Abstract
Systemic mastocytosis is a rare and recalcitrant disorder with nonspecific clinical features. Hence, a high index of suspicion is required. Here, we report the case of a 64 years old male presenting with chronic diarrhoea that was evaluated at different centres and treated with multiple lines of therapy. The diagnosis of aggressive systemic mastocytosis was finally clinched following a holistic work up that included a Jejunal biopsy and a laparoscopic lymph node biopsy. Treatment of this disorder is difficult, responses are transient and most patients will eventually relapse, as illustrated by this case. Cladribine, Interferon α, steroids and imatinib have limited success in the management of this disease. The role of stem cell transplant is uncertain.
Keywords: Systemic mastocytosis, Cladribine, Mast cell disorders
Introduction
Systemic mastocytosis (SM) is a clonal disorder characterised by the accumulation of abnormal mast cells in various tissues. The World Health Organisation classifies SM into seven distinct variants. Impaired organ function directly attributable to mast cell infiltration is the hallmark of aggressive systemic mastocytosis (ASM). ASM is rapidly progressive with a median survival of 3.5 years only [1]. Here we report the case of a 64 years old male presenting with chronic diarrhoea. After an extensive evaluation, the diagnosis of ASM was clinched, and he received treatment with an extended course of Cladribine. We shall also discuss the diagnosis and management of this recalcitrant disorder briefly.
Case Report
A 64-year-old male presented with low-grade fever, weight loss and diarrhoea of 9 months duration. The patient had lost over 20 kg of weight and was unable to eat due to fear of diarrhoea. Loose motions were very frequent (30–40 times/day), watery, and associated with cramping abdominal pain. The was no history of bleeding per rectum/malena, skin rashes, flushing or dizziness. He had been evaluated at two centres previously and had received multiple lines of treatment (anti-helminths, empirical anti-tubercular agents, symptomatic management). The patient was evaluated in our center after 5 months of therapy with anti-tubercular agents.
On examination, the patient was severely emaciated, pale, anicteric and had bilateral pitting pedal edema. The liver was enlarged (span 15 cm), and spleen was just palpable. Shifting dullness was noted. The patient did not have any significant lymphadenopathy or skin lesions.
Complete blood count revealed anaemia (92 g/L), thrombocytopenia (57 × 109/L) and neutropenia (1.4 × 109/L). Reticulocyte index was 1.1. Total leucocyte count was 4.2 × 109/L with 30 % lymphocytes, 22 % monocytes and 11 % eosinophils.
In addition, the patient had hypergammaglobulinemia (5.7 g/dL, polyclonal on immunofixation electrophoresis) and hypoalbuminemia (2.4 g/dL). Serum alkaline phosphatase and serum tryptase (126 μg/L, Normal-0-11.4 μg/L) were elevated. The patient underwent a computerized tomography (CT) scan of the thorax and abdomen. The CT scan revealed retroperitoneal and mesenteric lymphadenopathy (max 1.3 cm in size), splenomegaly (span 15.5 cm), ascites and edematous bowel loops. Also, the lumbar vertebrae (L1–L5, D11) had lytic lesions. Laparoscopic lymph node biopsy, bone marrow aspiration/biopsy and endoscopic biopsy from jejunum were done.
The lymph node (Fig. 1) showed preserved follicular architecture with paracortical expansion and prominent postcapillary venules in the interfollicular region. The paracortical expansion comprised of polyclonal plasma cells (both kappa and lambda positive), eosinophils and a nodular infiltrate of cells. These cells were small to medium with abundant clear cytoplasm and nuclear atypia. On immunohistochemistry (IHC), these cells were strongly positive for CD 117, CD 25 and tryptase and negative for CD20, CD3, CD5, CD10 and CD30. Jejunal biopsy (Figs. 2, 3) showed broadening and blunting of villi, with loose and focal dense infiltrates of mast cells (>30 cells/high power field).
Fig. 1.
