Abstract
Hemoglobin (Hb) J-Buda [α61(E10)Lys → Asn, AAG > AAT] is a very rare α-chain variant found in South-East Asia. We analyzed hematological parameters and provided a rapid molecular analysis method for detection of this hemoglobinopathy in two Thai women who had severe microcytic anemia with Hb and MCV <70 g/L and 80 fL, respectively. The HPLC revealed an abnormal Hb peak eluted ahead of HbA at retention time of 1.91–1.98 min. On CE, the abnormal Hb peak was observed at the electrophoretic zone 12 which corresponded to Hb Bart’s. The DNA sequencing revealed the AAG → AAT mutation at codon 61 for Hb J-Buda on one allele of the α1-globin gene. The developed Allele-specific PCR (ASPCR) showed the 455 bp amplified fragment from Hb J-Buda allele. Thus, understanding of hematological characterizations and the developed ASPCR for diagnosis of Hb J-Buda are essential for genetic counseling of this hemoglobinopathy.
Keywords: Hb J-Buda, Thai female, Hemoglobinopathy, Hematological parameters, Hemoglobin analysis
To the Editor,
Over 100 genetic forms of α-thalassemia and more than 220 mutation variants of β-thalassemia have been identified in the world with many community/region-specific mutations [1–3]. Hemoglobin (Hb) J-Buda [α61(E10)Lys → Asn, AAG > AAT] is an α-globin chain variant that results from a point mutation at codon 61 of the α1-globin gene on chromosome 16p [4]. Hb J-Buda was initially discovered in a Hungarian family [5]. It was previously reported in a Thai female [4]. We reported here the hematological parameters and molecular diagnostic aspects of Hb J-Buda in two Thai females.
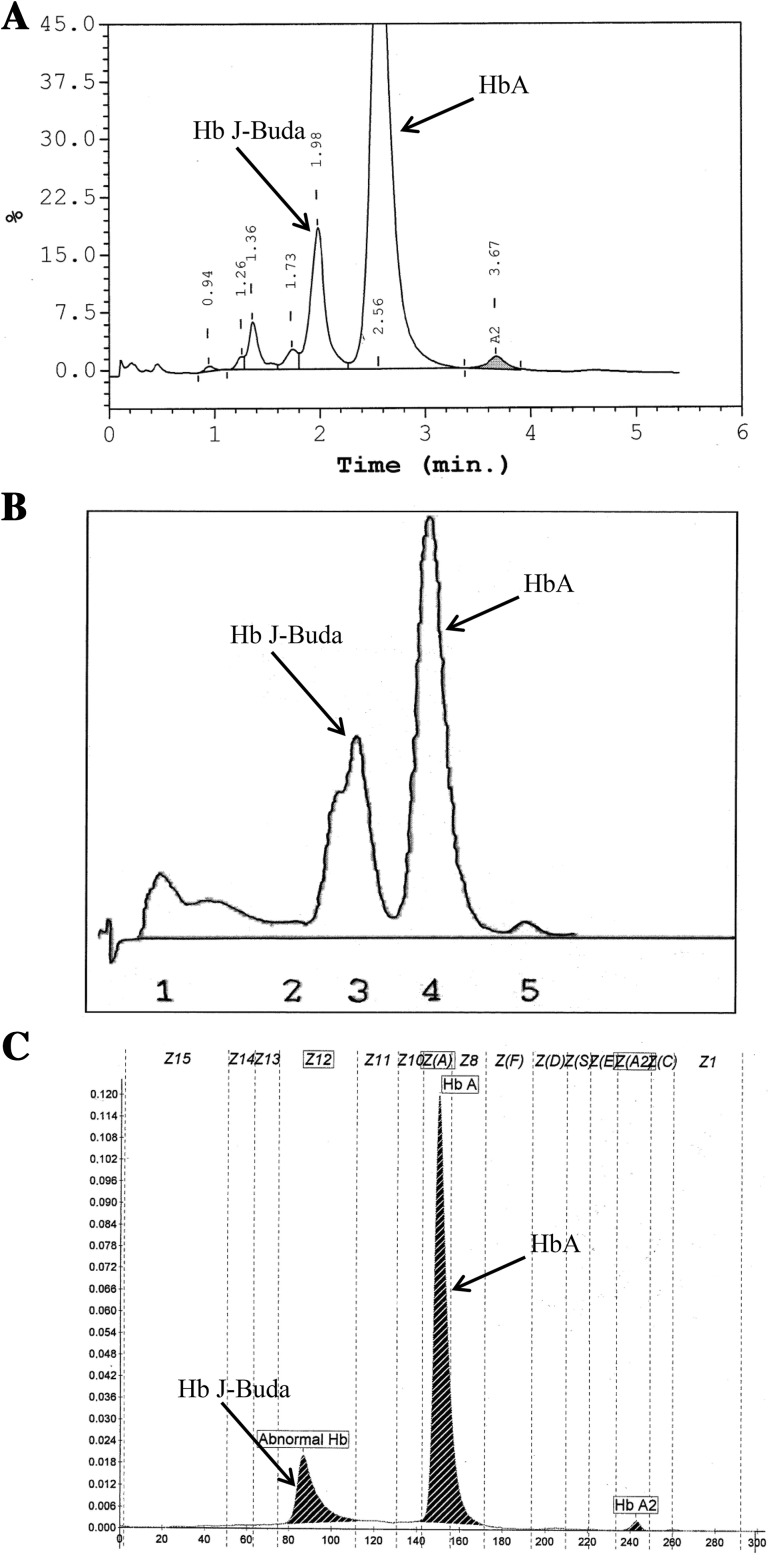
Their hematological parameters (Sysmex KX-21, Sysmex Corporation, Kobe, Japan) are shown in Table 1. Red blood cell counts (RBCs), Hb, hematocrit (Hct), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH) and mean corpuscular hemoglobin concentration (MCHC) were lower while the red cell distribution width (RDW) was higher than