Abstract
Human glucose-6-phosphate dehydrogenase deficiency (G6PD) is mostly caused by single nucleotide change in the G6PD gene which leads to single amino acid substitution. Previous trials suggested a few samples had decreased ratio of G6PD/6PGD(<1.00) but no mutation detected by multiple methods. In 138 cases of Chinese children with G6PD deficiency, RT-PCR combined with DNA Sequencing was performed to screen the mutations in the coding region and promoter region of G6PD gene. The mutation detection frequency by this method was 100 %, including a novel missense mutation (1088 A>T) and 13 mutations reported before. The novel mutation predicted an Asn-to-Ile substitution at codon 363, which was identified in a male infant patient. The variant caused by this mutation had reduced enzymatic activity, belonging to WHO Class I. Synonymous or missense mutation was not found in the proximal promoter region of the G6PD gene, which was consistent with earlier findings that G6PD deficiency was not associated with promoter mutations in the G6PD gene. RT-PCR combined with DNA Sequencing could be another alternative for clinically molecular diagnosis of G6PD deficiency.
Keywords: Glucose-6-phosphate dehydrogenase, G6PD deficiency, Gene, Mutation
To the Editor,
Glucose-6-phosphate dehydrogenase deficiency (G6PD) is a very common X-linked genetic disorder, and most deficient persons are asymptomatic. G6PD affects approximately 400 million people worldwide [1]. More than 180 mutations of G6PD deficiency have been identified, and most of these mutations are single nucleotide changes which lead to single amino acid substitutions [1]. Twenty-seven mutations within the coding region of the G6PD gene have been identified in the Chinese population [2]. Although plenty of methods were used to screen the mutations in G6PD, a few samples whose ratio of G6PD/6PGD were <1.00 had no mutation detected in previous trials [2–4]. We used RT-PCR combined with DNA sequencing to explore G6PD new mutations in the Chinese population. The results showed that mutation detection frequency by this method was 100 %, and a novel missense mutation (A1088T) was detected.
One hundred and thirty-eight children (male:female = 124:14) aged from 0.1 to 16 year with G6PD deficiency diagnosed between 2013 and 2014 were included in this study. Diagnostic testing was performed by using the Improved G6PD Nitroblue tetrazolium (NBT) Quantification Ratio Kit (Micky, Guangzhou, China), in triplicate. On the basis of the G6PD/6PGD ratio, sample which value was lower than 1.00 was identified as a deficient, and sample which value was higher than 1.00 as normal. This study was approved by the Bioethics Committee of Shenzhen Children’s Hospital and conducted in accordance with the guidelines of the Declaration of Helsinki. Informed consent was obtained from the patients and their guardians.
Total RNA and genomic DNA were isolated from peripheral blood samples by using QIAamp RNA Blood Mini Kit and FlexiGene DNA Kit (Qiagen, Hilden, Germany) respectively. The cDNA was generated with 1 µg of total RNA, random hexadeoxynucleotide primer and RAV-2 reverse transcriptase (Takara, Dalian, China). The coding region of human G6PD cDNA was amplified by PCR with primer pairs 5′-AGCGCAGACAGCGTCATG-3′/5′-GTAGTAGCAGCAGCGAGGG-3′. The PCR amplification was performed with EX-Taq polymerase and GC buffer(Takara) in a thermocycler(Biometra, Germany) for an initial denaturing cycle at 94 °C for 2 min, followed by 30 cycles (94 °C for 30 s, 59 °C for 30 s, 72 °C for 2 min), and a final extension step at 72 °C for 8 min. Genomic DNA was used to verify the novel mutation site and analyze mutations in the promoter region (from −509 bp to +26 bp). The promoter region of human G6PD DNA was amplified by PCR with primer pairs 5′-CCACGGATGGAACCCTGTC-3′/5′-CCGAAGTGTACGACCGTTTC-3′. The PCR products were then sequenced by sequencing company (Invitrogen, Guangzhou, China).
