Abstract
Chronic neutrophilic leukemia (CNL) is a rare entity amongst myeloproliferative neoplasms (MPNs). The classical presentation of CNL is with splenomegaly, mature neutrophilic leucocytosis and hyperuricemia. We herein report a case who presented with symptoms of acute gouty arthritis. Physical examination showed typical red, tender tophi in the right hand, right foot and both pinnae suggesting an acute episode of gout. During evaluation, moderate splenomegaly, mature neutrophilia, hyperuricemia and sub-nephrotic range range proteinuria were noted. Bone marrow examination and kidney biopsy was done. Final diagnosis of CNL with acute gouty arthritis and chronic renal thrombotic microangiopathy (TMA) was made. Although hyperuricemia is a common finding in MPNs but presentation of our case with symptoms of acute tophi and chronic TMA is atypical.
Case report
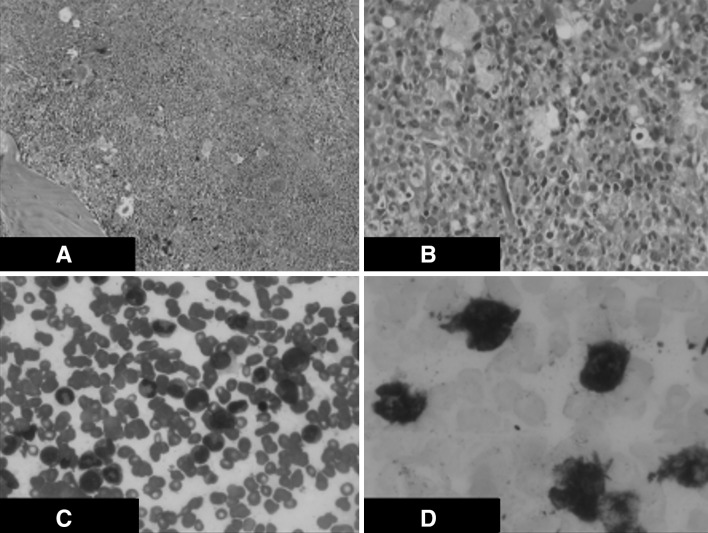
A-25-year-old gentleman with no previous comorbid illnesses presented to us in July 2010 with complaints of pain and swelling in multiple joints since the last 6 years. For past few years he used to have acute episodes of excruciating pain in the right great toe associated with redness of the overlying skin. General physical examination showed reddish, tender tophi in both pinnae, left 3rd metacarpal and right 1st metatarsal joints (Fig. 1a–c). X ray of the affected area showed erosion of the medial aspect of the right 1st metatarsal joint (Fig. 1d). Biochemical analysis showed elevated uric acid (15.6 mg/dL) with normal liver and renal function tests. Aspirate from left metacarpal swelling showed extensive deposition of needle shaped crystals confirming the diagnosis of gouty tophus (Fig. 2a, b). Abdominal examination was remarkable for splenomegaly 8 cm below the left costal margin. A complete blood count was performed that showed haemoglobin of 123 gm/L, platelet count of 320 × 109/L and total white blood cell count of 26 × 109/L with 83 % neutrophils, 10 % lymphocytes, 4 % basophils, 2 % eosinophils and 1 % monocytes on differential count. The neutrophils were morphologically normal and there were no immature cells in the peripheral blood. No evidence of chronic infections or occult malignancies that may be responsible for neutrophilia were identified on extensive investigation. A possibility of an MPN was considered in view of the symptom complex of hyperuricemia in addition to neutrophilic leukocytosis and splenomegaly. As depicted in the Table 1, our patient full filled all the criteria’s required to make a diagnosis of chronic neutrophilic leukemia (CNL). Bone marrow examination was hypercellular with granulocytic and megakaryocytic hyperplasia without dysplasia and with a significantly high leucocytic alkaline phosphate score 318 (normal range: 40–100) (Fig. 3a–d). BCR–ABL transcripts and JAK2V617F mutation were negative by polymerase chain reaction.
Fig. 1.
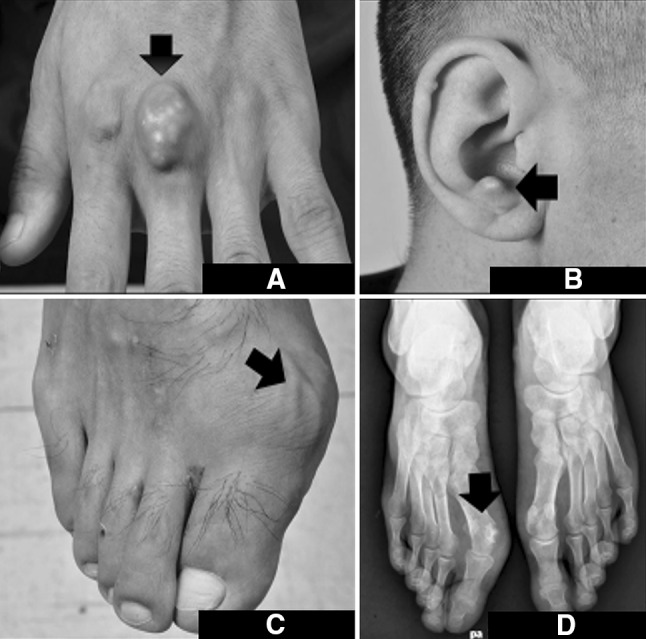
Composite figure shows tophaceous deposits at a Left 2nd metacarpophalangeal joint, b Tragus of right ear and c Right 1st metatarsophalangeal joint. d Plain X ray of the right foot shows erosion along with calcification (arrow) in the region of the left of 1st metatarsophalangeal joint
Fig. 2.