normal values. Their serum iron (SI) and transferrin saturation levels were reduced. Their HPLC and LPLC chromatograms showed a major peak of HbA, small peak of HbA2 and an unknown variant peak ahead of HbA with the retention time of 1.91–1.98 min on HPLC chromatogram (Fig. 1a) and 162–172 s on LPLC chromatogram (Fig. 1b). Hb analysis by capillary electrophoresis (CE) (CAPILLARYS™ 2, Sebia, Norcross, Georgia, USA) showed an abnormal Hb peak at the electrophoretic zone 12 which corresponded to Hb Bart’s (Fig. 1c). The levels of abnormal Hb, HbA, HbA2 and HbF analyzed by each method are presented in Table 1.
Table 1.
The characteristics and hematological parameters of two samples with Hb J-Buda
| Characteristics and parameters | Sample no. 1 | Sample no. 2 |
|---|---|---|
| Gender-age (years) | Female-73 | Female-72 |
| Red blood cell counts (1012/L) | 2.5 | 3.0 |
| Hemoglobin (g/L) | 50 | 62 |
| Hematocrit (L/L) | 0.16 | 0.23 |
| MCV (fL) | 64.2 | 74.0 |
| MCH (pg) | 20.1 | 20.4 |
| MCHC (g/L) | 312 | 276 |
| RDW (%) | 22.3 | 18.5 |
| Hb analysis by HPLC | ||
| Hb J-Buda (%) | 22.9 | 16.2 |
| HbA (%) | 69.7 | 75.0 |
| HbA2 (%) | 1.5 | 1.6 |
| HbF (%) | 1.5 | 0.0 |
| Hb analysis by LPLC | ||
| Hb J-Buda (%) | 20.9 | 18.0 |
| HbA (%) | 69.7 | 68.8 |
| HbA2 (%) | 1.0 | 1.2 |
| HbF (%) | 0.9 | 0.5 |
| Hb analysis by CE | ||
| Hb J-Buda (%) | 19.3 | 19.0 |
| HbA (%) | 71.7 | 79.8 |
| HbA2 (%) | 1.3 | 1.2 |
| HbF (%) | 0.0 | 0.0 |
| α-Globin genotype | ααJ-Buda/−α3.7 | ααJ-Buda/αα |
| SI (μg/dL) | 8 | 15 |
| TIBC (μg/dL) | 309 | 334 |
| Transferrin saturation (%) | 2.6 | 4.5 |
| Ferritin (ng/mL) | 25 | 45 |
Normal ranges for the measurements elaborated in the table are as follows: red blood cell counts 4.2–6.1 × 1012/L; hemoglobin 120–180 g/L; hematocrit 0.37–0.52 L/L; MCV 80–100 fL; MCH 27–31 pg; MCHC 320–360 g/L; RDW 11.5–14.5 %; HbA > 85 %; HbA2 1.5–3.5 %; HbF 0–1 %; SI 50–160 μg/dL; TIBC 240–400 μg/dL; transferrin saturation 20–55 %; ferritin 10–400 ng/mL
Fig. 1.