The sequencing results were analyzed with mutational database for G6PD deficiency (www.bioinf.org.uk/g6pd). The results revealed that there were 14 mutations, including 1376G>T (49), 1388G>A (40), 95A>G(17), 1024T>C (10), 392G>T (9), 871G>A (5), 563 C>T (1), 1360C>T (1), 487G>A(1), 592C>T (1), 835A>T(1), 406C>T(1), 1088A>T (1), 1376G>T and 95A>G(1) in the 138 G6PD deficiency cases. All the variants we detected were point mutations. The mutation detection frequency was 100 %. No synonymous or missense mutations were found in the promoter region.
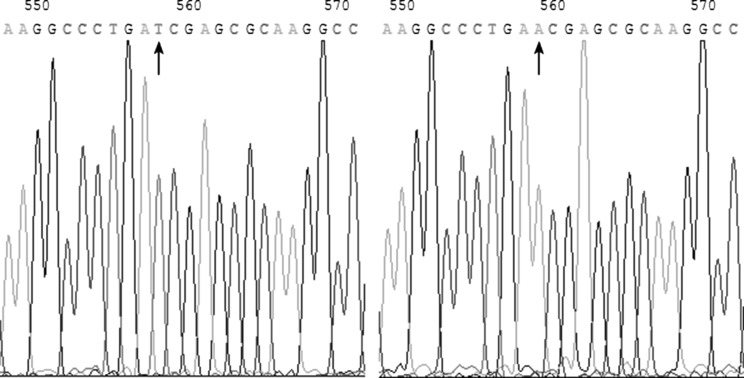
The 1088A>T that we found in this study was a novel missense mutation site which predicted an Asn-to-Ile substitution at codon 363. The 1088A>T (Fig. 1) was shown in a male infant patient aged 16-month-old. Both cDNA and DNA sequencing results showed that the other alleles were normal in the infant. The G6PD/6PGD ratio of the infant was the lowest one in all patient samples (0.37). The case history revealed that this infant with the novel G6PD variant presented because of acute hemolytic anemia (RBC 2.54 × 1012/L, Hb = 7.6 g/dl, MCV = 99.2 fl, MCH = 29.9 pg) and jaundice with a total bilirubin (TBIL) level of 1.88 mg/dl and indirect bilirubin (IBIL) level of 1.58 mg/dl. Blood smear showed irregular RBCs including teardrop cells and polychromatophils. G6PD activity of the infant was determined as 0.45 ± 0.10 IU/g Hb (mean ± 1 SD; n = 1, in triplicate) using the method recommended by the World Health Organization (WHO) [5], which was equivalent to 6.3 % of the normal activity (7.13 ± 1.92 IU/g Hb, for normal male in our laboratory). Therefore, this variant G6PD-1088T (363 Ile) belonged to Class I variant according to WHO criteria [5, 6].
Fig. 1.
The position 1088 of the coding region of G6PD cDNA (arrow) showed a T in the novel mutant by cDNA sequencing (left panel), an A in normal control subject (right panel)
The 1088 A>T mutation results in an amino acid substitution of asparagine for isoleucine at the polypeptide position 363. Isoleucine is a non-polar amino acid whereas asparagine is a polar amino acid, allowing for the possibility that this substitution may cause a conformational alteration within the enzyme molecule. On the basis of the X-ray structure of wild-type human G6PD (PDB accession code: 1H9A) [7], the analysis of amino acid mutation was carried out with the program named Swiss-PdbViewer on the level of three-dimensional structure. The result suggested that Asn > Ile amino acid substitution at codon 363 was in the dimer interface. The human G6PD enzyme has a balance between dimer and tetramer. It is indicated that a stable dimer is essential to retain activity in vivo [8]. Mutational database showed that three other missense mutations beside the nucleotide 1088 had been previously described, including 1081 G>A (361 Ala > Thr), 1082 C>T (361 Ala > Val) and 1089 G>A (363 Asn > Lys). These amino acid substitutions beside the amino acid 363 caused severe mutations (WHO Class I). G6PD Loma Linda (1089 G>A) is characterized by a mutation that is only 22 amino acids upstream from the putative NADP-binding site of the enzyme. The structure shows that point mutations causing severe deficiency predominate close to the structural NADP (+) and the dimer interface, primarily affecting the stability of the molecule. The literature further confirmed that the NADP-binding site was in a small region of exon 10 and suggested the possibility that this area was also concerned with the binding of glucose-6-P [9]. Another study suggested that the mutations in and around exons 10 and 11 cause variants of G6PD deficiency which was associated with chronic non-spherocytic haemolytic anaemia (class I). All the variants in the domain showed a striking reduction in thermal stability in vitro [1]. Therefore, analysis of amino acid mutation confirmed the variant 1088A>T (363 Ile > Asn) belonged to Class I variant.