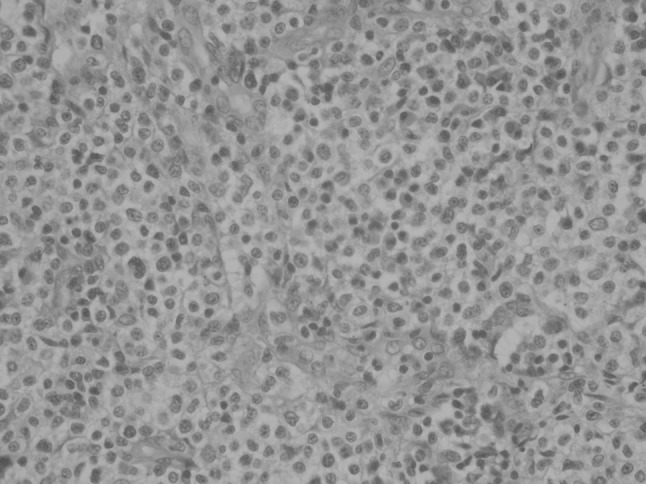
Lymph Node H&E
Fig. 2.
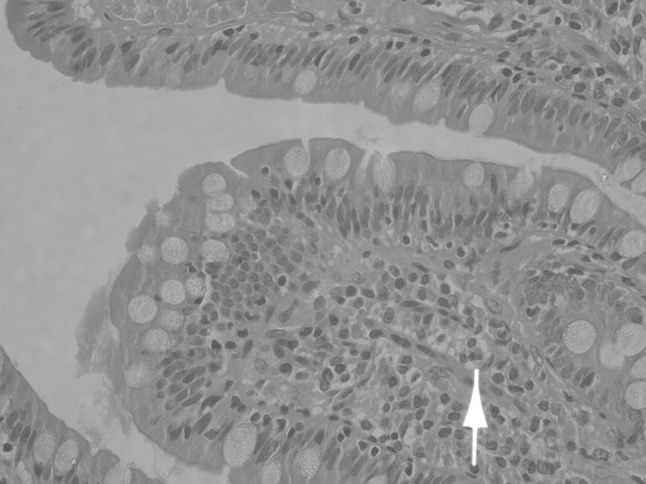
Jejunum H&E showing a mast cell infiltrate
Fig. 3.
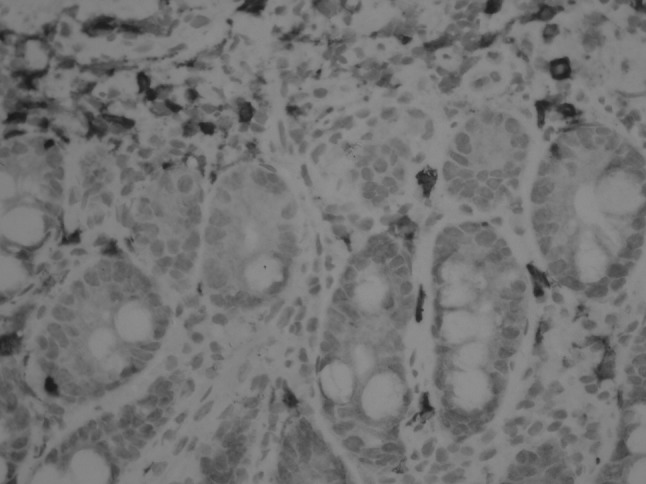
Jejunum IHC (CD117) showing a mast cell infiltrate
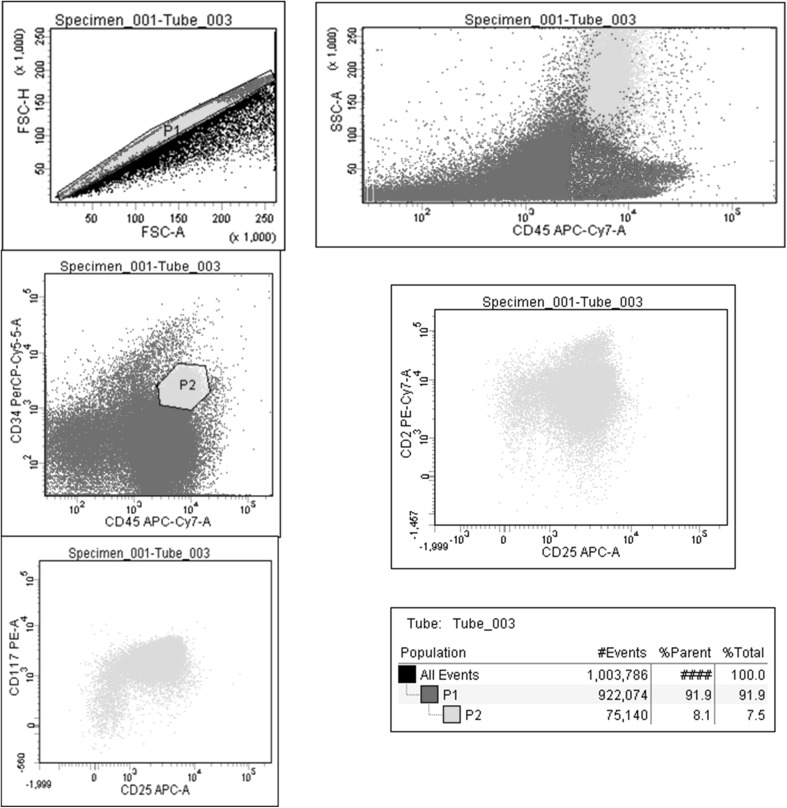
Bone marrow was hypercellular, M:E ratio was 3:1, and there was a significant increase in eosinophils. There were significant dys-granulopoiesis and dys-megakaryopoiesis, no ring sideroblasts. There was no appreciable increase in mast cells in the aspirate. Bone marrow reticulin was normal. On flow cytometry, a clonal population (8 %) of mast cells was identified. These cells positive for CD117, CD34, CD2 and CD25 (Fig. 4). The patient had a normal karyotype (46, XY), and Kit D816 V mutation was negative (on bone marrow sample).
Fig. 4.
Flow cytometry showing mast cells positive for CD25 and CD117
The patient was diagnosed as systemic mastocytosis with an associated clonal hematologic non–mast cell lineage disease (SM AHNMD—Myelodysplastic Syndrome-Refractory Cytopenia with Multilineage Dysplasia). He was initially treated with a combination of imatinib (400 mg OD), oral prednisolone and oral cromolyn sodium. The psychiatric consultation was also obtained because the severe psychological stress caused by very frequent loose motions. There was no response to imatinib and pancytopenia worsened after 4 weeks of therapy with imatinib. Hence, it was discontinued. The patient then received intravenous cladribine, 0.13 mg/kg for 5 days.
