Alpha gene number plays an important role in modulating the phenotype of thalassemia. It has been known that the coexistence of α-globin gene triplication (ααα) is an important modulator of the severity of β-thalassemia trait, exacerbating the phenotypic severity of β-thalassemia by causing more globin chain imbalance [1, 2]. Therefore characterization of α-globin gene mutations can be of great help in identification of hemoglobinopathies. Different frequencies of α-globin triplication have already been reported in many populations [3–6]. However, there has been no epidemiological study of α-globin gene triplication in Chinese. Here, we report the prevalence of α-globin triplication in a southern China population with high prevalence of β-thalassemia.
During a one-month period of June 2014, 1169 sequential cord blood samples at birth were collected from the delivery unit, Guangzhou Women and Children Medical Center, Guangdong, China. The samples were frozen and stored at −20 °C up to the time of molecular investigation. Genomic DNA was extracted from leukocytes using phenol–chloroform-based method. The multiplex Gap-PCR method described by Wang et al. was applied to screen for the presence of the αααanti3.7 and αααanti4.2 triplications [7]. For samples carrying either an αααanti3.7 or αααanti4.2 junction, multiplex ligation-dependent probe amplification (MLPA) was used to confirm the presence of extra copies of α-globin gene. MLPA can also discriminate between heterozygote and homozygote triplication. For samples with either an αααanti3.7 or αααanti4.2 junction, the two-round nested PCR strategies were also used to detect the HKαα or anti-HKαα allele according to previous studies [8, 9].
Among the 1169 cord blood samples of newborns with Chinese ethnicity, 11 were positive for αααanti3.7 junction and 9 positive for αααanti4.2 junction (Fig. 1). In the 9 samples positive for αααanti4.2, –α3.7 junction was found in 5 cases which were confirmed to carry the HKαα allele. Among the 11 cases positive for αααanti3.7, no –α4.2 junction was detected, thus excluding the presence of anti-HKαα allele. All of the 11 samples with simple αααanti3.7 triplication and 4 samples with simple αααanti4.2 triplication were also confirmed to have an extra copy of α-globin gene by MLPA (Fig. 2). Therefore the population-based prevalence of α-globin gene triplication was 0.9 % for αααanti3.7 and 0.3 % for αααanti4.2 triplication. The allelic frequencies of αααanti3.7 and αααanti4.2 triplications were 0.0045 and 0.0015, respectively.
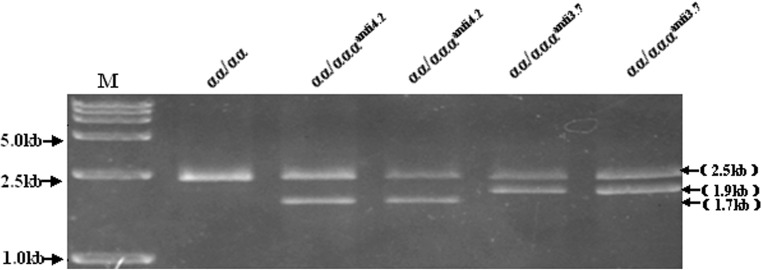
Fig. 1.
Multiplex Gap-PCR based molecular detection of α-globin gene triplications. Amplification of a 2.5-kb fragment of the LIS1 gene was included as an internal control. A 1.9-kb fragment was amplified for the anti-3.7 allele, and a 1.7-kb fragment for the anti-4.2 allele
Fig. 2.
MLPA analysis of the triplicated α cluster. The y-axis represents the ratio signal as compared to the normal control (ratio 1), on the x-axis the MLPA-probe numbers are ordered chronologically in 5′ to 3′ direction along the region. a simple αααanti3.7 allele; b simple αααanti4.2 allele; c simple HKαα allele
Triplication is a relatively common numerical change of α-globin gene. The reported frequencies of αααanti 3.7 triplication varied from 0.04 % in Sardinians, 0.36 % in American Blacks, 1 % in a multi ethnic population in The Netherland to 5 % in Greek Cypriots [3–6]. There was no data available for the prevalence of αααanti4.2 triplication, which might be due to its rare existence. In this study, we first reported the carrier frequencies of α-globin gene triplications in southern China. Corresponding to the more common of –α3.7 than –α4.2 deletion, the frequency (0.9 %) of αααanti3.7 triplication was far higher than that (0.3 %) of αααanti4.2 triplication. In total, we found a carrier frequency of 1.2 % in southern Chinese individuals for α-globin gene triplications. Since β-thalassemia is one of the most common monogenic disorders in southern China, it can be expected that the coexistence of triplicated α-globin gene and ß-thalassemia should be a cause of thalassemia intermedia in our locality [10]. Therefore the presence of triplicated α-globin genes should be considered in apparent ß-thalassemia carriers who were more anemic and symptomatic than expected. The information on the α-globin gene triplication is also of importance in terms of genetic counseling, especially in families with children affected by ß-thalassemia intermedia, while only one parent showed a picture of ß-thalassemia on hematological analysis.
