis of LGL leukemias. Though flow cytometry is ideal, IHC helps in the diagnosis. Normal T-LGL is a CD8+ T cell with CD2+, cytoplasmic and surface CD3+, CD4−, CD5+, CD7+, CD16−, CD56−, αβ T-cell receptor (TCR)+, and γδ TCR− [3].
Leukemic proliferation has an aberrant immunophenotype.CD5 and CD7 are most commonly downregulated antigens [11]. NK cell associated antigens like CD16 and CD57 are expressed in 80 and 100 % of the cases respectively. The absence of surface CD3 and positivity for CD56 and EBV helps to differentiate NK cells from T-LGL.
CD3+ CD8+, CD16+ CD57+ is the most common phenotype of T-LGL leukemia. They have been classified into three types based on the immunophenotype—(a) CD4+, (b) CD8+ with or without CD56 and (c) γδ T cell type. Types (a) and (c) have an indolent course.
The type (b) CD8+ group usually has an indolent course [2]. A subset of this subtype can have an aggressive course. This group has been found to have expression of CD56 which is considered the marker of rapid progression [12]. It is important to differentiate since therapy differs between these two groups. The immunophenotype in our patient was CD3+CD8+CD2+CD5+CD7dim+CD16+CD4−CD56−CD57−. Hence it falls in the category b subset with aggressive course but with the absence of CD56.
There are only few cases of aggressive variant of T-LGL leukemia reported in literature (16 to our knowledge) [7–10, 12, 13]. Most of them had expression of NK cell associated antigens. Macon et al. had classified them into NK cell like T cell leukemia based on NK cell antigen expression with TCR gene rearrangement at the molecular level. CD56 was positive in 92 % of their cases [13]. Only 2 cases of aggressive variant of CD56 negative T-LGL leukemia has been reported in literature [9, 10].
The case we have presented had an aggressive course with an abnormal uncommon immunophenotype of the T-LGL inspite of the absence of the markers of aggressiveness like CD56 and EBV. Hence we suggest that the T-LGL leukemia can have a variable course and aggressive therapy has to considered in the setting of worsening symptoms.
Conclusion
The rare features in our case of T-LGL leukemia are; the absence of autoimmune disease, presence of B symptoms, massive hepatomegaly and splenomegaly, very high LGL counts, pulmonary infiltration, rare immunophenotype and an aggressive clinical course despite the absence of markers of aggressiveness (CD56, EBV).This is the first case from India of a CD56 negative aggressive T-LGL leukemia to the best of our knowledge.
Acknowledgments
Conflict of Interest
None.
References
- 1.Zhang D, Loughran TP., Jr Large granular lymphocytic leukemia: molecular pathogenesis, clinical manifestations, and treatment. Hematology. 2012;2012:652–659. doi: 10.1182/asheducation-2012.1.652. [DOI] [PubMed] [Google Scholar]
- 2.O’Malley Dennis P. T-cell large granular leukemia and related proliferations. Am J Clin Pathol. 2007;127:850–859. doi: 10.1309/A8FHDA0VVRJ05GJP. [DOI] [PubMed] [Google Scholar]
- 3.Chan WC, Foucar K, Morice WG, Catovsky D. T-cell large granular lymphocytic leukemia. In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW, editors. World Health Organization classification of tumours of haematopoietic and lymphoid tissues. Lyon: IARC Press; 2008. pp. 272–273. [Google Scholar]
- 4.Rose MG, Berliner N. T-cell large granular lymphocytic leukemia and related disorders. Oncologist. 2004;9:247–258. doi: 10.1634/theoncologist.9-3-247. [DOI] [PubMed] [Google Scholar]
- 5.Morice WG, Kurtin PJ, Tefferi A, Hanson CA. Distinct bone marrow findings in T-cell granular lymphocytic leukemia revealed by paraffin section immunoperoxidase stains for CD8, TIA-1, and granzyme B. Blood. 2002;99:268–274. doi: 10.1182/blood.V99.1.268. [DOI] [PubMed] [Google Scholar]
- 6.Weidmann E. Hepatosplenic T cell lymphoma. A review on 45 cases since the first report describing the disease as a distinct lymphoma entity in 1990. Leukemia. 2000;14:991–997. doi: 10.1038/sj.leu.2401784. [DOI] [PubMed] [Google Scholar]
- 7.Lamy T, Bauer FA, Liu JH, Li YX, Pillemer E, Shahidi H, Gregory SA, Zambello R, Marcolongo R, Semenzato G, Loughran TP. Clinicopathological features of aggressive large granular lymphocyte leukaemia resemble Fas ligand transgenic mice. Br J Haematol. 2000;108(4):717–723. doi: 10.1046/j.1365-2141.2000.01934.x. [DOI] [PubMed] [Google Scholar]
- 8.Alekshun TJ, Tao J, Sokol L. Aggressive T-cell large granular lymphocyte leukemia: a case report and review of the literature. Am J Hematol. 2007;82:481–485. doi: 10.1002/ajh.20853. [DOI] [PubMed] [Google Scholar]
- 9.Falcao RP, Simoes BP, Garcia AB. Aggressive variant of morphologically typical T large granular lymphocytic leukaemia/lymphoma lacking NK cell markers. Acta Haematol. 2000;104:110–114. doi: 10.1159/000039762. [DOI] [PubMed] [Google Scholar]
- 10.Tordjman R, Macintyre E, Emile JF, Valensi F, Ribrag V, Burtin ML, Varet B, Brousse N, Hermine O. Aggressive acute CD3+, CD56− T cell large granular lymphocyte leukemia with two stages of maturation arrest. Leukemia. 1996;10(9):1514–1519. [PubMed] [Google Scholar]
- 11.Lundell R, Hartung L, Hill S, Perkins SL, Bahler DW. T-cell large granular lymphocyte leukemias have multiple phenotypic abnormalities involving pan—T-cell antigens and receptors for MHC molecules. Am J Clin Pathol. 2005;124:937–946. doi: 10.1309/PH7X78HF4FW4PRKW. [DOI] [PubMed] [Google Scholar]
- 12.Gentile TC, Uner AH, Hutchison RE, Wright J, Ben-Ezra J, Russell EC, Loughran TP., Jr CD3+, CD56+ aggressive variant of large granular lymphocyte leukemia. Blood. 1994;84(7):2315–2321. [PubMed] [Google Scholar]
- 13.Macon WR, Williams ME, Greer JP, Hammer RD, Glick AD, Collins RD, Cousar JB. Natural killer-like T-cell lymphomas: aggressive lymphomas of T-large granular lymphocytes. Blood. 1996;87:1474–1483. [PubMed] [Google Scholar]