The patient had a good clinical response to cladribine with complete resolution of diarrhoea, and the patient was able to eat again without fear of diarrhoea. Following the initial course, the patient was maintained on intravenous cladribine 5 mg/m2 once/week for four doses, followed by 5 mg/m2 once every 4–6 weeks for 13 doses. Eighteen months after the first cycle of cladribine, the patient complained of nausea, vomiting, poor appetite, loose motions and weight loss. CT thorax and abdomen were done which showed signs of progressive disease (new lymph nodes in the thorax). The patient was psychologically very distressed by the chronic nature of his disease, and he did not consent to further therapy, despite exhaustive counselling by the primary team and a psychiatrist. He was referred to palliative care and expired 19 months after the first course of cladribine.
Discussion
Systemic Mastocytosis has a varied clinical presentation with symptoms attributable to either mast cell degranulation, or infiltration of organs with neoplastic mast cells. It may involve virtually any tissue and commonly involved organ systems include skin, liver, bone, intestine and bone marrow. Given the varied presentation of this disease, a high index of suspicion and a thorough and systematic workup is required to establish the diagnosis of SM.
In this present case, there was an inappropriately high index of suspicion of tuberculosis without an attempt to establish a tissue based/bacteriological diagnosis, and the initial workup was fragmented and unsystematic. Hence, hence there was a delay in establishing the diagnosis.
Establishing the diagnosis of SM requires the presence of 1 major and one minor or 3 minor criteria (Table 1). Once a diagnosis of SM is established, two important questions arise: (1) does the patient have advanced disease and (2) is there an additional hematologic non–MC-lineage neoplasm.
Table 1.