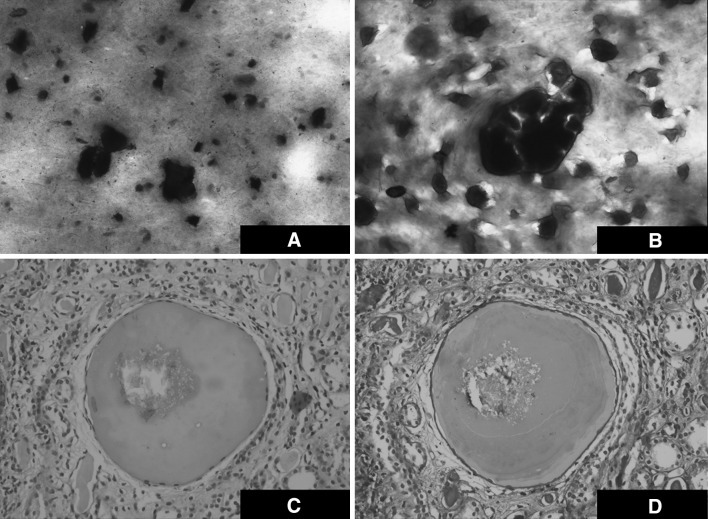
Fine needle aspirate of the tophus showing crystals a Giemsa Stain b Hematoxylin and Eosin stain. Renal biopsy showing glomerular sclerosis with evidence of chronic thrombotic Microangiopathy along with crystal deposition within the lumen of renal tubule c Hematoxylin and Eosin stain d PAS stain
Table 1.
Diagnostic criteria of CNL according to WHO 2008 classification and comparision with present case
| No. | Criterias | WHO diagnostic criteria | Our patient | |
|---|---|---|---|---|
| Values | Criteria fulfilled | |||
| 1. | Peripheral blood leukocytosis With segmented neutrophils | >25 109/l > 80 % | 45,700 83 % mature neutrophils | ✓ |
| 2. | Bone marrow biopsy | Hyper cellular with mature forms | 90–100 % cellular with mature forms | ✓ |
| 3. | Hepatosplenomegaly | To be present | 1. Hepatomegaly (3 cm below RCM) 2. Splenomegaly (12 cm below LCM) | ✓ |
| 4. | Physiologic neutrophilia | To be ruled out | Was not found | ✓ |
| 5. | BCR/ABL fusion gene | Must be absent | RQPCR for BCR-ABL (negative) | ✓ |
| 6. | PDGFRA, PDGFRB, or FGFR1 rearrangement | Must be absent | Negative | ✓ |
| 7. | Other myeloproliferative disease (PV,ET,PMF) | To be ruled out | JAK2V617F (negative) | ✓ |
| 8. | MDS or MDS/MPN syndrome | To be ruled out | No dysplasia noted | ✓ |
Fig. 3.
a. Bone marrow biopsy (×20)—hypercellular marrow spaces with panmyelosis and predominance of granulocytic series of cells. b Bone marrow biopsy (×10)—hypercellular marrow spaces (near 90–100 % cellarity). c Bone marrow aspirate (×20)—There is predominanace of mature polymorphs. The erythroid precursors, myeloid precursors are seen in adequate numbers. d LAP stain (×100 oil immersion)—High LAP score—majority of polymorphs show score of 3 or 4
The acute episode of gout was managed with indomethacin and colchicine. Once the acute episode settled, he was started on allopurinol for hyperuricemia and hydroxyurea (1500 mg/day) as a cytoreductive therapy. During evaluation, he was also found to have abnormal urinalysis with significant albuminuria (++++). 24 h urine examination was done which showed sub-nephrotic range proteinuria (2.3 g/total volume). The patient denied a kidney biopsy at the time and was discharged on the above medications.
After being lost to follow up for 4 years, during which time he continued to take hydroxyurea and allopurinol, the patient returned to seek medical care in February 2014. He did not have any new episodes of gouty arthritis in this period. General examination at this time revealed complete resolution of tophaceous swellings everywhere except the left 3rd metacarpal joint. The spleen was still palpable 4 cm below the left costal margin. Investigations were repeated which showed mild leukocytosis (14 × 109/L) with 70 % neutrophils, normal uric acid levels (6.80 mg/dL) and persistent sub-nephrotic proteinuria (3.3 g/total volume in 24 h collection). A renal biopsy was performed this time which showed glomerular sclerosis with evidence of chronic thrombotic microangiopathy (Fig. 2c, d). He was started on ramipril in addition to the other medications.