We also reported a carrier frequency (0.4 %) of the HKαα allele in our population. The HKαα is an α-globin gene cluster containing both –α3.7 and αααanti4.2 junctions [8]. This allele contains an intact α2 gene and a α1–α2 fusion gene (–α3.7) with neither decreased nor enhanced α-globin gene dosage. We have confirmed that compound heterozygosity for HKαα and an in cis deletion of double α genes presents as α-thalassemia trait [11]. It can also be speculated that carriers of β-thalassemia trait are unlikely to suffer any more deteriorative effects when combined with the HKαα allele since its imbalanced α/β ratio is unchanged.
Acknowledgments
This study was supported by grants from Guangzhou Health Bureau (20121A021012; 20131A011066), Guangdong, China.
Compliance with Ethical Standards
Ethical Statement
The authors declare that they have no competing interests. The work was carried out according to the principles of the Declaration of Helsinki and approved by the ethics committee of Guangzhou Women & Children Medical Center. Informed consent was obtained from the parents.
Footnotes
Man-Yu Wu and Jian-Ying Zhou have contributed equally to this study.
References
- 1.Goossens M, Dozy AM, Embury SH, et al. Triplicated α-globin loci in humans. Proc Natl Acad Sci USA. 1980;77:518–521. doi: 10.1073/pnas.77.1.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lie-Injo LE, Herrera AR, Kan YW. Two types of triplicated alpha-globin loci in humans. Nucleic Acids Res. 1981;9:3707–3717. doi: 10.1093/nar/9.15.3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goossens M, Dozy AM, Embury SH, et al. Triplicated α-globin loci in humans. Proc Natl Acad Sci USA. 1980;77:518–521. doi: 10.1073/pnas.77.1.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peres MJ, Romao L, Carreiro H, et al. Molecular basis of α-thalassemia in Portugal. Hemoglobin. 1995;19:343–352. doi: 10.3109/03630269509005826. [DOI] [PubMed] [Google Scholar]
- 5.Giordano PC, Bakker-Verwij M, Harteveld CL. Frequency of α-globin gene triplications and their interaction with β-thalassemia mutations. Hemoglobin. 2009;33:124–131. doi: 10.1080/03630260902827684. [DOI] [PubMed] [Google Scholar]
- 6.Nadkarni A, Phanasgaonkar S, Colah R, Mohanty D, Ghosh K. Prevalence and molecular characterization of α-thalassemia syndromes among Indians. Genet Test. 2008;12:177–180. doi: 10.1089/gte.2007.0080. [DOI] [PubMed] [Google Scholar]
- 7.Wang W, Ma ES, Chan AY, et al. Single-tube multiplex-PCR screen for anti-3.7 and anti-4.2 α-globin gene triplications. Clin Chem. 2003;49:1679–1682. doi: 10.1373/49.10.1679. [DOI] [PubMed] [Google Scholar]
- 8.Wang W, Chan AY, Chan LC, et al. Unusual rearrangement of the α-globin gene cluster containing both the -α3.7 and αααanti4.2 crossover junctions: clinical diagnostic implications and possible mechanisms. Clin Chem. 2005;51:2167–2170. doi: 10.1373/clinchem.2005.054189. [DOI] [PubMed] [Google Scholar]
- 9.Shang X, Li Q, Cai R, Huang J, Wei X, Xu X. Molecular characterization and clinical presentation of HKαα and anti-HKαα alleles in southern Chinese subjects. Clin Genet. 2013;83:472–476. doi: 10.1111/cge.12021. [DOI] [PubMed] [Google Scholar]
- 10.Chen W, Zhang X, Shang X, et al. The molecular basis of beta-thalassemia intermedia in southern China: genotypic heterogeneity and phenotypic diversity. BMC Med Genet. 2010;11:31. doi: 10.1186/1471-2350-11-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu MY, Li J, Li SC, Li R, Liao C, Li DZ. Compound heterozygosity for HKαα and an in cis deletion of double α genes presents as α-thalassemia trait. Hemoglobin. 2015;39:256–259. doi: 10.3109/03630269.2015.1039026. [DOI] [PubMed] [Google Scholar]