Abstract
Hemoglobin Constant Spring [Hb CS; α142, Term→Gln (TAA>CAA IN α2)] is often missed by routine laboratory testing, especially in subjects with co-inheritance of β-thalassemia or β-variants. We reported the case of a 1-year-old female with Hb H-CS disease who was born from a father with heterozygous of α-thalassemia-1 Southeast Asian type deletion and a mother with the combination of Hb CS and Hb E [β26 (B8) Glu→Lys, GAG>AAG] trait. A very tiny peak of Hb CS of the mother was easily ignored on the high performance liquid chromatography chromatogram while it was clearly seen on the capillary electrophoresis (CE) electrophoregram. Therefore, the CE is useful in screening for heterozygous Hb CS in a person with Hb E trait. This is of potential benefit for prevention of new cases of Hb H-CS disease.
Keywords: Capillary electrophoresis, Hemoglobin constant spring, Hb E, Hb H-CS, High performance liquid chromatography
Introduction
Hemoglobin Constant Spring [Hb CS; α142, Term→Gln (TAA>CAA IN α2)] is the most common non-deletional α-thalassemia among Southeast Asian (SEA) population. This abnormal hemoglobin results from point mutation at the stop codon of α2-globin gene (TAA→CAA) which leads to the addition of 31 amino acids to normal α-globin sequence [1, 2]. The heterozygote of Hb CS is clinically and hematologically normal. However, the homozygote shows a clinical picture as thalassemia intermedia with mild anemia, jaundice and hepatosplenomegaly. Moreover, co-inheritance of Hb CS with α-thalassemia-1 leads to Hb H-CS disease (–/αCSα), which has a more severe phenotype (e.g. thalassemia intermedia and splenomegaly) than deletional Hb H disease (–/−α3.7, –/−α4.2) [3–5]. The Hb CS mRNA is unstable, therefore the rate of α-globin chain synthesis is decreased [6]. Thus, Hb CS is often missed by routine laboratory testing, especially in the heterozygote [7, 8]. The previous studies showed that 76 % of heterozygous of Hb CS were detected by the high performance liquid chromatography (HPLC) [9, 10]. Moreover, Hb CS heterozygotes carrying β-thalassemia could not be detected by CE [11]. Thus, the missed diagnosis of Hb CS can be a cause of new case of Hb H-CS disease.
Case Report
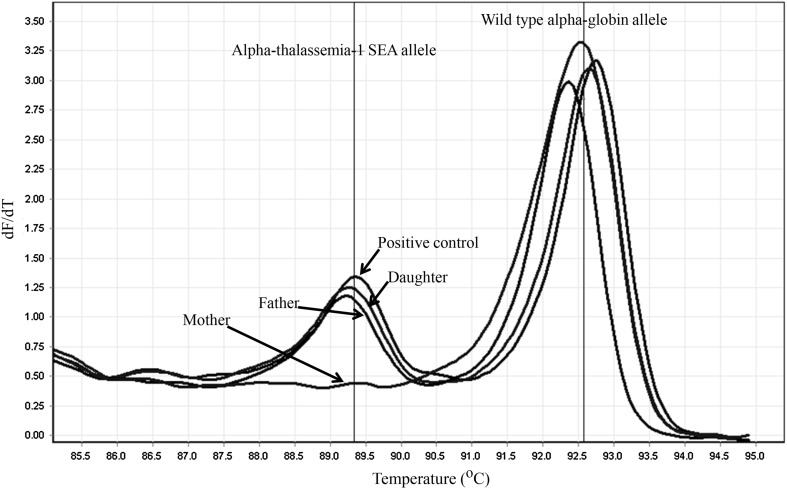
In June 2012, when a pregnant woman in her 15th week of gestation and her spouse were referred to the antenatal hemoglobinopathy screening clinic at Denchai Crown Prince Hospital, Den Chai, Phrae, Thailand. Their hematological parameters including hemoglobin, hematocrit, red cell counts, and red cell indices [mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC) and red blood cell distribution width (RDW)] were estimated with an automated blood counter (Sysmex KX-21, Sysmex Corporation, Kobe, Japan). The Hb E was screened by using dichlorophenol indophenol precipitation test (DCIP) (Thalcon-DCIP Thalassemia Screening, Surathin International Co, Ltd, Samutprakarn, Thailand). The characteristics and hematological data of the pregnant woman and her spouse are shown in Table 1. The positive result for DCIP test was found in the pregnant woman while her spouse had a lower MCV (71 fL) than the reference range (80–97 fL). Thus, their blood samples were submitted to the laboratory of the Associated Medical Sciences Clinical Service Center (AMS-CSC), Chiang Mai, Thailand for hemoglobinopathy and thalassemia diagnosis. At the AMS-CSC, their blood samples were analyzed for β-thalassemia and hemoglobinopathies using the high performance liquid chromatography (HPLC, VARIANT II, β-thalassemia Short Program, Bio-Rad Laboratories, Hercules, California, USA). Genomic DNA was extracted from whole blood samples by using the NucleoSpin® kit (Macherey-Nagel, KG., Duren, Germany) according to manufacturers’ instructions. The α-thalassemia-1 SEA and Thai type deletions were detected by using the real-time PCR with SYBR Green1 high resolution melting (HRM) analysis as previously described [12, 13]. The HPLC chromatograms of the pregnant woman and her spouse are shown in Fig. 1a and b, respectively. The Hb A2 and E were co-eluted at the same retention time of 3.78 min. The pregnant woman had Hb A2/E (27.3 %) in the range of Hb E trait while those of her spouse was observed in the normal level (<4.0 %). The DNA analysis of her spouse was positive for α-thalassemia-1 SEA type deletion by presenting the specific melting curve at Tm of 89.0–89.5 °C (Fig. 2) and it was negative for her DNA sample (Fig. 2). Thus, she was diagnosed as Hb E trait and her spouse was a carrier of α-thalassemia-1 SEA type deletion. The fetus had no risk for thalassemia majors including homozygous α-thalassemia-1 (Bart’s hydrops), homozygous β-thalassemia and β-thalassemia/Hb E disease. Therefore, the prenatal diagnosis was not performed. A girl was born at 39 weeks after a mother’s last menstrual period and her birth weight was 2850 g. Three days after delivery the girl was discharged. One year later, she came for a follow-up and her hematological analyzed data are shown in Table 1. She was diagnosed as anemic and received a transfusion of 150 mL of RBC concentrate. Her blood sample and together with the blood samples of her parents were sent to the AMS-CSC, Chiang Mai, Thailand for hemoglobinopathy and thalassemia diagnosis. The hemoglobin analysis was performed by using capillary electrophoresis (CE) (CAPILLARYS™ 2, Sebia, Norcross, Georgia, USA). Their CE electrophoregrams are shown in Fig. 1d–f. The specific peaks of Hb CS (Zone 2), Hb Bart’s (Zone 12) and Hb H (Zone 15) were observed in blood sample of the girl (Fig. 1f). The hemoglobin analysis of the girl was also analyzed by HPLC and the HPLC chromatogram is shown in Fig. 1c. The specific peaks of Hb CS, Hb Bart’s and Hb H were presented. In addition, the DNA analysis for detection of α-thalassemia-1 SEA and Thai type deletions was performed by using the real-time PCR with SYBR Green1 and HRM analysis system [12, 13]. The amplified fragments from both α-thalassemia-1 SEA allele and wild type α-globin gene allele with the specific melting curve at Tm of 89.0–89.5 and 92.0–93.0 °C, respectively were observed (Fig. 2). Thus, she was diagnosed as Hb H-CS disease. The peaks of Hb E and Hb CS were found on CE electrophoregram of her mother (Fig. 1d) while only the peaks of Hb A and A2 were found on CE electrophoregram of her father (Fig. 1e). These results indicated that the Hb H-CS disease was caused by an inheritance of Hb CS and α-thalassemia-1 SEA type deletion from the mother and father, respectively. The HPLC chromatograms at the initial hospitalization of her mother (Fig. 1a) and father (Fig. 1b) were reviewed and a very small peak of Hb CS on the HPLC chromatogram of the mother was doubted. Therefore, the DNA analysis for detection of Hb CS was performed in this family by using the amplification refractory mutation system (ARMS) as previously described [5, 14]. The 180 bp amplified fragment from Hb CS allele was observed in DNA samples of the girl and her mother (Fig. 3).
Table 1.