Representative of HPLC chromatogram (a), LPLC chromatogram (b) and CE electrophoregram (c) of sample with Hb J-Buda
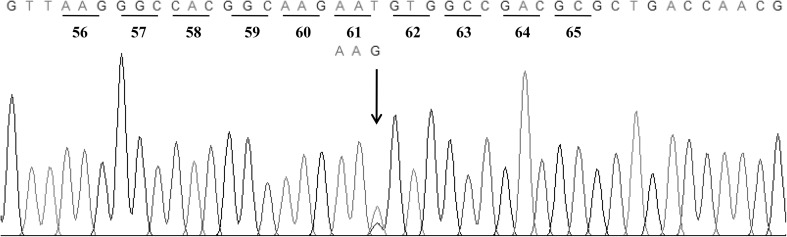
Analysis of α-globin gene by direct DNA sequencing showed a G to T transversion at the third position of codon 61 (AAG > AAT) of α1-globin gene (Fig. 2) that leads to a substitution of asparagine for lysine as described previously for Hb J-Buda [4]. The allele-specific PCR (ASPCR) for detection of Hb J-Buda was developed (Fig. 3). The Hb J-Buda specific forward primer, SP2 (5′-GTT AAG GGC CAC GGC AAG AAT-3′) was used with the reverse common primer B (5′-GAG GCC CAA GGG GCA AGA AGC AT-3′) to produce a 455 bp meanwhile the forward primer C1 (5′-TGG AGG GTG GAG ACG TCC TG-3′) and reverse common primer B were used to generate the 975 bp to serve as internal control fragment. The ASPCR mixture (50 μL) contains 50–200 ng genomic DNA, 30 pmol of primers C1 and SP2 and 60 pmol of primer B, 200 μM dNTPs and 1.5 unit Taq DNA polymerase (New England Biolabs Inc., Ipswich, MA, USA) in 10 mM Tris–HCl (pH 8.0), 50 mM KCl, 1.5 mM MgCl2 and 0.01 % gelatin. The amplification reaction was carried out on a Mycycler Thermalcycler (Cyclerus personalis, Bio-Rad, USA). After an initial heating at 94 °C for 3 min, the reaction was followed by 10 cycles of 94 °C for 30 s, 62 °C for 30 s, 68 °C for 2 min and 20 cycles of 94 °C for 30 s, 68 °C for 30 s, 72 °C for 2 min plus an additional of 20 s in every cycle. The amplified product was analyzed on 1.5 % agarose gel electrophoresis and visualized under UV light after ethidium bromide staining. The 455 bp amplified fragment from Hb J-Buda allele was observed in both samples (Fig. 3). Moreover, the multiplex Gap-PCR for detection of α-thalassemia-2 (−α3.7 and −α4.2 kb deletions) [6] was performed in these cases. The amplified fragment of 1779 bp specific for α-thalassemia-2 3.7 kb deletion was observed in the first sample (data not shown). Thus, she was diagnosed as double heterozygote for Hb J-Buda and α-thalassemia-2.
Fig. 2.
Representative of DNA sequence derived from sample with Hb J-Buda. The arrow indicates the heterozygote of Hb J-Buda nucleotide mutation in the α1-globin gene
Fig. 3.