Discussion
Chronic neutrophilic leukemia is a rare MPN. As per present guidelines, diagnosis requires the exclusion of both physiological and pathological causes of neutrophilia. BCR–ABL rearrangement, JAK2 mutation, PDGFRA, PDGFRB, or FGFR1 rearrangements need to be absent to label a case as CNL. This rare disease was first reported by Tuhoy et al. in 1920 [1]. Tanzer et al. introduced the term CNL in 1964 [2]. Recently a new term “True CNL” was proposed by Reilly et al. [3]. According to this series, previous cases which were labelled as CNL actually had neutrophilia which could have been categorised under categories such as “neutrophilic predominant chronic myeloid leukemia”, “plasma cell disorder related neutrophilia” or other commoner causes of leukemoid reaction. This emphasizes the need for a diligent search for alternative diagnoses that may explain leucocytosis.
Chronic neutrophilic leukemia is more commonly seen in older patients. Two of the major studies on CNL patients were done by Reilly et al. and Bohm et al., which had 33 and 14 cases of respectively [3, 4]. The average age in these groups was 62.5 and 64.7 years with a mean survival of 30 and 14.2 months respectively. Bohm et al. reported cerebral haemorrhage as the most common cause of death in his study. The only definitive treatment option available at present is allogeneic hematopoietic stem cell transplantation. Hydroxyurea reduces both leucocyte count as well as splenomegaly however this effect is often temporary. Interferon alpha has been shown to achieve long term remission in some cases [5].
Renal complications in MPNs are seen late in the disease course. Clinically, renal involvement in MPN presents as renal insufficiency and heavy proteinuria. Histologically renal biopsy specimens may show sclerosis, mesangial hypercellularity, focal segmental sclerosis, chronic TMA or intracapillary leukemic cell infiltration [6, 7]. Studies on MPN related glomerulopathy have proposed the role of cytokines such as platelet derived growth factor and tumour growth factor beta, intracapillary platelet aggregation and microvascular blood sludging as a crucial factor in development of renal complications [6, 7].
Reports of long term follow up of such patients are rare in literature. Out of 11 patients studied by Said et al., four patients progressed to ESRD [7]. Steroids, angiotensin converting enzyme inhibitors, angiotensin receptor II blockers and mycophenolate have been tried without much success and the ultimate outcome of this subset of patients is discouraging [3].
Conclusion
Despite existing diagnostic criteria, the diagnosis of CNL remains one of exclusion with detailed evaluation to rule out alternative causes of neutrophilic leukocytosis being mandatory prior to establishing the diagnosis. CNL is a special identity due to its rarity, diagnostic challenge and limited treatment options. The unique combination of hyperuricemia as a presenting symptom and renal involvement in our patient are unusual and depict one of the unusual presentations and complications of a rare and unusual MPN.
Acknowledgments
Ethical statement and conflict of interest
On behalf of all the co-authors (listed above) I would like to state that this work has not been submitted elsewhere in any other journal. Also there is no associated conflict of interest to be disclosed. No involvement or participation of human/animals requiring ethical clearance.
References
- 1.Tuohy EL. A case of splenomegaly with polymorphonuclear neutrophil hyperleukocytosis. Am J MedSci. 1920;160:18–25. doi: 10.1097/00000441-192007000-00003. [DOI] [Google Scholar]
- 2.Tanzer J, Harel P, Boiron M, Bernard J. Cytochemical and cytogenetic findings in a case of chronic neutriphilic leukaemia of mature cell type. Lancet. 1964;1(7329):387–388. doi: 10.1016/S0140-6736(64)92142-7. [DOI] [PubMed] [Google Scholar]
- 3.Reilly JT. Chronic neutrophilic leukaemia: a distinct clinical entity? Br J Haematol. 2002;116(1):10–18. doi: 10.1046/j.1365-2141.2002.03234.x. [DOI] [PubMed] [Google Scholar]
- 4.Bohm J, Schaefer HE. Chronic neutrophilic leukaemia: 14 new cases of an uncommon myeloproliferative disease. J Clin Pathol. 2002;55(11):862–864. doi: 10.1136/jcp.55.11.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meyer S, Feremans W, Cantiniaux B, et al. Successful alpha-2b-interferon therapy for chronic neutrophilic leukaemia. Am J Hematol. 1993;43:307–309. doi: 10.1002/ajh.2830430416. [DOI] [PubMed] [Google Scholar]
- 6.Bardy A, Tiple A, Rabant M, Kemeny JL, El Karoui K, Hermet M, et al. The myeloproliferative neoplasms-related glomerulopathy. Rev Med Interne. 2014;35(4):222–230. doi: 10.1016/j.revmed.2013.04.014. [DOI] [PubMed] [Google Scholar]
- 7.Said SM, Leung N, Sethi S, Cornell LD, Fidler ME, Grande JP, et al. Myeloproliferative neoplasms cause glomerulopathy. Kidney Int. 2011;80(7):753–759. doi: 10.1038/ki.2011.147. [DOI] [PubMed] [Google Scholar]