Acknowledgments
This study was funded by Science and Technology Project (Grant Number 201201004) and Technology innovation project (Grant Number CXZZ20130320172336579) from the Science Technology and Innovation Committee of Shenzhen Municipality.
Compliance with Ethical Standards
Conflict of interest
Xiaowen Chen, Rongyu Lv, Feiqiu Wen, Yunsheng Chen and Furong Liu declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Bioethics Committee of Shenzhen Children’s Hospital and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Footnotes
Xiaowen Chen and Rongyu Lv have contributed equally to this work.
References
- 1.Minucci A, Moradkhani K, Hwang MJ, Zuppi C, Giardina B, Capoluongo E. Glucose-6-phosphate dehydrogenase (G6PD) mutations database: review of the “old” and update of the new mutations. Blood Cells Mol Dis. 2012;48:154–165. doi: 10.1016/j.bcmd.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 2.Liu WL, Li F, He ZX, Jiang HY, Ai R. Glucose-6-phosphate dehydrogenase qingzhen: identification of a novel splice mutation (IVS5-1 G > A) Pediatr Blood Cancer. 2012;58:825–826. doi: 10.1002/pbc.23345. [DOI] [PubMed] [Google Scholar]
- 3.Chen X, Yue L, Li C, Li C. A novel G473A mutation in the glucose-6-phosphate dehydrogenase gene. Pediatr Blood Cancer. 2010;55:383–385. doi: 10.1002/pbc.22517. [DOI] [PubMed] [Google Scholar]
- 4.Jiang W, Yu G, Liu P, Geng Q, Chen L, Lin Q, et al. Structure and function of glucose-6-phosphate dehydrogenase-deficient variants in Chinese population. Hum Genet. 2006;119:463–478. doi: 10.1007/s00439-005-0126-5. [DOI] [PubMed] [Google Scholar]
- 5.WHO Working Group Glucose-6-phosphate dehydrogenase deficiency. Bull WHO. 1989;67:601–611. [PMC free article] [PubMed] [Google Scholar]
- 6.Frank JE. Diagnosis and management of G6PD deficiency. Am Fam Phys. 2005;72:1277–1282. [PubMed] [Google Scholar]
- 7.Naylor CE, Gover S, Basak AK. NADP+ and NAD+ binding to the dual coenzyme specific enzyme Leuconostoc mesenteroides glucose 6-phosphate dehydrogenase: different interdomain hinge angles are seen in different binary and ternary complexes. Acta Crystallogr D Biol Crystallogr. 2001;57:635–648. doi: 10.1107/S0907444901003420. [DOI] [PubMed] [Google Scholar]
- 8.Au SWN, Gover S, Lam VMS, Adams MJ. Human glucose-6-phosphate dehydrogenase: the crystal structure reveals a structural NADP+ molecule and provides insights into enzyme deficiency. Structure. 2000;8:293–303. doi: 10.1016/S0969-2126(00)00104-0. [DOI] [PubMed] [Google Scholar]
- 9.Beutler E, Kuhl W, Gelbart T, Forman L. DNA sequence abnormalities of human glucose-6-phosphate dehydrogenase variants. J Biol Chem. 1991;266:4145–4150. [PubMed] [Google Scholar]