The allele-specific PCR for identification of the Hb J-Buda. The locations and orientations of primers (SP2 and B) and (C1 and B) that produce fragments of 455 and 975 bp specific for Hb J-Buda and internal control fragment, respectively are indicated. Lane 1 Hb J-Buda carrier, lane 2 normal control, lanes 3 and 4 the samples 1 and 2, respectively. M represents the GeneRuler 100 bp DNA ladder
Accurate diagnosis of even asymptomatic hemoglobinopathies is sometimes essential for genetic counseling. The heterozygote of Hb J-Buda was reported in a Thai female [4] however a combination of this variant with other globin gene defects has not been observed previously in Thai population. Nevertheless, the Hb J-Buda has been found in association with Hb G-Pest [α74(EF3)Asp → Asn, GAC > AAC] [5, 7]. In this study, there was no peak of Hb G-Pest on HPLC and LPLC chromatograms or on a CE electrophoregram. Subjects who co-inherit Hb J-Buda and Hb G-Pest show erythrocytosis and abnormally high Hb and Hct while those are not found in subjects who are heterozygote of either Hb J-Buda or Hb G-Pest [5]. The co-inheritance of Hb J-Buda and α-thalassemia-2 3.7 kb deletion was discovered for the first time in the first sample. This co-inheritance with α-thalassemia-2 might be a cause of anemia. Hb, Hct, MCV and MCH of our cases were lower than those reported previously [4] due to concomitant iron deficiency. Therefore, hematological parameters in heterozygote of Hb J-Buda are affected by diseases. Generally, the heterozygote of Hb J-Buda has no deleterious effect on hematological parameters and could only result in a reduced positive charge of Hb molecule as compared to HbA. The reduction of positive charge of Hb molecule resulted from the replacement of lysine (Lys) by asparagine (Asn) in Hb J-Buda. Thus, Hb analysis by both HPLC and LPLC chromatograms and also on CE electrophoregram demonstrated the specifically abnormal peak of Hb J-Buda with <25 % level due to the defect in 1 of 4 α-globin genes. On CE electrophoregram, Hb J-Buda presents in the same migration time (zone 12) of Hb Bart’s. The CE separates molecules on the basis of their charge and their hydrodynamic volume [8] thus Hb J-Buda and Hb Bart’s might have the similar charge and hydrodynamic volume. Moreover, this variant has similar HPLC and LPLC patterns with Hb J Bangkok, Hb Beijing and Hb Thailand, which have been found in Thailand. Thus, there are potentially pitfall for misinterpretation. The ASPCR can, in the future, provide a rapid method for detection of Hb J-Buda.
Acknowledgments
The authors thank technicians at the Associated Medical Sciences Clinical Service Center, Associated Medical Sciences, Chiang Mai University, Chiang Mai, Thailand and the department of Medical Technology, Uttaradit hospital, Uttaradit, Thailand for their help and assistance. We are also grateful to Roscoe C. Butler Jr. and Kallayanee Rock for refinement of English language. This work was supported by a grant from University of Phayao, Phayao, Thailand.
Compliance with Ethical Standards
Conflict of interest
The authors report no conflicts of interest.
Ethical Standard
This study was approved by the Ethics Committee of the Faculty of Associated Medical Sciences, Chiang Mai University, Chiang Mai, Thailand.
Informed Consent
The informed consent was obtained from all individual participants included in the study.
References
- 1.Achoubi N, Asghar M, Saraswathy KN, Murry B. Prevalence of beta-thalassemia and hemoglobin E in two migrant populations of Manipur, North East India. Genet Test Mol Biomark. 2012;16(10):1195–1200. doi: 10.1089/gtmb.2011.0373. [DOI] [PubMed] [Google Scholar]
- 2.Giardine B, Borg J, Viennas E, Pavlidis C, Moradkhani K, Joly P, et al. Updates of the HbVar database of human hemoglobin variants and thalassemia mutations. Nucleic Acids Res. 2014;42(Database issue):D1063–D1069. doi: 10.1093/nar/gkt911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Piel FB, Weatherall DJ. The alpha-thalassemias. N Engl J Med. 2014;371(20):1908–1916. doi: 10.1056/NEJMra1404415. [DOI] [PubMed] [Google Scholar]
- 4.Itchayanan D, Svasti J, Srisomsap C, Winichagoon P, Fucharoen S. Identification of Hb J-Buda [alpha61(E10)Lys → Asn] in a Thai female. Hemoglobin. 1999;23(2):183–186. doi: 10.3109/03630269908996163. [DOI] [PubMed] [Google Scholar]
- 5.Hollan SR, Szelenyi JG, Brimhall G, Duerst M, Jones RT, Koler RD, et al. Multiple alpha chain loci for human haemoglobins: Hb J-Buda and Hb G-Pest. Nature. 1972;235(5332):47–50. doi: 10.1038/235047a0. [DOI] [PubMed] [Google Scholar]
- 6.Fucharoen S, Fucharoen G, Sanchaisuriya K, Pengjam Y. Molecular analysis of a thai beta-thalassaemia heterozygote with normal haemoglobin A2 level: implication for population screening. Ann Clin Biochem. 2002;39(1):44–49. doi: 10.1258/0004563021901720. [DOI] [PubMed] [Google Scholar]
- 7.Szelenyi JG, Horanyi M, Foldi J, Hollan SR. The unequal expression of human alpha-globin genes at the protein level. Biomed Biochim Acta. 1983;42(11–12):S187–S191. [PubMed] [Google Scholar]
- 8.Doelman CJ, Siebelder CW, Nijhof WA, Weykamp CW, Janssens J, Penders TJ. Capillary electrophoresis system for hemoglobin A1c determinations evaluated. Clin Chem. 1997;43(4):644–648. [PubMed] [Google Scholar]