The characteristics and hematological data of the family
| Parameters | Mother | Father | Daughter |
|---|---|---|---|
| Age (Years) | 20 | 33 | 1 |
| RBC counts (1012/L) | 4.4 | 5.9 | 3.5 |
| Hemoglobin (g/L) | 125 | 132 | 64 |
| Hematocrit (L/L) | 0.38 | 0.42 | 0.21 |
| MCV (fL) | 86.0 | 71.0 | 61.1 |
| MCH (pg) | 28.4 | 22.4 | 18.4 |
| MCHC (g/L) | 329 | 314 | 301 |
| RDW | 12.8 | 16.0 | 34.1 |
| Hb analysis by HPLC | |||
| Hb A (%) | 63.5 | 86.5 | 89.4 |
| *Hb A2/E (%) | 27.3 | 2.8 | 2.2 |
| Hb F (%) | 0.9 | 0.2 | 2.0 |
| Hb analysis by CE | |||
| Hb A (%) | 73.2 | 97.7 | 82.6 |
| Hb A2 (%) | 3.2 | 2.3 | 0.8 |
| Hb E (%) | 23.1 | 0.0 | 0.0 |
| Hb F (%) | 0.0 | 0.0 | 1.4 |
| Hb Bart’s (%) | 0.0 | 0.0 | 4.2 |
| Hb H (%) | 0.0 | 0.0 | 8.2 |
| Hb CS (%) | 0.5 | 0.0 | 2.8 |
| β-Globin genotype | β/βE | β/β | β/β |
| α-Globin genotype | αα/αCSα | –SEA/αα | –SEA/αCSα |
* Hb A2 and Hb E are co-eluted at the same retention time
Fig. 1.
The HPLC chromatogram of mother (a), father (b) and the proband (c) and CE electrophoregram of mother (d), father (e) and the proband (f)
Fig. 2.
The melting curve analysis for detection of α-thalassemia-1 SEA type deletion. The real-time PCR with SYBR Green1 and HRM analysis was performed in DNA samples of the family and positive control for α-thalassemia-1 SEA trait
Fig. 3.
Molecular analysis of Hb CS by ARMS PCR. The amplified fragments were separated by 2.0 % agarose gel electrophoresis and visualized under UV-light after ethidium bromide staining. M represents λ/Hind III size markers. The 391 bp is an internal control fragment and 180 bp is an amplified fragment from Hb CS allele. Lane 1 shows the analysis result of a normal individual, lane 2 heterozygous of Hb CS, lane 3–5 show the analysis results of the father, mother and proband, respectively
Discussion
The mRNA of Hb CS as well as the gene product is unstable and presented at a low level in peripheral blood [7, 8], especially the Hb CS level of the heterozygote, which usually range from 0.1 to 1.0 % [15]. In the previous study, it was reported that Hb CS heterozygotes carrying β-thalassemia could not be detected by CE and that is because of a very small amount of Hb CS in compound heterozygote where the expression of β-globin chain is reduced [11]. In contrary with the current study, Hb CS heterozygotes carrying Ηb Ε, another unstable hemoglobin variant of the β-globin chain which is frequently found in the Thai population, can be detected by CE but not by HPLC. Consistent with the previous study, the CE had higher sensitivity than HPLC for detection of Hb CS [16]. Thus, CE analysis is necessary in a couple which is discordant for thalassemia that is one partner has α-thalassemia-1 and the other has β-thalassemia or Hb E trait. However, the molecular analysis for detection of Hb CS should also be performed as a confirmation test.
In conclusion, the CE is useful in screening for heterozygous Hb CS in a person with Hb E trait. This is of potential benefit for prevention of new cases of Hb H-CS disease.
Acknowledgments
The authors thank technicians in the Associated Medical Sciences Clinical Service Center, Associated Medical Sciences, Chiang Mai University, Chiang Mai, Thailand for their help and assistance. We are also grateful to Roscoe C Butler Jr for editing the manuscript. This study was supported by Grants from the National Research Council of Thailand (NRCT).
Conflict of interest
The authors report no conflicts of interest.