Diagnostic criteria for systemic mastocytosis
| Major | Multifocal dense infiltrates of mast cells in bone marrow or other extracutaneous organ sections (>15 mast cells aggregating) |
| Minor | >25 % mast cells in tissue sections or bone marrow aspirate smears that are spindle-shaped, immature or have atypical morphology |
| C-kit point mutation at codon 816 V detected in bone marrow, blood, or another extracutaneous organ | |
| Expression of CD2 and/or CD25 in neoplastic mast cells | |
| Baseline serum tryptase persistently >20 ng/mL (not valid in presence of another non–mast cell clonal disorder) |
The presence of advanced disease mandates treatment with cytoreductive agents. Due to the rare and heterogeneous nature of this disease, prospective data are unavailable to guide therapy. Steroids, interferon (IFN-α) and cladribine have been used with limited success. In patients who progress slowly, it is possible to keep the disease under control for months to years with INF-α. Interferon is usually started at a dose of three million units thrice a week, increased to a maximum of five million units daily [2, 3]. However, the toxicity (myalgia, flu-like symptoms) of this treatment is often a limiting factor. The addition of prednisolone may reduce the toxicity of interferon without any significant improvement in response rates. In patients who have an inadequate response to interferon or those who progress rapidly, cladribine is the recommended treatment. Cladribine is usually dosed at 0.13 mg/kg, infused over 2 h for 5 days, repeated every 4–6 weeks [4, 5]. In clinical studies, cladribine as a single agent was effective in reducing the symptoms of the disease and the tumour burden. However, complete responses are rare, and most patients will relapse with a median duration of response of 19.5 months.
In patients who do not have a Kit D816 V mutation, imatinib may be a useful therapeutic alternative. It has been found to be effective in relieving symptoms, and there is an improvement in cytopenias, organomegaly and serum tryptase levels [6]. However, most responses are transient.
The role of stem cell transplant in ASM is not clear. A pilot study of three ASM patients who underwent non-myeloablative transplant did not result in long-term remission with all patients progressing after transplant [7].
To conclude ASM is a rare and recalcitrant disorder for which the current treatment options are unsatisfactory. Treatment with cytoreductive agents like interferon and cladribine result in transient responses. However, progression is inevitable. In our patient, we attempted to prolong the period of response by administering low dose cladribine over an extended period without success.
Prospective studies are required to refine the management of this difficult disorder.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
Human and Animal Rights Statement
This article does not contain any studies with human participants or animals performed by any of the authors.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study formal consent is not required.
References
- 1.Lim KH, Tefferi A, Lasho TL, Finke C, Patnaik M, Butterfield JH, et al. Systemic mastocytosis in 342 consecutive adults: survival studies and prognostic factors. Blood. 2009;113:5727. doi: 10.1182/blood-2009-02-205237. [DOI] [PubMed] [Google Scholar]
- 2.Kluin-Nelemans HC, Jansen JH, Breukelman H, Wolthers BG, Kluin PM, Kroon HM, et al. Response to interferon alfa-2b in a patient with systemic mastocytosis. N Engl J Med. 1992;326(9):619–623. doi: 10.1056/NEJM199202273260907. [DOI] [PubMed] [Google Scholar]
- 3.Hauswirth AW, Simonitsch-Klupp I, Uffmann M, Koller E, Sperr WR, Lechner K, et al. Response to therapy with interferon alpha-2b and prednisolone in aggressive systemic mastocytosis: report of five cases and review of the literature. Leuk Res. 2004;28(3):249–257. doi: 10.1016/S0145-2126(03)00259-5. [DOI] [PubMed] [Google Scholar]
- 4.Kluin-Nelemans HC, Oldhoff JM, Van Doormaal JJ, Van ‘t Wout JW, Verhoef G, Gerrits WB, et al. Cladribine therapy for systemic mastocytosis. Blood. 2003;102(13):4270–4276. doi: 10.1182/blood-2003-05-1699. [DOI] [PubMed] [Google Scholar]
- 5.Hermine O, Hirsh I, Damaj G, Granpeix C, Barète S, Suarez F et al. (2010) Long term efficacy and safety of cladribine in adult systemic mastocytosis: a French multicenter study of 44 patients. Blood (ASH annual meeting abstracts) 116: Abstract 1982
- 6.Vega-Ruiz A, Cortes JE, Sever M, Manshouri T, Quintás-Cardama A, Luthra R, et al. Phase II study of imatinib mesylate as therapy for patients with systemic mastocytosis. Leuk Res. 2009;33(11):1481–1484. doi: 10.1016/j.leukres.2008.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakamura R, Chakrabarti S, Akin C, Robyn J, Bahceci E, Greene A, et al. A pilot study of nonmyeloablative allogeneic hematopoietic stem cell transplant for advanced systemic mastocytosis. Bone Marrow Transpl. 2006;37(4):353–358. doi: 10.1038/sj.bmt.1705245. [DOI] [PubMed] [Google Scholar]