References
- 1.Fucharoen S, Winichagoon P. Hemoglobinopathies in southeast Asia. Hemoglobin. 1987;11(1):65–88. doi: 10.3109/03630268709036587. [DOI] [PubMed] [Google Scholar]
- 2.Laig M, Pape M, Hundrieser J, et al. The distribution of the Hb constant spring gene in Southeast Asian populations. Hum Genet. 1990;84(2):188–190. doi: 10.1007/BF00208939. [DOI] [PubMed] [Google Scholar]
- 3.Fucharoen S, Winichagoon P, Pootrakul P, Piankijagum A, Wasi P. Differences between two types of Hb H disease, alpha-thalassemia 1/alpha-thalassemia 2 and alpha-thalassemia 1/Hb constant spring. Birth Defects Orig Artic Ser. 1987;23(5A):309–315. [PubMed] [Google Scholar]
- 4.Tan JA, Kok JL, Tan KL, Wee YC, George E. Thalassemia intermedia in HbH-CS disease with compound heterozygosity for beta-thalassemia: challenges in hemoglobin analysis and clinical diagnosis. Genes Genet Syst. 2009;84(1):67–71. doi: 10.1266/ggs.84.67. [DOI] [PubMed] [Google Scholar]
- 5.Tangvarasittichai O, Jeenapongsa R, Sitthiworanan C, Sanguansermsri T. Laboratory investigations of Hb constant spring. Clin Lab Haematol. 2005;27(1):47–49. doi: 10.1111/j.1365-2257.2004.00658.x. [DOI] [PubMed] [Google Scholar]
- 6.Hunt DM, Higgs DR, Winichagoon P, Clegg JB, Weatherall DJ. Haemoglobin constant spring has an unstable alpha chain messenger RNA. Br J Haematol. 1982;51(3):405–413. doi: 10.1111/j.1365-2141.1982.tb02796.x. [DOI] [PubMed] [Google Scholar]
- 7.Ne W, Harano K, Harano T, et al. Hb Constant Spring [alpha 142, Term–>Gln (TAA>CAA in alpha2)] in the alpha-thalassemia of anemic patients in Myanmar. Hemoglobin. 2008;32(5):454–461. doi: 10.1080/03630260802341588. [DOI] [PubMed] [Google Scholar]
- 8.Singsanan S, Fucharoen G, Savongsy O, Sanchaisuriya K, Fucharoen S. Molecular characterization and origins of Hb constant spring and Hb Pakse in Southeast Asian populations. Ann Hematol. 2007;86(9):665–669. doi: 10.1007/s00277-007-0310-x. [DOI] [PubMed] [Google Scholar]
- 9.Liao C, Zhou JY, Xie XM, Li J, Li R, Li DZ. Detection of Hb constant spring by a capillary electrophoresis method. Hemoglobin. 2010;34(2):175–178. doi: 10.3109/03630261003680191. [DOI] [PubMed] [Google Scholar]
- 10.Panyasai S, Sukunthamala K, Pornprasert S. Molecular confirmatory testing of hemoglobin Constant Spring by real-time polymerase chain reaction SYBR Green1 with high-resolution melting analysis. Eur J Haematol. 2010;84(6):550–552. doi: 10.1111/j.1600-0609.2010.01437.x. [DOI] [PubMed] [Google Scholar]
- 11.Li YQ, Li R, Li DZ. Detection of Hb Constant Spring [alpha142, Term–>Gln, TAA>CAA (alpha2)] in heterozygotes combined with beta-thalassemia. Hemoglobin. 2013;37(2):197–200. doi: 10.3109/03630269.2013.768532. [DOI] [PubMed] [Google Scholar]
- 12.Pornprasert S, Phusua A, Suanta S, Saetung R, Sanguansermsri T. Detection of alpha-thalassemia-1 Southeast Asian type using real-time gap-PCR with SYBR Green1 and high resolution melting analysis. Eur J Haematol. 2008;80(6):510–514. doi: 10.1111/j.1600-0609.2008.01055.x. [DOI] [PubMed] [Google Scholar]
- 13.Pornprasert S, Punyamung M, Treesuwan K. Criteria for detection of alpha-thalassemia-1 Thai type deletion in routine laboratory. Clin Lab. 2013;59(11–12):1423–1427. doi: 10.7754/clin.lab.2013.121217. [DOI] [PubMed] [Google Scholar]
- 14.Pornprasert S, Panyasai S, Waneesorn J, Kongthai K, Singboottra P. Quantification of hemoglobin constant spring in heterozygote and homozygote by a capillary electrophoresis method. Int J Lab Hematol. 2012;34(2):143–147. doi: 10.1111/j.1751-553X.2011.01371.x. [DOI] [PubMed] [Google Scholar]
- 15.Liao C, Zhou JY, Xie XM, Li DZ. Screening for Hb constant spring in the Guangdong Province, South China, using the Sebia capillary electrophoresis system. Hemoglobin. 2011;35(1):87–90. doi: 10.3109/03630269.2010.547430. [DOI] [PubMed] [Google Scholar]
- 16.Waneesorn J, Panyasai S, Kongthai K, Singboottra P, Pornprasert S. Comparison between capillary electrophoresis and high performance liquid chromatography for detection and quantification of Hb constant spring [Hb CS; alpha142, Term–>Gln (TAA>CAA IN alpha2)] Hemoglobin. 2011;35(4):338–345. doi: 10.3109/03630269.2011.588140. [DOI] [PubMed] [Google Scholar]